Fritillaria taipaiensis P. Y. Li is a perennial herb belonging to the lily family. It has a bitter taste and a slightly cold nature. It is a traditional Chinese medicinal material used to clear heat and nourish the lungs, relieve cough and phlegm, as well as reduce swelling and discharge pus (Xu et al., 2019). Wild Fritillaria resources have become increasingly scarce with a recent increase in market demand F. taipaiensis P. Y. Li is a new species of Fritillaria (Qu et al., 2022), and hence its artificial cultivation is also the most successful at present. This plant has become a well-known medicinal material that meets the market demand for Fritillaria. The artificial cultivation technology has become mature after 40 years of development (Lang et al., 2024). Although the altitude range suitable for planting has remarkably expanded after the successful introduction of F. taipaiensis P. Y. Li, the cultivation environment remains limited (Liu et al., 2022). It is worth noting that the growth and development, nutrient accumulation, and medicinal quality of F. taipaiensis P. Y. Li are influenced by various soil elements and conditions, including potential phosphorus deficiency. Therefore, in-depth research is research is required to improve yield and quality, thereby satisfying the ever-growing demand for traditional Chinese medicine and high-quality F. taipaiensis P. Y. Li.
Phosphorus is an element essential for plant growth. It is involved in synthesizing many crucial organic compounds, including enzymes and coenzymes, and can promote photosynthesis and accelerate root development (Lambers et al., 2022). However, plants can absorb less than 3% of the soil’s phosphorus, and almost half of the arable land in China is deficient in phosphorus. The phosphorus fertilizers can only increase the amount of plant-absorbed phosphorus to some extent because phosphorus easily forms phosphates, which are difficult to dissolve in the soil. Hence, it is an ineffective form of phosphorus that plants cannot easily absorb (Ameen et al., 2019; Silva et al., 2023). This may also lead to soil erosion, soil compaction, and other problems, thereby severely damaging the ecological environment (Ye, 2024). Phosphorus-solubilizing fungi can convert soluble or insoluble phosphate in soil into organic phosphorus compounds, which plants easily absorb. These fungi reduce the soil pH by producing acetic acid, lactic acid, malic acid, and other organic acids, so that phosphate can be dissolved under acidic conditions and soil phosphorus can be activated. Therefore, the application of phosphorus-solubilizing fungi has become a critical method of overcoming the shortage of phosphorus in plants or its incomplete absorption by plants. When applied, these fungi can promote plant growth and development and accelerate the production of beneficial active substances in plants. Nowadays, the influence of rhizosphere microorganisms on plant growth is frequently explored (Saeed et al., 2021; Yuan et al., 2022; Wu et al., 2024). Investigating rhizosphere microorganisms is also markedly significant for biofertilizer development because it improves the soil environment and reduces chemical fertilizer-induced pollution (Niu et al., 2021; Zhang et al., 2024). At present, phosphorus-solubilizing bacteria are more widely used in crop production than phosphorus-solubilizing fungi. Ceci et al. (2018) reported that many bacteria lose their phosphorus-solubilizing ability during their isolation and purification, but fungi maintain their phosphorus-solubilizing effect. However, this phosphorus-enhancing mechanism of fungi and the interaction between beneficial fungi isolated from F. taipaiensis P. Y. Li rhizosphere soil and the plant itself have remained largely unexplored.
In this study, the effect of phosphorus-solubilizing fungi on the growth of F. taipaiensis P. Y. Li was explored. Different phosphorus-solubilizing fungal strains or their combinations were applied to F. taipaiensis P. Y. Li. The contents of photosynthetic pigments, various protective enzymes, polysaccharides, and proteins were considered indicators of the plant growth status. Therefore, we explored the effects of the applied fungi on the growth and development, photosynthesis, and antioxidant activities of F. taipaiensis P. Y. Li. The optimal strains or flora were selected through comparative analysis, which served as an effective reference value for the cultivation technology of F. taipaiensis P. Y. Li and biofertilizer development.
2 Materials and methods2.1 Test materialsThe bulbils of F. taipaiensis F. Y. Li used in this experiment were provided by the F. taipaiensis F. Y. Li Planting Base located in Hongchiba, Wuxi County, Chongqing Municipality (N31°36′26.07″, E108°49′18.75″), and were identified as four-year-old bulbils of F. taipaiensis F. Y. Li (belonging to the Liliaceae family) by Professor Zhou Nong of Chongqing Three Gorges University. These bulbils underwent a rigorous screening process to ensure uniformity in size and quality. The cultivation experiments were conducted between 2022 and 2023 at the F. taipaiensis F. Y. Li Planting Base within the Hongchiba Scenic Area. The test strains of phosphorus-solubilizing fungi used in this study were ESA3 (Aspergillus tubingensis), GYB1 (Aspergillus niger), and TBXB2 (Aspergillus nigerfunigatus). These strains were rigorously screened from 29 strains of phosphorus-solubilizing fungi isolated from the rhizosphere soil of F. taipaiensis P. Y. Li collected from ten different locations in China. The screening process first involved the use of the phosphorus-solubilizing ring method for initial selection, followed by the molybdenum-antimony resistance colorimetric method to further identify highly efficient phosphorus-solubilizing fungi. Ultimately, the three dominant strains were determined based on colony morphology and the amplified ITS sequence results. The test soil comprising yellow loam, river sand, and organic fertilizer (2:1:1) was obtained from the Chongqing Three Gorges University. The soil was sieved with a 2 mm sieve, sterilized at 121°C for 2 h, and left for 7 days before use.
2.2 Experimental designThe study was conducted in October 2022 at the Hongchiba F. taipaiensis P. Y. Li Planting Base in Wuxi County, Chongqing Municipality. The plants were potted in plastic flowerpots (diameter: 15 cm; height: 18 cm), which were wiped three times with 75% ethanol solution before planting. Seven treatment groups, namely S1, S2, S3, S4, S5, S6, and S7, and a control group (CK) were established in the experiment. The soil in the CK group was subjected to high-temperature sterilization without any fungal treatment. Ten replicates were established in each treatment group, and approximately 5 F. taipaiensis P. Y. Li plants were planted in each pot. The plants were placed in the same area to ensure that all the test samples were exposed to the same elevation, light, temperature, and humidity. In order to ensure the robustness and statistical significance of the experimental results, the fungal treatment solutions were applied twice in March and April of 2023, respectively. Each pot received 100 mL of fungal suspension of concentration 2 × 108 CFU/mL, whereas the control group received an equal amount of sterile water. During planting, the plants were maintained according to the conventional management method used for F. taipaiensis P. Y. Li. The inoculation methods were single plant, mixed two plants, and mixed three plants. A random grouping design was used to allocate plants to different inoculation methods, ensuring that any environmental or other confounding factors were evenly distributed between groups. Each treatment group had 10 biological replicates. Table 1 presents the specific inoculation methods.
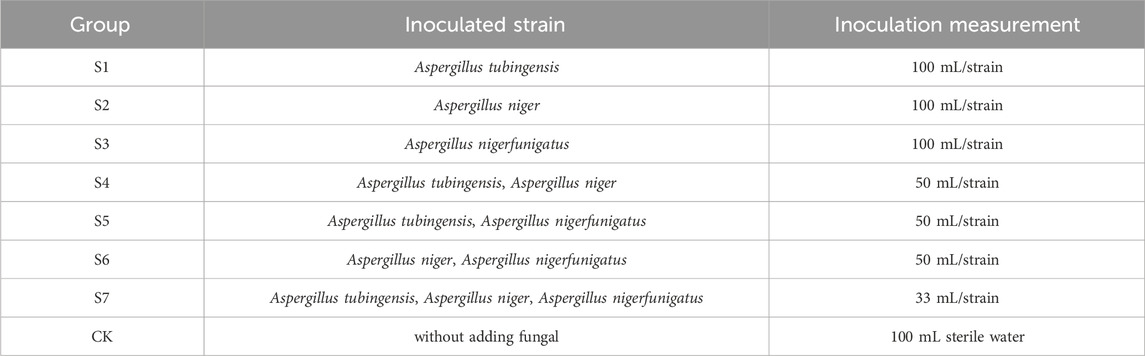
Table 1. Inoculated strains and inoculated amounts in different treatment groups.
2.3 Index measurement2.3.1 Measurement of growth indexes and photosynthetic parametersTo gather experimental data, in June 2023 during sunny weather, we randomly selected six plants from each parallel group and measured the growth index for each plant pot. The plant height was measured using a tape measure, and the plant height from its top to the soil matrix interface was used as the measurement standard. The root was measured using a tape measure, and the length from the root base to the longest root was established as the measurement standard. A leaf from each plant was randomly selected for measurement. The leaf area was measured using a portable laser leaf area scanner (CI-203, CID Bio-Science, Inc.) after completely unfolding the leaf. Four photosynthetic parameters, namely net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr), were evaluated using a photosynthesis analyzer (LI-6400, LI-COR, Inc., United States) between 11 a.m. and 1 p.m. when the stomatal opening of the leaves was the greatest, following the method outlined by Wu et al. (2021).
2.3.2 Determination of the contents of leaf photosynthetic pigmentsIn this experiment, six potted plants under sunny weather conditions were selected from each parallel group, and the growth index of one plant in each pot was measured. We applied the method proposed by Croft et al. (2017) to extract the chlorophyll from F. taipaiensis P. Y. Li leaves using a mixture containing certain proportions of acetone, water, and anhydrous ethanol. The absorbance value at a specified wavelength was determined using an ultraviolet spectrophotometer (UV-2450, Shimadzu Group, Japan). Also, chlorophyll a, chlorophyll b, and carotenoid contents were calculated.
2.3.3 Measurement of physiological and biochemical indexesIn this experiment, six potted plants under sunny weather conditions were selected from each parallel group, and the growth index of one plant in each pot was measured. The enzymatic activities within the leaf’s protective enzyme system were determined following the methodology described by Hayashi and Palmgren (2021). The peroxidase (POD) activity was measured using the guaiacol method, where an increase of 0.01 in absorbance at 470 nm per minute was considered as one unit of enzyme activity. The catalase (CAT) activity was measured via ultraviolet (UV) spectrophotometry, defining a unit of enzyme activity as a 0.1 decrease in absorbance at 240 nm (A240 nm). The superoxide dismutase (SOD) activity was determined by the nitro blue tetrazolium (NBT) photochemical reduction method, defining one unit of enzyme activity as 50% inhibition of NBT photochemical reduction. Additionally, malondialdehyde (MDA) and soluble sugar contents were assessed using the thiobarbituric acid colorimetric method. The soluble protein content was determined using the Thomas Brilliant Blue method, and free proline content was quantified by UV spectrophotometry. The soluble protein content was determined using the Coomassie brilliant blue method (Shams et al., 2024). The free proline content was determined using the UV method. The SOD activity was determined using the nitrogen blue tetrazole photochemical reduction method (Fernández-Marín et al., 2018; Shams et al., 2023). The POD activity was determined using the guaiacol method. The CAT activity was determined through UV spectrophotometry.
2.3.4 Differential analysis of protective enzyme system–related gene expression in Fritillaria taipaiensis P. Y. Li leavesIn this experiment, the TRIzol Plus RNA Purification Kit (Thermo Fisher Scientific, United States) and RNase-Free DNase Set (QIAGEN, Germany) reagents were used to isolate and purify RNA from F. taipaiensis P. Y. Li leaves through repeated high-speed freezing centrifugation. The RNA content, purity, and quality were determined through UV spectrophotometry and electrophoresis, with an optical density value (A260/A280) requirement of 1.8–2.1. Then, the RNA was reverse transcribed into first-strand cDNA using a reverse transcription kit (Thermo Fisher Scientific, United States), and the obtained cDNA was stored at −20°C for later use. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed on a real-time fluorescence quantitative instrument (CFX96, Bio-Rad, United States) using Taq Pro Universal SYBR qPCR Master Mix (Novogene, Wuhan, China) following the manufacturer’s protocols. The primer sequences designed for the highly specific fragment of the target genes, which could avoid the nonspecific amplification, are listed in Table 2. The fluorescence quantitative reaction system comprised 10 µL of 2 × Taq Pro Universal SYBR qPCR Master Mix, 1 μL of cDNA template, 0.4 μL each of forward and reverse primers, and an appropriate amount of water, with a total volume of 20 μL. The reaction conditions were as follows: 95°C for 2 min, 95°C for 20 s, and 58°C for 20 s, for 39 cycles. A melting curve was also plotted, and three biological replicates were set. Finally, the relative gene expression levels were calculated using the 2−△△Ct method using rpl16 as the reference gene (Adnan et al., 2011).
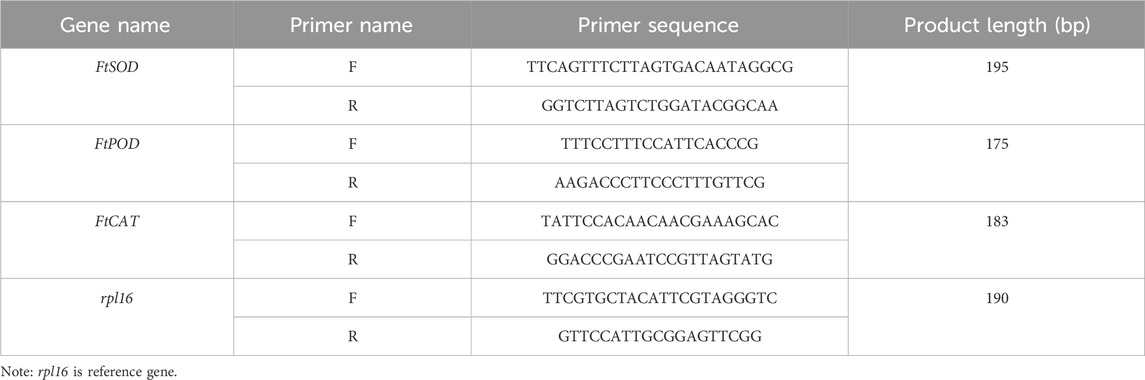
Table 2. Real-time PCR primers and conditions.
2.4 Data processingAll data and statistical analyses were performed using Microsoft Excel (Microsoft Office Professional 2021; version 2,407 Build 16.0.17830.20166) and SPSS statistical software (IBM SPSS Statistics for Windows, version 23.0). The Origin 2023 software package (OriginLab Corporation, United States) was used for plotting and visualization.
3 Results3.1 Effects of phosphorus-solubilizing fungi on the growth index of Fritillaria taipaiensis P. Y. LiThe growth index of F. taipaiensis P. Y. Li inoculated with different phosphorus-solubilizing fungi is shown in Table 3. The growth index in almost all treatment groups was significantly higher than that in the CK group. The stem thickness and plant height in the S5 group were the highest, which were 58.23% and 62.49% higher than those in the CK group, respectively. The two growth indexes in the S1, S2, and S6 groups were at the same level, but lower than those in the S5 group. The leaf thickness in the S1 group was the highest, 17.62% higher than that in the CK group. The leaf thickness in the S2 group was at the same level as that in the CK group. The leaf area in the S7 group was the largest, which was 141.34% higher than that in the CK group. The leaf area in the S5 group was 136.71% higher than that in the CK group. No significant difference was found between the S7 group and the S7 group. The leaf area in the S1 group was the smallest, which was only 44.49% higher than that in the CK group.
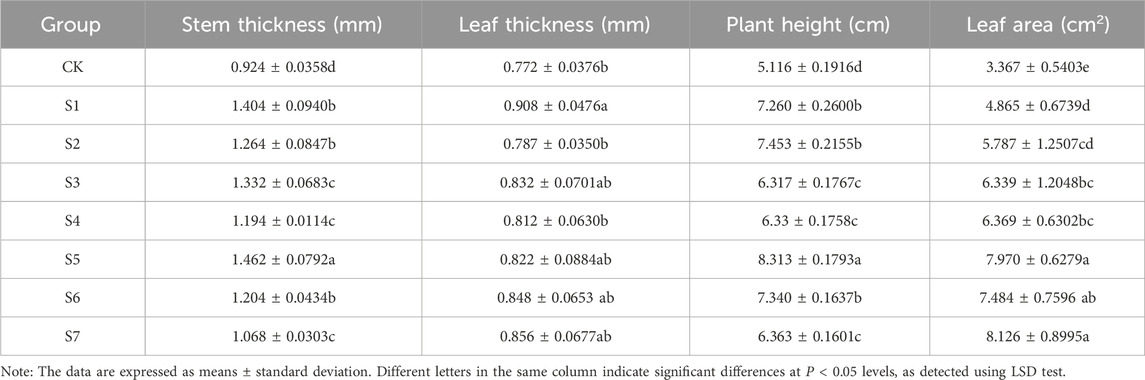
Table 3. Growth indexes of Fritillaria taipaiensis P. Y. Li plants under different treatments (±sd, n = 10).
3.2 Effects of phosphorus-solubilizing fungi on leaf area and photosynthetic parameters of Fritillaria taipaiensis P. Y. LiTable 4 presents the effects of the inoculation of different phosphorus-solubilizing fungi on the leaf area and photosynthetic parameters of F. taipaiensis P. Y. Li. The leaf area in the treatment groups was significantly higher than that in the CK group (P < 0.05). The leaf area of F. taipaiensis P. Y. Li inoculated with different fungal strains exhibited significant changes. The average middle lobe area in the S7 group inoculated with a mixture of the three strains was the highest, 155.53% higher than that in the CK group. In contrast, the leaf area in the S1 group was the smallest and was only 52.99% higher than that in the CK group. Inoculation with different phosphorus-solubilizing fungi exerted significant effects on plant photosynthesis in the same habitat.
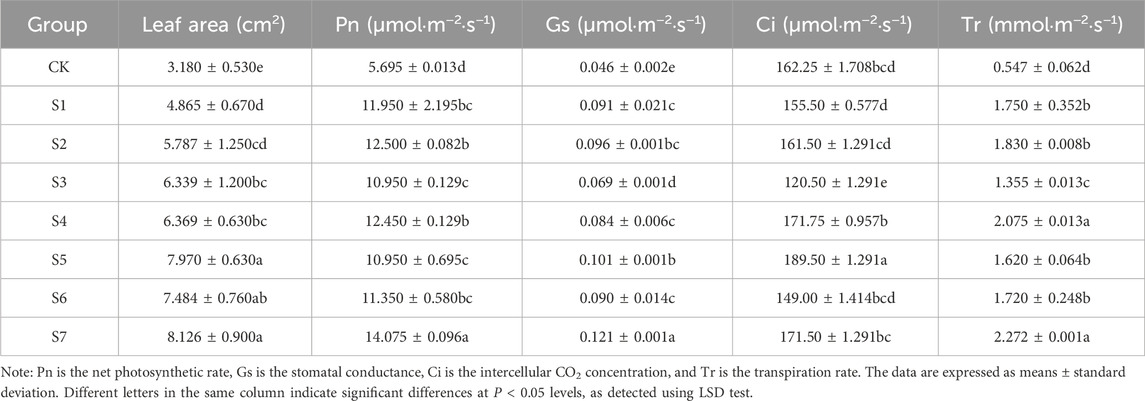
Table 4. Leaf area and photosynthetic parameters index measurement of Fritillaria taipaiensis P. Y. Li (±sd, n = 10).
Except for Ci, the average values of the other parameters were higher in the treatment groups than in the CK group. The Pn and Tr in the S7 group were the highest. They were 147.15% and 315.36% higher in the S7 group than in the CK group, respectively. The increase in Gs in the S5 group was the highest, 119.57% higher than that in the CK group. Other than the three single fungus groups S1, S2, and S3, all other mixed fungi groups displayed improved Ci value of the F. taipaiensis P. Y. Li leaves. The most obvious improvement was noted in the S5 group, which was 16.8% higher than that in the CK group. All groups displayed significant differences.
3.3 Effects of inoculation with different phosphorus-solubilizing fungi on photosynthetic pigment content in leavesAs shown in Table 5, the photosynthetic pigment content of leaves in some treatment groups was significantly higher than that in the CK group. The chlorophyll content was the highest in the S6 group, followed by the S3 and S7 groups. The chlorophyll content in the S6 group was 16.8% higher than that in the CK group. The chlorophyll b content in the S3 group was the highest, followed by the S6 group. It was 29.4% and 14.7% higher in the S3 and S6 groups, respectively, than in the CK group. The carotenoid content increased most significantly in the S1 group, followed by the S3 and S2 groups. The single fungus treatment groups exhibited a greater increase in the carotenoid content compared with the combined fungi treatment groups.
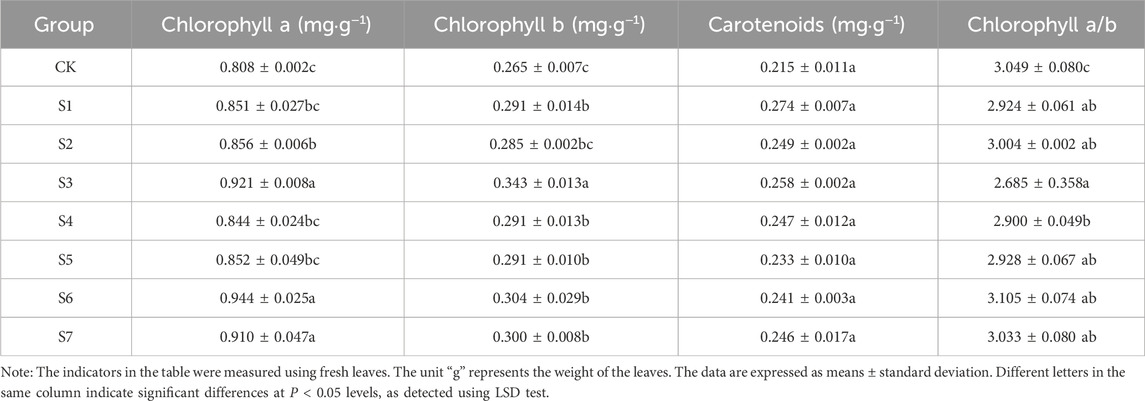
Table 5. Determination of photosynthetic pigment content in Fritillaria taipaiensis P. Y. Li (±sd, n = 10).
3.4 Effects of phosphorus-solubilizing fungi on MDA, soluble sugar, soluble protein, and free proline contents in Fritillaria taipaiensis P. Y. LiThe inoculation with phosphorus-solubilizing fungi significantly affected the MDA content in F. taipaiensis P. Y. Li (P < 0.05). The MDA content in all treatment groups was lower than that in the CK group. The content exhibited the largest reduction in the S7 group, followed by the S5 group. It was 44.83% and 37.93% lower in the S7 and S5 groups than in the CK group, respectively (Table 6).
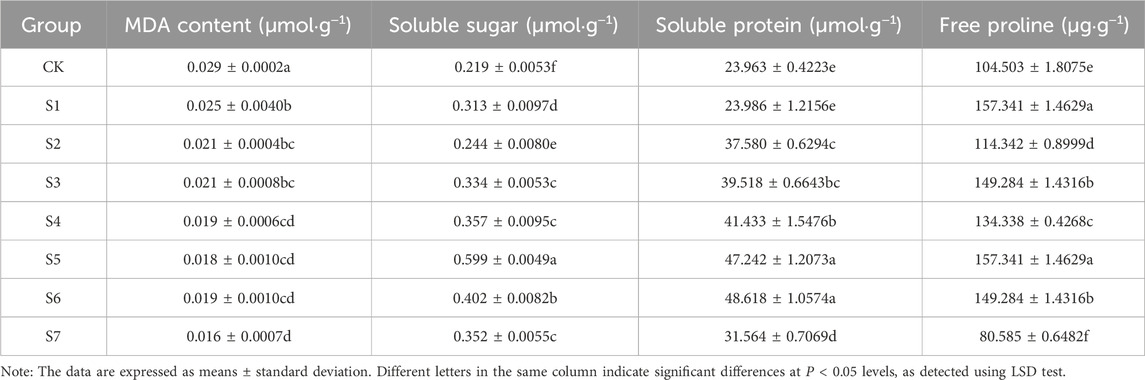
Table 6. Determination of MDA, soluble sugar, soluble protein and free proline in the leaves of Fritillaria taipaiensis P. Y. Li (±sd, n = 10).
The inoculation with phosphorus-solubilizing fungi also significantly improved the soluble sugar and protein contents in F. taipaiensis P. Y. Li (Table 5). All treatment groups exhibited an increase in these contents compared with the CK group. The soluble sugar content was 173.52% in the S5 group compared with the CK group. The increase in the soluble protein content was the largest in the S6 group, followed by the S5 group. This increase in the S6 group was 102.89% higher than that in the CK group. The weakest improvement effect was noted in the S1 and S2 groups. The soluble protein content in the S1 group was almost the same as that in the CK group.
The inoculation with different phosphorus-solubilizing fungi significantly affected the free proline content in F. taipaiensis P. Y. Li (P < 0.05). The free proline content in the treatment groups, except for the S7 group, was higher than that in the CK group. The S5 group had the most obvious improvement effect. The proline content in the S5 group was 52.84% higher than that in the CK group, followed by the S6 and S4 groups. The proline content increased by 42.85% and 28.55%, respectively, in the S6 and S4 groups compared with the CK group. The free proline content was 22.89% lower in the S7 group than in the CK group. This was speculated to be caused by the antagonism between A. niger and the other two fungi, which led to a decrease in the free proline content rather than an increase. Based on the aforementioned findings on soluble sugar and protein contents, we could reasonably infer that the effects of the three phosphorus-solubilizing fungi used individually were lower than those of the combined strains. This was because when the fungi worked together, it led to antagonism.
3.5 Effects of phosphorus-solubilizing fungi on the protective enzyme activity of Fritillaria taipaiensis P. Y. LiTable 7 shows the effects of the inoculation with phosphorus-solubilizing fungi on the activities of various protective enzymes of F. taipaiensis P. Y. Li. The protective enzyme activity values were higher in all treatment groups compared with the CK group. The enzyme activity was higher in most combined fungi treatment groups than in the single fungus treatment group. On analyzing all the measured data, we noted that the SOD activity enhancement effect in the S5 group was the best, followed by the S3 group. This effect was approximately 159.06% and 136.53% higher in the S5 and S3 groups than in the CK group, respectively. The POD enhancement effect in the S3 group was the best, which was approximately 86.00% higher than that in the CK group, followed by the S5 and S6 groups, which exerted the same enhancement effect. The POD activity enhancement effect did not increase but decreased in the S7 group and was 56.98% lower than that in the CK group. The CAT activity enhancement effect in the S6 group was the most obvious, which was 228.48% higher than that in the CK group, followed by the S5 and S7 groups. The three different combinations of strains more significantly improved the protective enzyme activity. The S3 group exerted a better promoting effect than the other two groups of single fungus. Among these, the S7 group reduced the POD enzyme activity. Thus, we speculated that A. niger and A. nigerfunigatus had a certain degree of antagonism, which also explained why the enhancement effect on the SOD and POD enzyme activities was not as expected in the S6 group.
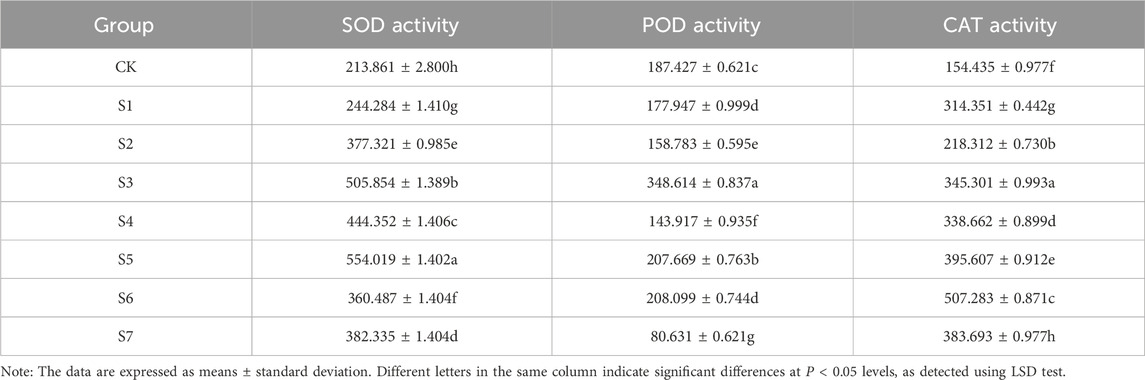
Table 7. Protective enzyme system of Fritillaria taipaiensis P. Y. Li (±sd, n = 10).
3.6 RT-qPCR analysis of genes related to the protective enzyme system of Fritillaria taipaiensis P. Y. Li treated with phosphorus-solubilizing fungiRT-qPCR was performed to determine the gene expression of three crucial protective enzymes in F. taipaiensis P. Y. Li. The gene expression levels of FtSOD, FtPOD, and FtCAT increased in all treatment groups compared with the control group. The gene expression of the three enzymes was higher in most combined strain groups than in the single-strain treatment groups. The FtSOD, FtPOD, and FtCAT activities increased 8.67, 7.65, and 6.08 times in the S5 group compared with the CK group, respectively. As shown in Figure 1, the effect on the gene expression levels was the best in the S5 group, followed by the combination of the S4 and S6 groups. FtPOD and FtCAT gene expression was higher, but the FtSOD gene expression was lower, in the S6 group than in the S4 group. The S7 group exhibited the lowest expression of genes related to the three enzymes. The FtSOD and FtPOD expression was lower in the S7 group than in the S3 single-strain treatment group.
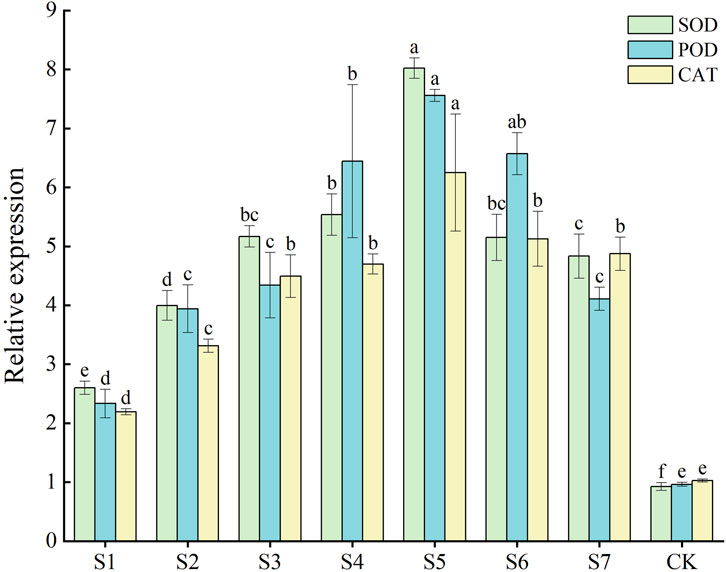
Figure 1. FtSOD, FtPOD, and FtCAT genes related to the protective enzyme system in leaves of Fritillaria taipaiensis P. Y. Li.
3.7 Correlation analysisIn this study, the correlation analysis was performed on the photosynthetic parameters (Pn, Gs, Ci, and Tr), MDA, soluble sugar, soluble protein, and free proline contents, the activity index of the three protective enzymes, and the relative gene expression levels of the three protective enzymes (Figure 2). As shown in Figure 2, the FtCAT gene expression was highly positively correlated with FtSOD (r = 0.97, P < 0.01) and FtPOD (r = 0.93, P < 0.01) genes expression, but highly negatively correlated with the MDA content (r = 0.92, P < 0.01). The FtPOD gene expression was highly positively correlated with the soluble protein content (r = 0.94, P < 0.01) and significantly positively correlated with the soluble sugar content (r = 0.82, P < 0.05). The FtSOD gene expression was highly positively correlated with the soluble sugar content (r = 0.87, P < 0.01) and soluble protein content (r = 0.85, P < 0.05). The MDA content was negatively correlated with Pn (r = 0.80, P < 0.05) and Gs (r = 0.78, P < 0.05).
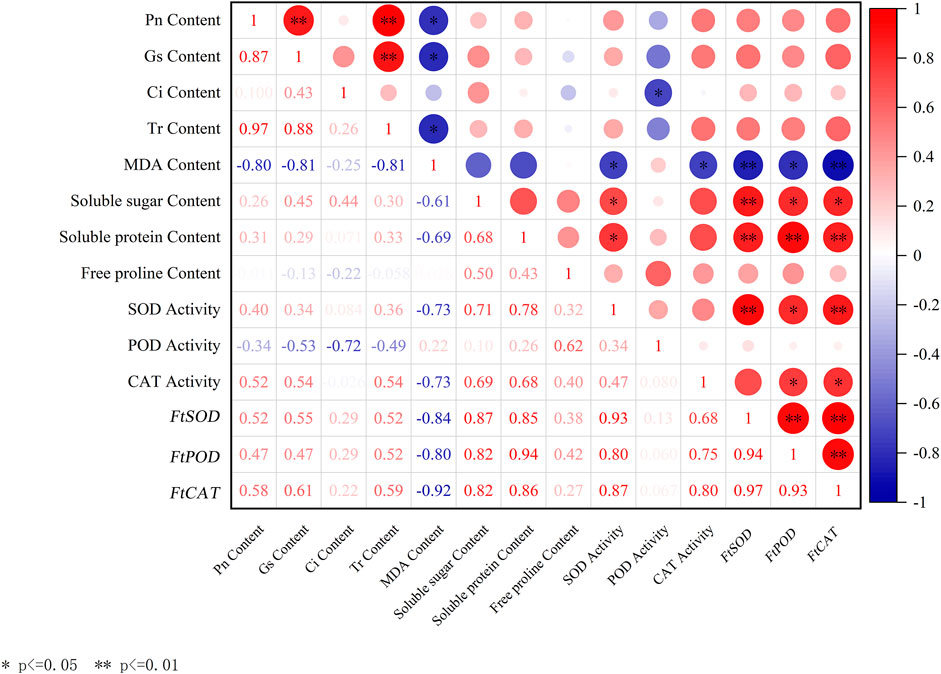
Figure 2. Correlation analysis between different indicators. Red in the figure indicates positive correlation, blue indicates negative correlation, and numbers indicate correlation coefficients.
4 DiscussionPhosphorus-solubilizing fungi are rhizosphere growth-promoting strains that can convert the phosphorus in the soil, which is difficult to dissolve, into a form that plants can absorb and use. The growth condition, oxidation resistance, stress resistance, and metabolism of plants can be improved by enhancing the phosphorus absorption capacity of plants. At the same time, fungi augment the soil structure and inhibit soil impoverishment (El-Maraghy et al., 2021; Iftikhar et al., 2024). In practice, phosphorus-solubilizing fungi applied for fertilizer production can improve the fertilizer utilization rate and alleviate environmental pollution caused by the excessive use of chemical fertilizers, which is also the practical significance of this study.
In this study, the leaf photosynthetic index of F. taipaiensis P. Y. Li was analyzed. Photosynthesis is the main metabolic pathway through which plants produce energy (Li et al., 2023). If photosynthesis is stronger, more organic matter accumulates in plants, promoting their growth. Plant photosynthesis occurs in the leaves. The leaf area size directly determines the light absorption area of the plant. The leaf area measurement can intuitively reflect plant growth (Lopez et al., 2024). In the present study, the leaf area was significantly higher in the treatment groups than in the control group. The leaf area in the S7 group was the largest, proving that phosphorus-solubilizing fungi significantly affected the growth of F. taipaiensis P. Y. Li. The photosynthetic pigment content was low in the S5 group, and the leaf area was relatively small. Pn, Gs, Ci, and Tr are also closely related to plant photosynthesis (Baruah et al., 2016; Ma et al., 2021; Guo et al., 2023; Ma et al., 2023). In this study, Ci was lower in some treatment groups than in the CK group. Under normal circumstances, the increase in Ci leads to stomatal closure and weakens transpiration because of stomatal limitation, reducing Gs and Tr (Wang et al., 2017). The Ci was lower in the single-strain treatment group than in the CK group. In contrast, the Ci in the combined strain treatment group was higher than that in the CK group. Gs and Tr were significantly higher in all treatment groups than in the CK group. The combined treatment improved the stomatal restriction of plants. Therefore, the net photosynthetic rate was significantly higher in these treatment groups than in the CK group. The aforementioned results indicated that using phosphorus-solubilizing fungi reduced stomatal limitation, thereby increasing the photosynthetic rate of plants. Chlorophyll and carotenoids in leaves primarily absorb sunlight and store energy for photosynthesis (Yuan et al., 2022). The strains used also exerted significant enhancement effects on photosynthetic pigments. The combined treatment groups exerted better enhancement effects on chlorophyll a and chlorophyll b, whereas the single treatment groups were more suitable for increasing the carotenoid content. An analysis of a series of photosynthetic parameters revealed that the phosphorus-solubilizing fungi promoted plant photosynthesis by reducing stomatal restriction and increasing the contents of light-absorbing pigments, which was beneficial for the growth of F. taipaiensis P. Y. Li plants.
MDA is the final product of membrane lipid peroxidation during plant aging. MDA content reflects the oxidation degree of membrane lipids and thus the stress resistance of plants (Zhao et al., 2022; Shams et al., 2024). The MDA content was significantly lower in all treatment groups than in the CK group. The MDA content in the S7 group was the lowest. This proved that phosphorus-solubilizing fungi significantly inhibited the production of free radicals and reactive oxygen species, reduced the damage caused by membrane lipid peroxidation to F. taipaiensis P. Y. Li plants, and thus promoted the normal growth and health of plants. Soluble sugar, soluble protein, and free proline are major osmoregulatory substances and nutrients in plants (Zhang et al., 2023; Peng et al., 2024). They participate in transport and metabolism in plants, protect plant cell membranes and organelles to maintain cell structure stability, and produce functional enzymes (Haider et al., 2023). In the present study, soluble sugar and soluble protein contents were all higher in the treatment groups than in the CK group. In contrast, the free proline content was significantly lower in the S7 group than in the CK group. Based on the experimental results, we concluded that phosphorus-solubilizing fungi positively impacted the physiology and biochemistry of F. taipaiensis P. Y. Li. However, the combined effect of the three fungi was not good or was antagonistic. Therefore, we do not recommend their combined application. Xu et al. (2022) also reported a similar effect, which might be caused by the excessively strong competitive interaction between A. niger and the other two co-existing fungi. However, this conclusion only suggested that the three fungi were antagonistic to each other. Pant et al. (2024), using scanning electron microscopy, showed that A. niger affected the mycelia of other fungi, leading to spore rupture of co-existing fungi and inhibited growth. This might be one of the reasons why the growth-promoting effect was inhibited when the three fungi were used together. Zhang et al. (2020) isolated the secondary metabolites of A. niger sporangium powder and obtained compounds with significant antagonistic effects on some bacteria. However, whether they had the same effect on fungi remains unclear.
SOD accelerates the transformation of superoxide anion into H2O2 and oxygen in plants. POD and CAT eliminate H2O2. The synergistic effect of these three enzymes maintains the free radical content in plants, which is the main function of antioxidant enzymes (Wang et al., 2023; Ali et al., 2024). SOD mainly scavenges free radicals and is the primary enzyme that fights against damage induced by oxygen free radicals to plant cells. The SOD content is the main index reflecting the plant antioxidant activity. POD and CAT can assist in scavenging H2O2 and toxic substances produced and fighting against aging (Xie et al., 2022; Liu et al., 2023; Ang et al., 2023). The activities of the three enzymes were significantly higher in the treatment groups than in the CK group. The experimental results largely in alignment with those obtained through RT-qPCR. Specifically, the gene expression levels of the genes encoding these protective enzymes exhibited a highly positively correlated with the activities of these enzymes. Notably, the S5 group showed the most pronounced enhancement in the expression levels of these genes. Furthermore, the activities of the three enzymes were relatively higher in the S5 group than in the other treatment groups. These findings collectively suggest that phosphorus-solubilizing fungi promoted the reaction of antioxidant enzymes in F. taipaiensis P. Y. Li and enhanced the expression of genes encoding these protective enzymes.
The emerging field of rhizosphere fungal research has revealed the significant potential of rhizosphere fungi to influence plant health and development, as evidenced by numerous studies on medicinal plants. Zheng et al. (2016) selected medicinal peonies cultivated at different growth stages across five geographical areas. They used Biolog and 454 pyrosequencing technology to assess overall microbial activity and fungal diversity, and explored the relationship between the true regional characteristics of medicinal plants and rhizosphere microorganisms. They reported the diversity and regional characteristics of medicinal peony rhizosphere soil fungi. Li et al. (2023) collected and cultured fungi from the roots and rhizosphere of Salvia miltiorrhiza, used culturomics and high-throughput sequencing to evaluate the fungal species diversity of S. miltiorrhiza roots and rhizosphere, and verified the predicted cellulose in metagenomic analysis. Tang et al. (2021) examined Fritillaria rhizosphere microorganisms. The results showed interactions between the growth of F. taipaiensis P. Y. Li, soil factors, and rhizosphere microorganisms. These studies highlighted the intrinsic link between the diversity of rhizosphere fungi, the accumulation of bioactive substances, and interactions between plants. They also illustrated the important roles of rhizosphere fungi in the health and performance of medicinal plants. A key aspect of cultivation and optimization. The impact of rhizosphere fungi on medicinal plant health, biomass production, and secondary metabolite biosynthesis has received widespread attention, particularly with the application of molecular biology and omics technologies in recent years (Yang et al., 2024). However, the extent to which rhizosphere fungi can contribute to the construction of a quality evaluation system for Chinese medicinal materials remains unclear. Our study only confirmed that different rhizosphere fungi or their combinations impacted the photosynthetic characteristics, basic physiological and biochemical indicators, and protective enzyme systems of F. taipaiensis P. Y. Li. However, further in-depth research is required to determine how these different fungal combinations affect plant production by producing different metabolites. In summary, a comprehensive analysis of the composition of rhizosphere fungi and their interactions with host plants can provide valuable insights for improving the quality of medicinal plants and promoting the efficient utilization of biological resources (Zhu et al., 2021). It provides a reference for the ecological cultivation of Chinese medicinal materials and ensuring the production of high-quality Chinese medicinal materials in the future by regulating rhizosphere microbial communities.
5 ConclusionPhosphorus-solubilizing fungi A. tubingensis and A. nigerfunigatus can promote plant photosynthesis by improving leaf stomatal conductance and increasing light-absorbing pigments. They also enhance the antioxidant enzyme activity, maintain osmotic pressure, and regulate a series of enzyme reactions in plants. Further, they help the plants in establishing a defense system against free radicals and toxic substances, enhance antioxidant activity, delay aging, and promote the growth and development of F. taipaiensis P. Y. Li. The effect of A. niger used alone was higher than that of A. niger used in combination with the other two fungi. The antagonistic reaction between the fungi may be a reason for this difference, but the exact cause of this antagonism remains unclear, requiring further investigation. Therefore, the development and promotion of the combination of A. tubingensis and A. nigerfunigatus during artificial cultivation can be a good technical approach to improve the quality of F. taipaiensis P. Y. Li, strengthen plants, enhance soil fertility, and improve the utilization rate of phosphorus. Additionally, it can reduce fertilizer pollution, improve soil conditions, and provide favorable conditions for further cultivation of F. taipaiensis P. Y. Li, creating a virtuous cycle in artificial cultivation.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributionsYW (1st author): Conceptualization, Methodology, Formal analysis, Validation, Writing–original draft, Writing–review and editing. LY: Conceptualization, Methodology, Validation, Writing–original draft, Writing–review and editing. YW (3rd author): Formal analysis, Writing–review and editing. JL: Methodology, Writing–review and editing. MY: Data curation, Investigation, Writing–original draft. QL: Writing–review and editing, Data curation. QM: Conceptualization, Writing–original draft, Writing–review and editing. NZ: Writing–review and editing, Writing–original draft, Supervision.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Chongqing Natural Science Foundation project (CSTB2023NSCQ--LMX0010) and the Open Project Program of Chongqing Key Laboratory of Development and Utilization of Genuine Medicinal Materials in Three Gorges Reservoir Area (Grant No. KFKT2022014).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAdnan, M., Morton, G., and Hadi, S. (2011). Hadi S. Analysis of rpoS and bolA gene expression under various stress-inducedenvironments in planktonic and biofilm phase using 2−△△CT method. Mol. Cell. Biochem. 357 (1-2), 275–282. doi:10.1007/s11010-011-0898-y
PubMed Abstract | CrossRef Full Text | Google Scholar
Ali, N., Rafiq, R., Wijaya, L., Ahmad, A., and Kaushik, P. (2024). Exogenous citric acid improves growth and yield by concerted modulation of antioxidant defense system in brinjal (Solanum melongena L.) under salt-stress. J. King. Saud. Univ. Sci. 36 (1), 103012. doi:10.1016/j.jksus.2023.103012
CrossRef Full Text | Google Scholar
Ameen, F., AlYahya, S. A., AlNadhari, S., Alasmari, H., Alhoshani, F., and Wainwright, M. (2019). Phosphate solubilizing bacteria and fungi in desert soils: species, limitations and mechanisms. Arch. Agron. Soil Sci. 65 (10), 1446–1459. doi:10.1080/03650340.2019.1566713
CrossRef Full Text | Google Scholar
Ang, Y., Guo, Y., Chen, H., Liu, R., Zhu, Q., Wang, L., et al. (2023). Interaction between plant phosphorus nutrition and abiotic stress responses and its potential application in agricultural production. J.Plant Nutr. Ferti. 29 (12), 2345–2359. doi:10.11674/zwyf.2023289
CrossRef Full Text | Google Scholar
Baruah, A., Baruah, K. K., and Bhattacharyya, P. (2016). Comparative effectiveness of organic substitution in fertilizer schedule: impacts on nitrous oxide emission, photosynthesis, and crop productivity in a tropical summer rice paddy. Water Air Soil Pollut. 227, 410–413. doi:10.1007/s11270-016-3111-9
CrossRef Full Text | Google Scholar
Ceci, A., Pinzari, F., Russo, F., Maggi, O., and Persiani, A. M. (2018). Saprotrophic soil fungi to improve phosphorus solubilisation and release: in vitro abilities of several species. Ambio 47, 30–40. doi:10.1007/s13280-017-0972-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Croft, H., Chen, J. M., Luo, X., Bartlett, P., Chen, B., and Staebler, R. M. (2017). Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 23 (9), 3513–3524. doi:10.1111/gcb.13599
PubMed Abstract | CrossRef Full Text | Google Scholar
El-Maraghy, S. S., Tohamy, A. T., and Hussein, K. A. (2021). Plant protection properties of the plant growth-promoting fungi (PGPF): mechanisms and potentiality. Curr. Res. Environ. Appl.Mycol. 11 (1), 391–415. doi:10.5943/cream/11/1/29
CrossRef Full Text | Google Scholar
Fernández-Marín, B., García-Plazaola, J. I., Hernández, A., and Esteban, R. (2018). “Plant photosynthetic pigments: methods and tricks for correct quantification and identification,” in Advances in plant ecophysiology techniques, 29–50. doi:10.1007/978-3-319-93233-0_3
CrossRef Full Text | Google Scholar
Guo, S., Ma, Z., Sem, B., Luo, L., Ren, Q., Chen, T., et al. (2023). Effects of slow-release fertilizer application at topdressing stage on leaf photosynthesis, leaf nutrition and fruit quality of walnut. J. Tarim. Univer. 35 (03), 62–69. doi:10.3969/j.issn.1009-0568.2023.03.008
CrossRef Full Text | Google Scholar
Haider, M. W., Nafees, M., Iqbal, R., Ali, S., Asad, H. U., Azeem, F., et al. (2023). Combined application of hot water treatment and eucalyptus leaf extract postpones senescence in harvested green chilies by conserving their antioxidants: a sustainable approach. BMC plant Biol. 23 (1), 576. doi:10.1186/s12870-023-04588-y
PubMed Abstract | CrossRef Full Text | Google Scholar
Iftikhar, A., Farooq, R., Akhtar, M., Khalid, H., Hussain, N., Ali, Q., et al. (2024). Ecological and sustainable implications of phosphorous-solubilizing microorganisms in soil. Discov. Appl. Sci. 6 (2), 33. doi:10.1007/s42452-024-05683-x
CrossRef Full Text | Google Scholar
Lang, J., Ye, M., Luo, Y., Wang, Y., Shi, Z., Kong, X., et al. (2024). Effects of potassium-solubilizing bacteria on growth, antioxidant activity and expression of related genes in Fritillaria taipaiensis P.Y.Li. Phyton 93 (4), 789–806. doi:10.32604/phyton.2024.049088
CrossRef Full Text | Google Scholar
Li, C., Wu, Y., Li, L., Zhao, C., Li, B., Wu, Y., et al. (2023). Different techniques reveal the difference of community structure and function of fungi from root and rhizosphere of Salvia miltiorrhiza Bunge. Plant Biol. 25 (6), 848–859. doi:10.1111/plb.13556
留言 (0)