Colorectal cancer (CRC) continues to be the third most commonly diagnosed cancer and the second leading cause of cancer-related deaths worldwide (1). Despite advancements in surgical techniques, targeted therapy, and immunotherapy for CRC patients, the clinical prognosis remains unsatisfactory. Currently, prognostic predictions for CRC primarily rely on the AJCC TNM staging system; however, long-term survival can vary even among patients with the same TNM stage (2). In order to enhance the long-term survival of patients with CRC, the utilization of robust prognostic biomarkers capable of identifying high-risk individuals could yield significant clinical benefits for tailoring individual postoperative follow-up plans and treatment strategies.
According to emerging evidence, the immune and nutritional status of hosts play a pivotal role in the progression and survival of cancer patients (3). Building upon these insights, several inflammation/nutrition indicators have been developed to forecast survival outcomes for cancer patients, including serum albumin (4), neutrophil-to-lymphocyte ratio (NLR) (5), and lymphocyte-monocyte ratio (LMR) (6). However, these biomarkers fall short in their capacity to accurately predict prognosis. The Naples prognostic score (NPS), which is calculated based on peripheral albumin level, total cholesterol level, NLR, and LMR, was therefore developed as a prognostic assessment tool for CRC patients undergoing surgery by Galizia et al. (7) in 2017. Subsequently, an increasing number of studies have investigated the association between the NPS and clinical outcomes in various malignancies (8–10).
To the best of our knowledge, no meta-analysis has been conducted to investigate the prognostic value of NPS in patients with CRC. Therefore, we undertook a meta-analysis to explore the association between the pretreatment NPS and long-term outcomes in CRC patients.
2 Methods2.1 Search strategyThe present systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11). Relevant studies from PubMed, Embase and Web of Science were examined up to July 1st, 2024. The following combination of key words was used to search the related studies: (“Naples prognostic score”) AND (((colorectal) or (colon) or (rectum) or(rectal)) and ((cancer) or (cancers) or (tumor) or (tumors)or (carcinoma))). Language restrictions were not applied during the search process. Additionally, the references of the included studies were thoroughly scanned for supplementary reports. The search was independently performed by two investigators (H-L and HY-P).
2.2 Study selectionThe inclusion criteria were as follows: (1) Studies investigating the association between the pretreatment NPS and survival outcomes in patients with colorectal cancer; (2) Hazard ratio (HR) along with a 95% confidence interval (CI) was either directly reported or could be calculated; (3) The specific cut-off value of NPS was clearly stated. The exclusion criteria were as follows: (1) Studies lacking separate data for colorectal cancer patients; (2) Case reports, reviews, conference papers, and letters were excluded; (3) Duplicate data.
2.3 Data extraction and quality assessmentTwo reviewers (H-L and HY-P) conducted the data extraction independently and cross-checked all the results. The extracted data encompassed essential information such as the first author, publication year, study interval, country, study design and sample size, cut-off value, clinicopathological features including age, sex, ASA (American Society of Anesthesiologists) score, BMI (Body mass index), tumor size, tumor differentiation, lymphovascular invasion, perineural invasion and tumor stage, as well as survival outcomes.
The quality assessment of included studies was performed using the Newcastle-Ottawa Scale (NOS) (12), which consists of predefined eight items. Each study received a final score ranging from 0 to 9 after thorough evaluation; scores between 7-9 were considered indicative of high-quality research.
2.4 Outcomes assessmentIn this study, the primary outcomes focused on survival measures, specifically overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS). The second outcome was to assess the correlation between the pretreatment NPS and clinicopathological features of colorectal cancer. It is worth noting that due to DFS and PFS shared similar endpoints, they were analyzed collectively as a single outcome measure (DFS), in accordance with previous recommendations (13, 14).
2.5 Statistical analysisThe mean difference (MD), risk ratio (RR), and HR along with their corresponding 95% CIs were utilized as the effect size for continuous variables, dichotomous variables and survival outcomes, respectively. For studies that reported median with range or inter-quartile range, data were converted into mean with standard deviation (SD) using the method reported by McGrath et al. (15). In cases where survival data were not directly reported in the literature, we extracted them from the survival curves using the methods described by Tierney et al. (16). Statistical heterogeneity among the included studies was assessed using I2 statistics. Random-effects models were employed to calculate effect sizes during the meta-analysis. Subgroup analysis and sensitivity analysis were conducted to evaluate the credibility of pooled results. Begg’s funnel plot was used to assess potential publication bias. A two-tailed P value <0.05 was considered statistically significant. All statistical analyses were performed using R software, version 4.2.1.
3 Results3.1 Study characteristicsThe databases yielded a total of 66 records, as depicted in Figure 1. After assessment of titles, abstracts, and full texts, eight studies (7, 17–23) were included in the present study. Tables 1 and 2 provided a summary of the basic information and clinical characteristics of these included studies, respectively. This study encompassed a total of 2571 patients from China, Japan, Korea, and Italy. The publication years ranged from 2017 to 2023 with sample sizes varying between 136 and 533 individuals. Among the included studies, seven focused on colorectal cancer while one specifically examined rectal cancer. Regarding primary treatment modalities, six studies involved surgery while neoadjuvant chemoradiotherapy was employed in one study and first-line chemotherapy in another study. All included studies evaluated OS, six assessed DFS, and one evaluated PFS. Notably, these studies demonstrated good quality with scores ranging from six to seven (Table 2, Supplementary Table S1).
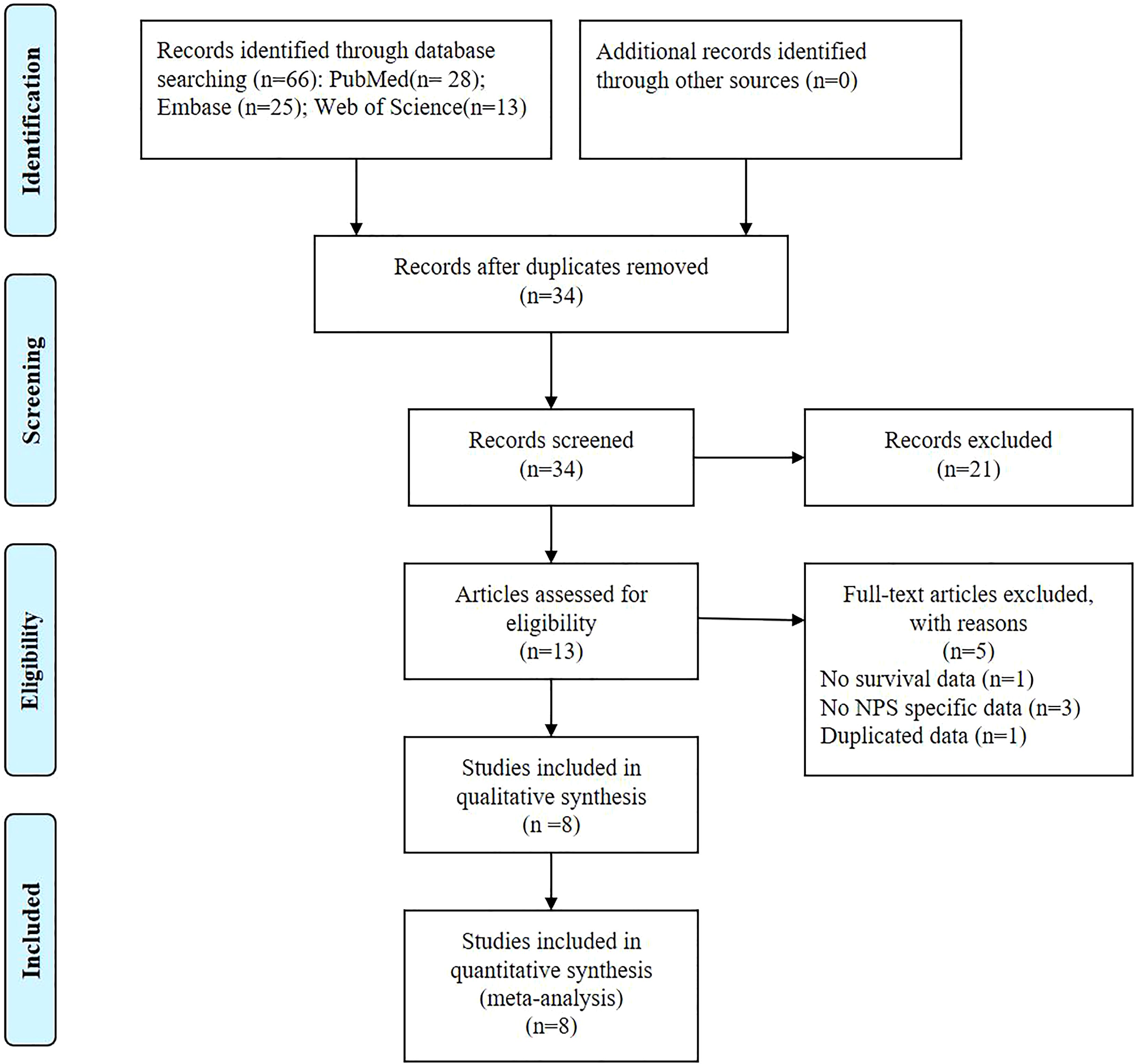
Figure 1. The PRISMA Flowchart of study selection.
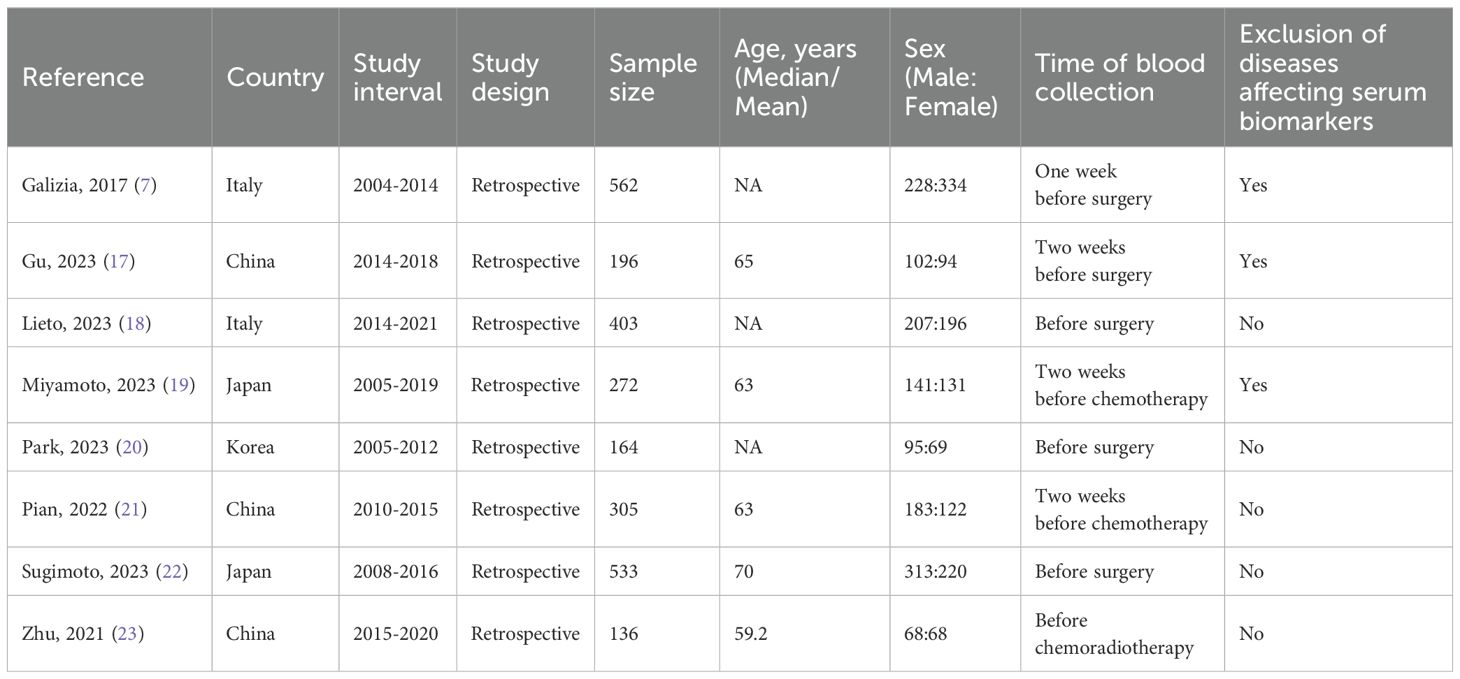
Table 1. Basic information of included studies.
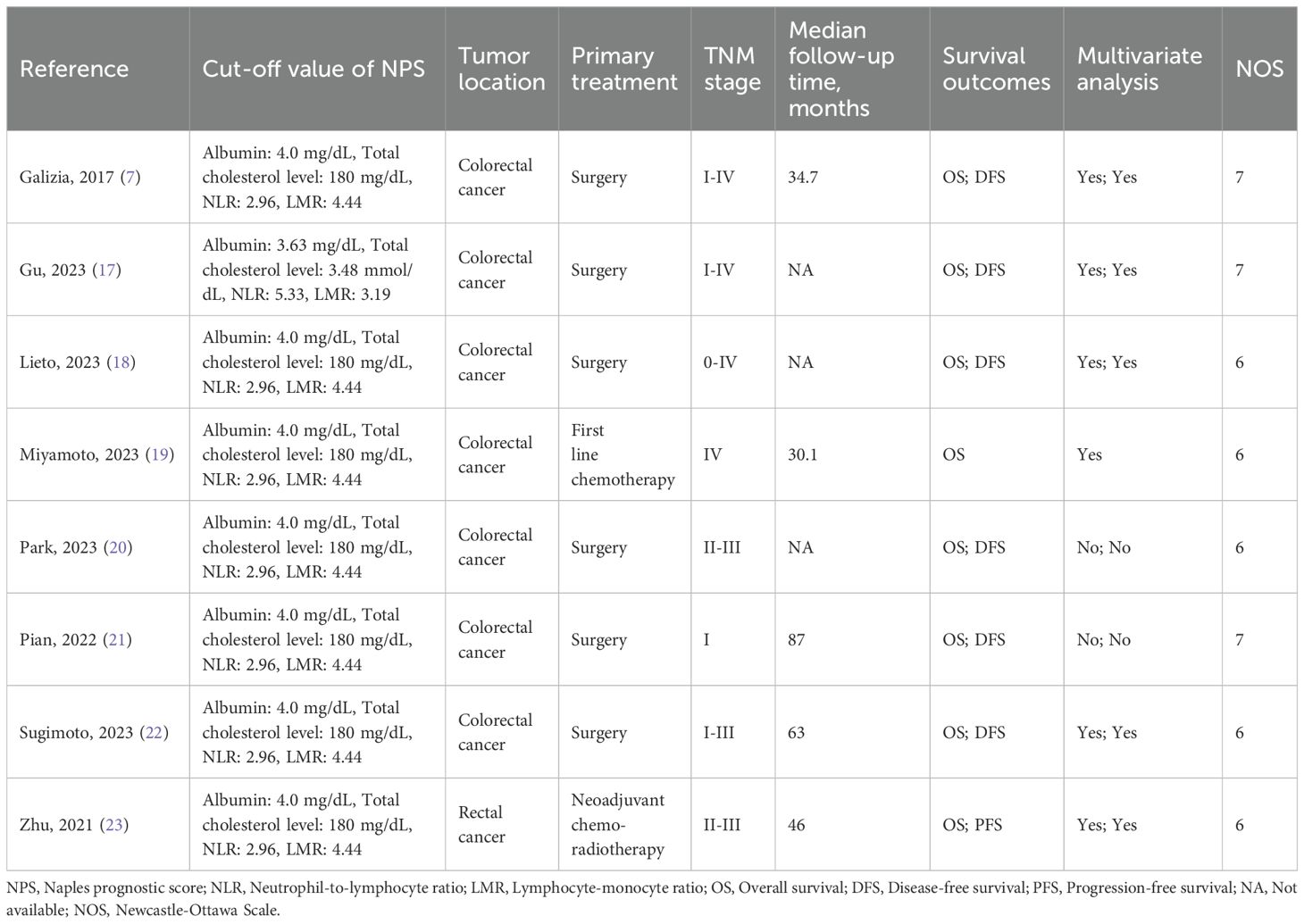
Table 2. Clinicopathological characteristics of included studies.
3.2 Relationship between the pretreatment NPS and OSThe association between the NPS and OS was investigated in eight studies involving 2571 patients. The pooled HR was 2.08 (95%CI: 1.74-2.48; P<0.01), indicating a significant correlation between high NPS and worse OS in CRC patients (Figure 2). Furthermore, subgroup analyses based on country, time of blood examination, exclusion of diseases effecting serum biomarkers, category of NPS, definition of NPS, tumor location, TNM stage, primary treatment, Multivariate analysis, and NOS were performed. As shown in Table 3 and Supplementary Figure S1, the results from all subgroup analyses consistently demonstrated that patients with high NPS had significantly reduced OS compared to those with low NPS group. Additionally, sensitivity analysis by omitting one study at a time showed no significant change in the overall outcome (Supplementary Figure S3A).
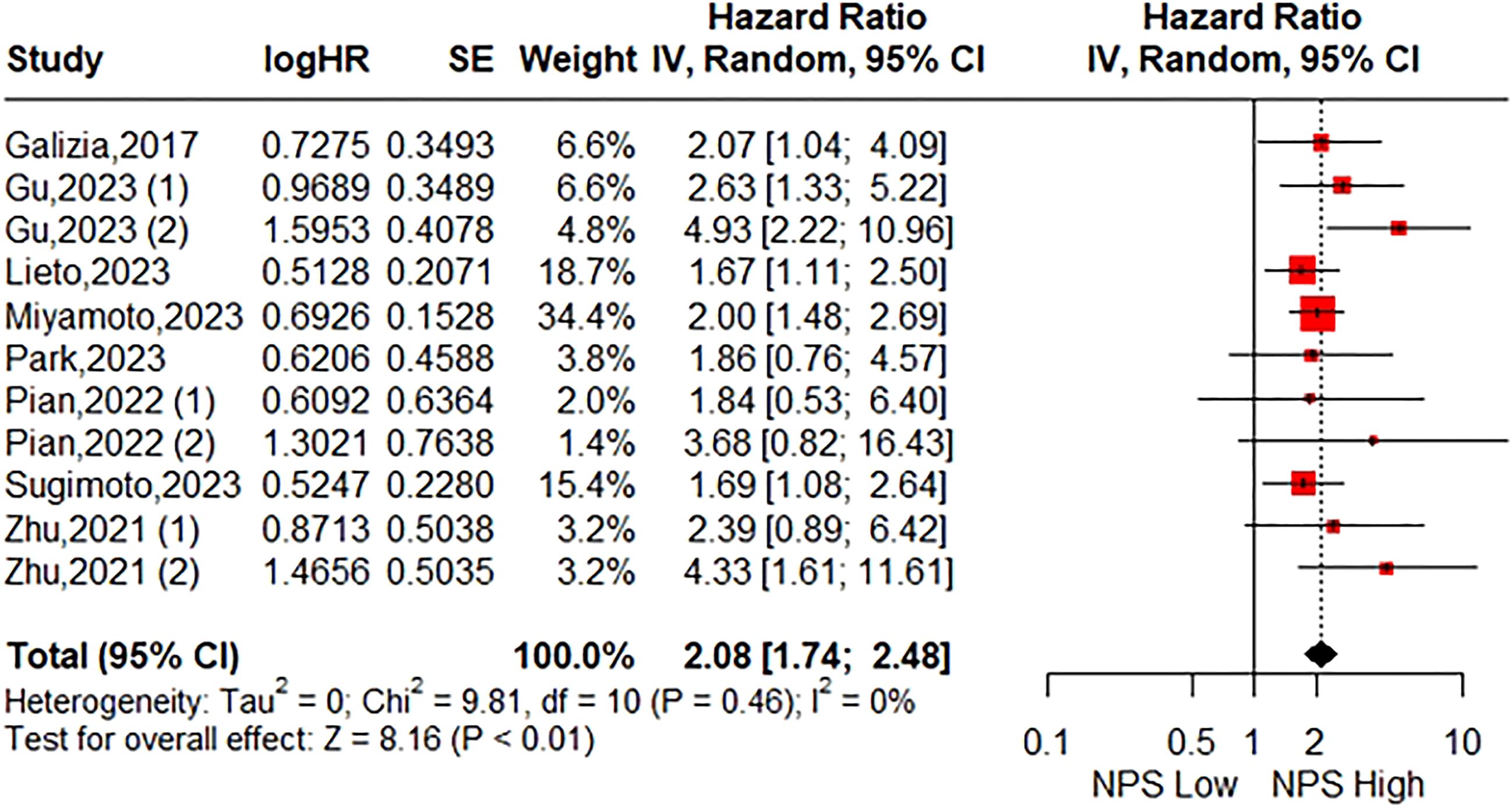
Figure 2. Forest plot assessing the relationship between the pretreatment NPS and OS.
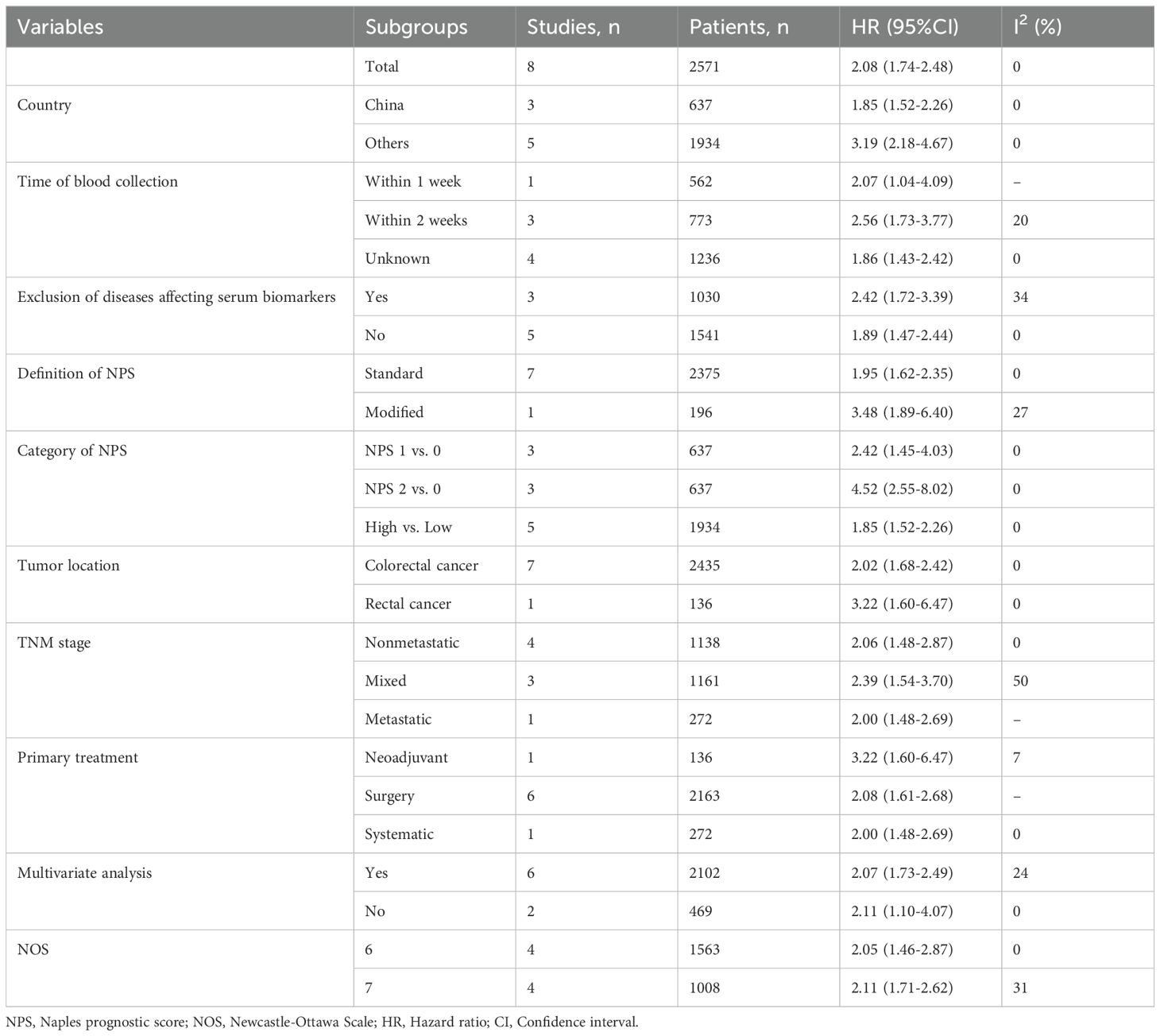
Table 3. Results of subgroup analyses of overall survival.
3.3 Relationship between the pretreatment NPS and DFSA total of seven studies consisting of 2299 patients reported on DFS. The pooled HR was 2.03 (95%CI: 1.49-2.77; P<0.01; I2 = 30%), indicating that a significant association between high NPS group and poorer DFS compared to the low NPS group (Figure 3). Similarly, Stratification by the same parameters based on the aforementioned parameters revealed that the incorporated results were almost consistent in each subgroup (Table 4, Supplementary Figure S2). Sensitivity analysis confirmed the stability of the pooled result (Supplementary Figure S3B).

Figure 3. Forest plot accessing the relationship between the pretreatment NPS and DFS.
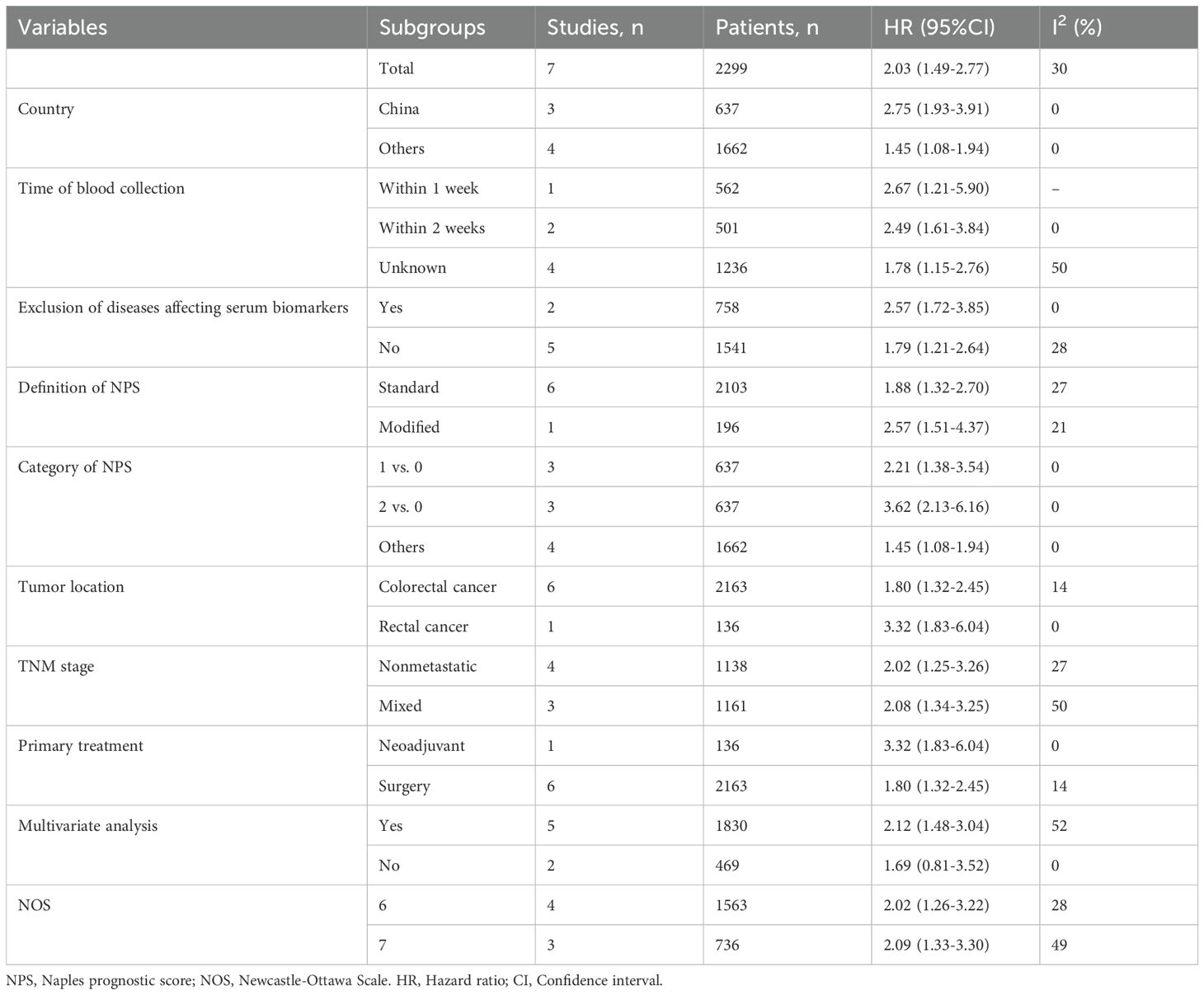
Table 4. Results of subgroup analyses of disease-free survival.
3.4 Relationship between the pretreatment NPS and clinicopathological factorsAltogether, four studies with 1029 patients reported a relationship between the pretreatment NPS and clinicopathological factors of colorectal cancer. As shown in Table 5 and Supplementary Figure S4, an increased NPS was markedly related to higher age (MD=-4.89; 95%CI: -9.13 to -0.64; I2 = 80%), lower BMI (MD=0.52; 95%CI: 0.02 to 1.01; I2 = 0%), and higher ASA score (RR=0.52; 95%CI: 0.36 to 0.75; I2 = 30%). However, NPS was not significantly associated with sex, tumor size, tumor differentiation, TNM stage, lymphovascular invasion and perineural invasion.
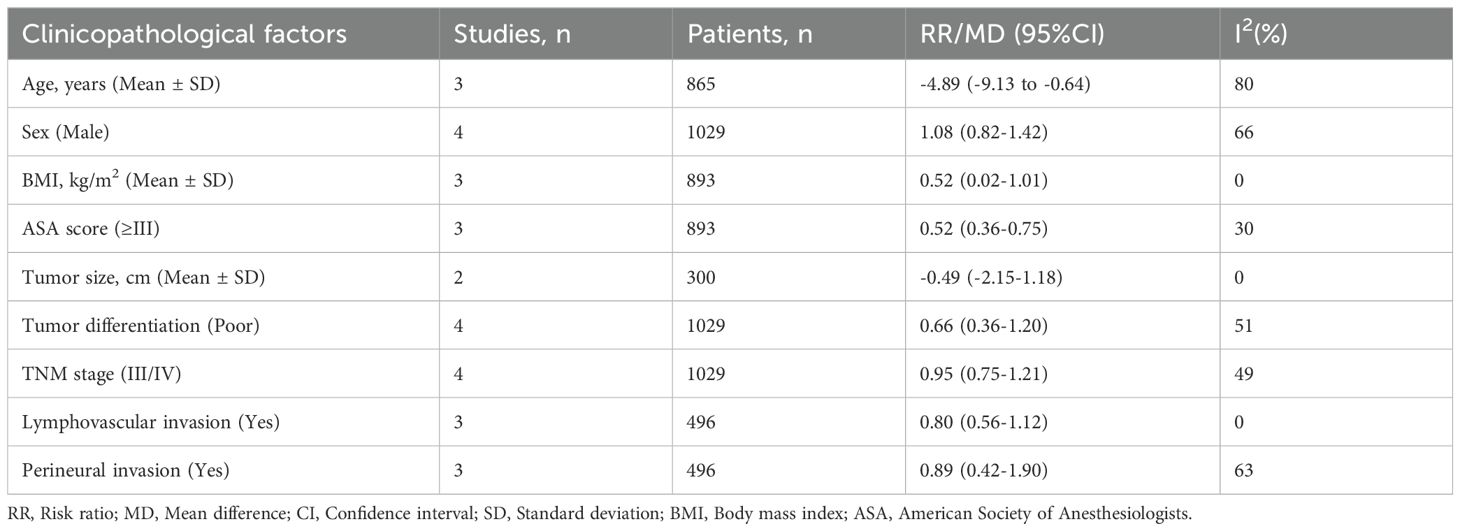
Table 5. The correlation between the pretreatment NPS and clinicopathological factors of colorectal cancer.
3.5 Publication biasThe Begg’s funnel plots were presented in Figure 4. According to the results of Begg’s test, no significant publication bias was observed in the current study regarding the association between NPS and OS (P=0.120) as well as DFS (P=0.592).
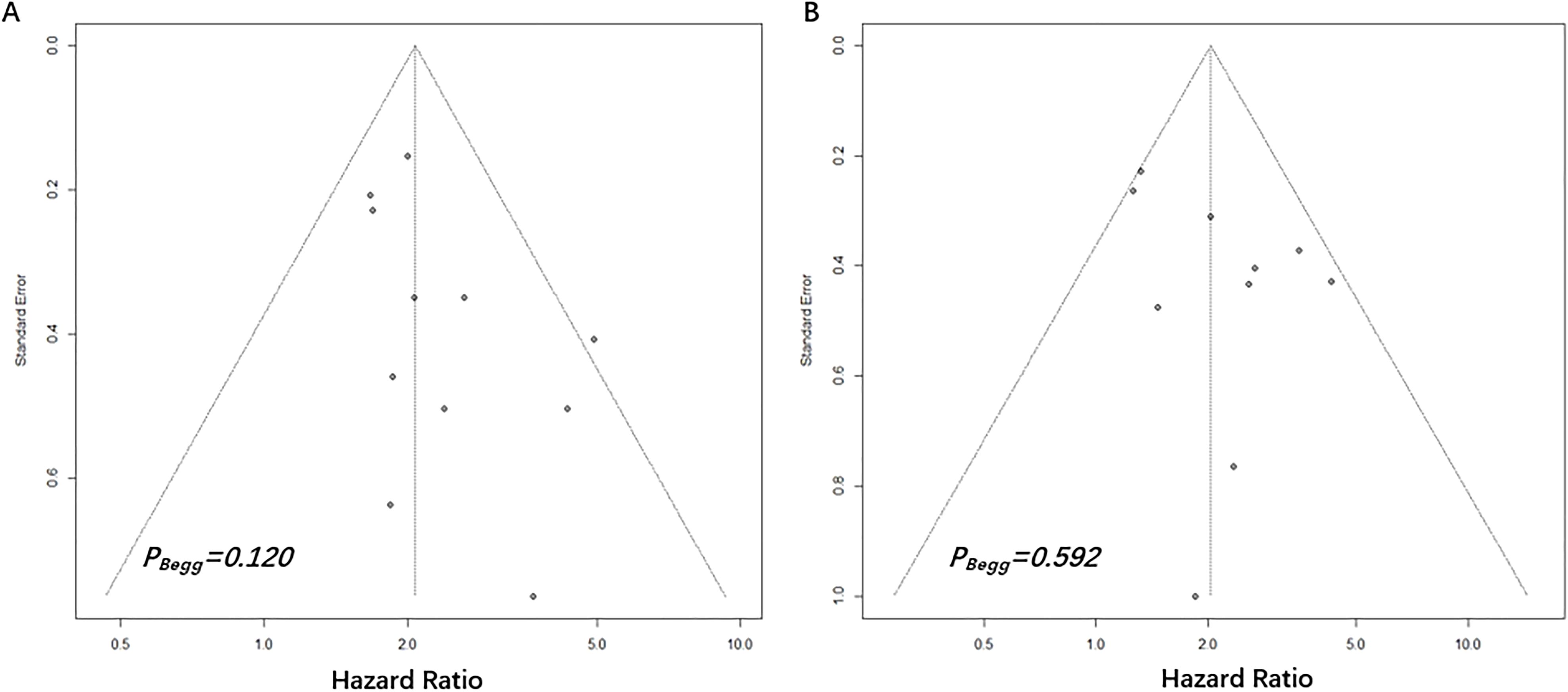
Figure 4. Begg’s funnel plots assessing publication bias between pretreatment NPS and OS (A) and DFS (B). The Begg’s P values were 0.120 and 0.592, respectively.
4 DiscussionUncontrolled inflammation and malnutrition are significant characteristics observed in cancer patients, which can result in a suboptimal response to medical treatment and deteriorating long-term outcomes (24, 25). Currently, there is substantial evidence confirming the utility of peripheral blood-based parameters as valuable biomarkers reflecting the inflammatory and nutritional status of cancer patients (26). The minimally invasive retrieval, cost-effectiveness, and objectivity associated with complete blood count-based biomarkers make them highly appealing to clinicians. Consequently, an increasing number of studies are focused on establishing clinically useful biomarkers.
In this context, the NPS was established as a potential tool to make clinical prognostic evaluation using four peripheral blood parameters: albumin level, total cholesterol level, NLR and LMR (7). Since then, the NPS has gradually gained popularity in assessing the prognosis of various cancers due to its easy availability and convenient calculation (27–29). Wang et al. (30) conducted a meta-analysis of seven studies and reported that elevated NPS levels are associated with poorer OS and DFS in patients with lung cancer. Another meta-analysis by Guo et al. (31) also confirmed the practical prognostic value of NPS in predicting the prognosis of esophageal cancer. Moreover, the clinical significance of NPS has been validated in other malignancies, including gastric cancer (27) and hepatocellular carcinoma (32). However, considering the heterogeneity among different types of cancers, it is crucial to investigate the applicability of NPS in colorectal cancer.
We conducted an extensive literature search and identified eight studies involving 2571 patients with CRC. Our pooled analyses revealed that patients in the high NPS group had a 2.08-fold increased risk of poor OS and a 2.03-fold increased risk of poor DFS compared to those with low NPS. By including a diverse range of patients from different ethnic backgrounds, disease stages, cut-off values of NPS and treatment options, we were able to investigate the utility of the pretreatment NPS as a screening metric for predicting survival outcomes in CRC patients. Subgroup analyses consistently demonstrated that the NPS could be considered an independent prognostic biomarker in CRC patients. Furthermore, our sensitivity analyses confirmed the robustness of these clinical findings, while Begg’s tests provided no evidence of publication bias. These results enhance the credibility of our conclusions.
The high discriminatory value of the NPS can be attributed to its combined utilization of various markers related to nutrition and inflammation, including albumin, cholesterol, neutrophils, monocytes, and lymphocytes. Firstly, albumin is a widely recognized indicator that reflects a patient’s nutritional status. Hypoalbuminemia has been shown to be significantly associated with poor wound healing, increased risk of infections, and reduced survival in cancer patients (33, 34). Additionally, serum albumin plays a crucial role in inhibiting the production of pro-inflammatory cytokines and enhancing cell-mediated immunity (35). Secondly, cholesterol is an essential component of cell membranes and plays a vital role in maintaining cellular function. Low levels of cholesterol have been suggested to promote tumor progression and worsen patient prognosis in various cancers (36, 37). This may be due to the requirement for cholesterol consumption by tumors for growth (38). Thirdly, as mature markers associated with inflammation, NLR and LMR have been extensively validated as predictors of short- and long-term adverse outcomes in malignancies (5, 6). The underlying mechanism involves neutrophils creating a favorable microenvironment for tumor cell proliferation while promoting tumor cell progression and invasion (39). Monocytes, differentiated into tumor-associated macrophages (TAMs), can induce apoptosis of T cells with antitumor functions and stimulate tumor angiogenesis (40, 41). Lymphocytes, particularly CD3+ and CD8+ T cells, in the inhibition of tumor cell growth through induction of tumor cell lysis and apoptosis have been well-documented, along with their association with improved long-term survival in cancer patients (42). Despite certain subgroups such as regulatory T cells and T helper 17 cells playing a pro-cancer role and being linked to poor prognosis, numerous clinical studies have consistently demonstrated a positive correlation between total lymphocyte count and cancer prognosis (42, 43).
The present meta-analysis had several limitations. Firstly, all of these studies were retrospective in nature, which may introduce selection bias and necessitate further investigation through prospective studies. Secondly, the majority of included studies originated from Asian countries, potentially limiting the generalizability of the NPS to Western populations. Thirdly, microsatellite instability (MSI) and microsatellite stability (MSS) colorectal cancer exhibit distinct characteristics in terms of their immune microenvironment. Although Lieto et al. (18) reported that preoperative NPS was an independent prognostic factor for overall survival in colorectal cancer regardless of the MSI status, further studies are still required to validate the prognostic significance of NPS in both subtypes. Lastly, the vast majority of included patients underwent surgery, therefore, the predictive value of NPS in the neoadjuvant therapy, first-line treatment, and even in later-line treatment still need to be further explored.
5 ConclusionOur findings suggest that the pretreatment NPS may serve as a valuable prognostic biomarker for patients diagnosed with colorectal cancer, as those in the high NPS group exhibit poorer overall survival and disease-free survival rates. Clinicians can utilize this informative indicator to stratify patients and develop personalized treatment plans. However, further research is necessary to validate the utility of this index in colorectal cancer.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributionsHL: Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. DZ: Investigation, Methodology, Resources, Software, Writing – review & editing. DJ: Methodology, Validation, Visualization, Writing – review & editing. HP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – review & editing. XY: Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1498854/full#supplementary-material
References1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74(3):229–63. doi: 10.3322/caac.21834
PubMed Abstract | Crossref Full Text | Google Scholar
2. Cai H, Li J, Chen Y, Zhang Q, Liu Y, Jia H. Preoperative inflammation and nutrition-based comprehensive biomarker for predicting prognosis in resectable colorectal cancer. Front Oncol. (2023) 13:1279487. doi: 10.3389/fonc.2023.1279487
PubMed Abstract | Crossref Full Text | Google Scholar
3. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis? Nutr Cancer. (2017) 69(8):1151–76. doi: 10.1080/01635581.2017.1367947
PubMed Abstract | Crossref Full Text | Google Scholar
4. Chiang JM, Chang CJ, Jiang SF, Yeh CY, You JF, Hsieh PS, et al. Pre-operative serum albumin level substantially predicts post-operative morbidity and mortality among patients with colorectal cancer who undergo elective colectomy. Eur J Cancer Care (Engl). (2017) 26(2). doi: 10.1111/ecc.12403
PubMed Abstract | Crossref Full Text | Google Scholar
5. Ouyang H, Xiao B, Huang Y, Wang Z. Baseline and early changes in the neutrophil-lymphocyte ratio (NLR) predict survival outcomes in advanced colorectal cancer patients treated with immunotherapy. Int Immunopharmacol. (2023) 123:110703. doi: 10.1016/j.intimp.2023.110703
PubMed Abstract | Crossref Full Text | Google Scholar
6. Chen XQ, Xue CR, Hou P, Lin BQ, Zhang JR. Lymphocyte-to-monocyte ratio effectively predicts survival outcome of patients with obstructive colorectal cancer. World J Gastroenterol. (2019) 25(33):4970–84. doi: 10.3748/wjg.v25.i33.4970
PubMed Abstract | Crossref Full Text | Google Scholar
7. Galizia G, Lieto E, Auricchio A, Cardella F, Mabilia A, Podzemny V, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis Colon Rectum. (2017) 60(12):1273–84. doi: 10.1097/DCR.0000000000000961
PubMed Abstract | Crossref Full Text | Google Scholar
8. Feng JF, Zhao JM, Chen S, Chen QX. Naples prognostic score: A novel prognostic score in predicting cancer-specific survival in patients with resected esophageal squamous cell carcinoma. Front Oncol. (2021) 11:652537. doi: 10.3389/fonc.2021.652537
PubMed Abstract | Crossref Full Text | Google Scholar
9. Li S, Wang H, Yang Z, Zhao L, Lv W, Du H, et al. Naples prognostic score as a novel prognostic prediction tool in video-assisted thoracoscopic surgery for early-stage lung cancer: a propensity score matching study. Surg Endosc. (2021) 35(7):3679–97. doi: 10.1007/s00464-020-07851-7
PubMed Abstract | Crossref Full Text | Google Scholar
10. Xiong J, Hu H, Kang W, Liu H, Ma F, Ma S, et al. Prognostic impact of preoperative naples prognostic score in gastric cancer patients undergoing surgery. Front Surg. (2021) 8:617744. doi: 10.3389/fsurg.2021.617744
PubMed Abstract | Crossref Full Text | Google Scholar
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
PubMed Abstract | Crossref Full Text | Google Scholar
12. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
PubMed Abstract | Crossref Full Text | Google Scholar
13. Pang HY, Yan MH, Chen LH, Chen XF, Chen ZX, Zhang SR, et al. Detection of asymptomatic recurrence following curative surgery improves survival in patients with gastric cancer: A systematic review and meta-analysis. Front Oncol. (2022) 12:1011683. doi: 10.3389/fonc.2022.1011683
PubMed Abstract | Crossref Full Text | Google Scholar
14. Sun KX, Xu RQ, Rong H, Pang HY, Xiang TX. Prognostic significance of the gustave roussy immune (GRIm) score in cancer patients: a meta-analysis. Ann Med. (2023) 55(2):2236640. doi: 10.1080/07853890.2023.2236640
PubMed Abstract | Crossref Full Text | Google Scholar
15. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. (2020) 29(9):2520–37. doi: 10.1177/0962280219889080
PubMed Abstract | Crossref Full Text | Google Scholar
16. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
PubMed Abstract | Crossref Full Text | Google Scholar
17. Gu J, Deng S, Jiang Z, Mao F, Xue Y, Qin L, et al. Modified naples prognostic score for evaluating the prognosis of patients with obstructive colorectal cancer. BMC Cancer. (2023) 23(1):941. doi: 10.1186/s12885-023-11435-8
PubMed Abstract | Crossref Full Text | Google Scholar
18. Lieto E, Cardella F, Wang D, Ronchi A, Del Sorbo G, Panarese I, et al. Assessment of the DNA mismatch repair system is crucial in colorectal cancers necessitating adjuvant treatment: A propensity score-matched and win ratio analysis. Cancers (Basel). (2023) 16(1):134. doi: 10.3390/cancers16010134
PubMed Abstract | Crossref Full Text | Google Scholar
19. Miyamoto Y, Akiyama T, Kato R, Sawayama H, Ogawa K, Yoshida N, et al. Prognostic significance of systemic inflammation indices by k-ras status in patients with metastatic colorectal cancer. Dis Colon Rectum. (2023) 66(8):e809–e17. doi: 10.1097/DCR.0000000000002392
PubMed Abstract | Crossref Full Text | Google Scholar
20. Park SH, Woo HS, Hong IK, Park EJ. Impact of postoperative naples prognostic score to predict survival in patients with stage II-III colorectal cancer. Cancers (Basel). (2023) 15(20):5098. doi: 10.3390/cancers15205098
PubMed Abstract | Crossref Full Text | Google Scholar
21. Pian G, Oh SY. Comparison of nutritional and immunological scoring systems predicting prognosis in T1-2N0 colorectal cancer. Int J Colorectal Dis. (2022) 37(1):179–88. doi: 10.1007/s00384-021-04043-0
PubMed Abstract | Crossref Full Text | Google Scholar
22. Sugimoto A, Fukuoka T, Shibutani M, Kasashima H, Kitayama K, Ohira M, et al. Prognostic significance of the naples prognostic score in colorectal cancer patients undergoing curative resection: a propensity score matching analysis. BMC Gastroenterol. (2023) 23(1):88. doi: 10.1186/s12876-023-02722-6
PubMed Abstract | Crossref Full Text | Google Scholar
23. Zhu J, Gao Q, Guo X, Liu Z, YAng B, Ji S, et al. Correlation analysis between naples prognostic score and treatment outcomes for locally advanced rectal cancer. Chin J Radiat Oncol. (2021) 30(12):1256–61. doi: 10.3760/cma.j.cn113030-20210706-00249
Crossref Full Text | Google Scholar
24. Zhu J, Wang D, Liu C, Huang R, Gao F, Feng X, et al. Development and validation of a new prognostic immune-inflammatory-nutritional score for predicting outcomes after curative resection for intrahepatic cholangiocarcinoma: A multicenter study. Front Immunol. (2023) 14:1165510. doi: 10.3389/fimmu.2023.1165510
PubMed Abstract | Crossref Full Text | Google Scholar
25. Pang H, Zhang W, Liang X, Zhang Z, Chen X, Zhao L, et al. Prognostic score system using preoperative inflammatory, nutritional and tumor markers to predict prognosis for gastric cancer: A two-center cohort study. Adv Ther. (2021) 38(9):4917–34. doi: 10.1007/s12325-021-01870-z
PubMed Abstract | Crossref Full Text | Google Scholar
26. Atasever Akkas E, Erdis E, Yucel B. Prognostic value of the systemic immune-inflammation index, systemic inflammation response index, and prognostic nutritional index in head and neck cancer. Eur Arch Otorhinolaryngol. (2023) 280(8):3821–30. doi: 10.1007/s00405-023-07954-6
PubMed Abstract | Crossref Full Text | Google Scholar
27. Aoyama T, Kato A, Hashimoto I, Maezawa Y, Hara K, Kazama K, et al. The naples prognostic score is an independent prognostic factor for gastric cancer patients who receive curative treatment. In Vivo. (2024) 38(2):890–6. doi: 10.21873/invivo.13515
PubMed Abstract | Crossref Full Text | Google Scholar
28. Chen S, Liu S, Xu S, Cao S, Han Z, Kong L, et al. Naples prognostic score is an independent prognostic factor in patients with small cell lung cancer and nomogram predictive model established. J Inflammation Res. (2022) 15:3719–31. doi: 10.2147/JIR.S371545
PubMed Abstract | Crossref Full Text | Google Scholar
29. Du CF, Gao ZY, Xu ZD, Fang ZK, Yu ZC, Shi ZJ, et al. Prognostic value of the naples prognostic score in patients with intrahepatic cholangiocarcinoma after hepatectomy. BMC Cancer. (2024) 24(1):727. doi: 10.1186/s12885-024-12502-4
PubMed Abstract | Crossref Full Text | Google Scholar
30. Wang YS, Niu L, Shi WX, Li XY, Shen L. Naples prognostic score as a predictor of outcomes in lung cancer: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. (2023) 27(17):8144–53. doi: 10.26355/eurrev_202309_33574
PubMed Abstract | Crossref Full Text | Google Scholar
31. Guo H, Wang T. Predictive role of naples prognostic score for survival in esophageal cancer: A meta-analysis. Med (Baltimore). (2024) 103(21):e38160. doi: 10.1097/MD.0000000000038160
PubMed Abstract | Crossref Full Text | Google Scholar
32. Xie YM, Lu W, Cheng J, Dai M, Liu SY, Wang DD, et al. Naples prognostic score is an independent prognostic factor in patients undergoing hepatectomy for hepatocellular carcinoma. J Hepatocell Carcinoma. (2023) 10:1423–33. doi: 10.2147/JHC.S414789
留言 (0)