The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has left an indelible mark on global health, society, and economies. While much attention has rightfully focused on acute illness and preventive measures, the aftermath of the virus is increasingly coming to light. Among the emerging concerns is the phenomenon known as Post COVID-19 condition (PCC).
PCC occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis (1).
At least 65 million people worldwide are estimated to have PCC (2).
The most prevalent PCC symptoms two-years after SARS-CoV-2 infection are fatigue and cognitive impairments (3–5). In a comprehensive neuropsychological evaluation patients with PCC showed significantly lower scores in domains of memory, language, processing speed, visuospatial function, executive function, and higher depressive and anxiety symptoms (6).
Recognizing the urgent need for targeted interventions to address the complex sequelae of PCC, the PoCoRe study was initiated as a collaborative effort across six German indoor rehabilitation centers specializing in neurologic or psychosomatic care. The centers adopted their therapeutic concept to the specific needs of the PCC patients (7).
The present study aims to elucidate the symptom burden of neuropsychological deficits in individuals grappling with PCC while also evaluating the efficacy of a multidisciplinary indoor rehabilitation program in ameliorating these challenges. By systematically assessing cognitive function, and functional outcomes before and after rehabilitation, this research aims to contribute valuable insights into the long-term management of PCC and inform the development of targeted interventions to support affected individuals on their path to recovery.
MethodsThe PoCoRe study is a prospective, non-randomized, controlled longitudinal study in Germany. A study protocol has been published previously (7).
The study took place in six indoor rehabilitation clinics specialized in neurological rehab and psychosomatic rehab. It was funded by the German pension found (Deutsche Rentenversicherung), so insured persons could take part from March 2022 to December 2023. All consecutively admitted rehabilitation patients who started rehabilitation as a result of PCC and who met the eligibility criteria as well as consent to participate in the study were included.
Eligibility criteria• SARS-CoV-2-infection and following PCC: Complaints that are still present more than 12 weeks after the onset of SARS-CoV-2 infection and cannot be explained otherwise.
• As a consequence of PCC at the time of the start of rehabilitation the presence of functional limitations that may threaten the ability to work.
• Written informed consent to participate in the study.
• Aged 18 years or above.
• Sufficient knowledge of the German language to participate in the study.
• Patients with ME/CFS were not excluded. However, we did not assess ME/CFS symptoms systematically within our study design.
InterventionsThe clinics and their treatment programs are reported previously (7). The specializations diverge in the duration and frequency of the therapies, but uniformly cover all essential symptom areas of the PCC with corresponding offers (psychoeducation, exercise, psychotherapy, pacing, breathing and relaxation therapy, cognitive training). Within in this framework, the treatment program was adapted to the individual needs of the patient, including adaptations for individuals affected by ME/CFS (see Supplementary Table 1 for comprehensive information).
Study variablesTrained psychologists conducted the study-related examinations/tests on admission to rehabilitation and on discharge. The participants completed the questionnaires autonomously (for a full listing of the questionnaires used, see the study protocol) (7).
Measures/outcomes Neuropsychology Montreal cognitive assessmentThe MoCa is a cognitive screening instrument. It is performed by healthcare professionals to detect early stages of dementia and mild cognitive impairment. It includes subtests for various cognitive abilities such as memory, language, contextual thinking, attention and concentration, behavior, arithmetic, temporal and spatial orientation and the ability to recognize complex shapes and patterns, while also taking into account educational background by awarding an extra point if they have not completed at least 12 years of education. Scores of 26 to 30 points are considered unremarkable, no limitations, 6 to 25 points indicate at least mild cognitive impairment, and 0 to 5 points are interpreted as extreme mental impairment (8).
TAP-testThe Test Battery for Attention (TAP) is a software package that offers a collection of different tests that can be used to measure the various aspects of attention in a differentiated way and also cover related aspects of visual perception. Used subtests are alertness, working memory, sustained attention, and divided attention. The battery we chose took about 45 min to complete (9) (see Table 1). Detailed information on the TAP is openly available openly available at https://www.psytest.net/en/test-batteries/tap/subtests.
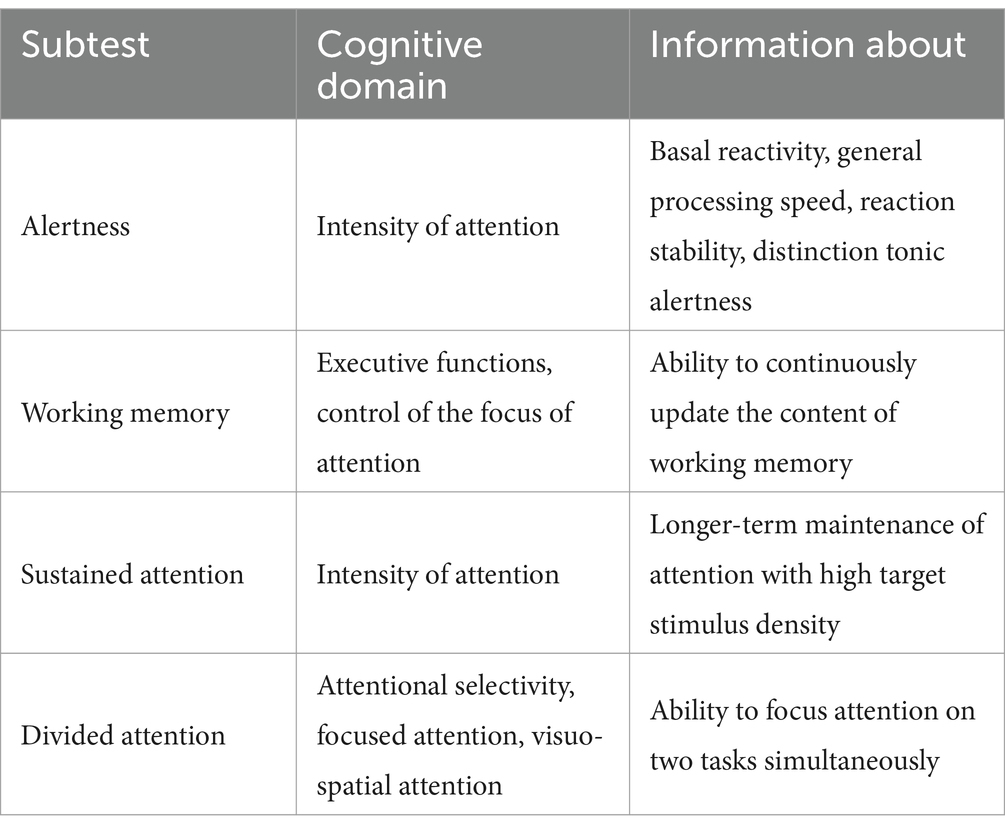
Table 1. Overview of the TAP subtests used.
Assessment of fatigue Fatigue scale for motor functioning and cognition (FSMC)The FSMC is a self-rating scale for assessment of subjective symptoms of fatigue and provides a differential quantification and grading of cognitive and motor fatigue. The FSMC was tested against several external criteria (e.g., cognition, motivation, personality and other fatigue scales) and provides satisfactory results with regard to the test quality criteria. Twenty items (10 for motor fatigue, 10 for cognitive fatigue) are rated using a five-point scale (strongly disagree to strongly agree) (10).
Data management and analysisThe above outcomes were assessed by patient questionnaires (FSMC, MoCA) and cognitive assessment (TAP) or extracted from rehabilitation discharge letters (e.g., primary and demographic data; socio-medical data). For more information on the full assessment included in the PoCoRe study, we have uploaded in Supplementary Table 2. If participants withdrew informed consent, the collected data was deleted. The patient data were stored anonymously in an electronic study-data file with a patient cipher, whereby the paper–pencil data were entered in an automated conversion procedure and partly manually. Paper-pencil questionnaires were stored in locked filing cabinets and electronic data were stored on secure servers. To ensure a safe and secure environment for the data collected, data transmission was encrypted using Secure Socket Layer (SSL) technology.
Statistical methodsStatistical analyses was conducted using R version 4.3.0 (9), including the stats package, and ggplot2 package (11). Descriptive analyses were performed concerning FSMC, TAP tests, MoCA and sociodemographic data. Indoor rehabilitation effects were calculated employing the Wilcoxon signed-rank test for FSMC and ANOVA for TAP test and MoCA. p-values were set to <0.05 and adjusted using the Bonferroni-Holmes method. For Wilcoxon Signed Rank Tests, the effect size r was calculated using the following formula: r = z/sqrt(N) (12).
In addition, sensitivity and specificity of the MoCA predicting results of the alertness testing was evaluated (13).
Ethics and study registrationAll participants provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the responsible ethics committees [reference numbers: University Hospital Regensburg (including Berlin and Gelderland Klinik): Z-2022-1749-8; Westerwaldklinik: Landesärztekammer Rheinland Pfalz: 2022–16,395; Konstanz and Gailingen: University of Constance 25/2022; Todtmoos: Landesärtzekammer Baden-Württemberg: B-F-2022-032]. The PoCoRe study was registered 03 February 2022 at https://studienanmeldung.zks-regensburg.de.
ResultsOut of the 1,086 recruited participants, a total of N = 701 participants were included into data analysis, based on having complete data in the alertness subtest of the TAP (admission and discharge). They had a mean age of 49.5 years (range: 21 to 65, SD = 10.10) and 70.9% were female. The initial infection occurred on average 29 months ago (range: 9 to 50, SD = 8.11).
There was no significant difference concerning age, FSMC values and TAP values on admission between the completed sample and the dropouts at discharge. Because of violated assumptions for parametric testing, the non-parametric Mann–Whitney U test was used to compare MoCA scores at admission. There was a statistically significant difference (U = 79913.00, p = 0.003) in MoCA scores between the completed sample (M = 26.53, SD = 2.61) and the dropout sample (M = 25.82, SD = 3.54) with a small effect size of r = 0.10. Within the complete sample, 177 out of 657 individuals (27%) showed a MoCA score below cut-off (<26). This proportion is significantly different from the 105 out of 277 individuals (38%) within the dropouts (Chi-squared = 10.602, df = 1, p = 0.0001).
Prevalence of Fatigue on admission was high, to discharge it decreased significantly with a mild effect size (see Table 2).
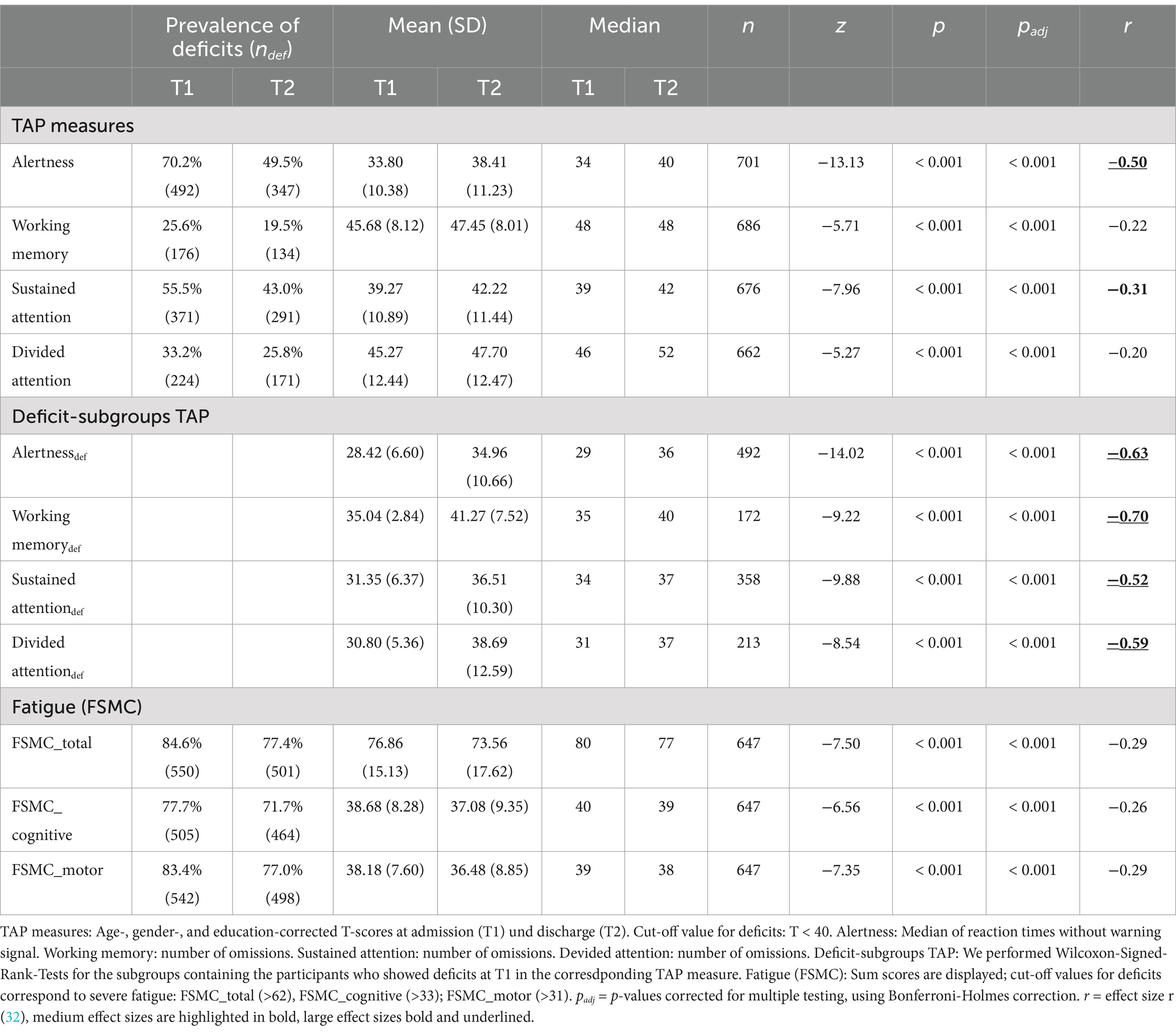
Table 2. Prevalence of deficits and Wilcoxon signed rank test comparing TAP and fatigue measures at admission (T1) and discharge (T2).
The results of the TAP subtests were converted to T values. T values lower than 40 were considered to be abnormal (14).
The reaction times of the alertness subtest scored abnormal in 70% of patients on admission and 50% on discharge (see Figure 1).
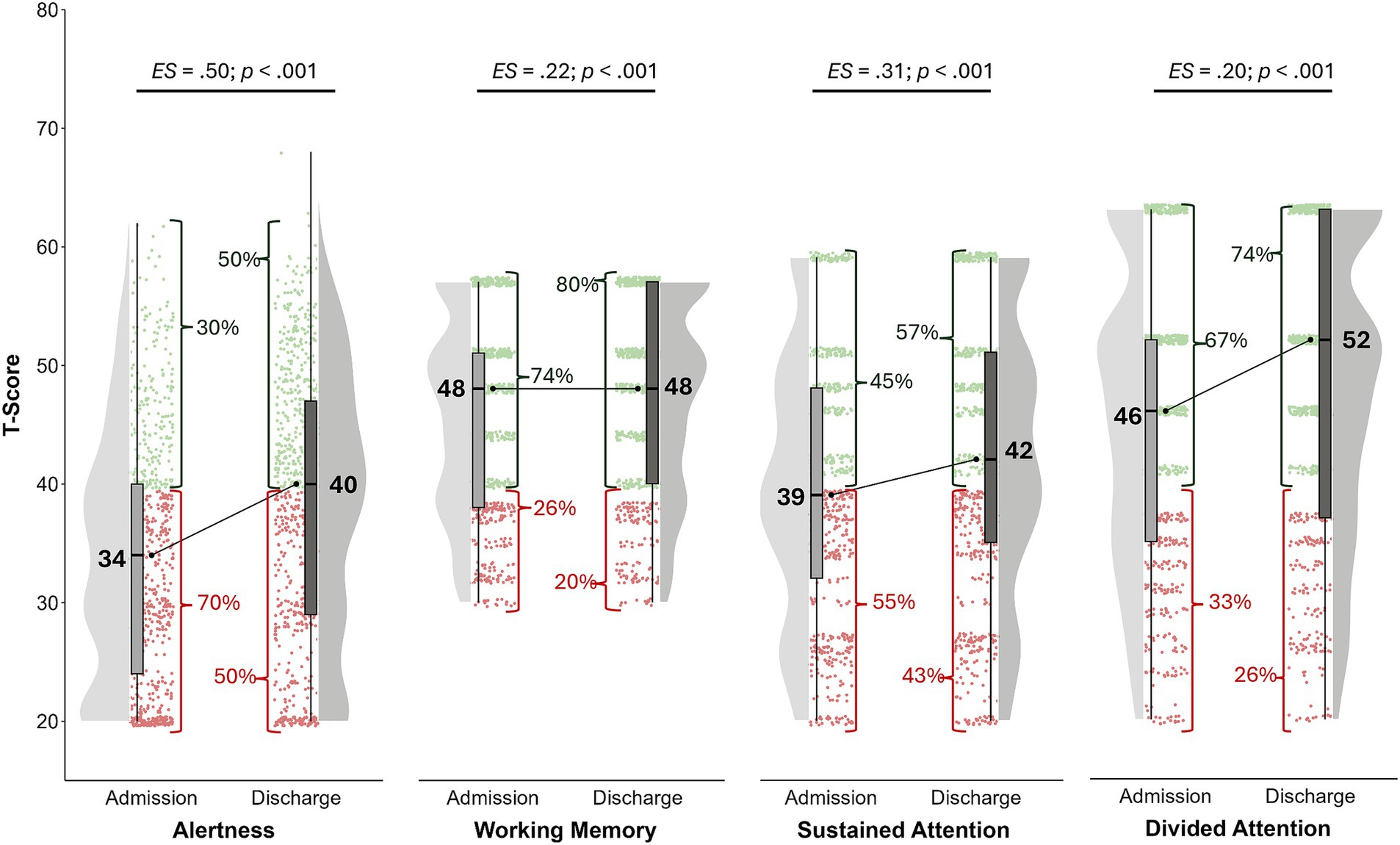
Figure 1. Age-, gender-, and education-corrected T-scores at admission (T1) und discharge (T2). Values in red represent impaired performance (T < 40), while green values are interpreted as at least average. Alertness: Median of reaction times without warning signal; nT1 = 701; nT2 = 701. Working memory: number of omissions; nT1 = 688; nT2 = 686. Sustained attention: number of omissions; nT1 = 681; nT2 = 676. Divided attention: number of omissions; nT1 = 674; nT2 = 662.
Sustained attention (depicted as the number of omitted answers) was abnormal in 55% on admission with a decrease to 43% on discharge. These differences were significant with mild effect sizes (see Figure 1).
Working memory was impaired in 26% at baseline and 20% at discharge. A significant and mild effect (see Figure 1).
Divided attention scored abnormal in 33% on admission with a decrease to 26% on discharge: a significant and small effect, too (see Figure 1).
The treatment effects for sustained attention, working memory and divided attention were even larger when we took only those participants into account scoring with abnormal results on admission with a T < 40 (see Table 2).
To evaluate the clinical relevance of the observed group-level improvements, we additionally calculated the proportion of individuals who improved on a clinically relevant level, according to critical differences in T-values (Tdiff) provided within the manual of the TAP (14).
At discharge, 37 participants (5.4%) showed clinically relevant improvements in working memory compared to admission (Tdiff ≥ 14.079), while 10 participants (1.5%) showed clinically relevant worsening. In divided attention, 98 participants (15.1%) showed clinically relevant improvements (Tdiff ≥ 13.060), while 32 participants (4.9%) showed clinically relevant worsening. In sustained attention, 119 participants (17.9%) showed clinically relevant improvements (Tdiff ≥ 10.312), while 52 participants (7.8%) showed clinically relevant worsening. At discharge, 341 participants (48.6%) showed clinically relevant improvements in alertness compared to admission (Tdiff ≥ 3.791), while 82 participants (11.7%) showed clinically relevant reductions in reaction times.
Improvements in alertness being the most prevalent finding on both the group and individual level, we performed further post-hoc analysis evaluating whether the patients significantly improving in alertness also significantly improve in fatigue measured by the difference in FSMC scores. As indicated by Wilcoxon Signed Rank Tests, patients who showed clinically relevant improvements in alertness showed higher improvements in overall fatigue and cognitive fatigue with small effect sizes, but no significant improvements in motor fatigue (see Table 3).
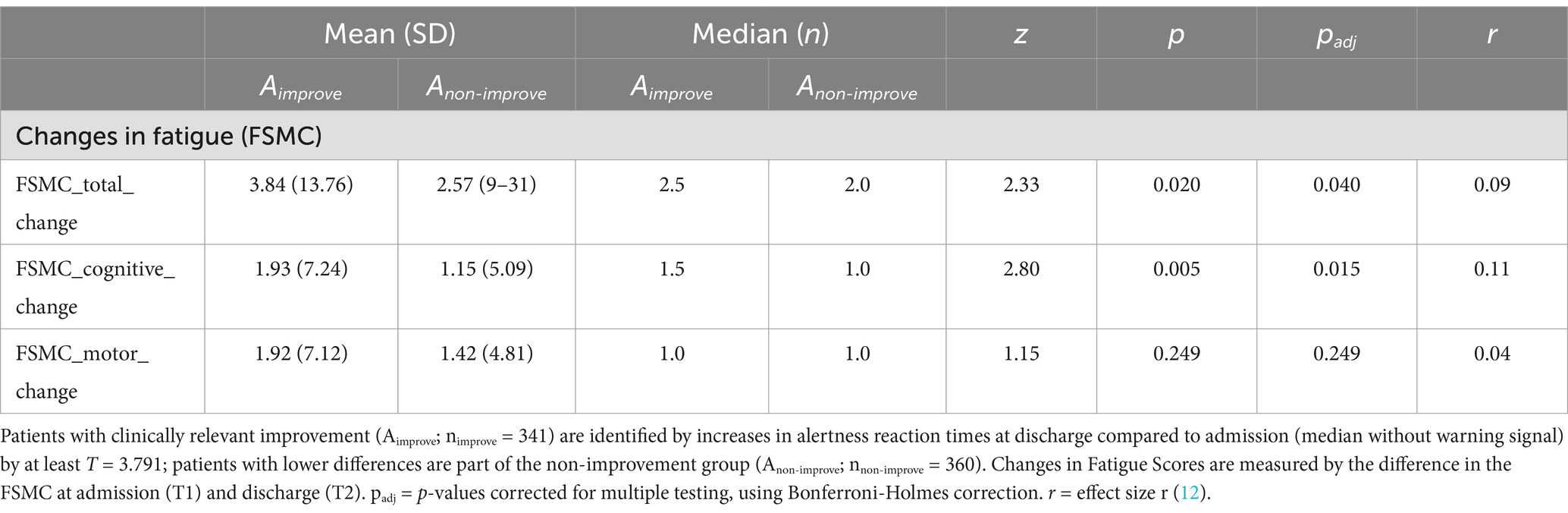
Table 3. Wilcoxon signed rank test comparing changes in fatigue scores (T1-T2) for patients clinically improving (Aimprove) and not improving (Anon-improve) in alertness.
We pooled the FSMC and TAP data by assuming the presence of neurocognitive deficits in the attention domain if two subdomains of the TAP test scored T < 40. Based on this assumption, 54% of PCC patients with severe fatigue were found to have coexisting attentional disorders.
Applying the MoCA test at admission showed suspicious results (cut off <26) in 177 out of 657 individuals (27%) of the participants, which normalized in 72 out of 115 (63%) at follow-up on discharge (see Figure 2).
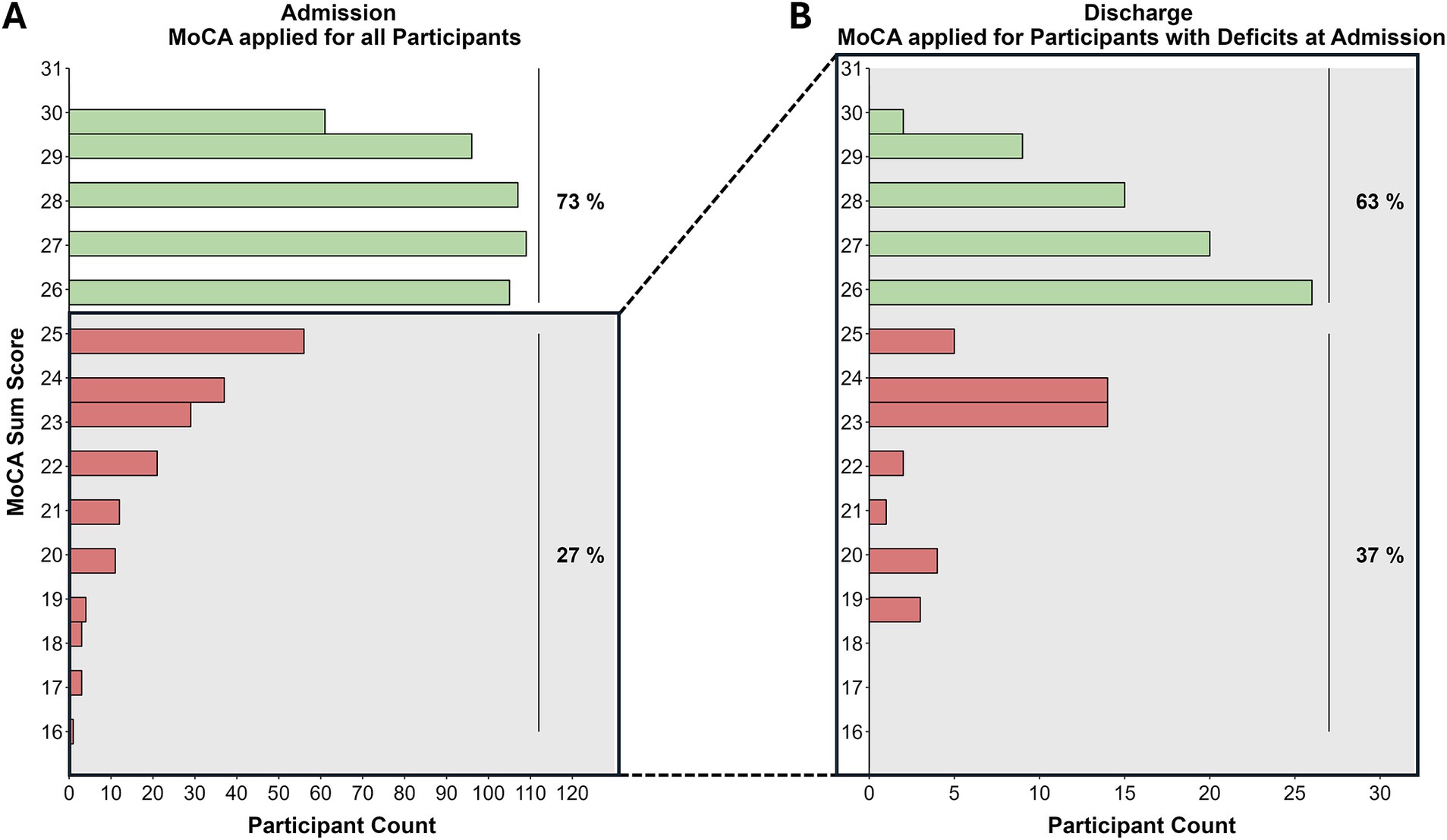
Figure 2. Distribution of MoCA sum scores at admission (A) and discharge (B). Green bars indicate normative MoCA scores (≥26), red bars indicate deficits. (A) nnormative = 480 (73%); nimpaired = 177 (27%). (B) nnormative = 72 (63%); nimpaired = 43 (37%).
To detect clinically relevant changes in MoCA scores over time, Krishnan et al. (15) calculated a reliable change index (16) of ±1.73 based on a healthy sample. Within our sample, 68 of 115 (59%) impaired patients at T1 (cutoff <26) showed an improvement of at least 2 points on the MoCA score, while 3 of 115 (3%) participants showed a clinically relevant worsening of the MoCA score (≤ −2).
For the evaluation of the specificity and sensitivity of the MoCA to detect neurocognitive deficits in attention domain, we compared the prevalence of abnormal MoCA sum score at T1 with the prevalence of neurocognitive deficits in attention domain at T1. The ladder we defined as the prevalence of deficits as showing impairments in at least two domains of the TAP. It should be noted, that we included only participants with complete MoCA and TAP data at T1 (N = 875) for this subanalysis. The MoCA test has a specificity of 83% to predict a deficit in attention, but only a sensitivity of 37%.
DiscussionWith the aim of assessing the prevalence of neuropsychological deficits in PCC and depicting potential changes throughout the course of indoor rehabilitation, we conducted a comprehensive, standardized assessment of attention at the beginning and end of a multidisciplinary rehabilitation program.
Performing subtests of the TAP, we measured a high prevalence of attention deficits, particularly in the domains alertness and sustained attention. Compared to admission, we found significant overall improvement in all subdomains at discharge, especially large effects among those admitted with impairments.
Our data adds to existing knowledge on overall cognitive slowing in post COVID patients (17), indicated by slow reaction times in alertness that were highly prevalent in our sample. Further, we provided evidence that a rehabilitation program can lead to improvements on a group level and clinically relevant individual improvements. On an individual level, we could show that patients with clinically relevant improvements in cognitive slowing were more likely to improve in overall and cognitive fatigue symptoms. While this effect was small, it adds to the findings on the interplay of cognitive slowing and fatigue symptoms.
Analyses on individual levels revealed that while a higher proportion of patients improve within the subtests of attention, some individuals (1.5–11.7%) show worse performance at discharge compared to admission. This proportion being rather small, this finding highlights the importance of individualized diagnostics, treatment plans, and evaluation, and might encourage further research to evaluate interventions on an individual basis in addition to group comparisons. This aligns with publications highlight the heterogeneity of the post COVID population and the need for comprehensive, interdisciplinary diagnostics and individualized treatment. This, however, acquires additional resources, as discussed by, e.g., Hayden (18).
Of the 27% of participants with suspicious scores on the MoCA at admission, 63% improved to a normative level during rehabilitation, also indicating a positive effect of the rehabilitation program in this subgroup. For the interpretation of this effect, it should be considered that we observed baseline differences in MoCA scores, with T2-dropouts showing lower performance than the analyzed complete sample. However, this group difference only had a small effect size (r = 0.10), suggesting a small potential bias.
The MoCA test did not prove effective as a screening tool for detecting attention deficits in the participating PCC patients. It was suspicious in only 27% of the cases, and thus only predicted the presence of attentional deficits (measured with the TAP) with a sensitivity of 37%.
Neuropsychological studies have found that the executive functions are particularly severely impaired in PCC patients (19). Others emphasize the importance of attention deficits (20, 21). In addition to the findings of Ariza et al. (22), our data show the importance of conducting a differentiated test of attention.
It should be noted that the same version of the MoCA and TAP was applied at admission and discharge, so that learning effects cannot be ruled out. However, these could be minimal since several weeks lie between the measurement points (23).
Furthermore, assessment was only possible for deficits in the attention domain; other neurological subdomains of the MoCA (e.g., memory) were not extensively explored. Further investigations could explore whether adjusted cut-off values in the MoCA, combined with items from other questionnaires, offer a pragmatic screening alternative.
Fatigue has a very high prevalence and amount in our cohort.
This is interesting because between 6 and 24 months after infection, about half of all fatigue cases resolve in a population-based study (24). Therefore our PCC patient cohort is considered to be a selected population. Our PCC patients were on average 29 months post-infection and can be considered chronic patients. Despite such a high degree of chronicity, a substantial and significant effect of indoor rehabilitation was observed. To our knowledge, this is the first prospective study to demonstrate such effects in a large patient population with PCC.
However, the impact on fatigue symptoms was less pronounced compared to attention deficits. Consequently, the improvement in self-reported cognitive fatigue and measured cognitive performance does not appear to align within our sample. Additionally, our findings revealed that neurocognitive deficits in the attention domain (defined as deficits in at least two subdomains of attention, as measured by the TAP) coexist with severe fatigue (FSMC) in 54% of cases. This suggests a significant overlap between attention deficits and severe fatigue; however, attention deficits alone do not provide a complete explanation for the presence of fatigue in our sample.
Other studies have similarly discovered that self-reported fatigue measures do not always correlate with reaction times (25). This discrepancy may be due to the measurement of two distinct constructs: While the FSMC assesses perceived fatigue symptoms as a stable trait, the TAP measures cognitive performance as a state.
In this context, the question of the influence of psychological, respectively, psychosocial factors naturally arises. Numerous studies show a certain psychological predisposition for the onset of a PCC (26–28). This question was examined in detail in a subset at one of the participating centers of our PoCoRe study, and Kupferschmitt and colleagues were able to demonstrate a significant improvement in the PHQ-9 (a measure of depression) as a result of indoor rehabilitation treatment at one of the participating centers. However, this did not correlate with the measured changes in the neuropsychological assessment (29).
In a highly nuanced combination of neuropsychological tests and the Patient-Health- Questionnaire- 9 to assess depressive symptoms, Morawa and colleagues found frequently deficient neuropsychological parameters. Clinically relevant depressive symptoms were associated with an elevated risk for an impairment regarding some cognitive functions (30).
It should be noted, however, that the participating clinics also addressed the psychological aspects of rehabilitation needs and developed and evaluated their own cognitive behavioral therapy (CBT) procedures for this clientele (31).
Overall, our study shows desired rehabilitation effects, meaning improvement in the tested neuropsychological domains and fatigue at discharge compared to admission. One limitation is that we cannot generalize this statement to severely affected individuals, as our sample consisted of individuals with a sufficiently good overall health status to be eligible for rehabilitation, due to the German healthcare system regulations.
Another limitation of our study is the absence of a control group, which means we cannot ascertain whether the improvements observed are attributable solely to the rehabilitation program or could also be attributed to spontaneous remission. However, since the PCC patients had already experienced considerable chronification, spontaneous remission is unlikely to be the cause of these improvements.
Another limitation, however, is the considerable drop-out rate among participants for neuropsychological testing at the time of discharge. Since there were no significant differences between this group and the group of participants with a complete TAP examination when comparing FSMC values and TAP examination results at T1, we believe that our results are still representative. For the interpretation of the improvements in MoCA-Scores, it should be considered that we observed baseline differences in MoCA scores, with T2-dropouts showing lower performance than the analyzed complete sample. However, this group difference only had a small effect size (r = 0.10), suggesting a small potential bias. Still, potential training effects cannot be excluded.
The reasons for discontinuation were not exclusively due to the refusal of the rehabilitants to undergo a second TAP examination, but also were related to other reasons, for example expiration of funding commitments on the part of the payers or personal reasons that made it necessary to discontinue rehabilitation.
In the future, it seems desirable to combine the data collected here with further data on the psychological outcomes of the participants. In addition, the data indicate the need for intensive neuropsychological assessment and therapy as part of rehabilitation. The aftercare situation is particularly worrying as many as 50% of patients are discharged from rehabilitation with persistent neurocognitive deficits in the attention domain and were searching for further intensive neuropsychological training required. A catamnesis of the PoCoRe cohort 6 months after discharge from rehabilitation will show how the rehabilitation procedures and other contextual factors have affected quality of life and social and occupational participation.
ConclusionIn a large cohort we have shown the high prevalence of neuropsychological deficits and fatigue among PCC patients who generally benefited from indoor rehabilitation in specialized centers. Given the so far unresolved overlap with self-reported fatigue, depressive symptoms, and other factors such as sleep quality, a comprehensive neuropsychological assessment is essential for developing an individualized treatment plan. Screening instruments need to be evaluated carefully for adequate sensitivity. This underscores the need for improved neuropsychological care in both rehabilitation and outpatient settings.
Data availability statementThe datasets presented in this article are not readily available they are available on request and after verification. Requests to access the datasets should be directed to Thilo Hinterberger, VGhpbG8uSGludGVyYmVyZ2VyQHVrci5kZQ==.
Ethics statementThe study was approved by the responsible ethics committees (reference numbers: University Hospital Regensburg (including Berlin and Gelderland Klinik): Z-2022-1749-8; Westerwaldklinik: Landesärztekammer Rheinland Pfalz: 2022–16,395; Konstanz and Gailingen: University of Constance 25/2022; Todtmoos: Landesärtzekammer Baden-Württemberg: B-F-2022-032). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsMJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CK: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. CH: Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. SK: Data curation, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. AK: Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. IM: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Software, Writing – original draft, Writing – review & editing. NW: Formal analysis, Writing – original draft, Writing – review & editing, Data curation. GS: Investigation, Writing – original draft, Writing – review & editing. TL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. VK: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. TH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Deutsche Rentenversicherung (German pension fund) funded this study.
AcknowledgmentsWe would like to thank Sara Tholl, Haris Tüter, Melanie Berger, Imke Jana Hrycyk, Sebastian Indin for their expert and careful performance of the neuropsychological tests and data collection. We would also like to thank Ariel Schoenfeld, who provided valuable advice on the design of the study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1486751/full#supplementary-material
References1. Soriano, JB, Murthy, S, Marshall, JC, Relan, P, and Diaz, JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. (2022) 22:e102–7. doi: 10.1016/s1473-3099(21)00703-9
Crossref Full Text | Google Scholar
2. Davis, HE, McCorkell, L, Vogel, JM, and Topol, EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
PubMed Abstract | Crossref Full Text | Google Scholar
3. Fernandez-de-Las-Peñas, C, Notarte, KI, Macasaet, R, Velasco, JV, Catahay, JA, Ver, AT, et al. Persistence of post-COVID symptoms in the general population two years after SARS-CoV-2 infection: a systematic review and meta-analysis. J Inf Secur. (2024) 88:77–88. doi: 10.1016/j.jinf.2023.12.004
PubMed Abstract | Crossref Full Text | Google Scholar
4. Jaywant, A, Gunning, FM, Oberlin, LE, Santillana, M, Ognyanova, K, Druckman, JN, et al. Cognitive symptoms of post-COVID-19 condition and daily functioning. JAMA Netw Open. (2024) 7:e2356098. doi: 10.1001/jamanetworkopen.2023.56098
PubMed Abstract | Crossref Full Text | Google Scholar
5. Hampshire, A, Azor, A, Atchison, C, Trender, W, Hellyer, PJ, Giunchiglia, V, et al. Cognition and memory after Covid-19 in a large community sample. N Engl J Med. (2024) 390:806–18. doi: 10.1056/NEJMoa2311330
PubMed Abstract | Crossref Full Text | Google Scholar
6. Bonner-Jackson, A, Vangal, R, Li, Y, Thompson, N, Chakrabarti, S, and Krishnan, K. Factors associated with cognitive impairment in patients with persisting sequelae of COVID-19. Am J Med. [ahead of print] (2024). doi: 10.1016/j.amjmed.2024.01.021
PubMed Abstract | Crossref Full Text | Google Scholar
7. Kupferschmitt, A, Hinterberger, T, Montanari, I, Gasche, M, Hermann, C, Jöbges, M, et al. Relevance of the post-COVID syndrome within rehabilitation (PoCoRe): study protocol of a multi-Centre study with different specialisations. BMC Psychol. (2022) 10:189. doi: 10.1186/s40359-022-00892-8
PubMed Abstract | Crossref Full Text | Google Scholar
8. Nasreddine, ZS, Phillips, NA, Bédirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
PubMed Abstract | Crossref Full Text | Google Scholar
9. Scherwath, A, Poppelreuter, M, Weis, J, Schulz-Kindermann, F, Koch, U, and Mehnert, A. Psychometric evaluation of a neuropsychological test battery measuring cognitive dysfunction in cancer patients--recommendations for a screening tool. Fortschr Neurol Psychiatr. (2008) 76:583–93. doi: 10.1055/s-2008-1038248
Crossref Full Text | Google Scholar
10. Penner, IK, Raselli, C, Stöcklin, M, Opwis, K, Kappos, L, and Calabrese, P. The fatigue scale for motor and cognitive functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler. (2009) 15:1509–17. doi: 10.1177/1352458509348519
PubMed Abstract | Crossref Full Text | Google Scholar
11. Wickham, H. ggplot2. Cham: Springer International Publishing (2016).
12. Maier-Riehle, B, and Zwingmann, C. Effect strength variation in the single group pre-post study design: a critical review. Rehabilitation (Stuttg). (2000) 39:189–99. doi: 10.1055/s-2000-12042
PubMed Abstract | Crossref Full Text | Google Scholar
13. Hilgers, R-D, Bauer, P, and Scheiber, V. Einführung in die medizinische Statistik. Berlin, Heidelberg: Springer Berlin Heidelberg (2003).
14. Zimmermann, P, and Fimm, B. TAP Testbatterie zur Aufmerksamkeitsprüfung, Version 2.3.1. Psychologische Testsysteme. (2017).
15. Krishnan, K, Rossetti, H, Hynan, LS, Carter, K, Falkowski, J, Lacritz, L, et al. Changes in Montreal cognitive assessment scores over time. Assessment. (2017) 24:772–7. doi: 10.1177/1073191116654217
Crossref Full Text | Google Scholar
16. Jacobson, NS, and Truax, P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. (1991) 59:12–9. doi: 10.1037/0022-006X.59.1.12
PubMed Abstract | Crossref Full Text | Google Scholar
17. Martin, EM, Rupprecht, S, Schrenk, S, Kattlun, F, Utech, I, Radscheidt, M, et al. A hypoarousal model of neurological post-COVID syndrome: the relation between mental fatigue, the level of central nervous activation and cognitive processing speed. J Neurol. (2023) 270:4647–60. doi: 10.1007/s00415-023-11819-7
Crossref Full Text | Google Scholar
19. Ariza, M, Cano, N, Segura, B, Adan, A, Bargalló, N, Caldú, X, et al. COVID-19 severity is related to poor executive function in people with post-COVID conditions. J Neurol. (2023) 270:2392–408. doi: 10.1007/s00415-023-11587-4
PubMed Abstract | Crossref Full Text | Google Scholar
20. García-Sánchez, C, Calabria, M, Grunden, N, Pons, C, Arroyo, JA, Gómez-Anson, B, et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav. (2022) 12:e2508. doi: 10.1002/brb3.2508
PubMed Abstract | Crossref Full Text | Google Scholar
21. Delgado-Alonso, C, Valles-Salgado, M, Delgado-Álvarez, A, Yus, M, Gómez-Ruiz, N, Jorquera, M, et al. Cognitive dysfunction associated with COVID-19: a comprehensive neuropsychological study. J Psychiatr Res. (2022) 150:40–6. doi: 10.1016/j.jpsychires.2022.03.033
PubMed Abstract | Crossref Full Text | Google Scholar
22. Ariza, M, Cano, N, Segura, B, Adan, A, Bargalló, N, Caldú, X, et al. Neuropsychological impairment in post-COVID condition individuals with and without cognitive complaints. Front Aging Neurosci. (2022) 14:1029842. doi: 10.3389/fnagi.2022.1029842
PubMed Abstract | Crossref Full Text | Google Scholar
23. Claros-Salinas, D, Bratzke, D, Greitemann, G, Nickisch, N, Ochs, L, and Schröter, H. Fatigue-related diurnal variations of cognitive performance in multiple sclerosis and stroke patients. J Neurol Sci. (2010) 295:75–81. doi: 10.1016/j.jns.2010.04.018
PubMed Abstract | Crossref Full Text | Google Scholar
24. Hartung, TJ, Bahmer, T, Chaplinskaya-Sobol, I, Deckert, J, Endres, M, Franzpötter, K, et al. Predictors of non-recovery from fatigue and cognitive deficits after COVID-19: a prospective, longitudinal, population-based study. EClinicalMedicine. (2024) 69:102456. doi: 10.1016/j.eclinm.2024.102456
PubMed Abstract | Crossref Full Text | Google Scholar
25. Stoll SEG, MS, Watolla, D, Bauer, I, Lunz, V, Metsch, M, Kath, P, et al. Fatigue und Fatigability bei Patienten mit Multipler Sklerose vor und nach kognitiver Belastung versus Entspannung – eine Pilotstudie. Neurol Rehabil. (2021) 27:23–30. doi: 10.14624/NR2101003
Crossref Full Text | Google Scholar
26. Premraj, L, Kannapadi, NV, Briggs, J, Seal, SM, Battaglini, D, Fanning, J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. (2022) 434:120162. doi: 10.1016/j.jns.2022.120162
Crossref Full Text | Google Scholar
27. Renaud-Charest, O, Lui, LMW, Eskander, S, Ceban, F, Ho, R, Di Vincenzo, JD, et al. Onset and frequency of depression in post-COVID-19 syndrome: a systematic review. J Psychiatr Res. (2021) 144:129–37. doi: 10.1016/j.jpsychires.2021.09.054
Crossref Full Text | Google Scholar
29. Kupferschmitt, A, Jöbges, M, Randerath, J, Hinterberger, T, Loew, TH, and Köllner, V. Attention deficits and depressive symptoms improve differentially after rehabilitation of post-COVID condition - a prospective cohort study. J Psychosom Res. (2023) 175:111540. doi: 10.1016/j.jpsychores.2023.111540
PubMed Abstract | Crossref Full Text | Google Scholar
30. Morawa, E, Krehbiel, J, Borho, A, Herold, R, Lieb, M, Schug, C, et al. Cognitive impairments and mental health of patients with post-COVID-19: a cross-sectional study. J Psychosom Res. (2023) 173:111441. doi: 10.1016/j.jpsychores.2023.111441
PubMed Abstract | Crossref Full Text | Google Scholar
31. Huth, D, Bräscher, AK, Tholl, S, Fiess, J, Birke, G, Herrmann, C, et al. Cognitive-behavioral therapy for patients with post-COVID-19 condition (CBT-PCC): a feasibility trial. Psychol Med. (2024) 54
留言 (0)