Parkinson’s disease (PD) is a common neurodegenerative disease that affects approximately 2–3% of the world’s population aged 65 years and over (1), and is characterized by the depletion of dopaminergic neurons in the substantia nigra (SN), leading to a subsequent dopamine deficiency in the striatum (2). PD is primarily known for its motor impairments caused by the gradual depletion of dopaminergic neurons. Nonetheless, individuals with PD also endure a wide range of disabling non-motor symptoms, such as anosmia, constipation, autonomic dysfunction, psychiatric manifestations and cognitive impairment (3), these symptoms are not directly linked to the degree of motor impairment and may even manifest prior to the appearance of motor symptoms.
Over the past few years, significant progress has been made by researchers in understanding the pathology of the disease. Although the precise mechanisms underlying PD pathology are complex and involve multiple factors, several molecular pathways have been identified as playing a role, such as the accumulation of misfolded α-synuclein, mitochondrial dysfunction, oxidative stress, inflammation, and elevated iron levels (4–7).
Research has demonstrated that iron plays a pivotal role in the cell death process known as ferroptosis (8), and iron deposition has long been suspected as a contributing factor in the damage of dopaminergic neurons (9). The accumulation of iron in the brains of healthy individuals usually increases with age (10), but this increase is notably less pronounced compared to that seen in patients with PD. As a powerful pro-oxidant, excess iron causes an increase in reactive oxygen species, disrupts mitochondrial function, and ultimately leads to cell death through iron-related mechanisms (11, 12). Furthermore, iron overload can facilitate the aggregation of α-synuclein (6). Quantitative susceptibility mapping (QSM) is a Magnetic Resonance Imaging (MRI) technique that can be employed to quantify iron content (13). Anatomical landmarks, particularly those found in deep gray matter regions, can be easily recognized using QSM. QSM exhibits increased precision in measuring iron concentrations in tissues, coupled with improved dependability and reproducibility (14). Additionally, the magnetic susceptibility values (MSVs) obtained through QSM provide useful quantifiable metrics for evaluations and comparisons among different groups.
In this study, we used QSM to determine iron content in the deep gray matter of PD brain, with the aim of evaluating iron deposition in different stages of PD and its association with motor and non-motor symptoms of PD.
2 Materials and methods 2.1 Participants and agreementsFrom November 2021 to November 2023, a total of 56 patients were consecutively recruited from the Department of Neurology, Affiliated Hospital of Nantong University. The diagnosis of PD was made according to the MDS clinical diagnostic criteria for idiopathic PD (15) and was evaluated by two neurologists specializing in movement disorders. Additionally, 29 healthy controls (HCs), matched for age and gender, were included in the study, with no known history of clinically overt neurological or psychiatric disease. Patients were excluded from the study if they presented with the following conditions: (1) secondary parkinsonism, parkinson-plus syndromes and hereditary parkinson syndromes; (2) a history of neurological diseases, such as severe head trauma or stroke; (3) poor image quality; (4) general contraindications for MRI scanning, including claustrophobia, pacemaker, or implanted metal parts. (5) PD patients had comorbidities that could influence iron accumulation or clinical symptom expression. The Ethics Committees of the Affiliated Hospital of Nantong University granted approval for this study, and prior to participation, written informed consent was obtained from all subjects.
2.2 Clinical evaluationsAll patients with PD were assessed within 1 week before QSM by a series of rating scales for evaluating the motor and non-motor symptoms. Severity of PD was assessed by Hoehn and Yahr (H-Y) stage scores, and patients with a stage ≤2 were grouped into the early stage PD group, while those with a stage >2 were grouped into the advanced stage PD group. The third part of the MDS-Unified PD Rating Scale (UPDRS-III) (16), served as the metric to determine the extent of motor symptoms. Additionally, the Mini-Mental Status Examination (MMSE) was used to assess cognitive impairment, while the Hamilton Anxiety Scale (HAMA) and the Hamilton Depression Scale (HAMD) were utilized to measure anxiety and depression, respectively.
2.3 MRI acquisitionAll participants underwent MRI scans using a 3 T MR scanner (Signal 750w; GE Healthcare, USA) with a multi-echo gradient recalled echo (GRE) sequence. All PD patients discontinued dopamine agonists for one week prior to the scan. To prevent head movement and minimize scanner noise, foam pads and earplugs were used. In addition to the GRE sequence for QSM, routine brain MRI scans included T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), and T2-weighted fluid attenuated inversion recovery (T2-FLAIR), which would be helpful for anatomical guidance and lesion exclusion. The scan parameters for QSM were set as follows: echo time (TE) of first echo = 3.3 ms; echo spacing = 2.3 ms; total number of echoes = 16; repeat time (TR) = 32.5 ms; flip angle (FA) = 20°; field of view (FOV) = 256 × 256 mm2; matrix = 256 × 256; layer thickness = 1 mm; acceptance bandwidth = 62.50 Hz/Px; imaging time = 3 min 42 s.
2.4 MRI post processingThe phase images with multiple echoes were unwrapped using a Laplacian-based unwrapping technique (17). To eliminate the background field from the unwrapped phase image, the V-SHARP (variable-kernels sophisticated harmonic artifact reduction for phase data) method was applied in conjunction with a binary brain mask (18). Additionally, we utilized the STAR-QSM technique to further reduce streaking artifacts in the image (19). The QSM scans encompassed the bilaterally symmetric regions of the substantia nigra (SN), red nucleus (RN), globus pallidus (GP), putamen (PT) and caudate nucleus (CN) (Figure 1). Cerebrospinal fluid was chosen as the reference region for QSM of the brain. Two experienced neuroradiologists, blinded to the subjects’ identity and clinical details, manually traced the ROIs using AW Volume Share 5.0 software. First, we opened the QSM images in the software and adjusted the contrast of the acquired images. Then, we selected the most prominent layer, along with two adjacent layers of each nucleus, and performed manual segmentation. Finally, the software performed additional calculations to determine the average one-sided MSV of the three layers. Subsequently, the mean values from both sides were utilized as regional MSVs for further analysis. The unit for these MSVs is ppm (parts per million).
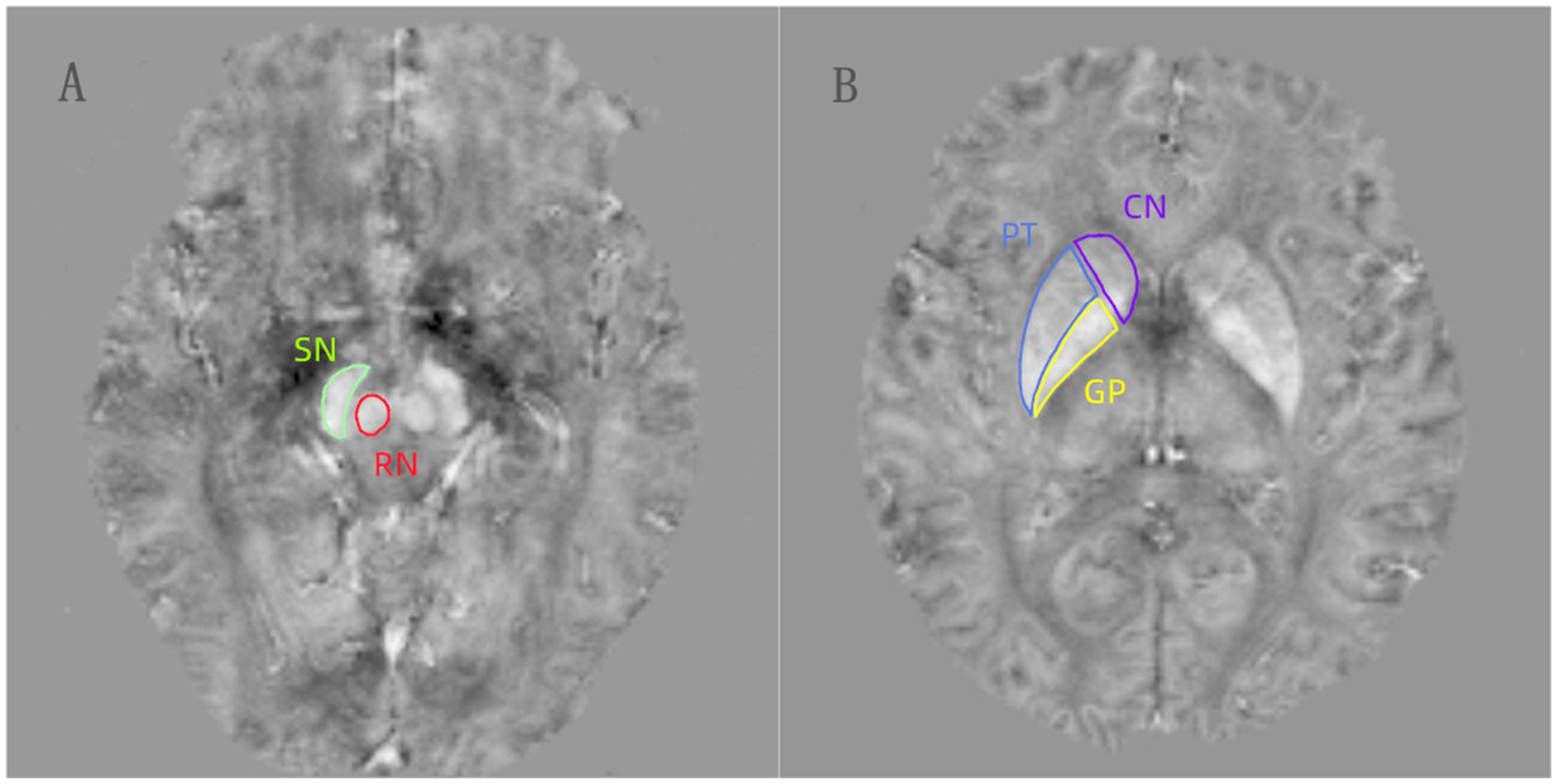
Figure 1. (A,B) The regions of interests (ROIs) were manually drawn for the substantia nigra (SN), red nucleus (RN), globus pallidus (GP), putamen (PT), and caudate nucleus (CN) on the QSM images.
2.5 Statistical analysisAll datas analyzed in this study were processed using IBM SPSS 25.0 software. Normality tests were conducted on the continuous variables. Variables exhibiting a normal distribution were presented using mean ± standard deviation. Variables that deviated from a normal distribution, on the other hand, were represented by the median and interquartile range. Categorical variables were expressed as frequencies and percentages. In this study, age, UPDRS-III scores, HAMA scores, and regional MSVs all exhibited a normal distribution, while the disease duration, HAMD scores, and MMSE scores all followed a skewed distribution.
For continuous variables, the two datasets were compared using either the independent samples t-test (when the data followed a normal distribution) or the Mann–Whitney U-test (when the distribution was skewed). Pearson’s chi-square tests were used to compare categorical variables. Spearman or pearson correlation analysis was used to evaluate the correlations between MSVs and clinical scores. p < 0.05 was considered to be statistically significant.
3 Results 3.1 Demographic dataDemographic and clinical characteristics were summarized in Table 1. Compared to the HCs, the PD group exhibited statistically significant differences in the MSVs of the SN and GP, and the intercomparisons of MSVs between the two groups were performed controlling for age and gender. Whereas, no significant differences were observed in the MSVs of the RN, PT, and CN.
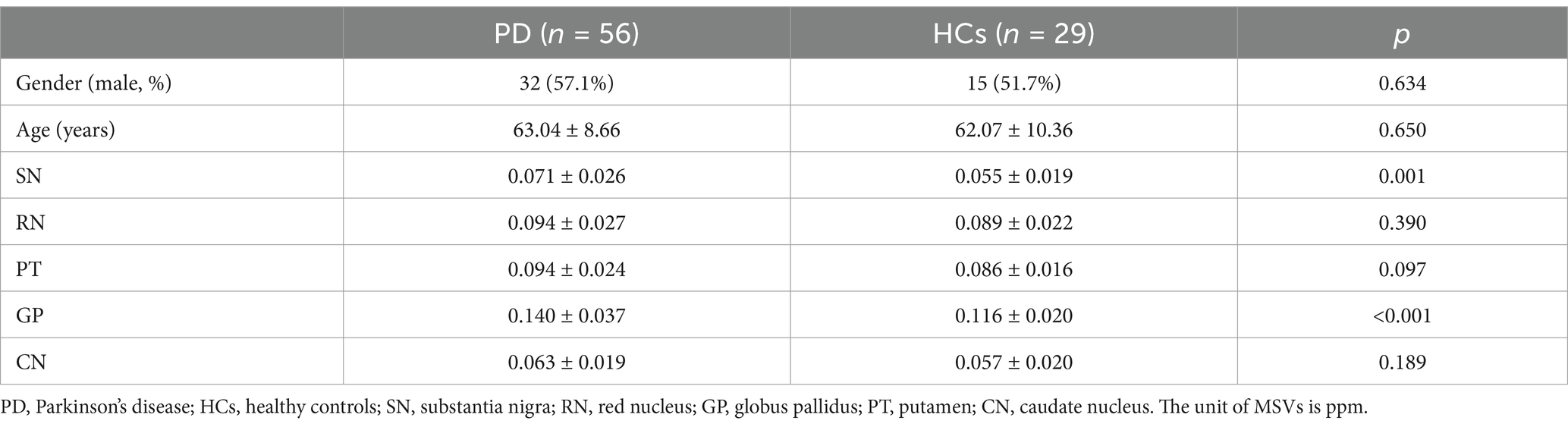
Table 1. Baseline demographic and clinical characteristics of participants.
3.2 Comparison of MSVs in different brain ROIs between early PD and advanced PDTable 2 summarizes the comparison between PD patients with early and advanced disease stages, indicating that patients in the advanced stage had higher ages, longer disease durations, and higher scores on the UPDRS-III, HAMA, and HAMD compared with patients in the early stage. Furthermore, significant differences could be seen when referring to iron deposition in SN, PT, GP, and CN, which is compliant with the progressiveness of PD. However, no marked differences in MSVs of RN were found between the two groups.

Table 2. Comparisons of clinical characteristics and regional MSVs between Early PD and Advanced PD.
3.3 Correlation analysis between clinical characteristics and regional MSVs in patients with PDIn the PD patients recruited for the current study, Table 2 and Figure 2 demonstrate a significant correlation between regional MSVs and the scores of the UPDRS-III, HAMA, HAMD, and MMSE. The results turned out that the MSVs of SN were significantly and positively correlated with scores of UPDRS-III, HAMA and HAMD (r = 0.310, p = 0.020; r = 0.273, p = 0.042; r = 0.342, p = 0.010, respectively). Similarly, MSVs of GP also had a notable correlation with scores of HAMA and HAMD (r = 0.275, p = 0.040; r = 0.415, p = 0.001). And the MSVs of PT was significantly correlated with the HAMD scores (r = 0.360, p = 0.006). We also observed a significant negative correlation between the MMSE scores and the MSVs of PT and GP (r = −0.268, p = 0.046; r = −0.305, p = 0.022).
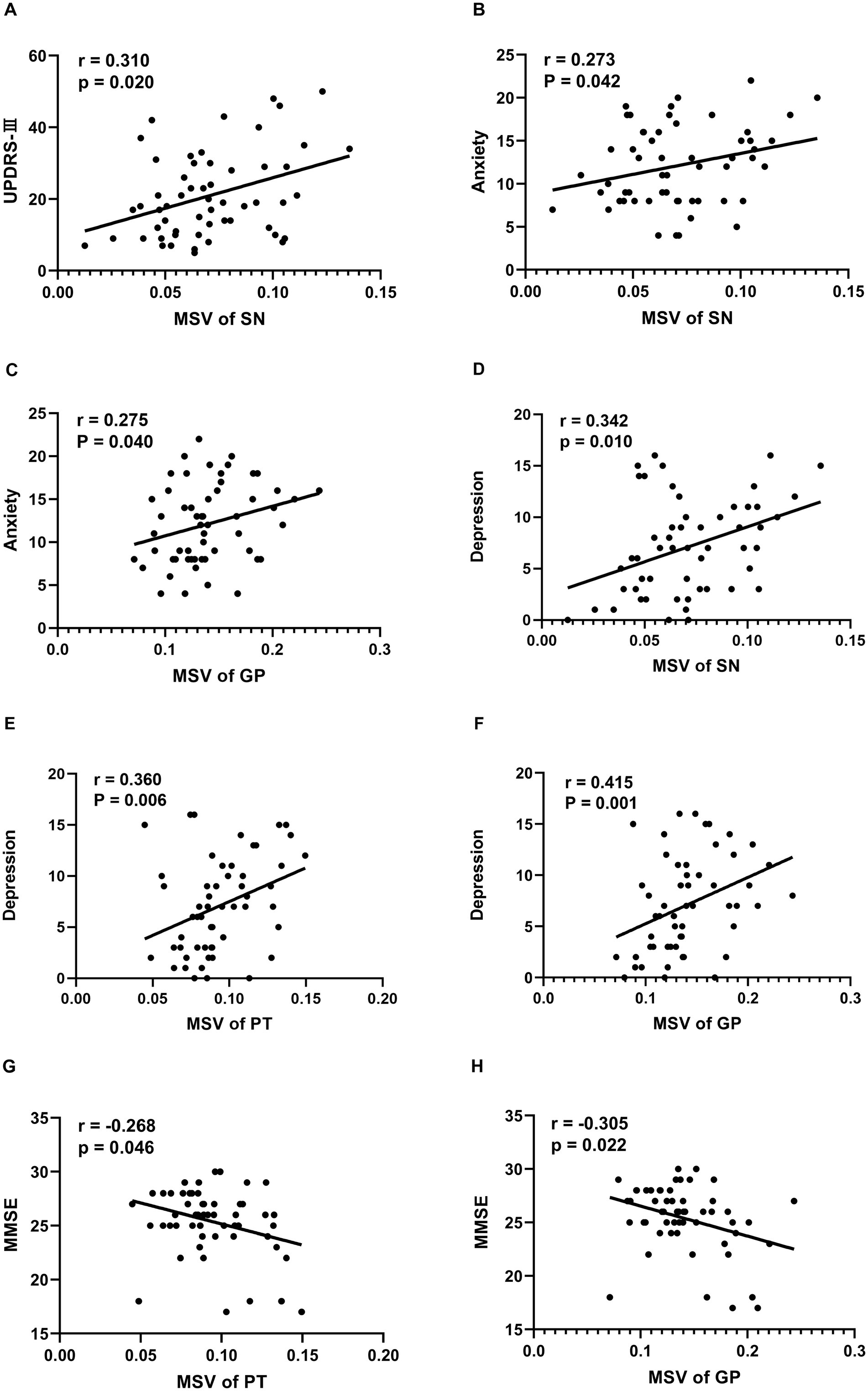
Figure 2. Correlation between clinical Characteristics and regional MSVs in PD patients. (A) Correlation between UPDRS-III scores and MSVs of SN. (B) Correlation between anxiety scores and MSVs of SN. (C) Correlation between anxiety scores and MSVs of GP. (D) Correlation between depression scores and MSVs of SN. (E) Correlation between depression scores and MSVs of PT. (F) Correlation between depression scores and MSVs of GP. (G) Correlation between MMSE scores and MSVs of PT. (H) Correlation between MMSE scores and MSVs of GP. The unit of MSVs is ppm.
4 DiscussionUntil now, the precise contributing factors in the development of PD remain elusive. It has been shown that in patients with PD, excess iron deposition has been observed in the deep gray matter structures of the brain, including the basal ganglia (comprising the GP, PT, and CN), the midbrain (including the RN and SN), and the dentate nucleus. Using QSM analyses to map the MSV in the nigrostriatal system, we found increased iron accumulation in SN and GP in PD patients compared with the HCs. As the disease progressed into the advanced stages, iron deposition extended to be more severe in the SN, GP, PT, and CN regions than in the early stages.
Several studies have documented the presence of unusual iron accumulations in certain deep brain nuclei of individuals with PD (20, 21). However, there is some inconsistency in these findings, which might stem from the limited sensitivity of the detection techniques and the relatively small number of participants. Studies have indicated that the SN is a crucial structure that has a significant impact on the pathophysiology of individuals with PD (22). A study utilizing QSM demonstrated that the concentration of iron in the SN of PD patients is significantly increased, exceeding the normal levels found in HCs. Furthermore, PD patients who exhibit more severe iron accumulation in the SN tend to score higher on the UPDRS motor section (20), which is consistent with our study.
The process of iron deposition in PD is complex, encompassing various mechanisms that affect iron distribution, transportation, storage, and circulation, ultimately resulting in iron accumulation within the SN and other parts of the brain (6). Previous studies have indeed demonstrated the existence of a diverse array of neural circuits, projections, and interconnected neural networks between the SN and the striatum. The dysfunction of dopaminergic striatal pathways is intricately linked to the elevated iron content in the SN (23). The cortical–limbic-striatal circuitry is also a functional brain network involved in neuromodulation. These intricate connections play a crucial role in the regulation and modulation of various motor and non-motor functions in the brain (24).
The potential associations between non-motor symptoms in PD and the underlying nigrostriatal pathology are not well understood. Increasing evidence suggests that a nigrostriatal dopamine deficiency in PD is linked not only to motor symptoms but also to non-motor symptoms (25, 26). Our study revealed that there is a notable correlation between iron deposition within the deep gray matter and non-motor symptoms (including anxiety, depression, and cognitive impairment), which was less frequently discussed in previous studies.
Anxiety is an emotional state that can arise from the anticipation of a potential or envisioned future danger or threat (27). Dopamine is a crucial neurotransmitter found in the central nervous system and plays a significant role in regulating human emotions. The degeneration of dopaminergic neurons located in the SN is confirmed to be the main pathological feature of PD, and alterations in dopaminergic activity within the limbic cortico-striato-thalamocortical circuits might also explain the high prevalence of anxiety among PD patients (28). Elevated iron levels within the neural circuits linked to fear in the brain are associated with anxiety in PD (29). Avila et al. (30) indicated that dopamine interacting with D2 or D4 receptors in the GP might play a role in the manifestation of anxiety. Our study revealed that PD patients with greater iron deposition in the SN and GP exhibited more severe anxiety symptoms. This iron accumulation contributes to cellular and tissue damage, potentially explaining the development of anxiety in PD.
Correlation analysis in our study provided some objective evidence that iron deposition within the SN, GP, and PT was actually correlated with the depression symptoms of PD patients. PD patients with depression exhibited a more significant loss of dopaminergic neurons in the SN compared to those without depression (31). This suggests that the SN is a crucial area for mood modulation in patients with PD (32). Moreover, in PD patients with depression, there is a noticeable widespread degeneration of dopaminergic terminals specifically within the striatum, and more prominently in the dorsal caudate nucleus (33). Hamilton et al. (34) observed that the raclopride binding potential increased in the ventral striatum bilaterally and in the right dorsal striatum of depressed participants. Furthermore, the connectivity between these regions and cortical targets, such as the cortico-striatal-pallido-thalamic circuit, was decreased.
Dopamine also plays a pivotal role in modulating hippocampal-dependent mnemonic processes, exerting distinct effects on multiple facets of memory and cognition (35). A previous study showed that striatal iron accumulations were correlated with dopaminergic deficits and neurophysiological signs in patients with PD (36). Our study found that there is a positive correlation between the iron deposition in the GP, PT and the severity of cognitive impairment in patients with PD. A recent study revealed that age-related alterations occur in the dopaminergic innervation of the striatum, resulting in decreased cognitive performance (37). Stögbauer et al. (38) found that the degeneration of dopaminergic neurons in the striatum, particularly those located in the executive subregion, plays a significant role in causing cognitive impairments observed in PD.
The current study has several limitations. First, the sample size utilized was comparatively small, thus future research endeavors involving more extensive cohorts are warranted to yield stronger and more reliable insights. Second, the MSV may be influenced by other factors such as calcium, copper, or lipid, although these effects are minor compared with those of iron. Third, the cross-sectional design of this study limits our ability to reach conclusions about the specific role of striatal iron in the progression of PD, therefore, more long-term studies are needed. Additionally, our current post-processing software requires manual segmentation to delineate the ROIs and lacks automatic segmentation functionality. In the future, we plan to select a more advanced post-processing software to achieve automatic segmentation and minimize result errors.
In summary, we documented the substantial accumulation of iron in the deep gray matter of patients with PD. The progressive pattern of iron deposition in the SN and GP might occur throughout the entire course of PD. Furthermore, there was a potential correlation between iron content in the SN, GP, and PT and certain clinical symptoms, such as motor impairments, anxiety, depression, and cognitive impairment. These findings offer a non-invasive biomarker for tracking disease progression and potentially contribute to a deeper understanding of the clinicopathological mechanisms underlying PD.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by Ethics Committees of the Affiliated Hospital of Nantong University (Ethical Approval Code: 2018-k026). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsHZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. Q-HJ: Methodology, Project administration, Resources, Supervision, Writing – review & editing. Z-ZJ: Conceptualization, Resources, Software, Writing – review & editing. L-HS: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Research Project of Jiangsu Provincial Health Commission (ZD2022040).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1442903/full#supplementary-material
References1. Poewe, W, Seppi, K, Tanner, CM, Halliday, GM, Brundin, P, Volkmann, J, et al. Parkinson disease. Nat Rev Dis Primers. (2017) 3:17013. doi: 10.1038/nrdp.2017.13
PubMed Abstract | Crossref Full Text | Google Scholar
2. García-Sanz, P, Aerts, JMFG, and Moratalla, R. The role of cholesterol in α-Synuclein and Lewy body pathology in GBA1 Parkinson's disease. Mov Disord. (2021) 36:1070–85. doi: 10.1002/mds.28396
PubMed Abstract | Crossref Full Text | Google Scholar
4. Moradi Vastegani, S, Nasrolahi, A, Ghaderi, S, Belali, R, Rashno, M, Farzaneh, M, et al. Mitochondrial dysfunction and Parkinson's disease: pathogenesis and therapeutic strategies. Neurochem Res. (2023) 48:2285–308. doi: 10.1007/s11064-023-03904-0
PubMed Abstract | Crossref Full Text | Google Scholar
5. Chakrabarti, S, and Bisaglia, M. Oxidative stress and Neuroinflammation in Parkinson's disease: the role of dopamine oxidation products. Antioxidants (Basel). (2023) 12:955. doi: 10.3390/antiox12040955
Crossref Full Text | Google Scholar
8. Yao, Z, Jiao, Q, Du, X, Jia, F, Chen, X, Yan, C, et al. Ferroptosis in Parkinson's disease -- the iron-related degenerative disease. Ageing Res Rev. (2024) 101:102477. doi: 10.1016/j.arr.2024.102477
Crossref Full Text | Google Scholar
9. Masaldan, S, Bush, AI, Devos, D, Rolland, AS, and Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic Biol Med. (2019) 133:221–33. doi: 10.1016/j.freeradbiomed.2018.09.033
PubMed Abstract | Crossref Full Text | Google Scholar
10. Lu, LN, Qian, ZM, Wu, KC, Yung, WH, and Ke, Y. Expression of Iron transporters and pathological hallmarks of Parkinson's and Alzheimer's diseases in the brain of young, adult, and aged rats. Mol Neurobiol. (2017) 54:5213–24. doi: 10.1007/s12035-016-0067-0
PubMed Abstract | Crossref Full Text | Google Scholar
13. Eskreis-Winkler, S, Zhang, Y, Zhang, J, Liu, Z, Dimov, A, Gupta, A, et al. The clinical utility of QSM: disease diagnosis, medical management, and surgical planning. NMR Biomed. (2017) 30:30. doi: 10.1002/nbm.3668
PubMed Abstract | Crossref Full Text | Google Scholar
15. Postuma, RB, Berg, D, Stern, M, Poewe, W, Olanow, CW, Oertel, W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
PubMed Abstract | Crossref Full Text | Google Scholar
16. Goetz, CG, Tilley, BC, Shaftman, SR, Stebbins, GT, Fahn, S, Martinez-Martin, P, et al. Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340
PubMed Abstract | Crossref Full Text | Google Scholar
17. Li, W, Avram, AV, Wu, B, Xiao, X, and Liu, C. Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed. (2014) 27:219–27. doi: 10.1002/nbm.3056
PubMed Abstract | Crossref Full Text | Google Scholar
19. Wei, H, Dibb, R, Zhou, Y, Sun, Y, Xu, J, Wang, N, et al. Streaking artifact reduction for quantitative susceptibility mapping of sources with large dynamic range. NMR Biomed. (2015) 28:1294–303. doi: 10.1002/nbm.3383
PubMed Abstract | Crossref Full Text | Google Scholar
20. Ghassaban, K, He, N, Sethi, SK, Huang, P, Chen, S, Yan, F, et al. Regional high Iron in the substantia Nigra differentiates Parkinson's disease patients from healthy controls. Front Aging Neurosci. (2019) 11:106. doi: 10.3389/fnagi.2019.00106
PubMed Abstract | Crossref Full Text | Google Scholar
21. Guan, X, Lancione, M, Ayton, S, Dusek, P, Langkammer, C, and Zhang, M. Neuroimaging of Parkinson's disease by quantitative susceptibility mapping. NeuroImage. (2024) 289:120547. doi: 10.1016/j.neuroimage.2024.120547
PubMed Abstract | Crossref Full Text | Google Scholar
23. Biondetti, E, Santin, MD, Valabrègue, R, Mangone, G, Gaurav, R, Pyatigorskaya, N, et al. The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson's disease. Brain. (2021) 144:3114–25. doi: 10.1093/brain/awab191
PubMed Abstract | Crossref Full Text | Google Scholar
24. Zhang, Y, Larcher, KM, Misic, B, and Dagher, A. Anatomical and functional organization of the human substantia nigra and its connections. eLife. (2017) 6:6. doi: 10.7554/eLife.26653
PubMed Abstract | Crossref Full Text | Google Scholar
25. Song, B, Feldmann, JW, Cao, S, Feitosa, M, Kong, Y, Kim, W, et al. A Pitx3-deficient developmental mouse model for fine motor, olfactory, and gastrointestinal symptoms of Parkinson's disease. Neurobiol Dis. (2022) 170:105777. doi: 10.1016/j.nbd.2022.105777
PubMed Abstract | Crossref Full Text | Google Scholar
26. Liu, R, Umbach, DM, Tröster, AI, Huang, X, and Chen, H. Non-motor symptoms and striatal dopamine transporter binding in early Parkinson's disease. Parkinsonism Relat Disord. (2020) 72:23–30. doi: 10.1016/j.parkreldis.2020.02.001
PubMed Abstract | Crossref Full Text | Google Scholar
28. Thobois, S, Prange, S, Sgambato-Faure, V, Tremblay, L, and Broussolle, E. Imaging the etiology of apathy, anxiety, and depression in Parkinson's disease: implication for treatment. Curr Neurol Neurosci Rep. (2017) 17:76. doi: 10.1007/s11910-017-0788-0
PubMed Abstract | Crossref Full Text | Google Scholar
29. Chen, K, Zhang, L, Mao, H, Chen, K, Shi, Y, Meng, X, et al. The impact of iron deposition on the fear circuit of the brain in patients with Parkinson's disease and anxiety. Front Aging Neurosci. (2023) 15:1116516. doi: 10.3389/fnagi.2023.1116516
PubMed Abstract | Crossref Full Text | Google Scholar
30. Avila, G, Picazo, O, Chuc-Meza, E, and García-Ramirez, M. Reduction of dopaminergic transmission in the globus pallidus increases anxiety-like behavior without altering motor activity. Behav Brain Res. (2020) 386:112589:112589. doi: 10.1016/j.bbr.2020.112589
PubMed Abstract | Crossref Full Text | Google Scholar
31. Fischer, NM, Hinkle, JT, Perepezko, K, Bakker, CC, Morris, M, Broen, M, et al. Brainstem pathologies correlate with depression and psychosis in Parkinson's disease. Am J Geriatr Psychiatry. (2021) 29:958–68. doi: 10.1016/j.jagp.2020.12.009
PubMed Abstract | Crossref Full Text | Google Scholar
32. Saari, L, Heiskanen, L, Gardberg, M, and Kaasinen, V. Depression and Nigral neuron density in Lewy body Spectrum diseases. Ann Neurol. (2021) 89:1046–50. doi: 10.1002/ana.26046
PubMed Abstract | Crossref Full Text | Google Scholar
34. Hamilton, JP, Sacchet, MD, Hjørnevik, T, Chin, FT, Shen, B, Kämpe, R, et al. Striatal dopamine deficits predict reductions in striatal functional connectivity in major depression: a concurrent (11)C-raclopride positron emission tomography and functional magnetic resonance imaging investigation. Transl Psychiatry. (2018) 8:264. doi: 10.1038/s41398-018-0316-2
PubMed Abstract | Crossref Full Text | Google Scholar
35. Duszkiewicz, AJ, McNamara, CG, Takeuchi, T, and Genzel, L. Novelty and dopaminergic modulation of memory persistence: a tale of two systems. Trends Neurosci. (2019) 42:102–14. doi: 10.1016/j.tins.2018.10.002
PubMed Abstract | Crossref Full Text | Google Scholar
36. Uchida, Y, Kan, H, Sakurai, K, Inui, S, Kobayashi, S, Akagawa, Y, et al. Magnetic susceptibility associates with dopaminergic deficits and cognition in Parkinson's disease. Mov Disord. (2020) 35:1396–405. doi: 10.1002/mds.28077
PubMed Abstract | Crossref Full Text | Google Scholar
37. Li, H, Hirano, S, Furukawa, S, Nakano, Y, Kojima, K, Ishikawa, A, et al. The relationship between the striatal dopaminergic neuronal and cognitive function with aging. Front Aging Neurosci. (2020) 12:41. doi: 10.3389/fnagi.2020.00041
PubMed Abstract | Crossref Full Text | Google Scholar
38. Stögbauer, J, Rosar, F, Dillmann, U, Faßbender, K, Ezziddin, S, and Spiegel, J. Striatal dopamine transporters and cognitive function in Parkinson's disease. Acta Neurol Scand. (2020) 142:385–91. doi: 10.1111/ane.13320
留言 (0)