Mycoplasma pneumoniae pneumonia (MPP) refers to pulmonary inflammation caused by an M. pneumoniae (MP) infection, which can affect bronchi, bronchioles, alveoli, and the pulmonary interstitium. According to MPP’s clinical classification, it can be divided into mild (MMPP), severe (SMPP), and fulminant M. pneumoniae pneumonia (FMPP) (1–3). In mild cases, the alveolar spaces are mostly infiltrated by neutrophils, while in severe cases, there is infiltration by lymphocytes, plasma cells, and macrophages; thickening and edema of the alveolar walls; exudation of fibrin and polypoid tissue in the alveolar cavity; necrosis and shedding of epithelial cells in the bronchus and bronchioles; destruction of cilia; and proliferation of fibroblasts, resulting in fibrotic changes and thus affecting gas exchange (2). Approximately 10% of children with COVID-19 have diffusion dysfunction after 3 months of follow-up, and the decline is more obvious in children with severe symptoms (4). However, one study has shown that carbon monoxide diffusion volume (DLCO) can return to normal 6 months after pneumococcal pneumonia in children (5). The improvement in pulmonary function examinations in children with MPP is of great significance for monitoring disease outcomes and improving the prognosis (6, 7). Previous studies have shown that pulmonary ventilation dysfunction in children with MPP can manifest as obstructive, restrictive, and mixed types (6, 8). However, there is no relevant study on whether pulmonary ventilation dysfunction in children with MMPP is different from that in children with SMPP, and there are relatively few studies on the changes in diffusion pulmonary function of children with MPP of different severities. Understanding the differences in lung function and diffuse lung function indicators between mild and severe M. pneumoniae pneumonia is beneficial for monitoring the severity of the disease and disease prognosis in clinical practice and providing guidance for disease diagnosis and treatment. This study retrospectively analyzed data from 97 children diagnosed with M. pneumoniae pneumonia, compared the ventilation lung function and diffusion lung function-related indicators in children with MMPP and SMPP, and further explored the changes in lung function in the children infected with M. pneumoniae.
2 Methods 2.1 Study populationThe data from 97 children (aged 6–13 years) with MPP who were hospitalized in the Children's Respiratory Department of Chengdu Women's and Children's Central Hospital from June 2023 to December 2023 were collected from electronic records by a researcher according to the Chinese “Guidelines for the Diagnosis and Treatment of Mycoplasma Pneumoniae in Children (2023 Edition)” (2). They were divided into an MMPP group and an SMPP group. The studies involving human participants were reviewed and approved by the ethics board of Chengdu Women and Children Center Hospital (Ethical number: [2021]203). Written informed consent to participate in this study was provided by the participants’ legal guardians.
The inclusion criteria for MMPP were according to clinical manifestations, chest imaging changes, and pathogenic examination as follows: (1) MP antibody titer of single serum ≥1:160 [particle agglutination (PA) method] or, during the course of the disease, the MP antibody titer in double serum increased four-fold or more; (2) positive for MP DNA or RNA (through a throat swab examination); (3) failed to reach SMPP diagnostic thresholds.
The inclusion criteria for SMPP were those who met one of the following criteria: (1) persistent high fever (above 39°C) ≥5 days or fever ≥7 days, with no downward trend of heat peak; (2) suffering from wheezing, shortness of breath, dyspnea, chest pain, hemoptysis, etc.; (3) experiencing extrapulmonary complications but the criteria for critical illness are not met; (4) in a resting state, inhaled air results in a pulse oxygen level ≤0.93; (5) presenting with one of the following imaging manifestations: (i) a single lung lobe ≥2/3 is involved with uniform high-density consolidation or two or more lung lobes have high-density consolidation (regardless of the size of the involved area), which can be accompanied by moderate to large pleural effusion and can also be accompanied by localized bronchiolitis; (ii) single lung diffuse or bilateral lobar bronchiolitis ≥4/5, which can be combined with bronchitis, and there is the formation of mucus plug leading to atelectasis; (6) the clinical symptoms worsen progressively, and the imaging shows that the range of lesions progressed more than 50% within 24–48 h; (7) C-reactive protein (CRP), lactate dehydrogenase (LDH), and/or D-dimer levels increase significantly.
The exclusion criteria were as follows: (1) Children with chronic inflammation of the respiratory system, bronchial asthma, immune system defects or suppression, chronic diseases of other organs, etc.; (2) children who had recently been treated with immunomodulators or inhibitors; (3) children with underlying diseases or major diseases; (4) children who met the diagnostic criteria of critical M. pneumoniae infection according to the “Guidelines for the Diagnosis and Treatment of Mycoplasma Pneumoniae in Children (2023 Edition)” as critical children may have unstable vital signs that make it difficult to cooperate with lung function tests and may also have other comorbidities that could affect the results of this study; (5) children with incomplete medical records.
2.2 MethodsThe clinical manifestations, laboratory examinations, imaging characteristics, and pulmonary function tests, including forced spirometry, lung volume measurement, and lung carbon monoxide diffusion capacity tests, of children were retrospectively analyzed. The laboratory examinations included CRP, LDH, D-dimer, and other indicators.
2.3 Pulmonary function measurementThe pulmonary ventilation function of the children was measured using a MasterScreen Pulmonary Function Testing System produced by Jaeger in Germany. The measured parameters were forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), forced expiratory flow at 25%, 50%, or 75% vital capacity (FEF25, FEF50, or FEF75), maximum mid expiratory flow (MMEF), functional residual capacity (FRC), total lung volume (TLC), residual volume (RV), residual to total lung volume ratio (RV/TLC), and DLCO [hemoglobin (Hb) correction was performed for anemic patients]. The above data were converted to Z-scores using the Global Lung Initiative reference equation.
2.4 Statistical analysisData analysis was performed using SPSS 26.0 statistical software. If the quantitative data followed a normal distribution (x ± s), two independent sample t-tests were used for inter-group comparisons. Quantitative data that were not normally distributed were described by the median and upper and lower quartiles (M, P25–P75), and compared between groups using the rank sum test of two independent samples (Mann–Whitney U test). Categorical data were described by percentages and compared between groups by the Chi-square test. P < 0.05 was considered statistically significant.
3 Results 3.1 Comparative demographic informationThere were 44 children in the MMPP group, with 24 boys and 20 girls aged 6–13 years. There were 53 cases in the SMPP group, with 27 boys and 26 girls aged 6–13 years. There was no statistical difference in sex and age composition ratio (P > 0.05), as shown in Table 1.
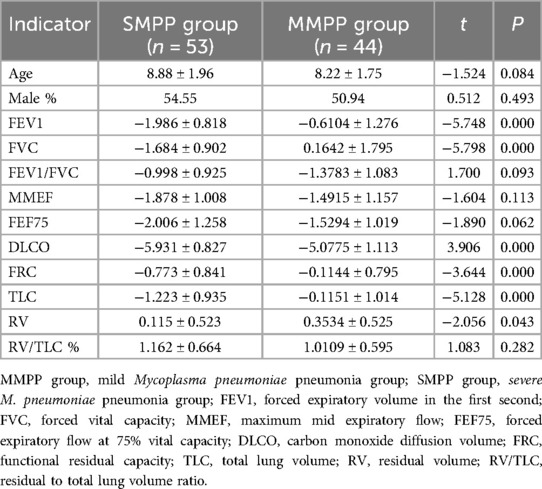
Table 1. Comparison of the Z-scores transformed from pulmonary ventilation function and lung diffusion function indexes in the SMPP and MMPP groups.
3.2 Comparison of pulmonary ventilation functionThe results of the Z-scores, transformed from the raw pulmonary ventilation function and lung diffusion function data, in the two groups are shown in Table 1. In the MMPP group, all the indexes were higher than normal except for the reduction in DLCO, while in the SMPP group, FVC, FEV1, FEF75, MMEF, and DLCO were lower than normal. FVC, FEV1, FEF75, and DLCO in the SMPP group were −1.684 ± 0.902, −1.986 ± 0.818, −2.006 ± 1.258, and −5.931 ± 0.827, which were lower than −0.6104 ± 1.276, 0.1642 ± 1.795, −1.5294 ± 1.0193 and −5.078 ± 1.113 in the MMPP group, respectively, P < 0.05 (Figure 1). These results suggested that the MMPP group had diffuse dysfunction and the SMPP group had restrictive ventilation function reduction and diffuse dysfunction. However, there was no statistically significant difference in FEV1/FVC and MMEF between the two groups.
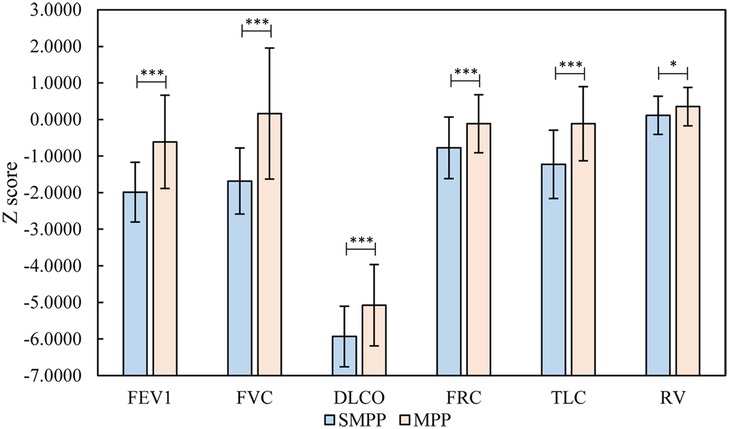
Figure 1. Relevant analysis of the results (FVC, FEV1/FVC, DLCO, FRC, TLC, RV) of the SMPP and MMPP groups. *P < 0.05; **P < 0.01; ***P < 0.001. MMPP group, mild Mycoplasma pneumoniae pneumonia group; SMPP group, severe M. pneumoniae pneumonia group; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; DLCO, carbon monoxide diffusion volume; FRC, functional residual capacity; TLC, total lung volume; RV, residual volume.
4 DiscussionOur study found that DLCO in the MMPP and SMPP groups was lower than the normal value, and DLCO in the SMPP group was significantly lower than that in the MMPP group. None of the children with SMPP had a decrease in blood oxygen saturation, but the DLCO of these children was significantly lower than the normal value. This indicates that there was abnormal gas exchange before the clinical manifestations of obvious hypoxia in these children, but it may still have been in the compensatory period. In addition, we also found that FVC, FEV1, and FEF75 in the SMPP group were lower than normal values and significantly lower than those in the MMPP group. FEV1/FVC were in the normal range, suggesting that the SMPP group had restrictive ventilation function reduction and this was more serious than that in the MMPP group. The FEF75 and MMEF in the SMPP group were lower than normal values, suggesting that SMPP can cause large and small airway dysfunction.
Respiratory system infection is a common disease in childhood. Lower respiratory tract infection, represented by pneumonia, is the main cause of death in children under 5 years old (9). MPP is one of the most common types of pneumonia in children. M. pneumoniae infection accounts for 10%–40% of the pathogens that cause community-acquired pneumonia (2) and up to 50% (3) in outbreak years. In recent years, the incidence of SMPP has increased year by year. During the COVID-19 pandemic, the incidence of M. pneumoniae infection decreased significantly but it has increased significantly again since 2023 (8). In some cities in China, children with M. pneumoniae infection accounted for nearly 25% of respiratory tract infections, of which SMPP accounted for 19.5%–23.1% (10). Another study showed that nearly 40% of children with MPP in China will develop SMPP (11) despite appropriate antimicrobial treatment, which poses a serious threat to these children's lives and health. Pneumonia in young children may increase the risk of asthma and impaired lung function (12). Childhood pneumonia is a major risk factor for adult chronic obstructive pulmonary disease (13). In addition, there are also relevant studies that show that MP infection is closely related to childhood bronchial asthma and is a significant cause of bronchial asthma (14–16). Therefore, detecting the changes in pulmonary function in children with MPP is helpful for the early detection of the disease. This study further explored the changes in pulmonary diffusion function after M. pneumoniae infection by examining the pulmonary ventilation and pulmonary diffusion functions of children with MPP, comparing the pulmonary diffusion and ventilation functions of children with SMPP and MMPP, and exploring their clinical significance.
This study found that FVC, FEV1, and FEF75 in the SMPP group were lower than normal values and significantly lower than those in the MMPP group, suggesting that the SMPP group had restrictive ventilation function reduction and this was more serious than the MMPP group. The FEF75 and MMEF in the SMPP group were lower than normal, suggesting that SMPP can cause large and small airway dysfunction, which is consistent with Yong et al. (17). This may be due to the specific antigen to Mycoplasma stimulating the body to produce inflammatory mediators, thus causing airway smooth muscle spasm and increased vascular permeability, leading to mucosal edema and increased secretion of mucus. This then causes congestion, edema, and erosion of tissues, reducing lung compliance, increasing elastic resistance, and reducing pulmonary surfactant, thus causing airway stenosis. The increase in airway resistance decreases the ventilation function of the large and small airways (18). A study on bacterial pneumonia in children showed that FEV1/FVC in severe pneumonia was 60.74 ± 2.83%, and the FEV1% was 60.29 ± 2.64%, which were significantly lower than the levels of the mild and control groups. However, the study did not provide information on small airway function (19).
Pulmonary diffusion function refers to the ability of certain alveolar gases to diffuse from the alveolar cavity to the capillaries and then through the alveolar-capillary membrane to the blood and combine with Hb (20). In clinical practice, the one-breath lung carbon monoxide diffusion function test (the DLCO single breath method, DLCO-SB), established by Ogilvie et al. (21), is the most commonly used. Common reasons for a decline in pulmonary diffusion function include (1) a reduction of the alveolar membrane area due to consolidation, atelectasis, or lobectomy; (2) increased alveolar membrane thickness due to pulmonary edema, formation of a transparent alveolar membrane, or pulmonary fibrosis; (3) ventilation/blood flow ratio (VA/Q) imbalance due to a pulmonary embolism, chronic obstructive emphysema, or pulmonary hypertension; and (4) a decrease in hemoglobin content due to blood system diseases, chronic renal insufficiency, or hemorrhagic anemia. Our study found that the DLCO of the SMPP group was lower than the normal value and was significantly lower than that of the MMPP group. The mechanism for this may be that MP adheres to and settles in respiratory epithelial cells after infection, damaging the cell membrane and then releasing community-acquired respiratory distress syndrome toxin (CARDs TX), hydrogen sulfide (H2S), hydrogen peroxide (H2O2), and other substances. The above substances cause cell dissolution, epithelial cell swelling, and necrosis (22), resulting in a reduction of the alveolar-capillary membrane area. Furthermore, after Mycoplasma infection, the airway ciliary activity is inhibited, and the formation of an alveolar hyaline membrane and the infiltration and edema of inflammatory cells in the alveolar wall lead to the thickening of the alveolar membrane and a decline in lung compliance (23). Some studies have shown that the airway epithelial cells are more damaged in SMPP than in MMPP (24). Furthermore, inflammatory cell infiltration is more serious in SMPP than in MMPP and there is fibrosis in the lumen and wall in the later stages, leading to airway distortion and occlusion. This may be why DLCO in the SMPP group decreased more significantly than that in the MMPP group. Marc et al. (5) also showed that 48% of the children with M. pneumoniae pneumonia in their study had a lower DLCO than normal. Furthermore, these children's diffuse lung function did not recover 6 months after being diagnosed with the disease. Similarly, 10% of children with COVID-19 have diffusion dysfunction, which lasts for more than 3 months, and the decline of diffusion function is more obvious in children with severe symptoms (4). Buchvald and Nielsen examined the pulmonary diffusion capacity of 20 children with hypersensitive pneumonitis and the results showed that the pulmonary diffusion capacity of these children decreased. During follow-up, it was found that the clinical symptoms of most of the children improved and their pulmonary diffusion capacity gradually recovered (25).
In addition, in our study, we found that the children with SMPP did not have a decrease in blood oxygen saturation but the DLCO of these children was significantly lower than the normal value. This indicates that there was abnormal gas exchange before the clinical manifestations of obvious hypoxia in these children but it may still be in the compensatory period when it is difficult to diagnose. A Norwegian study on COVID-19 also showed that the blood oxygen saturation of patients with severe COVID-19 was in the normal range even when their pulmonary diffusion function decreased (26). In addition, the DLCO in the SMPP group decreased more significantly than that in the MMPP group, suggesting that a decrease in DLCO can be used as an early warning indicator for the development of MMPP into SMPP. The dynamic detection of diffusion function in children may help to diagnose the disease early, improve the prognosis, and prevent the occurrence of chronic lung disease. However, this conclusion needs further verification in the future. Because most of the children in this study live in different places and lung function tests are expensive, it is difficult to dynamically monitor the prognosis of their pulmonary ventilation function and discern when their diffusion function returns to normal. However, we will attempt to follow up on the lung function of these patients in the future to obtain more data.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by the ethics board of Chengdu Women and Children Center Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsLW: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. QL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. JH: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. RL: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. YD: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TA: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Chengdu Women’s and Children’s Central Hospital (High-level clinical research projects, (2022LC02).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. (2015) 372:835–45. doi: 10.1056/NEJMoa1405870
PubMed Abstract | Crossref Full Text | Google Scholar
2. National Health Commission. Guidelines for the diagnosis and treatment of Mycoplasma pneumoniae in children (2023 edition). Infect Dis Inf. (2023) 36(4):291–7. doi: 10.19871/j.cnki.xfcrbzz.2024.01.015
Crossref Full Text | Google Scholar
3. Respiratory Group of Pediatric Branch of Chinese Medical Association, Editorial Committee of Chinese Journal of Practical Pediatrics. Expert consensus on diagnosis and treatment of Mycoplasma pneumoniae in children (2015 edition). Chin J Pract Pediatr. (2015) 30(17):1304–8. doi: 10.3760/cma.j.issn.2095-428X.2015.17.006
Crossref Full Text | Google Scholar
4. Öztürk GK, Beken B, Dogan S, Akar HH. Pulmonary function tests in the follow-up of children with COVID-19. Eur J Pediatr. (2022) 181(7):2839–47. doi: 10.1007/s00431-022-04493-w
PubMed Abstract | Crossref Full Text | Google Scholar
5. Marc E, Chaussain M, Moulin F, Iniguez JL, Kalifa G, Raymond J, et al. Reduced lung diffusion capacity after Mycoplasma pneumoniae pneumonia. Pediatr Infect Dis J. (2000) 19(8):706–10. doi: 10.1097/00006454-200008000-00007
PubMed Abstract | Crossref Full Text | Google Scholar
6. Liu FJ, Gong CH, Qin JJ, Fu Z, Liu S. Changes in pulmonary function in infants and young children with Mycoplasma pneumoniae pneumonia. Zhongguo Dang Dai Er Ke Za Zhi. (2020) 22(2):118–23. doi: 10.7499/j.issn.1008-8830.2020.02.007
PubMed Abstract | Crossref Full Text | Google Scholar
7. Ma X, Ding MJ, Zhao XX, Sun J, Yang JZ, Han YL. Features of lung dysfunction in children with Mycoplasma pneumoniae pneumonia with different chest imaging findings. Zhongguo Dang Dai Er Ke Za Zhi. (2014) 16(10):997–1000.25344179
PubMed Abstract | Google Scholar
8. Xiang M, Mingjie D, Xiuxia Z, Jing S, Jinzhi Y, Yuling H, et al. Characteristics of pulmonary function changes in children with Mycoplasma pneumoniae pneumonia with different chest imaging manifestations. Chin J Contemp Pediatr. (2014) 10:997–1000. doi: 10.7499/j.issn.1008-8830.2014.10.008
Crossref Full Text | Google Scholar
9. Jinrong L, Chengsong Z, Shunying Z. Interpretation of the diagnosis and treatment guidelines for children’s community acquired pneumonia (2019 edition). Chin J Pract Pediatr. (2020) 35(3):3. CNKI:SUN:ZSEK.0.2020-03-005
10. Zhang XB, He W, Gui YH, Lu Q, Yin Y, Zhang JH, et al. Current Mycoplasma pneumoniae epidemic among children in Shanghai: unusual pneumonia caused by usual pathogen. World J Pediatr. (2024) 20(1):5–10. doi: 10.1007/s12519-023-00793-9
PubMed Abstract | Crossref Full Text | Google Scholar
11. Jin J, Wang L, Zhang MZ. Clinical features and bronchoscopy treatment for 378 patients of Mycoplasma pneumoniae pneumonia in children. Chongqing Med. (2015) 44:4815–7. doi: 10.3969/j.issn.1671-8348.2015.34.022
Crossref Full Text | Google Scholar
12. Collaro AJ, McElrea MS, Marchant JM, Chatfield MD, Sondergeld P, Perret JL, et al. The effect of early childhood respiratory infections and pneumonia on lifelong lung function: a systematic review. Lancet Child Adolesc Health. (2023) 7(6):429–40. doi: 10.1016/S2352-4642(23)00030-5
PubMed Abstract | Crossref Full Text | Google Scholar
13. Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. (2018) 6(7):535–44. doi: 10.1016/S2213-2600(18)30100-0
PubMed Abstract | Crossref Full Text | Google Scholar
14. Liu X, Wang Y, Chen C, Liu K. Mycoplasma pneumoniae infection and risk of childhood asthma: a systematic review and meta-analysis. Microb Pathog. (2021) 155:104893. doi: 10.1016/j.micpath.2021.104893
PubMed Abstract | Crossref Full Text | Google Scholar
15. Gorina LG, Krylova NA, Rakovskaya IV, Geppe NA, Gamova NA, Barkhatova OI. Mechanisms of long-term persistence of mycoplasmas in children with asthma. Microorganisms. (2023) 11(7):1683. doi: 10.3390/microorganisms11071683
PubMed Abstract | Crossref Full Text | Google Scholar
16. Bian C, Li S, Huo S, Yang B, Wang P, Li W, et al. Association of atopy with disease severity in children with Mycoplasma pneumoniae pneumonia. Front Pediatr. (2023) 11:1281479. doi: 10.3389/fped.2023.1281479
PubMed Abstract | Crossref Full Text | Google Scholar
17. Yong W, Junwei C, Lihao L. Dynamic observation of lung function in children with severe Mycoplasma pneumonia. China Contemp Med. (2016) 23(7):114–6.
18. Hao Z, Yufen W. Effects of different respiratory tract infectious diseases on lung function in children. Chin J Pract Pediatr. (2019) 34(22):1693–7.
19. Jiaying S, Ting W, Gang L. Distribution characteristics of pathogenic bacteria in children with bacterial pneumonia and difference of inflammatory indexes in children with different severity. Chin J Pathog Biol. (2023) 18(12):1452–6. doi: 10.13350/j.cjpb.231217
Crossref Full Text | Google Scholar
20. Pulmonary Function Specialized Group of the Respiratory Disease Branch of the Chinese Medical Association. Guidelines for pulmonary function tests - lung diffusion function tests. Chin J Tuberc Respir. (2015) 38(3):164–9. doi: 10.3760/cma.j.issn.1001-0939.2015.03.003
Crossref Full Text | Google Scholar
21. Ogilvie CM, Forster RE, Blakemore WS, Morton JW. A standardized breath holding technique for the clinical measurement of the diffusing capacity of the lung for carbon monoxide. J Clin Invest. (1957) 36(1 Part 1):1–17. doi: 10.1172/JCI103402
PubMed Abstract | Crossref Full Text | Google Scholar
22. Minmin L, Xiaoming W. Research progress on the correlation between Mycoplasma pneumoniae infection and childhood asthma. Med Rev. (2015) 21(19):3524–5. doi: 10.3969/j.issn.1006-2084.2015.19.025
Crossref Full Text | Google Scholar
23. Jiang W, Qian L, Liang H, Tian M, Liu F, Zhao D. Relationships between the varied ciliated respiratory epithelium abnormalities and severity of Mycoplasma pneumoniae pneumonia. Scand J Infect Dis. (2014) 46(7):486–92. doi: 10.3109/00365548.2014.885658
PubMed Abstract | Crossref Full Text | Google Scholar
24. Liu J, He R, Zhang X, Zhao F, Liu L, Wang H, et al. Clinical features and "early" corticosteroid treatment outcome of pediatric Mycoplasma pneumoniae pneumonia. Front Cell Infect Microbiol. (2023) 13:1135228. doi: 10.3389/fcimb.2023.1135228
PubMed Abstract | Crossref Full Text | Google Scholar
25. Buchvald F, Nielsen KG. Long term lung function and diffusion capacity in children with previous hypersensitivity pneumonitis. Paediatr Respir Rev. (2013) 14:63. doi: 10.1016/S1526-0542(13)70084-8
PubMed Abstract | Crossref Full Text | Google Scholar
26. Lerum TV, Aaløkken TM, Brønstad E, Aarli B, Ikdahl E, Lund KMA, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. (2021) 57(4):2003448. doi: 10.1183/13993003.03448-2020
留言 (0)