Acute lymphoblastic leukemia (ALL) is the most prevalent childhood cancer, occurring in approximately 25% of all children aged 0–14 years (1). When treated based on risk adaptation using multi-agent chemotherapy protocols, children show remarkable improvements in survival rates, with an overall survival (OS) rate >90% (2).
Relapse is the leading cause of ALL treatment failure, particularly in patients with B-cell ALL. The relapse rate in patients with ALL ranges from 15%–20%, and the OS rate after relapse ranges from 40 to 70%. All relapses can be classified according to the site as isolated bone marrow relapse, extramedullary relapse, or combined bone marrow with extramedullary relapse, whereas timing can range between early (occurring within 18 months of diagnosis) and late (after 36 months) (3). The addition of blinatumomab, a bispecific antibody targeting CD3 and CD19, in patients with high-risk relapsed B-ALL led to better outcome (4, 5).
Only 5%–17% of ALL relapses are asymptomatic, with no difference in survival rates between relapses detected using abnormal cell counts and symptomatic relapses (6, 7).
Data on the sensitivity and specificity of routine blood count surveillance for detecting relapse and its effects on disease outcomes and complications are scarce. We aimed to evaluate the efficacy of routine blood testing using a systematic method of assessing normal and abnormal results.
MethodsWe conducted a retrospective cohort study between 2014 and 2021 in patients diagnosed with ALL aged 1–14 years treated at our institution. For B-cell ALL, our center risk stratifies, treats, and assesses responses according to the Children's Oncology Group (COG) AALL0932 and AALL1131 for standard risk (SR) and high risk (HR), respectively (8, 9). For T-cell ALL, we treat patients according to AALL0434 (10) with modifications (11). Risk and relapse patients were stratified according to COG AALL1331 (4), and patients were identified from the hospital database. All patients with leukemia who had been treated and completed therapy in complete response and were followed up at our institution were included. Patients with Down syndrome or inherited cytopenia were excluded from this study. Data abstracted from electronic health records included patient demographics; leukemia subtype; risk stratification; response and outcome; follow-up laboratory testing results, including white blood cell count, hemoglobin level, platelet count, and absolute neutrophil count; relapse site and time; and post-relapse complications. Follow-up data were collected after primary treatment completion, with the last date of chemotherapy used as the date of completion.
The patients were followed up according to the institutional guidelines, which included clinic visits and laboratory tests every 1 month for the first 6 months, every 2 months for the second 6 months of the first year and every 3–4 months for the second and third years, every 6 months thereafter for the fourth year, and annually thereafter. This study was approved by the institutional review board (No: NRJ21J-205-08).
The patients were classified into full laboratory surveillance (100% adherence to surveillance protocol), partial surveillance (<100% adherence), and no surveillance groups. The relapse groups were classified as asymptomatic (relapse diagnosed using routine blood tests) and symptomatic (diagnosed clinically). The clinical outcomes related to relapse or complications were analyzed for each category.
For each surveillance laboratory component, we assessed whether the test results were normal or abnormal based on the Common Terminology Criteria for Adverse Events version 5.5.
The patients were considered to have abnormal results if their test abnormalities were grade 2 or higher.
Statistical analysesBaseline patient characteristics are expressed as absolute frequency (%) and interquartile range (IQR) values. Categorical data were compared using Fisher's exact tests, and continuous data are presented as medians and IQRs and compared using the Mann–Whitney U test. The value of blood count in detecting relapse was assessed using sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV). Number needed to screen (NNS) is defined as the number of patients that need to be screened for the duration to detect relapse event. Statistical tests were performed using GraphPad Prism version 9.0.0.
ResultsBetween January 2014 and December 2021, 187 patients with ALL met the eligibility criteria for this study. The median age of the patients was 12 (range, 10–16) years, and 161 (86.10%) had B-cell ALL. In total, 113 (60.4%) patients had National Cancer Institute Standard-risk (SR). Ninety-three (49.4%) patients were assigned to the SR treatment group, whereas 74 (39%) patients were assigned to the High-risk (HR) group. Most patients [130 (69.5%)] had favorable cytogenetics, and 158 (84.4%) patients attained negative minimal residual disease by the end of induction. No cases of treatment-related leukemia were identified in our cohort (Table 1).
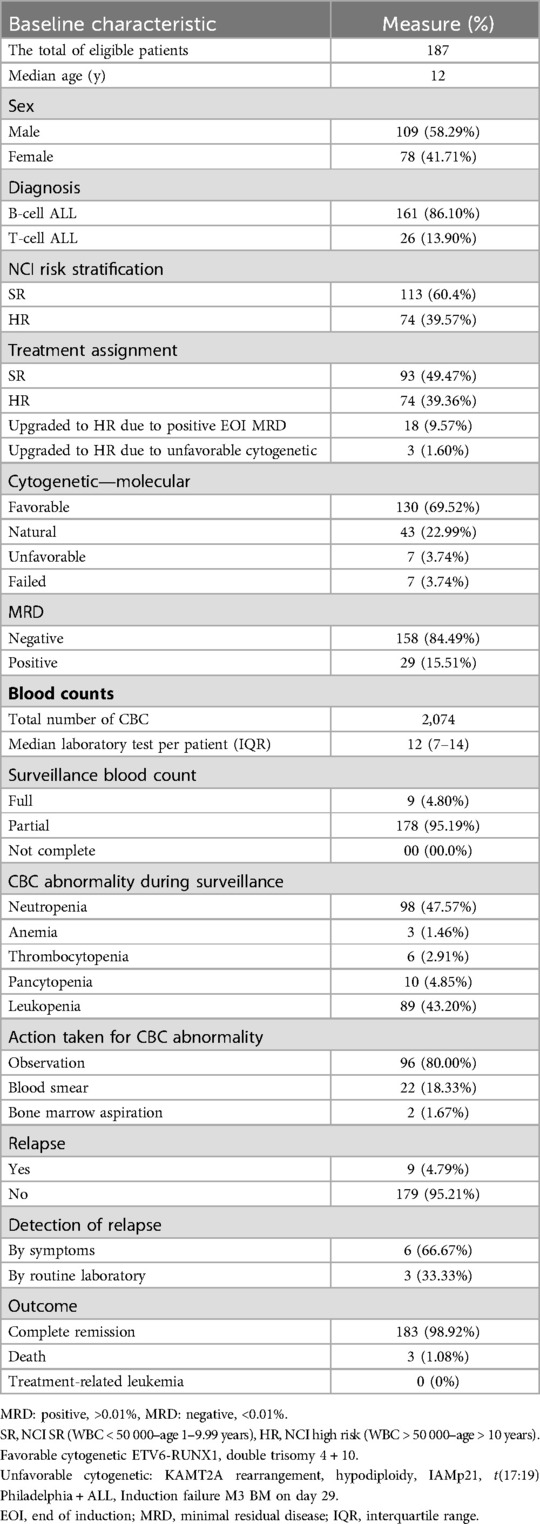
Table 1. Patient and disease characteristics.
Disease surveillanceThe total complete blood count (CBC) tests performed for the whole cohort were 2074; the median laboratory test per patient was 12 (IQR, 7–14). Laboratory abnormalities were detected in 201 tests; the most common abnormalities detected were neutropenia 98 (47.57%) and leukopenia 89 (43.2%). Most patients [96 (80%)] were observed; 22 (18.3) and 2 (1.6%) patients underwent blood smear and bone marrow assessment, respectively.
Most patients [178 (95.1%)] underwent partial surveillance; nine (4.79%) patients relapsed at a median follow-up period of 18 months. Of the total 187 patients, only three relapses were identified through routine blood tests (Table 1). The calculated number needed to screen (NNS) to detect a relapse was 63.
The most frequent abnormal laboratory results were thrombocytopenia [n = 3 (33%)] and pancytopenia [n = 3 (33%)]. The median blast percentage at relapse was 70% (IQR, 52%-76%) (Table 2). Comparing the blast percentage between the two groups at initial presentation of relapse, the symptomatic and asymptomatic blast percentages were 57% (IQR, 32%-72%) and 76.5% (IQR, 70%-83%) (p = 0.0397), respectively.
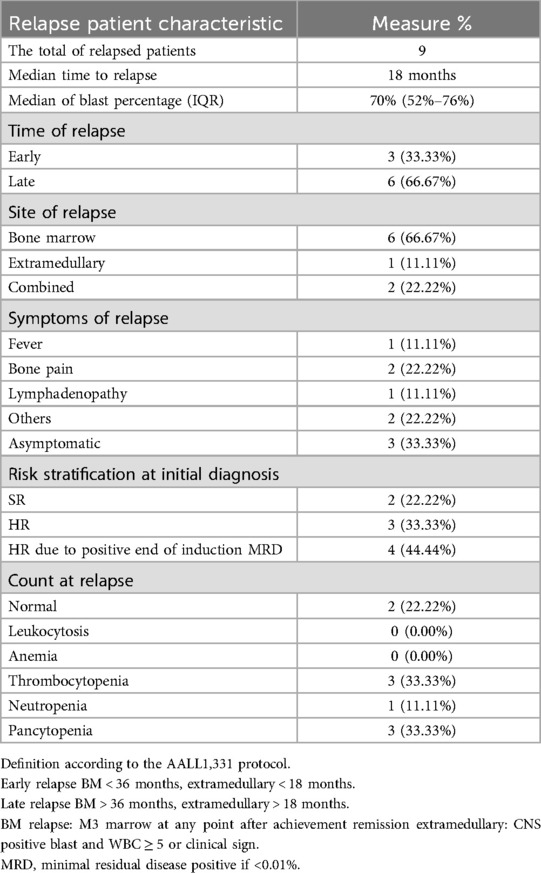
Table 2. Relapse characteristics.
The sensitivity and specificity of neutropenia, leukopenia, thrombocytopenia, anemia, and pancytopenia were 11.11% and 47.9%, 0% and 50%, 33.3% and 98.31%, 0% and 99.4%, and 33.3% and 96.07%, respectively. PPV and NPV were assessed for each abnormal blood count and are summarized in Table 3.
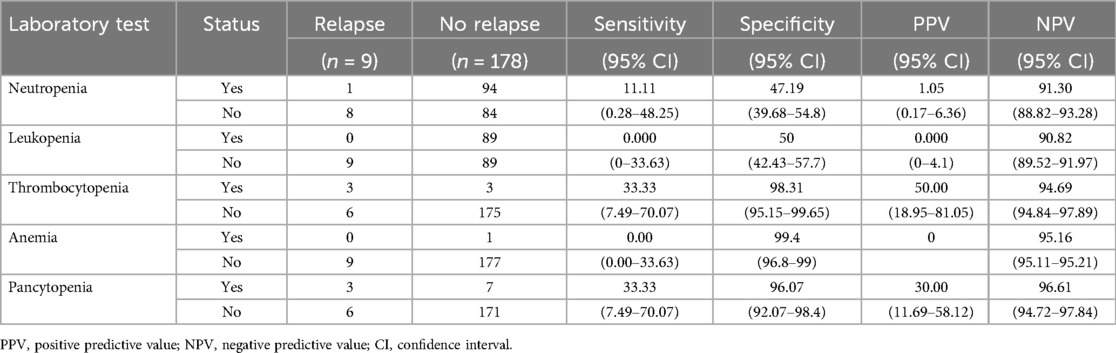
Table 3. Measurement of screening accuracy.
Relapse characteristics and outcomesOut of the nine patients with relapse, 66.6% (n = 6) had late relapse. Bone marrow relapse was the most common relapse (66.6%), and most relapse occurred in the high-risk group [n = 7 (77.7%)]. No predominant symptoms were associated with symptomatic relapse.
Treatment-related complications, including infection, tumor lysis syndrome, and medication toxicity, were comparable between the two groups (Table 4).
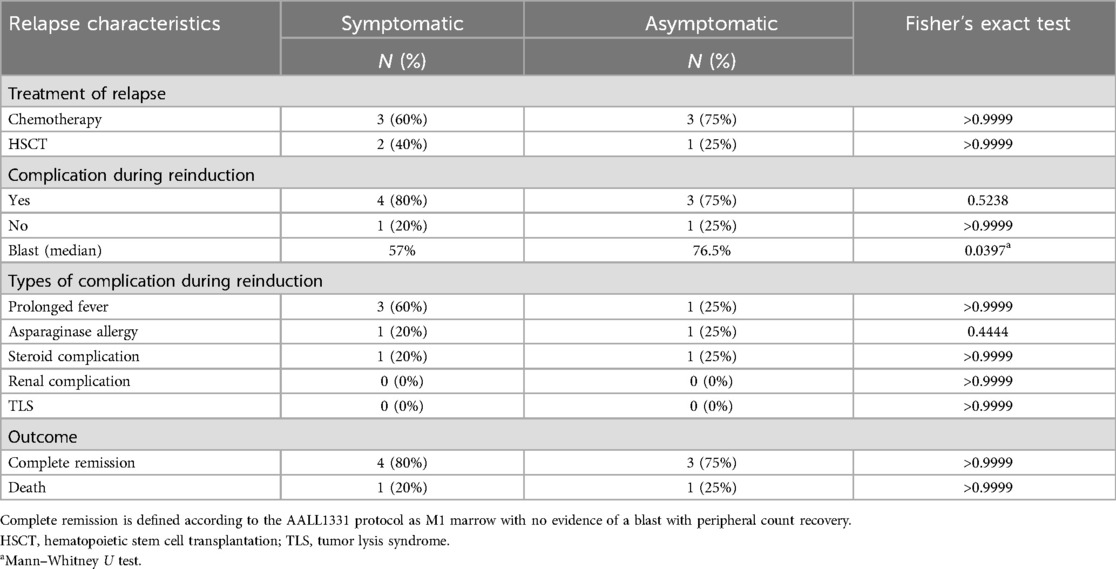
Table 4. Relapse treatment and outcomes.
Seven patients achieved remission after reinduction therapy, and three patients underwent hematopoietic stem cell transplantation. Seven patients were alive at the median follow-up period of 30 (IQR, 12–36) months (Table 4).
DiscussionTreatment outcomes of children with ALL have improved over the last few years (2). An ideal surveillance protocol should encompass modalities capable of detecting most cancer types commonly found in at-risk individuals. Additionally, the selected modalities should demonstrate effectiveness in the early detection of asymptomatic tumors that can be successfully managed with minimal treatment-related morbidities. Moreover, surveillance protocols must be assessed to demonstrate that early detection of asymptomatic tumors ultimately leads to improved overall patient survival (12). Several attempts to identify relapsed acute lymphoblastic leukemia earlier through surveillance failed to show its effects on outcomes (6, 7, 13). The outcomes of patients with relapse largely rely on disease phenotype, timing of relapse, sites of relapse, and response to therapy (3). It is imperative to assess whether the surveillance tool used to identify relapse is sensitive and specific and can detect disease at low burden, enhance outcomes, and minimize complications. Our data showed that relapse was uncommon after therapy completion. Only nine of our patients relapsed after treatment completion. Our patient population underwent a large number of post-treatment tests, with an average of 12 tests per patient (range, 7–14), but only three patients showed evidence of suspected relapse based on blood count, no patient developed treatment-related leukemia, and in patients with non-relapse, or persistent cytopenia required intervention.
In our cohort, 39% of patients were classified as high risk indicating a significant proportion at risk of relapse.
Abnormal blood counts following therapy for acute lymphoblastic leukemia (ALL) were prevalent, with 57% of our patients' exhibiting abnormalities in one or more CBC tests. The sensitivity, specificity, and positive predictive value (PPV) of the blood tests were low. However, the negative predictive values, were high ranging from 90.0%–96.6% across different CBC parameters, underscores the reliability of CBC as low-risk method for ruling out relapses. Nevertheless, the high likelihood of benign abnormal results may substantially impact the quality of life (QoL) for patients and their families, contributing to increased anxiety and prompting additional testing. No differences were observed in treatment-related complications or responses. We observed a slightly higher blast percentage in asymptomatic relapse cases than in symptomatic relapse cases. This observation may indicate the presence of subtle symptoms and signs that were not readily apparent to either the family or treating physician. This study highlights the necessity for a balanced approach to surveillance that weighs the benefits of early detection against the psychological effects of frequent testing for both high-risk and standard-risk groups. Our study highlights the limitations of routine blood testing for detecting relapse in children with ALL and underscores the importance of developing more sensitive and specific diagnostic methods. The comparable clinical outcomes and treatment-related complications between symptomatic and asymptomatic relapse cases challenge the need for post-treatment surveillance. Furthermore, considering the limitations of routine blood testing in detecting relapse, an important question arises as to whether clinical judgment alone can replace laboratory assessment in relapse surveillance. Our study has some limitations, including the inherent limitations of retrospective studies, a small sample size, and the fact that the effects of such extensive surveillance on QoL was not evaluated. A prospective study evaluating the effectiveness of clinical assessment as a stand-alone approach versus a combined approach involving laboratory assessment and evaluate their effects on patients' QoL, with the aim of enhancing the post-treatment evaluation and management of children with ALL.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by King Abdullah International Medical Research Center, institutional review board (No: NRJ21J-205-08). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsSA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AT: Data curation, Writing – review & editing. AA: Data curation, Writing – review & editing. NE: Formal Analysis, Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsALL, acute lymphoblastic leukemia; CBC, complete blood count; COG, Children's oncology group; HR, high risk; IQR, interquartile range; NPV, negative predictive value; PPV, positive predictive value; QoL, quality of life; SR, standard risk.
References1. Kakaje A, Alhalabi MM, Ghareeb A, Karam B, Mansour B, Zahra B, et al. Rates and trends of childhood acute lymphoblastic leukaemia: an epidemiology study. Sci Rep. (2020) 10:6756. doi: 10.1038/s41598-020-63528-0
PubMed Abstract | Crossref Full Text | Google Scholar
2. Hunger SP, Loh ML, Whitlock JA, Winick NJ, Carroll WL, Devidas M, et al. Children’s oncology group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. (2013) 60:957–63. doi: 10.1002/pbc.24420
PubMed Abstract | Crossref Full Text | Google Scholar
4. Brown PA, Ji L, Xu X, Devidas M, Hogan LE, Borowitz MJ, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. (2021) 325:833–42. doi: 10.1001/jama.2021.0669
PubMed Abstract | Crossref Full Text | Google Scholar
5. Locatelli F, Zugmaier G, Rizzari C, Morris J, Gruhn B, Klingebiel T, et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. (2021) 325(9):843–54. doi: 10.1001/jama.2021.0987
PubMed Abstract | Crossref Full Text | Google Scholar
6. Gandhi M, Rao K, Chua S, Saha V, Lilleyman J, Shankar A. Routine blood counts in children with acute lymphoblastic leukaemia after completion of therapy: are they necessary? British J Haematol. (2003) 122:451–3. doi: 10.1046/j.1365-2141.2003.04453.x
PubMed Abstract | Crossref Full Text | Google Scholar
7. Rubnitz JE, Hijiya N, Zhou Y, Hancock ML, Rivera GK, Pui C-H. Lack of benefit of early detection of relapse after completion of therapy for acute lymphoblastic leukemia. Pediatr Blood Cancer. (2005) 44:138–41. doi: 10.1002/pbc.20166
PubMed Abstract | Crossref Full Text | Google Scholar
8. Angiolillo AL, Schore RJ, Kairalla JA, Devidas M, Rabin KR, Zweidler-McKay P, et al. Excellent outcomes with reduced frequency of vincristine and dexamethasone pulses in standard-risk B-lymphoblastic leukemia: results from children’s oncology group AALL0932. J Clin Oncol. (2021) 39:1437–47. doi: 10.1200/JCO.20.00494
PubMed Abstract | Crossref Full Text | Google Scholar
9. Salzer WL, Burke MJ, Devidas M, Chen S, Gore L, Larsen EC, et al. Toxicity associated with intensive postinduction therapy incorporating clofarabine in the very high-risk stratum of patients with newly diagnosed high-risk B-lymphoblastic leukemia: a report from the children’s oncology group study AALL1131. Cancer. (2018) 124:1150–9. doi: 10.1002/cncr.31099
PubMed Abstract | Crossref Full Text | Google Scholar
10. Winter SS, Dunsmore KP, Devidas M, Wood BL, Esiashvili N, Chen Z, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: results from the children’s oncology group AALL0434 methotrexate randomization. J Clin Oncol. (2018) 36:2926–34. doi: 10.1200/JCO.2018.77.7250
PubMed Abstract | Crossref Full Text | Google Scholar
11. Jastaniah W, Elimam N, Abdalla K, AlAzmi AA, Aseeri M, Felimban S. High-dose methotrexate vs. capizzi methotrexate for the treatment of childhood T-cell acute lymphoblastic leukemia. Leuk Res Rep. (2018) 10:44–51. doi: 10.1016/j.lrr.2018.10.001
PubMed Abstract | Crossref Full Text | Google Scholar
12. Dobrow MJ, Hagens V, Chafe R, Sullivan T, Rabeneck L. Consolidated principles for screening based on a systematic review and consensus process. CMAJ. (2018) 190:E422–9. doi: 10.1503/cmaj.171154
PubMed Abstract | Crossref Full Text | Google Scholar
13. Jensen KS, Oskarsson T, Lähteenmäki PM, Flaegstad T, Schmiegelow K, Vedsted P, et al. Detection mode of childhood acute lymphoblastic leukaemia relapse and its effect on survival: a Nordic population-based cohort study. Br J Haematol. (2021) 194:734–44. doi: 10.1111/bjh.17555
留言 (0)