Fungi produce secondary metabolites that enhance their environmental fitness, but these compounds are by definition not required for axenic survival (Avalos and Limón, 2022). The most famous fungal secondary metabolite is the antibiotic penicillin. Used in medicine to treat bacterial infections, this metabolite in nature allows its producer to gain a competitive advantage over bacteria by blocking their cell wall biosynthesis and thus suppressing their growth (Yocum et al., 1980). Mycotoxins are a well-studied subgroup of fungal secondary metabolites due to their toxic effects on humans and animals (Bennett and Klich, 2003). Additionally, the United Nations Food and Agriculture Organization has estimated that, in the United States and Canada alone, mycotoxins cause losses of up to $5 billion U.S. annually (Eskola et al., 2020).
Two infamous mycotoxins that are frequent contaminants in some agricultural products are aflatoxins and fumonisins. Aflatoxin was the first mycotoxin identified. It was associated with the Turkey X disease outbreak in the United Kingdom in 1960 where 100,000 poults were killed upon consumption of contaminated peanut meal (Pickova et al., 2021). Aflatoxins are primarily produced by Aspergillus flavus and Aspergillus parasiticus and were shown to be the most carcinogenic naturally occurring compounds known with demonstrated toxicity in the parts per billion range (Trucksess and Diaz-Amigo, 2011). Currently, aflatoxin is under strict regulation in the United States and European Union to limit how much enters the food chain targeted for human consumption (20 parts per billion (ppb)) and animal feed (50-100 ppb for poultry). Fumonisin, a mycotoxin primarily produced by Fusarium spp., has also been associated with a plethora of health problems in animals. Currently, there are only recommended guidelines for the permissible amount of fumonisin in food and feed, with the standard for poultry broiler feed set at 100 parts per million (ppm) (Alshannaq and Yu, 2017).
In addition to addressing the toxicity of mycotoxins to animals, several studies have examined other roles these compounds may have that could provide a selective advantage to the producing fungi. When confronted with oxidative stress, A. flavus increases production of aflatoxin (Fountain et al., 2016b, a). Strains of A. flavus that produced more aflatoxin have demonstrated increased resistance to oxidative stress (Fountain et al., 2015). It has been hypothesized that aflatoxin acts as an antioxidant to scavenge free radicals, thus protecting the fungus from the oxidative stress it typically encounters upon colonizing plants (Finotti et al., 2021). In F. verticillioides, there is evidence that fumonisin can be a virulence factor aiding in infection and symptom development in susceptible lines of corn (Glenn et al., 2008; Blacutt et al., 2018; Gao et al., 2020). Fumonisin B1 has been reported to have antifungal activity against Alternaria alternata, Pencillium expansum, and Fusarium graminearum, the later being a competing pathogen in corn in some regions (Keyser et al., 1999).
Both A. flavus and F. verticillioides are common colonizers of corn, infecting and contaminating the crop with their harmful mycotoxins. Numerous studies have found both fungal species occupying the same field as recently reviewed by Chen et al (Chen et al., 2023). Several of these studies have also found both aflatoxin and fumonisin in the same kernels indicating that both fungi can colonize the same host simultaneously. Examination of the interactions between these two fungi revealed that direct competition impacts their growth and the production of their respective mycotoxins, but none have examined the direct effect the individual mycotoxins have in their competition (Shu et al., 2017; Chen et al., 2021; Lanubile et al., 2021). Based on the hypothesis that A. flavus and F. verticillioides encounter each other’s mycotoxins when competing to colonize corn kernels, we investigated the specific role of aflatoxin and fumonisin in this dual fungus competitive interaction. To achieve this, we performed direct competition assays with wild-type toxin producing strains compared to interactions with non-toxigenic mutant strains of A. flavus (ΔaflR) and F. verticillioides (Δfvfum1). The direct effect of each mycotoxin on its competitor was also assayed focusing on growth, mycotoxin production, and the expression of selected genes impacting secondary metabolism.
2 Materials and methods2.1 Fungal strains used and growth conditionsWild-type strains NRRL3357 and FRCM3125 (FGSC7600) were used for A. flavus and F. verticillioides, respectively. The aflatoxin non-producing strain, a ΔaflR (ΔaflR::ptrA) mutant in the NRRL3357 background was generated for this study, while the fumonisin non-producing strain was from a previous study that has a defective FvFum1 (FVEG_00316) gene (derived from FRC M3125, Δfvfum1::hyp) (Proctor et al., 1999; Desjardins et al., 2002). Stocks of each strain were stored in 30% glycerol at -80°C. To maintain the stock and/or generate spores for inoculum, cultures were grown on Potato Dextrose Agar (PDA) (Neogen, Lasing, Michigan, USA) or double strength 5/2 agar (100 mL V8 juice, 40 g agar, pH 5.2 per liter of medium) unless specified differently (Chang et al., 1993).
2.2 Construction and confirmation of an Aspergillus flavus ΔaflR strainTo construct an Aspergillus flavus ΔaflR strain, 1.5 kb DNA fragments containing upstream and downstream sequences flanking the aflR (F9C07_7811) coding region were amplified by PCR from gDNA of the A. flavus NRRL3357 wild-type strain utilizing the primers P1/P2 and P3/P4, respectively. The 2.0 kb pyrithiamine resistance gene (ptrA) was PCR amplified from the commercial pPTRI vector (TaKaRa Bio Inc., Shiga, Japan) using primers P5/P6. Fusion PCR was carried out with the primers P7/P8 as previously described (Szewczyk et al., 2006) to generate a 4.8 kb aflR deletion cassett. Protoplast and CaCl2-PEG mediated fungal transformation of the aflR deletion cassette into the NRRL3357 wild-type strain was carried out as previously described (Chang, 2008). Transformants were selected on Czapek-dox (BD Difco, Franklin Lakes, NJ, USA) regeneration plates supplemented with 0.1 µg/L pyrithiamine hydrobromide (Sigma-Aldrich, Burlington, MS, USA). Colonies displaying resistance to pyrithiamine hydrobromide were subcultured. Fungal mycelium used for genomic DNA isolation was cultivated by inoculating 106 spores/mL into 50 mL of PDB (PDB; EMD, Darmstadt, Germany) medium. The cultures were incubated at 250 rpm at 30°C for 24 h prior to harvesting mycelium and extracting genomic DNA using a Zymo Quick-DNA Fungal/Bacterial Miniprep Kit (Zymo Research, Irvine, CA, USA). Diagnostic PCRs were carried out by using either OneTaq 2X Master Mix (New England BioLabs, Ipswich, MA, USA) or Phire PCR Master Mix (Thermo Scientific, Watham, MA, USA) with location-specific primers to confirm the knockout mutants of aflR gene. Thermocycler settings used were set according to manufactures recommendations. All primers utilized in creating this strain are listed in Supplementary Table S1.
2.3 Direct confrontation assaysUsing standard 100 mm disposable Petri plates with 25 ml of PDA, A. flavus and F. verticillioides strains were point inoculated 40 mm apart and equidistant from the center of the plate. 5 µl of a spore suspension (106 spores/ml) was used for plate spot inoculations. Plates were incubated for up to seven days in the dark at 28°C. For mycotoxin quantification, cultures were grown for five days, which allowed for sufficient growth without colonies making direct contact. After incubation cultures were photographed and cores were taken if required for mycotoxin analysis. For each pairing, five 5 mm diameter plugs were taken with a sterile cork borer from five locations across the plate: from the center in between colonies, 5 mm from the colony edge closest (proximal) to the center of the plate, and 5 mm from the colony edge farthest (distal) from the center. Proximal and distal sample cores were collected from both fungi on each plate. Controls were monocultures of the wild-type A. flavus or F. verticillioides. Cores from the edge of the wild-type colonies were used for comparison to both the distal and proximal plugs in the dual interactions. All five cores from each sample were submerged in 10 ml of 50% acetonitrile (+ 5% formic acid) and extracted overnight. Samples were then diluted to a total concentration of 30% acetonitrile before being analyzed for aflatoxin and fumonisin by Liquid Chromatography Mass Spectrometry (LC/MS).
2.4 Mycotoxin exposure assaysStocks of both aflatoxin B1 and fumonisin B1 were purchased from Cayman Chemical (Niles, Illinois, USA). Aflatoxin stocks were created using a 50:50 mixture of acetone:methanol while fumonisin was dissolved in sterile water. Various concentrations of each mycotoxin, up to 100 µg/ml, were added to 1 ml molten PDA in 24-well plates. 5 µl of a spore suspension (106 spores/ml) from the wild-type strain of A. flavus or F. verticillioides was placed in each well. Cultures were grown for 72 h, photographed, and the entire contents of each well were harvested for quantification of aflatoxin or fumonisin content by LC/MS as stated above.
2.5 Gene expression analysis of response to mycotoxinsTo measure the response of each fungus to its competitor’s mycotoxin, wild-type strains of either A. flavus or F. verticillioides were inoculated into 3 ml of Potato Dextrose Broth (PDB, Neogen) at a final concentration of 105 spores/ml. At the time of inoculation, aflatoxin B1 was added to F. verticillioides cultures and fumonisin B1 was added to A. flavus cultures both at a concentration of 20 µg/ml. Cultures were grown at 28°C in the dark and shaken at 250 rpm for up to 96 h. At 72 h and 96 h, cultures were destructively sampled with mycelia separated from culture supernatants. A. flavus mycelium was collected by filtering culture through sterile Miracloth (Millipore Sigma). 1.5 ml of F. verticillioides cultures were collected into prechilled 1.7 ml microcentrifuge tubes and then pelleted at 8000 rpm at 4°C. Collected mycelia was then flash frozen in liquid nitrogen and immediately stored at -80°C until ready for RNA extraction. Additional separate biological replicates (mycelia and supernatant) were collected for analysis of aflatoxin and fumonisin production. Equal volumes of 100% acetonitrile (+ 5% formic acid) were added to the cultures and extracted overnight. Extracts were diluted to 30% acetonitrile with water and analyzed via LC/MS.
For extracting RNA from F. verticillioides mycelia, the PureLink RNA Mini Kit (Invitrogen, Waltham, MA, USA) was used following manufacturer instructions. Homogenization was performed using a MP Biomedical (Santa Ana, CA, USA) FastPrep with Lysing Matrix D tubes. For A. flavus, RNA extraction was done using the manufacturer protocol for a TRIzol extraction with cleanup done using the RNeasy Mini Kit (Qiagen). RNA quantity and quality was determined using an Agilent 2100 Bioanalyzer with RIN values above 6 acceptable for further processing. RNA was treated with RQ1 DNAse (Promega, Madison, WI, USA) following the manufacturer protocol. To create cDNA, a Moloney murine leukemia virus (MMLV) (Promega) reverse transcriptase was used on the DNAse treated RNA. qRT-PCR was performed on a Bio-Rad CFX96 Real-Time System using SYBR green dye for fluorescence detection. Gene expression was normalized to expression of the 18S ribosomal RNA gene for A. flavus and the β-Tubulin gene (FVEG_04081) for F. verticillioides. Expression was assessed by the 2-ΔΔCT method (Sherif et al., 2023). All primer sequences used in qRT-PCR analysis are listed in Supplementary Table S2.
2.6 Statistical analysisAll statistical analyses were done using R version 4.4.1. An analysis of variance (ANOVA) was performed on data generated in each experiment alongside a Tukey multiple-comparison test. To determine significance between treatments a P value of <0.05 was set.
3 Results3.1 Fusarium verticillioides inhibits Aspergillus flavus growth through fumonisinInitially, wild-type strains of A. flavus and F. verticillioides were confronted with each other on PDA. Inhibition of A. flavus growth was observed as a sharp line of demarcation, beyond which it did not grow (Figure 1). To determine if aflatoxin and/or fumonisin were involved in this interaction, nonproducing mutants were utilized. For this study, an aflatoxin non-producing aflR deletion strain was created as described in the Methods section above (Supplementary Figure S1A). Pyrithiamine resistant transformants were further confirmed with diagnostic PCR (Supplementary Figures S1B–E). For a fumonisin non-producing strain of F. verticillioides, a strain with a disruption of the FvFUM1 gene from previous work was utilized (Proctor et al., 1999; Desjardins et al., 2002). The aflatoxin non-producing mutant (ΔaflR) confronted with wild-type F. verticillioides showed an even sharper demarcation line than observed with wild type A. flavus, suggesting that aflatoxin diminishes the competitive edge of F. verticillioides over A. flavus (Figure 1). Likewise, when confronted with the fumonisin non-producing mutant (Δfvfum1) the growth of wild-type A. flavus was clearly less inhibited, and its colony made direct contact with the fumonisin non-producing mutant. Additionally, when non-toxigenic competitor strains are confronted, the result is similar to the A. flavus wild-type vs Δfvfum1 interaction with the colonies making contact. These results indicate that both aflatoxin and fumonisin are, in part, responsible for competitor growth inhibition in these interactions.
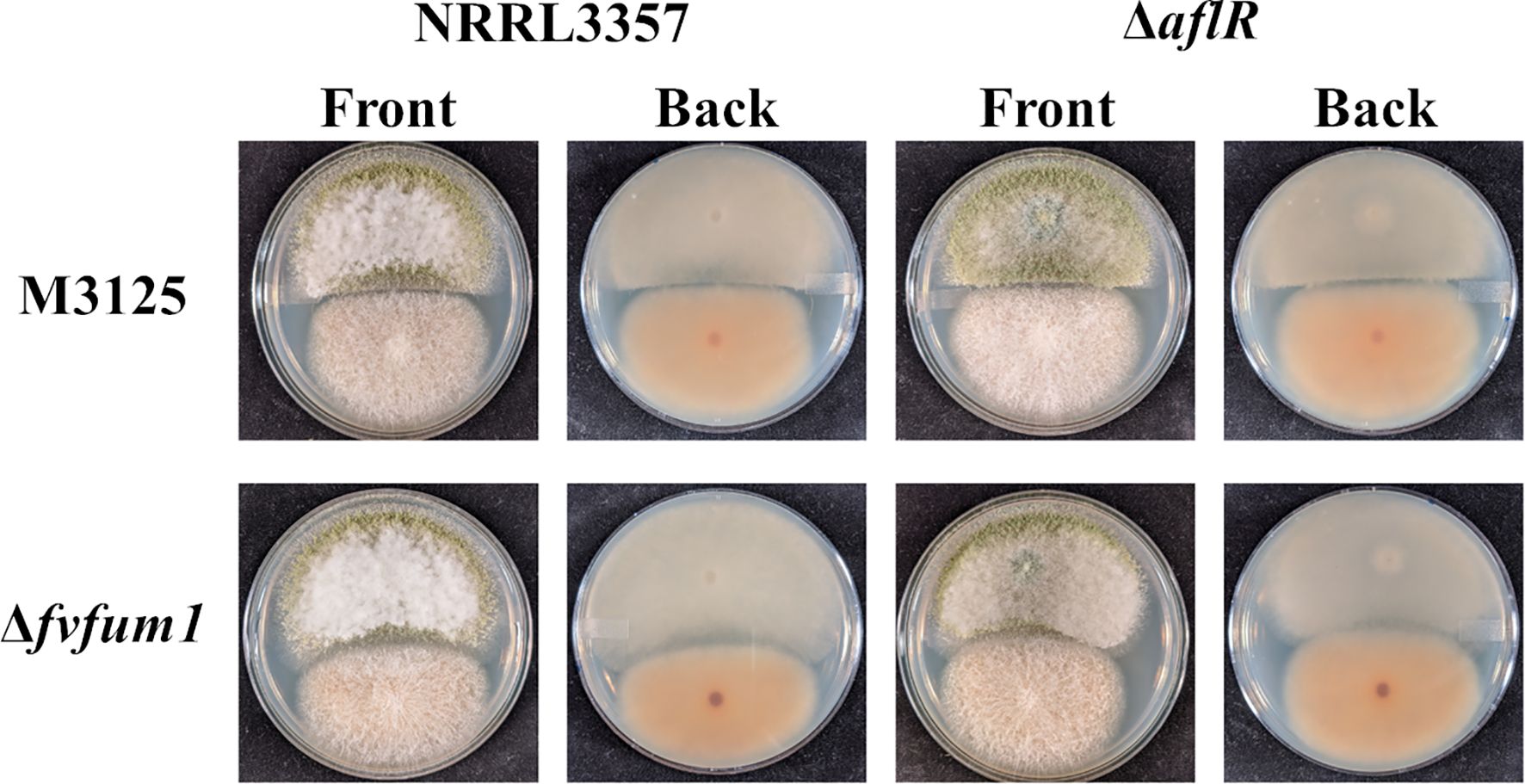
Figure 1. Fumonisin plays a role in F. verticillioides inhibition of A. flavus growth. Wild-type and/or atoxigenic mutant (ΔaflR and/or Δfvfum1) strains of both A. flavus (Af) and F. verticillioides (Fv) were point inoculated onto PDA and grown for 7 days at 28°C in the dark. Experiment was done three different times with three biological replicates.
3.2 Aflatoxin suppresses production of fumonisin in F. verticillioidesWith the demonstrated effect of mycotoxin production on their competitor’s growth, we investigated the effect each mycotoxin had on its competitor’s mycotoxin production. Using the same strains as above, F. verticillioides and A. flavus were point inoculated equidistant from each the plate center and grown for 5 days to prevent direct contact (Figure 2A). Agar plugs were collected from three locations: between colonies, the proximal edge of each colony closest to its competitor, and the distal edge (Figure 2B). This was done to determine if the effects of mycotoxin exposure were localized to one area or if a colony-wide response was caused. In response to the competitor fungus, both A. flavus and F. verticillioides secreted mycotoxins at higher levels than monoculture controls (Figures 2C, D).
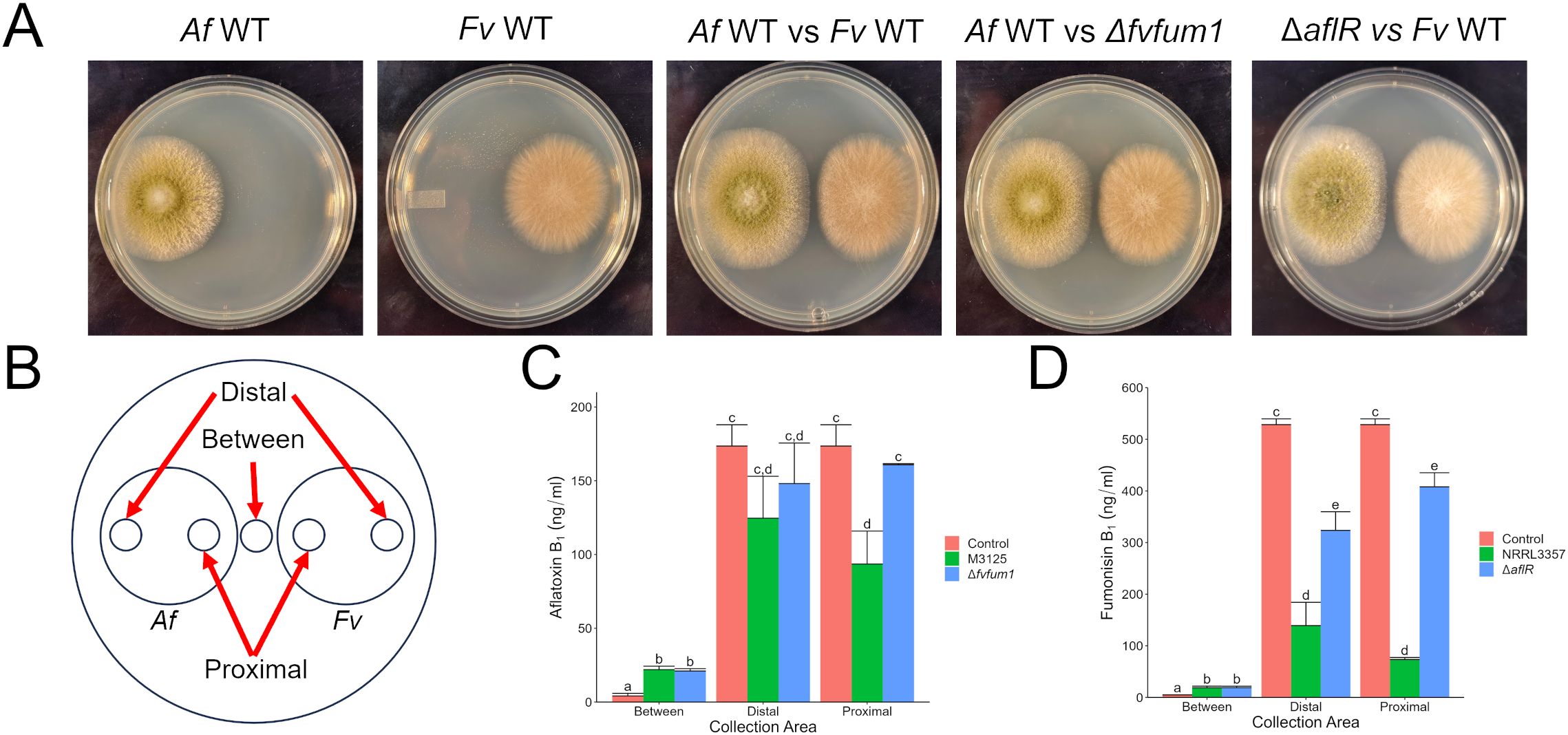
Figure 2. In direct competition with A. flavus, aflatoxin suppresses fumonisin production by F. verticillioides. (A) A. flavus (Af) and F. verticillioides (Fv) strains grown for 5 days at 28°C in the dark. (B) Collection sites from fungal cultures for mycotoxin analysis via LC/MS. (C) Aflatoxin B1 production by A. flavus in response to F. verticillioides wild-type and Δfvfum1 strains. (D) Fumonisin B1 analysis of F. verticillioides culture in response to A. flavus wild-type and ΔaflR strains. The controls used in C & D were the monocultures shown in (A) Different letters above each column indicate that the values are statistically different (P<0.05) based on results of an ANOVA run with Tukey test comparison. Experiment was three done different times with three biological replicates.
Compared to monoculture controls, when A. flavus encountered a wild-type strain of F. verticillioides, aflatoxin production was reduced proximally as compared to the other treatments. No statistical differences were found at the distal colony edge (Figure 2C). After exposure to aflatoxin producing strains, fumonisin production by F. verticillioides was suppressed at both proximal and distal collection sites (Figure 2D). At distal collection sites, no aflatoxin was detected (data not shown) indicating the response is not a localized effect but is rather colony-wide. Additionally, the ΔaflR strain also caused slight inhibition of fumonisin production, suggesting other metabolites produced in these interspecies interactions likely play roles.
3.3 Antagonistic mycotoxins directly influence gene expression of competitor regulators of secondary metabolismFilamentous fungi like Aspergillus and Fusarium are known to produce a plethora of secondary metabolites. Some of these metabolites have been characterized, but there are apparently more that have yet to be identified. To focus our efforts, we decided to evaluate the direct effects of purified aflatoxin B1 (AFB1) and fumonisin B1 (FB1) on F. verticillioides and A. flavus, respectively. Both mycotoxins are commercially available in relatively pure form (>98%) and are also the primary forms of the respective toxins. Testing FB1 on A. flavus demonstrated the same phenotypes as observed with the wild type fumonisin producer, with FB1 inhibiting A. flavus growth and aflatoxin production. At 24 h with a 100 µg/ml dose of FB1, the A. flavus spores were yet to germinate; at 72 h the cultures had active vegetative growth. This indicates that FB1 was fungistatic providing a head start but not fungicidal towards A. flavus at concentrations up to 100 µg/ml (Figure 3). The original phenotype of fumonisin suppression when exposed to wild type A. flavus also held true with large amounts (up to 100 µg/ml) of AFB1 suppressing fumonisin production but having little to no effect on F. verticillioides colony growth (Figure 4).
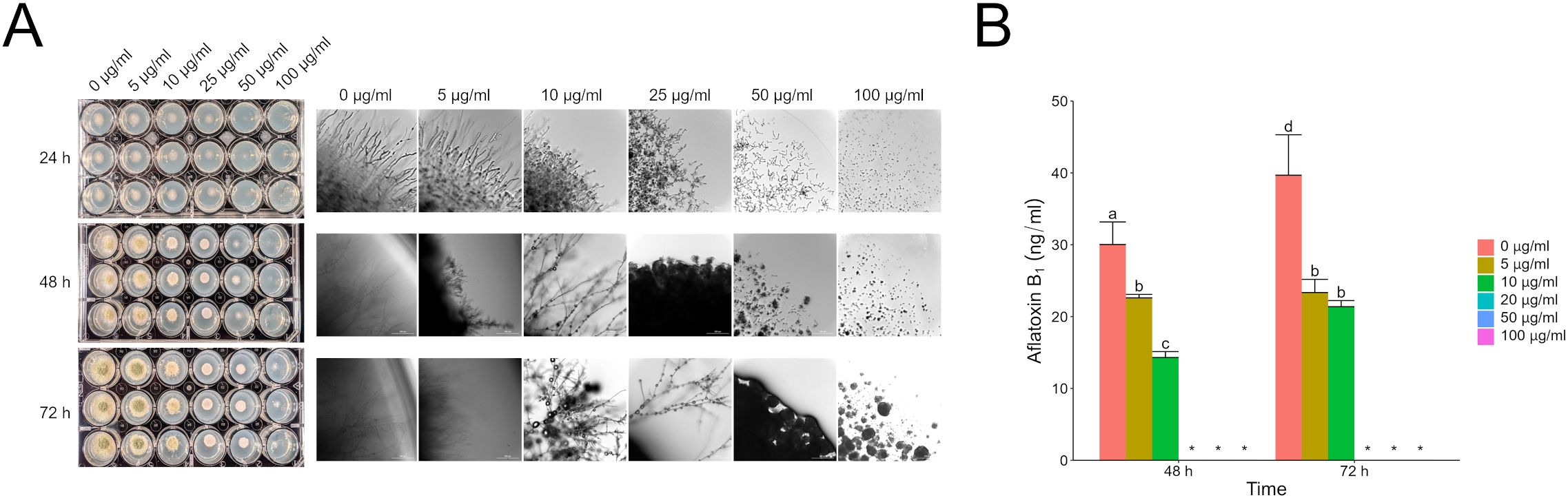
Figure 3. Direct effect of fumonisin B1 on A. flavus growth and aflatoxin B1 production. (A) A. flavus culture treated with increasing concentrations of FB1 as shown in (B). Cultures were grown in 24-well plates for 72 hours at 28°C in the dark with micrographs taken every 24 hours. (B) LC/MS analysis of AFB1 content in culture at 48 and 72 hours. Different letters above each column indicate that the values are statistically different (P<0.05) based on results of an ANOVA run with Tukey test comparison. In panel B, “*” indicates samples that were not detected or quantifiable by LC/MS. Experiment was done three different times with three biological replicates.
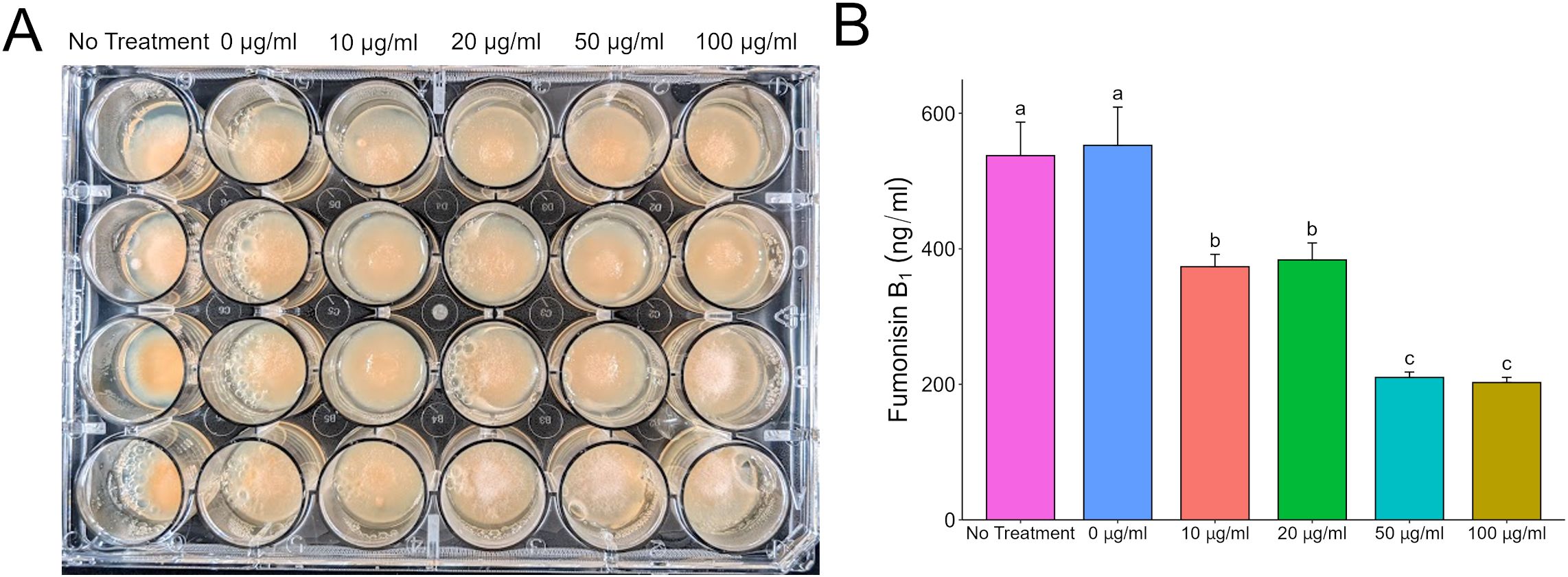
Figure 4. Direct effect of aflatoxin B1 on F. verticillioides growth and fumonisin B1 production. (A) F. verticillioides treated with increasing concentrations of AFB1 up to 100 µg/ml and grown in 24-well plates for 72 hours at 28°C in the dark. Samples labeled as “0 µg/ml” are samples that have a volume of 1% acetone:methanol (50:50) with no aflatoxin added to represent the carrier used to administer aflatoxin to the F. verticilliodes as a 0 µg/ml treatment. The “No Treatment” sample do not have the acetone:methanol carrier added to the samples. (B) LC/MS analysis of FB1 content in culture at 72 hours. Different letters above each column indicate that the values are statistically different (P<0.05) based on results of an ANOVA run with Tukey test comparison. Experiment was done three different times with three biological replicates.
After validating the effect of the individual mycotoxins, we assessed the expression of selected mycotoxin regulatory and biosynthetic genes using subinhibitory doses of each against its appropriate competitor. Mycotoxins were administered at the time of inoculation. Liquid cultures were grown for up to 96 h, with separate full cultures (mycelia and supernatant) destructively sampled at 72 and 96 h for both RNA extraction and LC/MS analysis. Shaken A. flavus broth cultures exposed to fumonisin had significant reduction in aflatoxin production compared to control cultures without added fumonisin (Figure 5A). Surprisingly, no significant statistical differences in expression of aflR, which encodes the key activating transcription factor in the aflatoxin biosynthetic gene cluster, was found. However, there was a significant decrease in expression of aflM at 96 h (Figures 5B, C). The aflatoxin biosynthetic cluster gene, aflM (previously known as ver-1), encodes versicolorin dehydrogenase and has been effectively used to show activation of the aflatoxin biosynthetic pathway (Calvo et al., 2004; Raruang et al., 2020). The decrease in expression was not temporally correlated with a decrease in aflatoxin. Outside the biosynthetic cluster, two global regulators of morphological development and secondary metabolism, veA and laeA, were also assessed (Figures 5D, E) (Calvo et al., 2016). In A. flavus samples exposed to fumonisin B1 expression of veA was suppressed at 72 h, while expression in the control reached the same level 24 h later at the 96 h timepoint. The gene laeA was more consistent in expression that in the fumonisin treated samples it showed a significant decrease in expression at both time points.
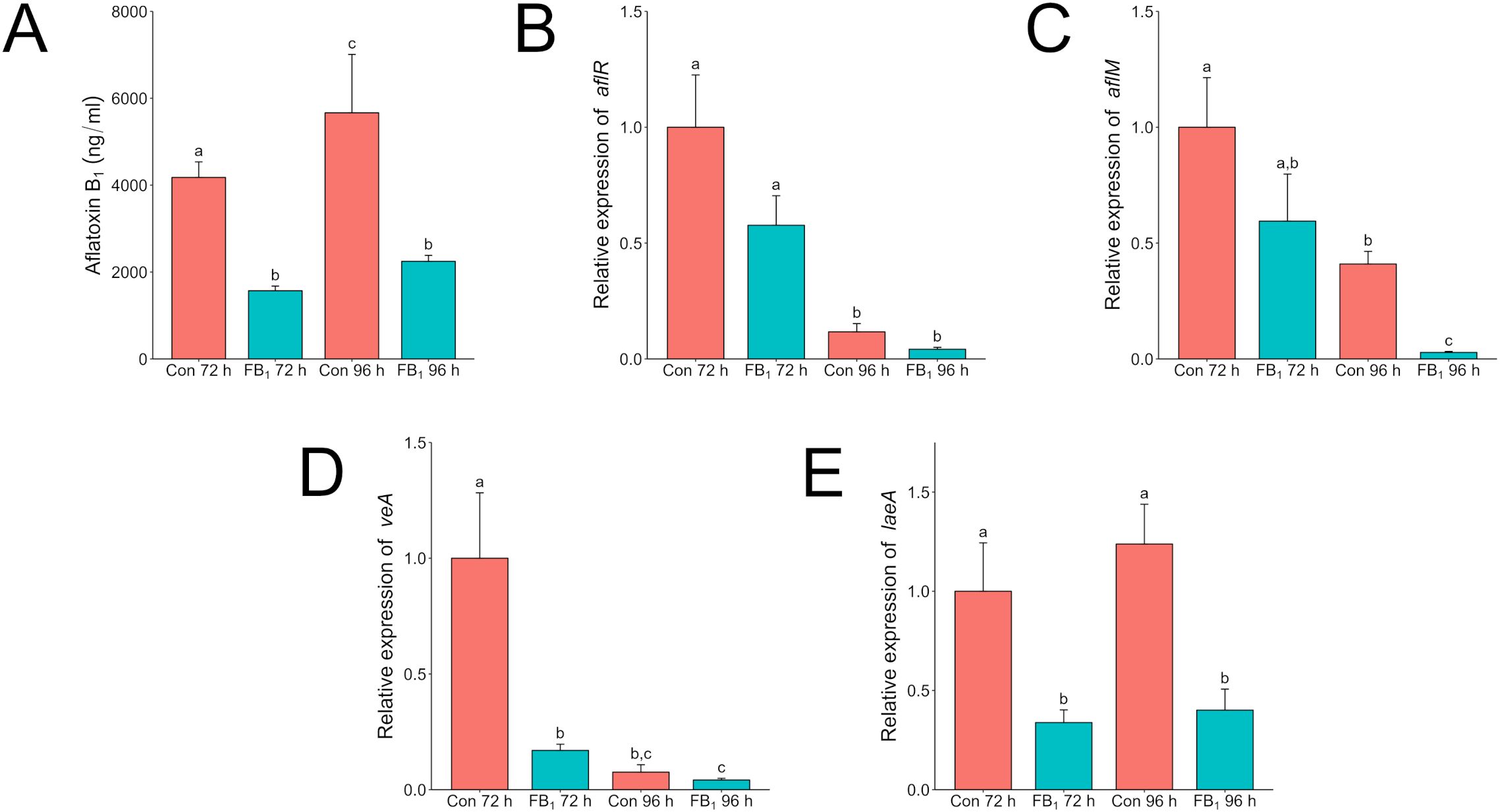
Figure 5. Fumonisin B1 impacts aflatoxin production and the expression of velvet regulators veA and laeA in A. flavus in liquid culture. A. flavus wild type was grown in 3 ml of PDB with or without 20 µg/ml fumonisin B1 for 72 or 96 h. The “Con” or control treatment are cultures that do not have any fumonisin added and “FB1” are cultures with 20 µg/ml of fumonisin B1 added. (A) Complete samples, mycelia and supernatant, were collected at 72 and 96 h and analyzed via LC/MS for aflatoxin B1 content. Mycelia were also collected from additional tubes for RNA extraction. Relative expression of aflR (B), aflM (C), veA (D), and laeA (E) was analyzed by qRT-PCR. Expression values were normalized to the 72-hour control. Different letters above columns indicate that the values are statistically different (P<0.05) based on results of an ANOVA run with Tukey test comparison. Experiment was done three different times with four biological replicates.
In contrast to results on solid medium, F. verticillioides liquid shaken cultures produced more fumonisin at 72 and 96 h when dosed with aflatoxin than when not (Figure 6A). In response to aflatoxin treatment expression of the fumonisin biosynthetic gene cluster, a polyketide synthase encoding gene (FvFUM1 (FVEG_00316)), increased over time while the gene for zinc-binding dehydrogenase (FvZBD1) decreased (Figures 6B, C). FvFUM1 encodes the key polyketide synthase located in the fumonisin biosynthetic cluster and is directly involved in fumonisin biosynthesis, whereas FvZBD1 was traditionally not considered part of the cluster but in F. verticillioides is located directly adjacent and has been demonstrated to suppress fumonisin production when expressed at a high level (Proctor et al., 1999; Gao et al., 2020). This trend corresponded with decreasing amounts of aflatoxin remaining in the cultures, indicating that fumonisin production increased as aflatoxin was degraded either by F. verticillioides or by some other means (Supplementary Table S3). Like A. flavus, F. verticillioides also contains copies of both velvet regulators veA (FvVE1) and laeA (FvLAE1), whose expression was examined in this study (Figures 6D, E) (Calvo et al., 2016). At both 72 h and 96 h post inoculation, FvVE1 expression was suppressed by aflatoxin in comparison to the control treatment. In the presence of aflatoxin, activation of FvLAE1 was induced early at 72 h, whereas the control reached a similar level of expression only after 96 h.
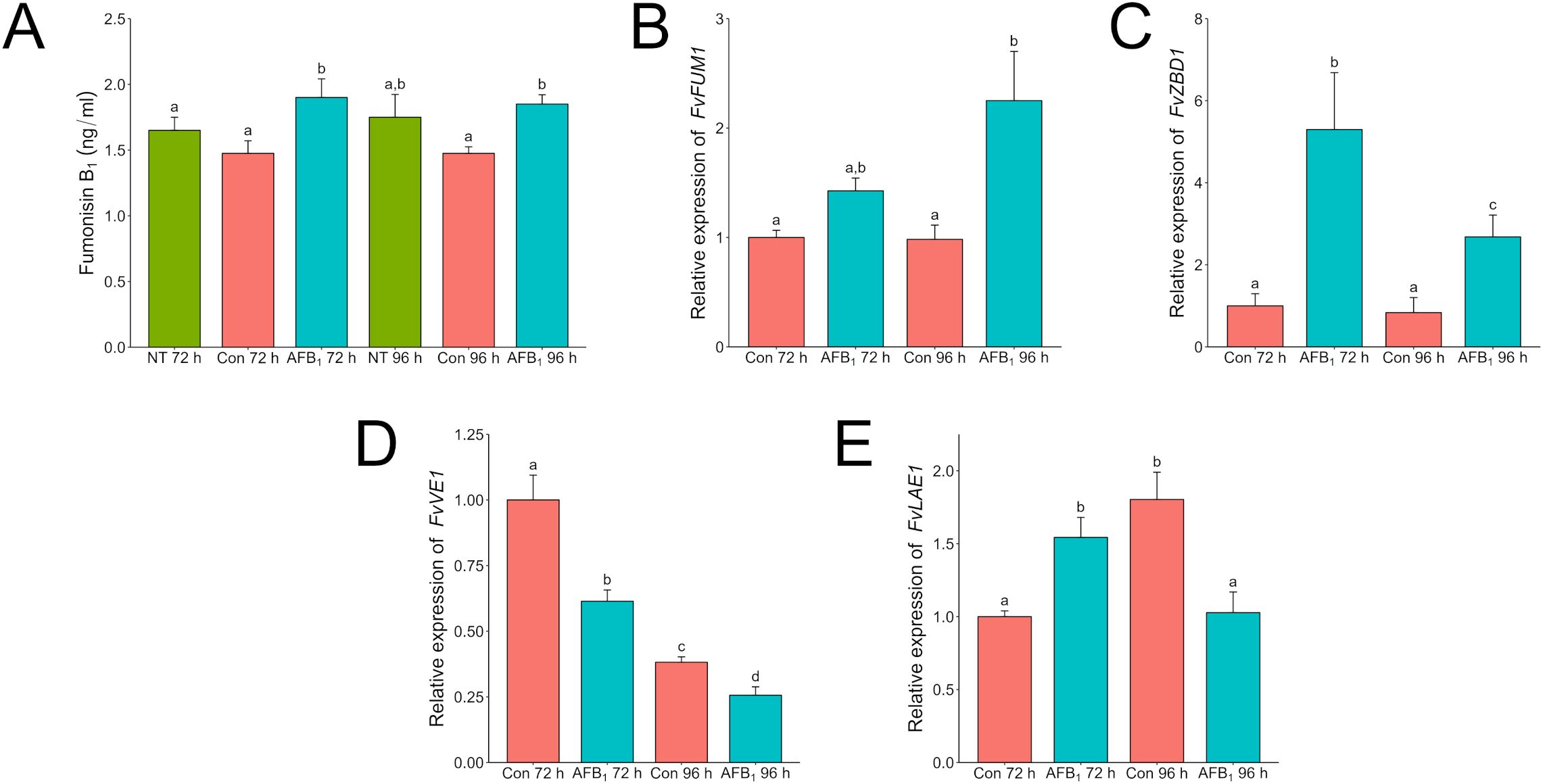
Figure 6. Aflatoxin B1 influences Fumonisin B1 production likely through induction of FvZBD1 in F. verticillioides. F. verticillioides wild-type was grown in 3 ml of PDB with or without 20 µg/ml Aflatoxin B1 for 96 h. Samples labeled as “Con” are samples that have a volume of 1% acetone:methanol (50:50) with no aflatoxin added to represent the carrier used to administer aflatoxin to the F. verticilliodes as a 0 µg/ml treatment. The “No Treatment” sample do not have the acetone:methanol carrier added to the samples. Labels with “AFB1” are cultures with 20 µg/ml of aflatoxin B1 added. (A) Complete samples, mycelia and supernatant, were collected at 72 and 96 h and analyzed via LC/MS for aflatoxin B1. Mycelia from duplicate tubes were collected for RNA extraction. “NT” is a no treatment control. Samples labeled as “Control” are samples that have a volume of 1% acetone:methanol (50:50) with no aflatoxin added to represent the carrier used to administer aflatoxin to the F. verticilliodes as a 0 µg/ml treatment. Relative expression of FvFUM1 (B), FvZBD1 (C), FvVE1 (D), and FvLAE1 (E) was analyzed by qRT-PCR. Expression values were normalized to the 72-hour control. Different letters above each column indicate that the values are statistically different (P<0.05) based on results of an ANOVA run with Tukey test comparison. Experiment was done three different times with four biological replicates.
4 DiscussionAs recently reviewed by Chen et al. (2023), at least thirty studies over the last few decades across the globe have identified co-occurrences of both A. flavus and F. verticillioides in maize. Initially, these studies were relegated to warmer climates such as those in Africa, but as temperatures rise due to climate change, discovery of these fungal co-occurrences are spreading to traditionally more cooler climates. Several direct interaction studies under in-vivo and in-vitro conditions have been performed with the results varying greatly based on the experimental conditions (Camardo Leggieri et al., 2019; Chen et al., 2021; Lanubile et al., 2021). However, the reports overall agree that during competition each fungus influences the other’s production of their primary mycotoxin, but they never focused on the role the individual mycotoxins might have in these interactions.
The aim of this work was to determine the roles of mycotoxins of primary concern as regulators of competitive interactions between these two frequent commensal mycotoxigenic corn colonizing fungi. Here, we characterized the response to aflatoxin and fumonisin on its competitor’s growth and mycotoxin production. With null producing mutants, our results demonstrated that F. verticillioides inhibits the growth of A. flavus via fumonisin. Despite the inhibition caused by fumonisin, aflatoxigenic cultures of A. flavus still produce enough aflatoxin to inhibit fumonisin production in the F. verticillioides wild-type colony compared to the non-producing ΔaflR cultures. Fumonisin also inhibited aflatoxin accumulation in the reciprocal conditions, but this only occurred together with direct growth reduction. Consistent with the results of this report, work performed by Camardo Leggieri et al. (2019) and Chen et al. (2021) found that the presence of a competing fungus had significant effects on production of mycotoxins in A. flavus and F. verticillioides with coculture on plates and in planta showing reductions in both aflatoxin and fumonisin.
Exposing A. flavus spores to fumonisin B1 showed that, while toxic, the metabolite is not fungicidal. Even at a high dose of 100 µg/ml, while severely delayed, the spores eventually germinated followed by vegetative growth. Analysis of the fumonisin B1 content after adding it to liquid cultures of A. flavus demonstrated that fumonisin B1 concentration decreased by more than half that added (Supplementary Table S3). This decrease occurred by unknown means, but likely involved degradation by A. flavus itself. Regardless of how, this toxin decrease may explain the recovery seen with cultures exposed solely to fumonisin compared to F. verticillioides co-cultures where fumonisin continues to be actively produced, keeping A. flavus suppressed. This may also explain observations in the field, as it is likely to be more common that one species will establish itself on a host before a second can. With the initial establishment, the first species likely starts producing its secondary metabolites earlier; this allows it a competitive edge over other microbes. F. verticillioides was similarly antifungal and inhibitory towards Fusarium graminearum with fumonisin required for the effect (Sherif et al., 2023). The authors hypothesized that fumonisin accumulation in the seeds protects them from utilization by saprotrophic fungi giving a fitness advantage to fumonisin producing fungi. With A. flavus being a known opportunistic pathogen and saprotroph, the data here is consistent with the author’s hypothesis.
In terms of gene expression, fumonisin does not seem to have a direct effect on the aflatoxin biosynthetic gene cluster members. Even though expression of aflM was lower than the control at 96 h, this gene and aflR were unaffected at 72 h where aflatoxin production was already lower by nearly half in the treated sample compared to the control. In direct exposure to F. verticillioides or fumonisin, aflatoxin reduction only occurred at the proximal edge of interacting colonies or when exposed to high concentrations of fumonisin commensurate with visible growth inhibition (Figure 2). Thus, the effect of fumonisin treatment on key gene expression and on aflatoxin production appears to be a secondary effect of A. flavus growth inhibition.
In A. flavus, both VeA and LaeA are involved in secondary metabolism regulation and are noteworthy for their roles in regulation of aflatoxin biosynthesis by manipulating aflR (Amaike and Keller, 2009). Expression of both veA and laeA were suppressed in the fumonisin treatment. The interactions between proteins VeA, LaeA, and AflR are nuanced with imbalances in stoichiometry and self-regulating feedback loops impacting expression (Bok and Keller, 2004; Wang et al., 2022). Despite this, laeA expression was strongly suppressed when exposed to fumonisin B1.
Amaike and Keller (2009) speculated that the deletion of laeA in A. flavus resulted in defects in density based morphological development governed by an oxylipin quorum-like sensing system. Alterations in fungal quorum sensing through oxylipins may cascade into other defensive metabolic changes impacting fungal competition. Multiple oxylipins have been identified in both Aspergillus and Fusarium spp. that stimulate or inhibit production of mycotoxins (Liu et al., 2023). In the non-aflatoxigenic ΔaflR strain, suppression of fumonisin production by F. verticillioides was detected, albeit more weakly. This suggests that other A. flavus secondary metabolites, beyond aflatoxin, are produced that combat F. verticillioides. These other compounds may also be activated by changes in the expression of global regulators like VeA and LaeA.
For humans and animals, due to the high toxicity of consumed aflatoxin, regulations from the U.S. Food and Drug Administration place content limits in the part per billion (ppb) range for food (20 ppb) and feed (100 ppb). Under aflatoxin exposure up to 100 ppm, F. verticillioides appeared resistant to visible effects on colony growth. Interestingly, fumonisin production was severely inhibited by aflatoxin producing strains of A. flavus in a colony-wide response.
When F. verticillioides was exposed to 20 µg/ml of AFB1, fumonisin was still produced and found at higher levels than in the solvent control treatment. Expression of FvFum1 increased by more than 50% from 72 h to 96 h in the dosed samples. Intriguingly, this corresponded to a reduction of more than 50% in FvZBD1 expression over the same time frame. In a previous study, the gene FvZBD1 was identified as a highly induced negative regulator of fumonisin production in F. verticillioides (Gao et al., 2020). In that work, the xenobiotic compound pyrrocidine, produced by the corn kernel colonizing fungus and potential biological control agent, Sarocladium zeae, induced FvZBD1 4000-fold, coincident with dramatic suppression of fumonisin production in exposed F. verticillioides. Further deletion of FvZBD1 resulted in high fumonisin accumulation, consistent with its role as a suppressor. It appears that, like pyrrocidine, aflatoxin acts in a similar way suppressing fumonisin production via FvZBD1 induction albeit at a reduced capacity. Analysis of aflatoxin in those samples revealed that the total amount of AFB1 was reduced by 24% at 72 h and 37% at 96 h (Supplementary Table S3), suggesting F. verticilloides is degrading or biotransforming aflatoxin to better survive. This tolerance was also seen in solid agar where the amount of fumonisin produced in response to an aflatoxin concentration of 50 µg/ml was not significantly different than a dose twice as much at 100 µg/ml (Figure 4). Strangely though when F. verticillioides is directly exposed to an aflatoxin producing A. flavus the suppression of fumonisin (Figure 2) is much greater than when it is exposed to a high direct dose of aflatoxin (100 µg/ml). This is surprising because based on LC/MS data the A. flavus colony is only producing aflatoxins at levels around or below 100 ng/ml. This would indicate that F. verticillioides can handle high acute exposures of aflatoxin but suffers from chronic exposure such as when growing near a producing colony. It is worth noting that this effect may be due to additional secondary metabolites produced by A. flavus which may have a synergistic effect with aflatoxin on F. verticillioides.
Like Aspergillus, Fusarium spp. have functional homologs of the velvet complex including veA (FvVE1) and laeA (FvLAE1). FvVE1 and FvLAE1 are both positive regulators of fumonisin production, and as with A. flavus, the expression of these genes were also examined [ (Butchko et al., 2012) (Myung et al., 2012)]. Expression of FvVE1 was lower at both 72 h and 96 h in aflatoxin treated samples compared to the control. On the other hand, FvLAE1 in the treated sample had an earlier activation at 72 h. Suppression of FvVE1, while significant, does not appear to be repressed to the extent FvZBD1 is. Butchko et al. (2012) presented microarray data that demonstrated that FvZBD1 is positively regulated by FvLAE1. The interaction between FvZBD1 and the velvet complex, including FvLAE1, has not been thoroughly investigated, it is possible genetic regulation involving the velvet complex plays a role in both organisms’ response to the other.
5 ConclusionA. flavus and F. verticillioides are known to interact while colonizing corn seed, but there has been a lack of knowledge regarding the specifics of this interaction. Here, we focused on the role aflatoxin and fumonisin play when A. flavus and F. verticillioides directly compete. Our results indicate that fumonisin suppresses the growth of A. flavus and the expression of the global secondary metabolism regulator laeA. This is an interesting result as it appears when A. flavus encounters F. verticillioides one of its responses is to start secreting more aflatoxin. This response does appear to be defensive in nature since aflatoxin induces expression of the fumonisin repressing gene FvZBD1. Our results indicate that both aflatoxin and fumonisin had an effect on the expression of the velvet complex in both competing fungi. The velvet complex is known for its global role in regulating fungal secondary metabolism. This suggests that fumonisin and aflatoxin are just the opening salvos when these fungi battle for the same resources or host.
Data availability statementAll relevant data is presented in the publication. Data requiring upload to a third party (like sequencing data) was not used in this study.
Author contributionsTS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JH: Formal analysis, Investigation, Methodology, Writing – review & editing. TM: Formal Analysis, Investigation, Methodology, Writing – review & editing. QW: Formal analysis, Investigation, Writing – review & editing. JL: Methodology, Validation, Visualization, Writing – review & editing. AG: Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. SG: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by USDA Agricultural Research Service congressionally appropriated funds, ARS project number: 6040-42000-046-000D.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1513134/full#supplementary-material
ReferencesBlacutt, A. A., Gold, S. E., Voss, K. A., Gao, M., Glenn, A. E. (2018). Fusarium verticillioides: advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 108, 312–326. doi: 10.1094/PHYTO-06-17-0203-RVW
PubMed Abstract | Crossref Full Text | Google Scholar
Butchko, R. A., Brown, D. W., Busman, M., Tudzynski, B., Wiemann, P. (2012). Lae1 regulates expression of multiple secondary metabolite gene clusters in Fusarium verticillioides. Fungal Genet. Biol. 49, 602–612. doi: 10.1016/j.fgb.2012.06.003
PubMed Abstract | Crossref Full Text | Google Scholar
Calvo, A. M., Bok, J., Brooks, W., Keller, N. P. (2004). veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol. 70, 4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004
PubMed Abstract | Crossref Full Text | Google Scholar
Calvo, A. M., Lohmar, J. M., Ibarra, B., Satterlee, T. (2016). “18 velvet regulation of fungal development,” in Growth, Differentiation and Sexuality. Ed. WENDLAND, J. (Springer International Publishing, Cham).
Camardo Leggieri, M., Giorni, P., Pietri, A., Battilani, P. (2019). Aspergillus flavus and Fusarium verticillioides Interaction: Modeling the Impact on Mycotoxin Production. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02653
PubMed Abstract | Crossref Full Text | Google Scholar
Chang, P. K., Cary, J. W., Bhatnagar, D., Cleveland, T. E., Bennett, J. W., Linz, J. E., et al. (1993). Cloning of the Aspergillus parasiticus apa-2 gene associated with the regulation of aflatoxin biosynthesis. Appl. Environ. Microbiol. 59, 3273–3279. doi: 10.1128/aem.59.10.3273-3279.1993
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, X., Abdallah, M. F., Landschoot, S., Audenaert, K., De Saeger, S., Chen, X., et al. (2023). Aspergillus flavus and Fusarium verticillioides and Their Main Mycotoxins: Global Distribution and Scenarios of Interactions in Maize. Toxins 15, 577. doi: 10.3390/toxins15090577
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, X., Landschoot, S., Detavernier, C., De Saeger, S., Rajkovic, A., Audenaert, K. (2021). Cross-talk between Fusarium verticillioides and Aspergillus flavus in vitro and in planta. Mycotoxin Res. 37, 229–240. doi: 10.1007/s12550-021-00435-x
PubMed Abstract | Crossref Full Text | Google Scholar
Desjardins, A. E., Munkvold, G. P., Plattner, R. D., Proctor, R. H. (2002). FUM1–a gene required for fumonisin biosynthesis but not for maize ear rot and ear infection by Gibberella moniliformis in field tests. Mol. Plant Microbe Interact. 15, 1157–1164. doi: 10.1094/MPMI.2002.15.11.1157
PubMed Abstract | Crossref Full Text | Google Scholar
Eskola, M., Kos, G., Elliott, C. T., Hajslova, J., Mayar, S., Krska, R. (2020). Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 60, 2773–2789. doi: 10.1080/10408398.2019.1658570
PubMed Abstract | Crossref Full Text | Google Scholar
Finotti, E., Parroni, A., Zaccaria, M., Domin, M., Momeni, B., Fanelli, C., et al. (2021). Aflatoxins are natural scavengers of reactive oxygen species. Sci. Rep. 11, 16024. doi: 10.1038/s41598-021-95325-8
PubMed Abstract | Crossref Full Text | Google Scholar
Fountain, J. C., Bajaj, P., Nayak, S. N., Yang, L., Pandey, M. K., Kumar, V., et al. (2016a). Responses of aspergillus flavus to oxidative stress are related to fungal development regulator, antioxidant enzyme, and secondary metabolite biosynthetic gene expression. Front. Microbiol. 7, 2048. doi: 10.3389/fmicb.2016.02048
PubMed Abstract | Crossref Full Text | Google Scholar
Fountain, J. C., Bajaj, P., Pandey, M., Nayak, S. N., Yang, L., Kumar, V., et al. (2016b). Oxidative stress and carbon metabolism influence Aspergillus flavus transcriptome composition and secondary metabolite production. Sci. Rep. 6, 38747. doi: 10.1038/srep38747
PubMed Abstract | Crossref Full Text | Google Scholar
Fountain, J. C., Scully, B. T., Chen, Z. Y., Gold, S. E., Glenn, A. E., Abbas, H. K., et al. (2015). Effects of hydrogen peroxide on different toxigenic and atoxigenic isolates of aspergillus flavus. Toxins (Basel) 7, 2985–2999. doi: 10.3390/toxins7082985
留言 (0)