With a discerning and inquisitive interest, we read the paper “BAFF Blockade Attenuates DSS-Induced Chronic Colitis via Inhibiting NLRP3 Inflammasome and NF-κB Activation” published in Frontiers in Immunology (1). In this study, Zhang et al. present compelling evidence for the pivotal role of BAFF (B cell activating factor) in inflammatory bowel disease (IBD). Their findings suggest that BAFF neutralization ameliorates colitis by mitigating inflammation and suppressing NF-κB and NLRP3-related signaling pathways, thus offering a promising therapeutic target for IBD treatment. The author’s present provides valuable insights into the molecular mechanisms underlying IBD pathogenesis and highlights the potential of BAFF blockade as a novel therapeutic approach. In general, this is an excellent piece of research. However, there are a few points in the paper that require further discussion and critical examination.
2 Results and discussionIn Zhang’s report, the murine cell line RAW264.7 was used in vitro experimental analysis. The authors reported that BAFF blockade significantly reduced ASC by western blot in LPS-induced RAW264.7 cells (Figure 8A). It is widely acknowledged that the RAW264.7 murine cell line lacks the expression of ASC, which can be attributed to epigenetic silencing, particularly DNA methylation (2–7). Therefore, the detection of ASC protein expression in ASC-deficient RAW264.7 cells in the present study made us confused.

Figure 8. From Zhang, Ying et al. “BAFF Blockade Attenuates DSS-Induced Chronic Colitis via Inhibiting NLRP3 Inflammasome and NF-кB Activation.” Frontiers in immunology vol. 13 783254. 7 Mar. 2022.
A further apparent problem is that in the section of MATERIALS & METHODS, the primary antibody anti-ASC, used in the western blot experiment, is produced by the Cell Signaling Technology company. As illustrated in Figure 1, the image from the Cell Signaling Technology website depicts a Western blot analysis of extracts from J774A.1 and Raw 264.7 cells utilizing ASC antibody (#67824, #37953). According to the instructions, ASC antibody (either #67824 or #37953) has explicitly stated that ASC protein in Raw264.7 cells cannot be detected (server as a negative control). Moreover, this ASC-deficient characteristic of RAW264.7 cells has been widely exploited in a multitude of studies as a cellular model to investigate ASC-independent inflammasome pathways or to examine the effects of ASC exogenous expression (4, 6, 8–12). For instance, Sun et al. demonstrated (5) that propofol treatment of RAW264.7 cells did not result in caspase-1 and gasdermin D cleavage. The exogenous expression of ASC in RAW264.7 cells was found to be a prerequisite for propofol-induced pyroptosis. To foster studies on the ASC adaptor, InvivoGen company has developed RAW-ASC cells (Cat. Code: raw-asc), which were generated by stable transfection of the murine ASC gene into the murine RAW 264.7 macrophage cell line, which is naturally ASC-deficient.
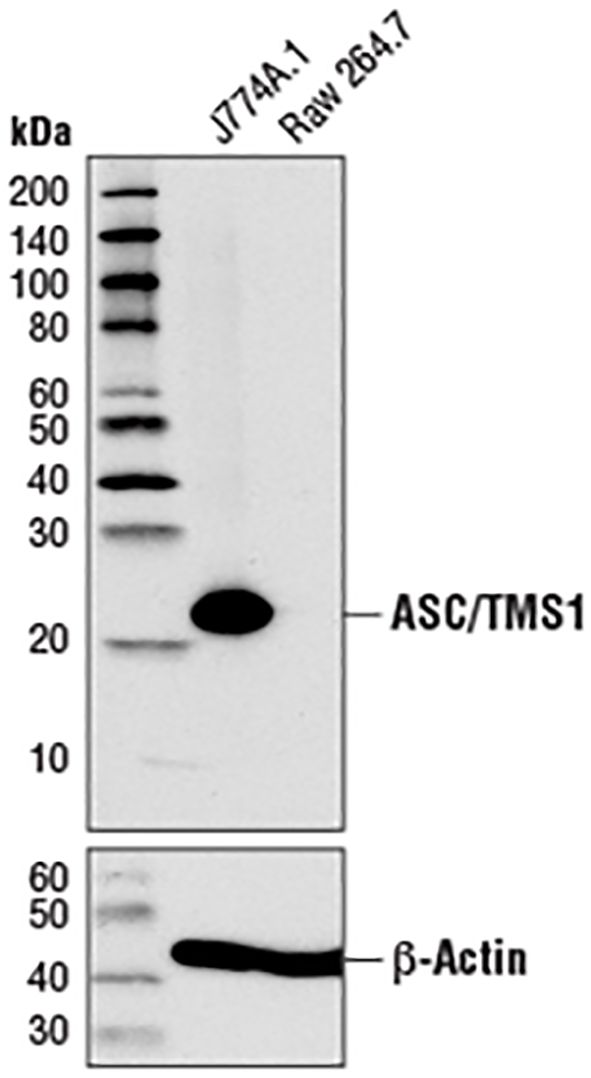
Figure 1. Western blot analysis of extracts from J774A.1 and RAW264.7 cells using ASC (D2W8U) rabbit monoclonal antibody (#67824) and β-actin (D6A8) rabbit monoclonal antibody (#8457). The data were downloaded from the website of Cell Signaling Technology company.
This discrepancy may be partially attributed to contamination of the samples and the use of incorrect reagents that cross-react with proteins unrelated to ASC in cellular extracts. An additional possibility is that the cultures examined may not contain the original RAW 264.7 cell line, potentially due to contamination with other cell types during the process of culturing and passaging. It may be necessary for the authors to confirm the identity of the RAW 264.7 cell line through the use of short tandem repeat analysis or other appropriate methods.
3 ConclusionIn conclusion, Zhang et al. have made a valuable contribution to our understanding of the role of BAFF in IBD pathogenesis. This work paves the way for new avenues of research and potential treatment strategies in inflammatory bowel diseases. Although the methodology and results of the study are praiseworthy, there is a need to reinforce the conclusions. It would be beneficial to address the discrepancy regarding ASC detection in RAW264.7 cells, which are known to lack ASC expression, in order to enhance the reliability of the in vitro findings. Furthermore, the validation of pivotal outcomes through the utilization of ASC-expressing RAW264.7 cell lines may facilitate the generation of more conclusive evidence regarding the impact of BAFF on the complete NLRP3 inflammasome.
Author contributionsJG: Conceptualization, Funding acquisition, Writing – review & editing. CL: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the grants from the National Natural Science Foundation of China (No.82404670), GuangDong Basic and Applied Basic Research Foundation (No.2023A1515110466), Shenzhen Nanshan District Health System Science and Technology Major Project Outstanding Youth Fund (No. NSZD2024035).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Zhang Y, Tao M, Chen C, Zhao X, Feng Q, Chen G, et al. BAFF blockade attenuates DSS-induced chronic colitis via inhibiting NLRP3 inflammasome and NF-kappaB activation. Front Immunol. (2022) 13:783254. doi: 10.3389/fimmu.2022.783254
PubMed Abstract | Crossref Full Text | Google Scholar
2. Guan X, Sagara J, Yokoyama T, Koganehira Y, Oguchi M, Saida T, et al. ASC/TMS1, a caspase-1 activating adaptor, is downregulated by aberrant methylation in human melanoma. Int J Cancer. (2003) 107:202–8. doi: 10.1002/ijc.11376
PubMed Abstract | Crossref Full Text | Google Scholar
3. Butts B, Gary RA, Dunbar SB, Butler J. Methylation of apoptosis-associated speck-like protein with a caspase recruitment domain and outcomes in heart failure. J Card Fail. (2016) 22:340–6. doi: 10.1016/j.cardfail.2015.12.004
PubMed Abstract | Crossref Full Text | Google Scholar
4. He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. (2015) 25:1285–98. doi: 10.1038/cr.2015.139
PubMed Abstract | Crossref Full Text | Google Scholar
5. Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z, et al. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis. (2019) 10:542. doi: 10.1038/s41419-019-1761-4
PubMed Abstract | Crossref Full Text | Google Scholar
6. Traughber CA, Deshpande GM, Neupane K, Bhandari N, Khan MR, McMullen MR, et al. Myeloid-cell-specific role of Gasdermin D in promoting lung cancer progression in mice. iScience. (2023) 26:106076. doi: 10.1016/j.isci.2023.106076
PubMed Abstract | Crossref Full Text | Google Scholar
7. Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. (2008) 180:7147–57. doi: 10.4049/jimmunol.180.11.7147
PubMed Abstract | Crossref Full Text | Google Scholar
8. Chen X, He WT, Hu L, Li J, Fang Y, Wang X, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. (2016) 26:1007–20. doi: 10.1038/cr.2016.100
PubMed Abstract | Crossref Full Text | Google Scholar
10. Zhang P, Liu Y, Hu L, Huang K, Hong M, Wang Y, et al. NLRC4 inflammasome-dependent cell death occurs by a complementary series of three death pathways and determines lethality in mice. Sci Adv. (2021) 7(42):eabi9471. doi: 10.1126/sciadv.abi9471
PubMed Abstract | Crossref Full Text | Google Scholar
11. Zhang L, Tian J, Ma L, Duan S. Mechanistic insights into severe pulmonary inflammation caused by silica stimulation: The role of macrophage pyroptosis. Ecotoxicol Environ Saf. (2023) 258:114975. doi: 10.1016/j.ecoenv.2023.114975
PubMed Abstract | Crossref Full Text | Google Scholar
12. Hirata Y, Cai R, Volchuk A, Steinberg BE, Saito Y, Matsuzawa A, et al. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr Biol. (2023) 33:1282–94.e5. doi: 10.1016/j.cub.2023.02.060
留言 (0)