Parkinson’s disease (PD) is the most prevalent movement disorder, affecting an estimated 6 million individuals globally (1). The clinical manifestations of PD are multifaceted, encompassing both motor symptoms—such as tremors, rigidity, postural gait abnormalities, and bradykinesia—and non-motor symptoms (NMSs), including cognitive impairment (2). The etiology of PD remains complex, with genetic variations emerging as pivotal contributors to its pathogenesis (3). In recent years, a growing body of evidence has implicated numerous novel single nucleotide polymorphisms (SNPs) in PD, highlighting the essential role of these genetic variants in the molecular mechanisms that increase susceptibility to the disease (4).
An increasing body of research suggests that glutamate-mediated excitotoxicity plays a role in common neurological disorders, including PD (5, 6). The N-methyl-D-aspartate (NMDA) receptors, which are glutamate receptors, consist of NR1 (GRIN1) and NR2 (GRIN2) subtypes. The specific NR2 subunit (A, B, C, or D) determines the distinct physiological characteristics of NMDA receptors (7). Variation in the GRIN2A gene is the most common genetic cause of developmental and epileptic encephalopathy with spike-and-wave activation in sleep (DEE-SWAS) (8). N-methyl-D-aspartate receptor 2B (GRIN2B) is recognized as a key contributor to excitotoxicity-induced neuronal loss (9). Furthermore, GRIN2B dysfunction has been implicated in motor and cognitive deficits in α-synuclein-induced mouse models, and treatment with a GRIN2B inhibitor has been shown to rescue motor impairments in PD (10). However, the contribution of GRIN2B SNPs to PD and the impact of GRIN2B variations on PD development remain controversial. Some studies have indicated that GRIN2B SNPs reduce the risk of PD (11), while others have found that GRIN2B SNPs increase the risk of PD and the occurrence of impulse control behaviors (ICBs) in PD patients (12, 13). Several studies have suggested no correlation between GRIN2B SNPs and PD (13–15). The association between the GRIN2B genotype and the susceptibility of PD patients in China has not been well explored, and the influence of GRIN2B SNPs on PD progression has yet to be reported. Importantly, the impact of GRIN2B SNPs on the progression of PD has not been documented. Therefore, our study aimed to reveal the influence of the GRIN2B rs219882 genotype on PD susceptibility, age of onset, and the progression of motor and cognitive symptoms in a longitudinal cohort of individuals from southern China.
2 Materials and methods 2.1 ParticipantsThis longitudinal study, conducted between 2017 and 2022, included a total of 165 individuals with sporadic PD from the Department of Neurology at Shanghai Dongfang Hospital (Figure 1). A control group comprising 154 healthy individuals, matched for sex and age, was recruited from the Zhoujiadu Community Health Service Center in the Shanghai Pudong New Area. All PD patients were diagnosed by neurologists who adhered to the clinical diagnostic criteria established by the UK Parkinson’s Disease Society Brain Bank (16). The inclusion criteria for the patients were as follows: (1) ability to provide informed consent; (2) diagnosis of idiopathic PD; (3) age between 30 and 90 years; and (4) ethnic Han Chinese descent. The exclusion criteria included the following: (1) parkinsonism attributable to other causes such as drugs, stroke, or toxins; (2) a familial history of PD; (3) the presence of other neurological disorders, including multiple system atrophy and PD dementia; and (4) a history of cerebrovascular diseases or other severe systemic conditions. The recruitment process and study protocols were approved by the Institutional Review Board (IRB) of Shanghai East Hospital (approval number: 2022135). All participants provided written informed consent in accordance with the ethical standards of the Declaration of Helsinki.
Figure 1. A flow chart of our study. PD, Parkinson’s disease; N-methyl-D-aspartate receptor 2B (GRIN2B).
2.2 Clinical assessmentsTo evaluate the impact of GRIN2B SNPs on motor and cognitive progression in PD, a longitudinal follow-up study was conducted. Initially, 165 PD patients were enrolled, with 89 participants available and eligible for follow-up. The average follow-up interval for all patients was 3.53 years (standard deviation, SD = 1.04), with each patient visiting at least once. Comprehensive baseline and follow-up clinical data were obtained through face-to-face interviews. The patients were categorized into early-onset PD (EOPD) with an onset age of less than 50 years and late-onset PD (LOPD) with an onset age of over 50 years. Information on anti-PD medications was collected, and the levodopa equivalent daily dose (LEDD) was calculated, as previously described (17). Consistent with prior studies, PD patients were classified into three subtypes based on the symptomatology: tremor-dominant (TD) PD (mean tremor score/PIGD score ≥ 1.5), postural instability and gait disturbances (PIGD)-dominant PD (mean tremor score/PIGD score ≤ 1.0), and intermediate PD (1.0 < mean tremor score/PIGD score < 1.5) (18, 19). Motor symptom severity was assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) parts II and III and the Hoehn–Yahr (H–Y) stage. Specific motor features, including tremor (UPDRS items 20 and 21), rigidity (item 22), bradykinesia (items 23–26 and 31), and axial impairment (items 27–30), were further evaluated (20). Non-motor symptoms (NMSs) were assessed using the Non-Motor Symptoms Questionnaire (NMSQuest). Cognitive performance was evaluated using the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). Emotional status was measured using the Hamilton Depression Rating Scale (HAM-D) and Hamilton Anxiety Rating Scale (HAM-A), while sleep quality was assessed using the Parkinson’s Disease Sleep Scale (PDSS). Neuropsychological assessments were conducted by highly trained specialist neurologists.
2.3 SNP genotypingThe GRIN2B phenotype of PD patients in our study was assessed using iPLEX® reagents and the MassARRAY® System (Sequenom Company) (21). Specifically, the PCR amplification primers and single-base extension primers for the SNP sites of GRIN2B were synthesized using genotyping tools and the MassARRAY Assay Design developed by the Sequenom Company, San Diego, CA, USA. The forward primer sequence was F:5’-ACGTTGGATGGCCTTCCCACCATTAATCTG-3′, and the reverse primer sequence was R:5’-ACGTTGGATGTGTGGAAGTAGCTGGGTATA-3′. Then, the peripheral blood samples were collected, and the DNA was extracted using a DNA extraction kit. The DNA was resuspended to a final concentration of 50 ng/μl and stored at −20°C. PCR amplification was performed by multiplex PCR in 384-well plates, with a total volume of 5 μL per reaction. The iPLEX assay was performed at the end of the PCR reaction. The PCR products were treated with shrimp alkaline phosphatase (SAP) to remove free deoxynucleotide triphosphates (dNTPs) from the system. Clean resin was used to desalt the iPLEX reaction products, optimizing MALDI-TOF mass spectrometric analysis. Then, the reaction products were put onto a 384-element SpectroCHIP bioarray. MassARRAY Workstation software was used to process and analyze the iPLEX SpectroCHIP bioarray. The genotypes of GRIN2B SNPs were determined through differences in the molecular weight of the PCR products.
2.4 Declaration of generative AI and AI-assisted technologies in the writing processThe authors declare that Generative AI was used in the creation of this manuscript. The authors used ChatGPT 4.0 to improve the readability and language of the manuscript. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the published article.
2.5 Statistical analysisAll statistical analyses were conducted using IBM SPSS Statistics version 26.0. Categorical data were described using frequencies and percentages. To assess the representativeness of the study population, the Hardy–Weinberg equilibrium (HWE) was tested. The chi-squared (χ2) test was applied to compare the allele and genotype frequencies between the groups. Logistic regression analysis was performed to calculate the odds ratios (ORs) and their corresponding 95% confidence intervals (95% CIs). Both linear and logistic regression analyses were conducted to compare the baseline clinical characteristics across the different groups.
In longitudinal studies, linear mixed-effects models are often employed to analyze repeated measures, effectively managing missing data and accounting for individual variability. Consequently, these models were used to explore the association between the GRIN2B genotypes and the progression of motor and cognitive symptoms, as assessed through the UPDRS motor scores and MoCA scores. The fixed effects included the GRIN2B genotype, disease duration (as the timescale), and their interaction while adjusting for age, sex, and LEDD or education levels. The model incorporated random intercepts for each participant’s ID and random slopes for time to account for the correlations within repeated measures over time and among individuals.
3 Results 3.1 Demographics and clinical features of the participantsA total of 165 patients with PD and 154 healthy controls were enrolled in our study, comprising a total of 319 participants. The demographic and clinical data of these individuals are presented in Table 1. The distribution of sex, age, and years of education between the PD patients and healthy controls was found to be statistically similar. Among the PD patients, 43 (25.5%) individuals were classified as early-onset, with an average disease duration of 4.43 years (standard deviation, SD = 0.27) across all participants with PD. The mean follow-up duration was 3.53 years (SD = 0.08). At baseline, the median Hoehn–Yahr (H–Y) stage was 2.0, with an interquartile range of 1.5 to 2.5, and the average UPDRS III score was 21.55 (SD = 0.27). In addition, the mean MoCA score was 24.46 (SD = 0.34), and the MMSE score was 28.43 (SD = 0.18).
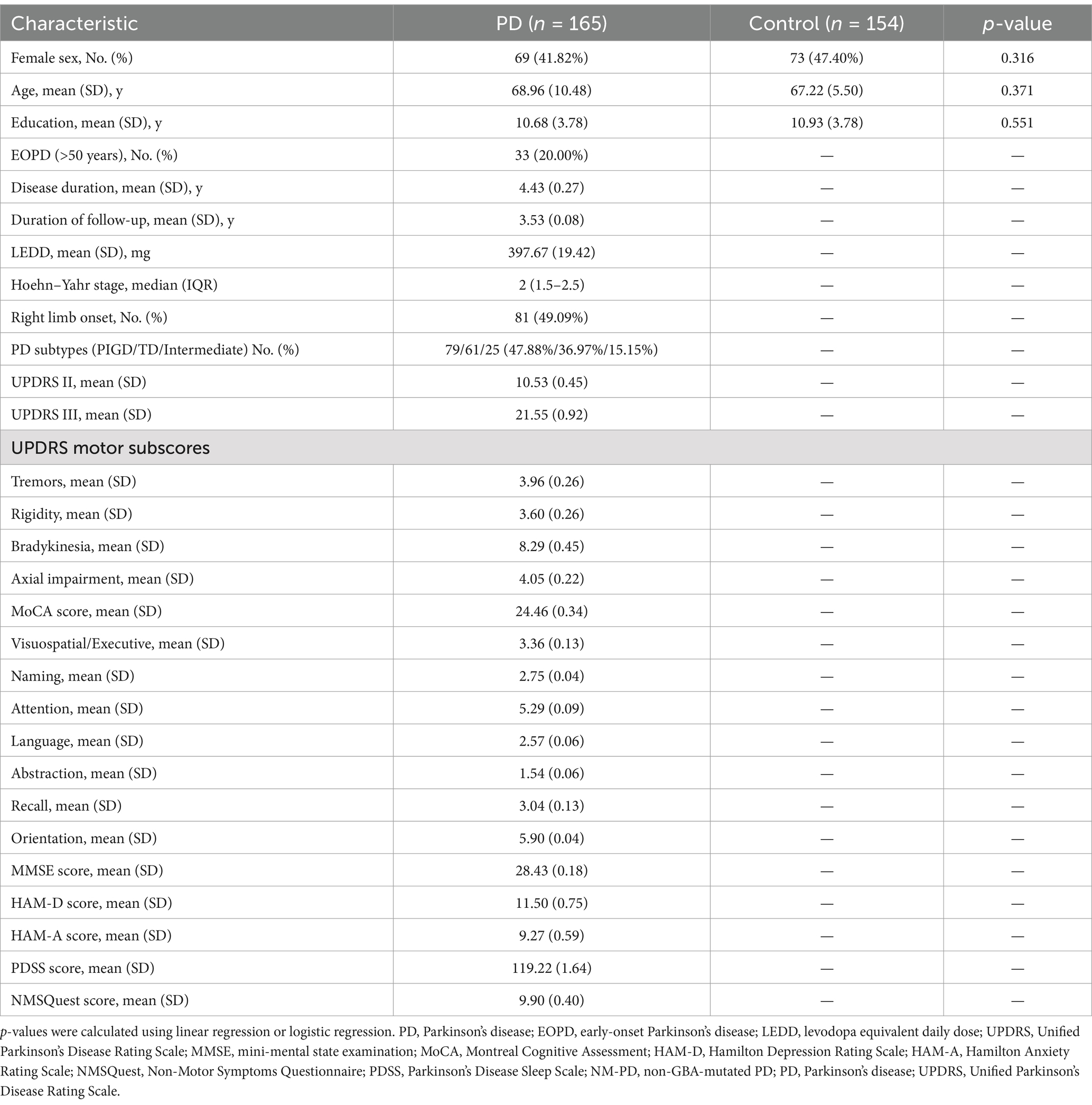
Table 1. Demographic data of the patients with sporadic PD and healthy controls.
3.2 Correlation analysis between GRIN2B rs219882 and PD riskThe genotype and allele frequencies of the GRIN2B rs219882 polymorphism in both the PD and control groups are detailed in Table 2. The analysis for the HWE revealed no significant deviation in the allele frequencies within the PD group (p = 0.881) or the control group (p = 0.900), indicating that our study population was representative. Notably, the frequency of the GG genotype and the G allele was significantly higher in PD patients compared to the controls (OR = 2.27, 95% confidence interval (CI): 1.13–4.59, Pgenotype = 0.021; OR = 2.42, 95% CI: 1.72–3.40, Pallele = 0.001). Furthermore, under a recessive model, PD patients with the GG + GT genotype showed a higher proportion compared to the healthy controls (OR = 1.98, 95% CI: 1.05–3.72, p = 0.032; adjusted OR = 1.99, 95% CI: 1.05–3.77), while no significant difference was observed between the two groups under a dominant model. These results suggest that the G allele of the GRIN2B rs219882 polymorphism is associated with PD susceptibility and that the G allele and GG + GT genotype may constitute risk factors for the development of PD.
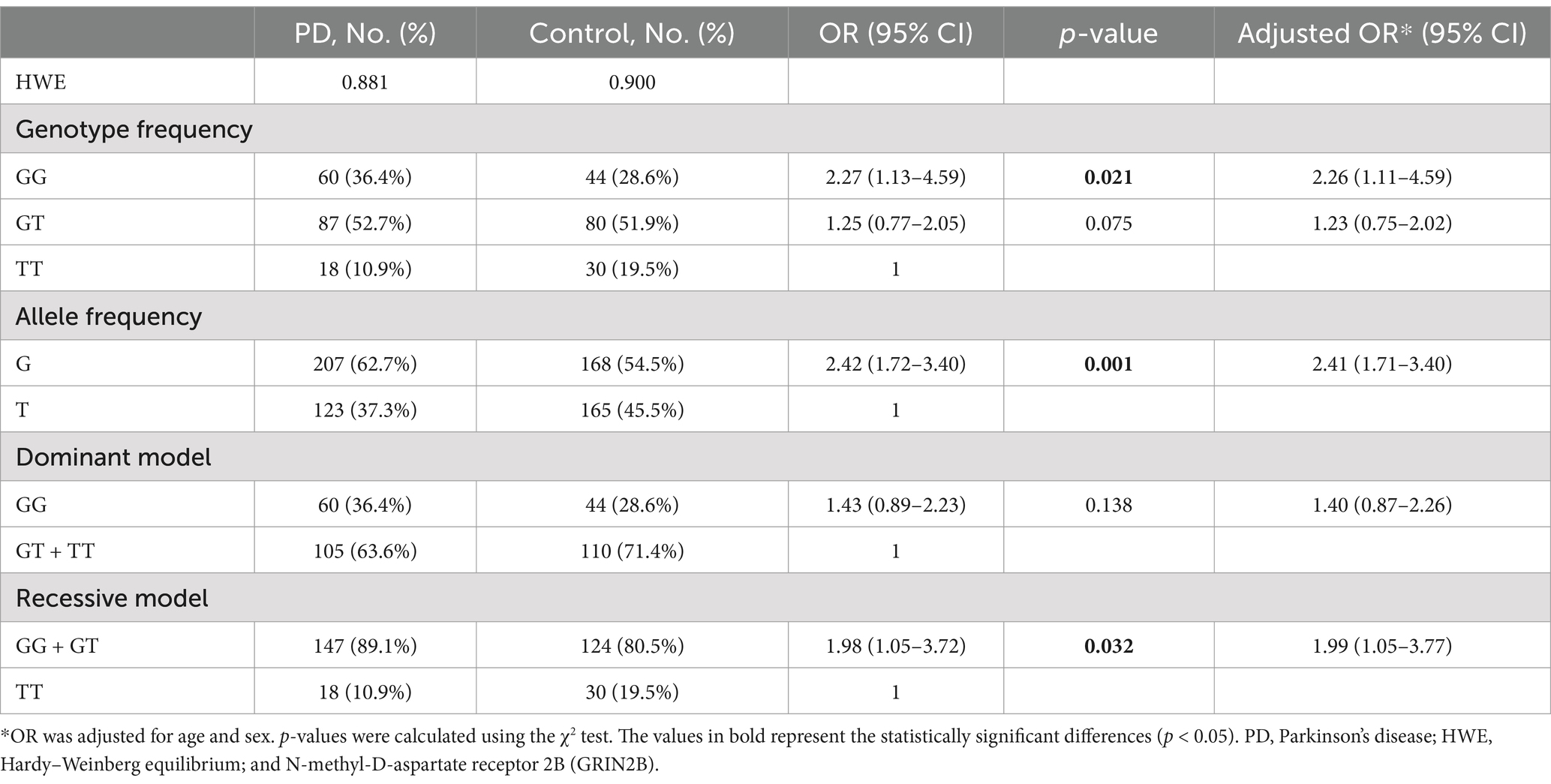
Table 2. Genotype and allele frequency of the GRIN2B rs219882 (T > G) polymorphism among the patients with sporadic PD and controls in China.
3.3 Association analysis between GRIN2B rs219882 and the age onset of PDWe conducted a stratified analysis based on the age of onset of PD, as presented in Table 3. In the LOPD subgroup, the frequency of the G allele was significantly higher in PD patients compared to the controls (OR = 1.55, 95%CI: 1.09–2.20, p = 0.014). In addition, under a dominant model, the frequency of the GG genotype was more prevalent in the PD group than in the controls (OR = 1.70, 95% CI: 1.01–2.85, p = 0.045). Most notably, a higher proportion of the GG + GT genotype was observed in the PD group compared to the healthy controls in a recessive model (OR = 2.11, 95% CI: 1.04–4.25, p = 0.035). Collectively, these findings suggest that the G allele and the GG + GT genotype may be associated with an increased susceptibility to PD, particularly in the context of LOPD.
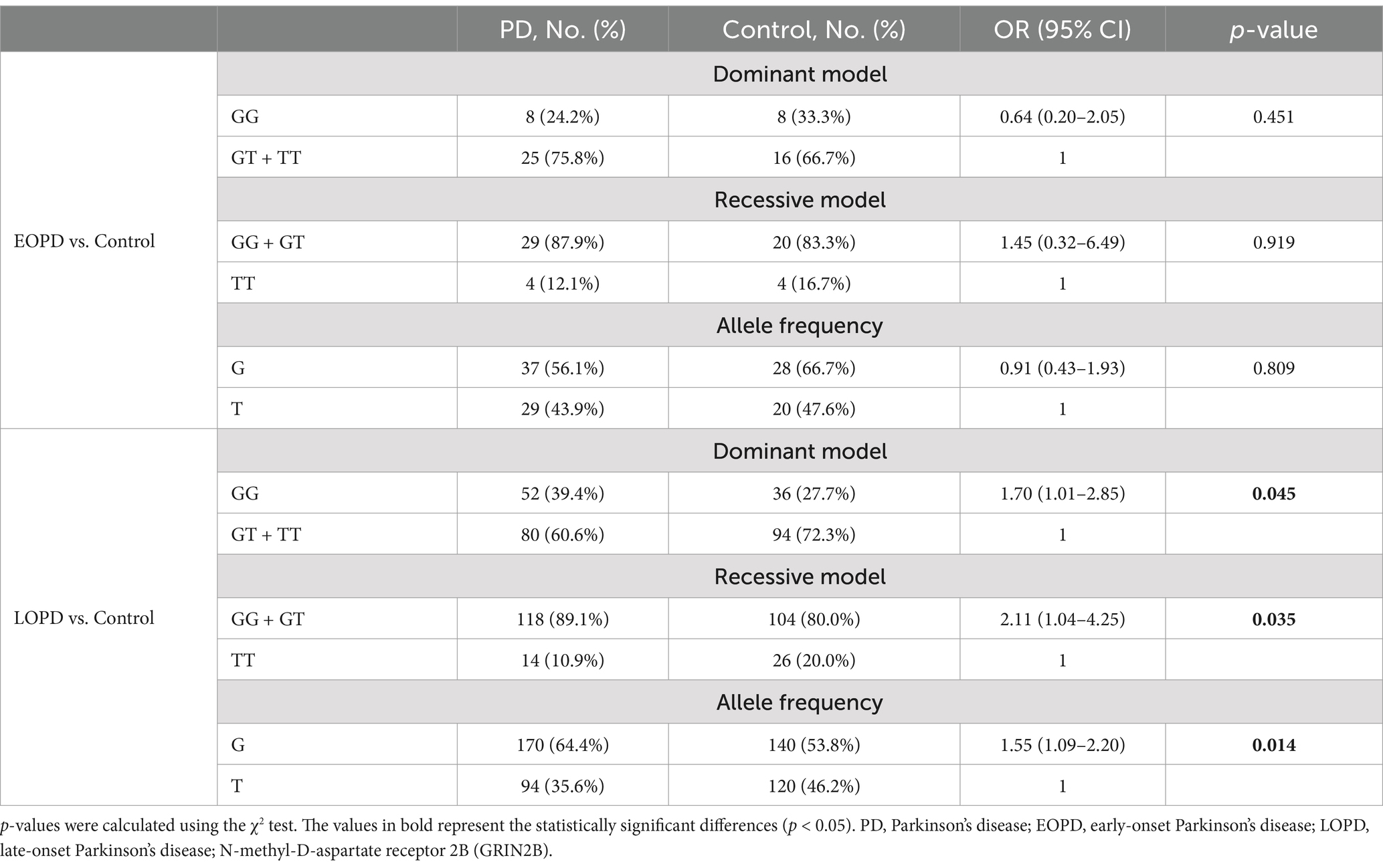
Table 3. Distributions of the genotype and allele frequencies of GRIN2B rs219882 (T > G) in the sporadic PD and control groups stratified by sex or age at onset.
3.4 Effect of GRIN2B rs219882 on motor or cognitive declineWe conducted a detailed longitudinal cohort study to examine whether the GRIN2B rs219882 genotype frequency influences the progression of motor and cognitive functions in PD. We performed a detailed longitudinal cohort study of 89 PD patients and the baseline clinical features of 165 PD patients are summarized in Table 3. The mean follow-up time was 3.53 (1.06) years for the GG + GT carriers and 3.51 (0.88) years for the TT carriers. At baseline, the distributions of age, sex, disease duration, education, LEDD, H–Y stages, and cognitive impairment were similar between the two groups. Interestingly, the individuals with the GG + GT genotype exhibited higher UPDRS motor scores (p = 0.008), particularly in rigidity (p = 0.003), bradykinesia (p = 0.012), and axial impairment (p = 0.045), with no significant difference observed in the tremor scores.
Linear mixed-effects models were used to examine the correlation between the GRIN2B genotype and the progression rate of UPDRS motor scores and its subparts, adjusting for sex, age at baseline, and LEDD at follow-up (Table 4; Figure 2). The interaction with time revealed the influence of the genotype status on the slope (annual change in the scores of the UPDRS motor and its subparts). The estimated progression rate (standard error, SE) of the UPDRS motor score was 6.19 (2.56) points/year for PD patients with the GG genotype, 5.99 (2.44) points/year for those with the GT genotype, and 5.42 (2.47) points/year for those with the TT genotype. Notably, the progression rate for the patients with the GG + GT genotype [6.10 (2.54) points/year; p = 0.022] was significantly faster than that of those with the TT genotype. The analysis of the subparts of the UPDRS motor scores revealed that the SE rate of change in the rigidity and axial impairment scores was significantly faster in the GG + GT carriers compared to the TT carriers [1.83 (0.58) points/year; Prigidity = 0.003; 1.20 (0.55) points/year; Paxial impairment = 0.033]. These findings suggest that the GG + GT genotype is associated with a faster motor decline, particularly in terms of rigidity and axial impairment.
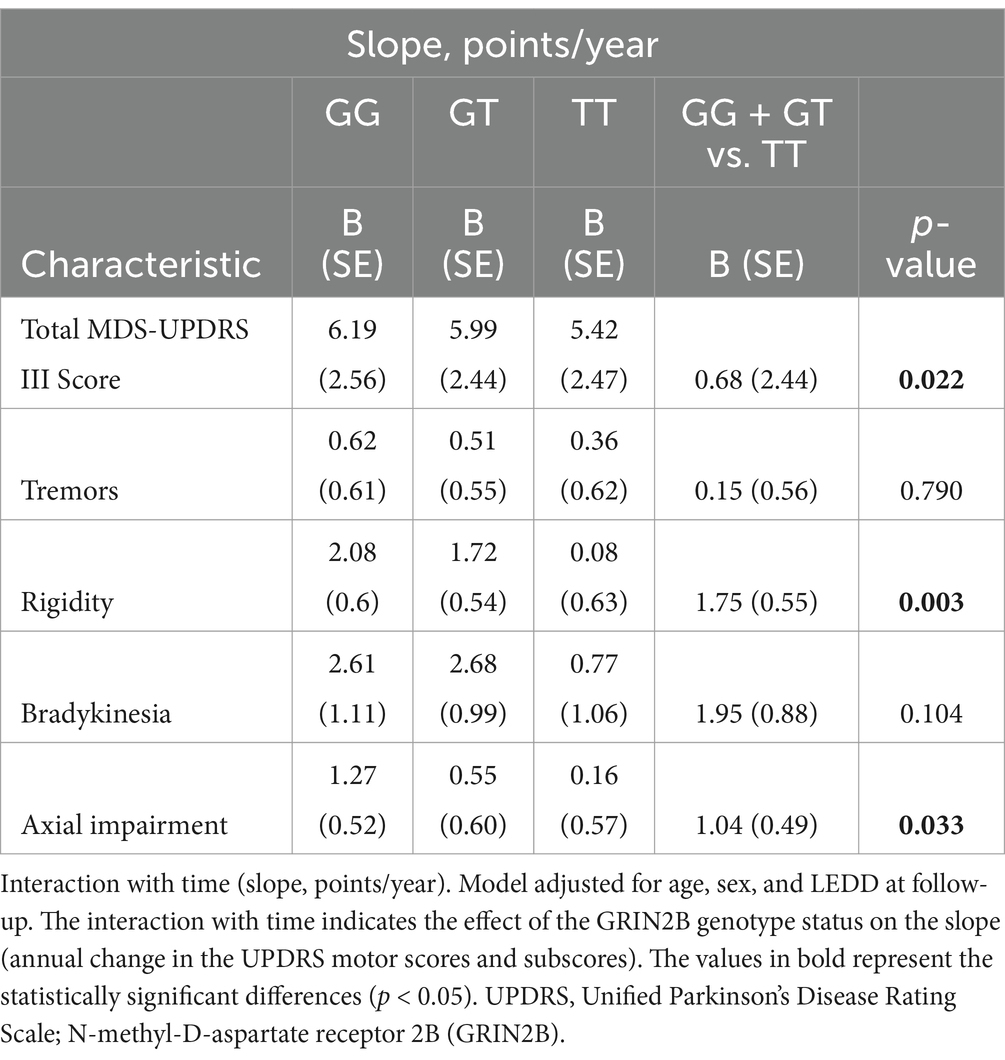
Table 4. Association between GRIN2B rs219882 and motor progression among the groups.
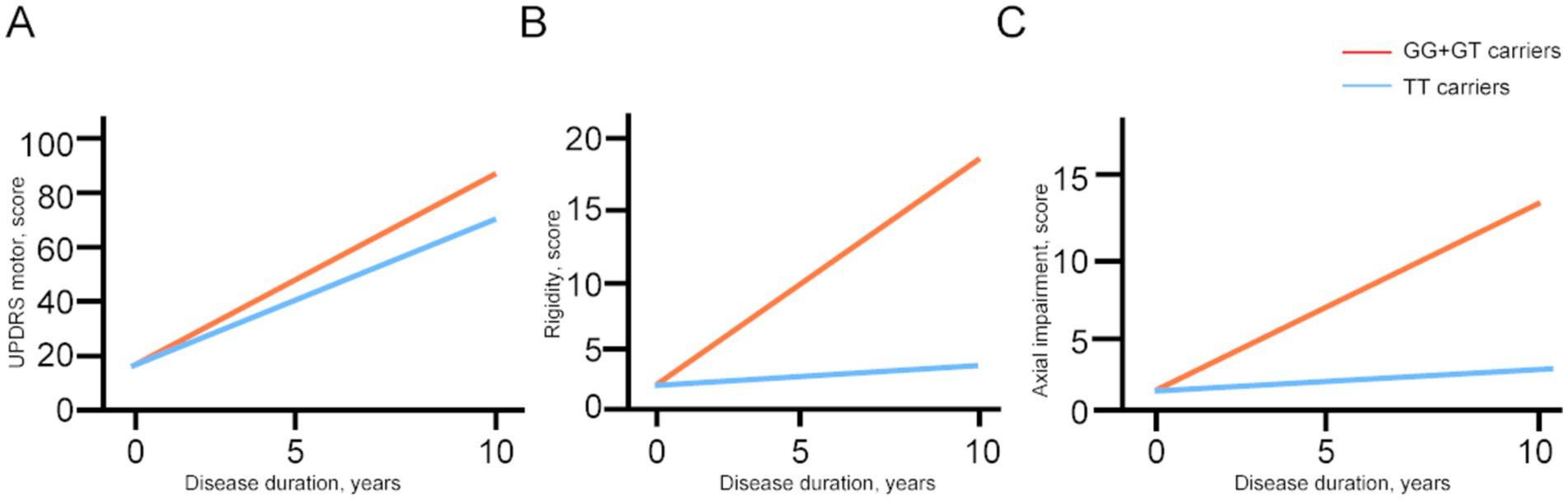
Figure 2. The longitudinal trajectories of the mean UPDRS motor scores and subparts among the GRIN2B genotypes. UPDRS, Unified Parkinson’s Disease Rating Scale; N-methyl-D-aspartate receptor 2B (GRIN2B). Rigidity scores, bradykinesia scores, and axial impairment scores were evaluated using the item 22, items 23–26, 31, items 27–30 respectively.
To further ascertain the impact of the GRIN2B rs219882 genotype on cognitive decline, we employed a linear mixed-effects model to examine the rate of change in the MoCA scores and its seven subscale scores, adjusting for age, sex, and years of formal education at baseline (Table 5). Notably, the rate of deterioration in the recall score for the patients with the GG genotype was 0.58 points/year higher than that for the carriers of the TT genotype (p = 0.030). However, no significant differences were observed in the progression rates of the MoCA and its subscale scores.
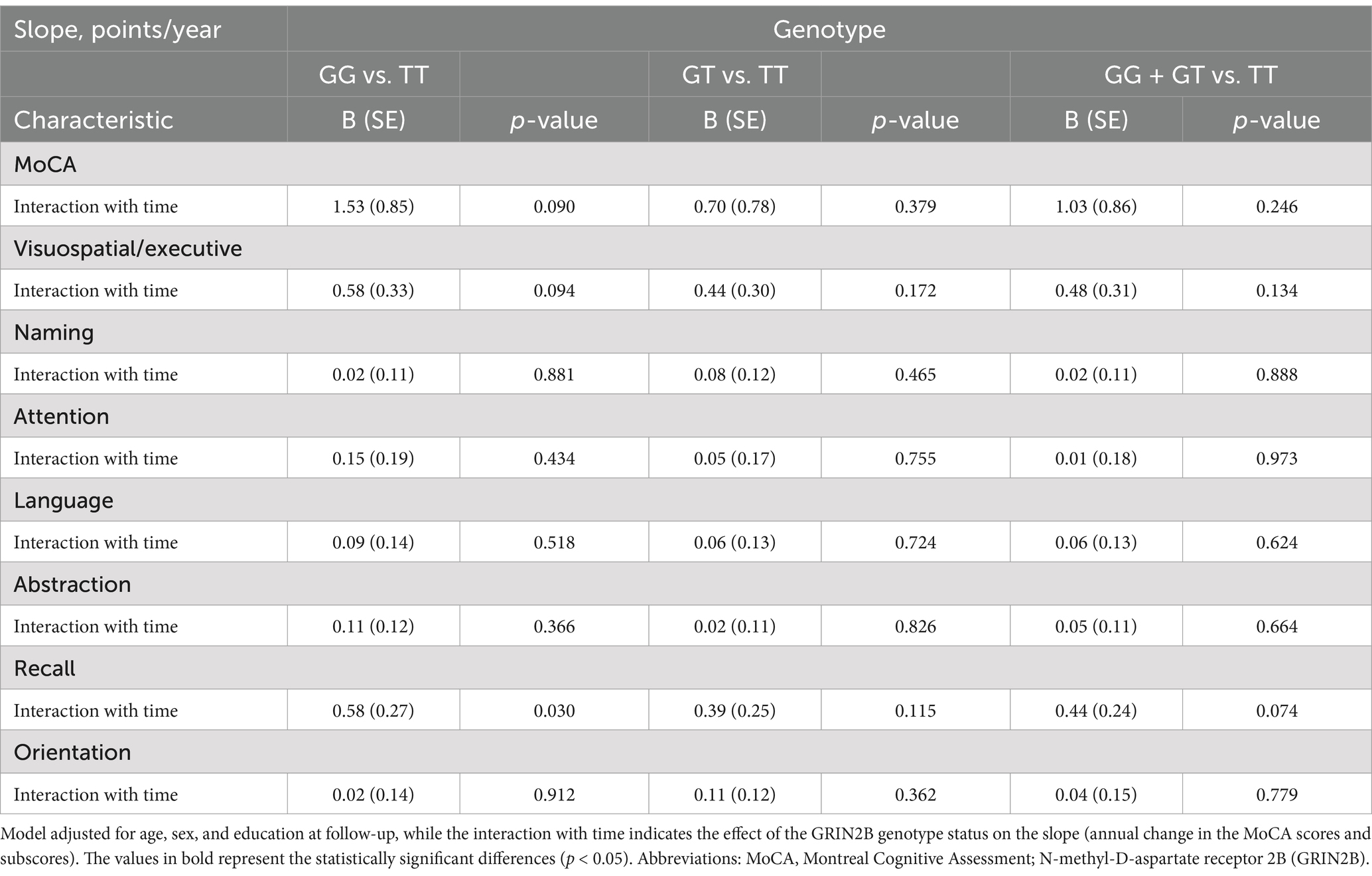
Table 5. Association between GRIN2B rs219882 and cognitive progression among the groups.
4 DiscussionTo the best of our knowledge, our research is the first to highlight the relationship between GRIN2B SNPs and the motor progression of PD within a longitudinal Chinese cohort. Our study revealed that the GRIN2B rs219882 G allele is associated with PD susceptibility, particularly in LOPD. Notably, the individuals with the GRIN2B GG + GT genotype exhibited a more rapid motor decline, especially in terms of rigidity and axial impairment, compared to the TT genotype carriers.
We found that the risk of PD in the G allele carriers was 2.4 times higher than that in the non-carriers. The GRIN2B rs219882T > G variant, an intron variant located within gene regions, likely contributes to transcriptional dysfunction (22). Elevated GRIN2B levels are implicated in PD pathogenesis through TNF-α-associated neuroinflammation, which may account for our findings (23). Notably, the G allele frequency in the healthy controls (0.455) in our study is consistent with that of East Asian populations in the dbSNP database (0.425), suggesting strong statistical power for this SNP in our cohort. Furthermore, our study demonstrated that the G allele significantly influenced the age of PD onset. The rs219882 variant was initially identified in a genome-wide association study (GWAS) of patients with sporadic LOPD (22). Our findings are consistent with this research, showing a higher frequency of the G allele in LOPD individuals compared to the healthy controls. LOPD is characterized by more severe dopaminergic damage and motor impairment, as well as a higher rate of progression compared to EOPD, due to distinct underlying molecular mechanisms (24, 25). Our results suggest that the pathogenic mechanisms of GRIN2B variations may share similarities with those of LOPD.
The UPDRS motor score, a standardized measure for evaluating motor impairment severity, is well-established for its reliability and validity (26, 27) and is frequently used in clinical research (28). However, few studies have utilized the UPDRS to assess the rate of motor progression. In our PD cohort, the GG + GT carriers experienced a faster rate of motor decline compared to the TT carriers, as estimated by the UPDRS scores. Moreover, we found that the GG carriers had a more rapid motor decline than the GT carriers, suggesting a correlation between the rate of motor decline and the severity of the GRIN2B variant. This suggests that GRIN2B variant severity is likely involved in GRIN2B function regulation, contributing to PD development and progression. Consistent with our findings, previous animal studies have confirmed that GRIN2B dysfunction impacts the progression of motor dysfunction in PD (10, 23, 29). As the GRIN2B G allele is associated with a faster rate of motor progression, we further explored the potential association between the GRIN2B genotype and specific motor features. We found that the GG + GT carriers had faster progression rates in the rigidity and axial impairment scores than the TT carriers. NMDA antagonists have been shown to alleviate rigidity in a PD mouse model (30, 31). Interestingly, we also found that the rate of bradykinesia decline was faster in the GT carriers than in the TT carriers. It has been reported that co-administration of levodopa with NMDA antagonists can potentiate the effect of levodopa and reduce levodopa-induced dyskinesias (30, 32). Our results indicate that GRIN2B variations play a pivotal role in the rigidity or dyskinesia symptoms of PD.
An intriguing finding was that the GG carriers lost 0.58 points in recall per year, which was significantly faster than the TT carriers. GRIN2B is crucial for synaptic and cognitive function in an α-synuclein-induced mouse model, possibly due to an imbalance in calcium homeostasis (10, 33). However, the difference in total MoCA scores between the GG and TT carriers was not significant. Further studies are needed to elucidate the association between GRIN2B and cognitive progression.
The strengths of this study include the following: (1) demonstrating a novel association between the GRIN2B rs219882 G allele and PD susceptibility and age of onset in a Chinese population and (2) being the first to discover that GRIN2B GG + GT carriers experience rapid motor progression, characterized by more severe rigidity and axial impairment, in a longitudinal study of PD patients from China. Our research may provide theoretical support for predicting the rapid progression of motor and cognitive impairments in patients with LOPD. Consequently, patients with LOPD could potentially predict the progression of their motor and cognitive functions by detecting GRIN2B gene polymorphisms, thereby guiding their clinical treatment. However, there are limitations to this study. Complete genotype testing is required to mitigate potential confounding factors from prevalent genes associated with PD, such as LRRK2, APOE, and PRKN. In addition, this retrospective study had missing data and inhomogeneous follow-up times. To address these issues, we performed linear mixed-effects models to account for potential confounders and handle the missing data. Finally, our study was a single-center follow-up research with a relatively small sample size. Therefore, it is necessary to conduct multi-center studies with larger populations to validate our findings.
5 ConclusionOur study represents a pioneering effort in examining the role of GRIN2B in the prevalence and motor and cognitive functional progression of PD within the Chinese population. Our findings reveal that PD patients with GG + GT genotypes experience a more rapid decline in motor function, particularly in the progression of rigidity and axial impairment. Notably, GG genotype carriers exhibit a more rapid decline in recall function. These insights are of significant importance for understanding the impact of GRIN2B variants on the pattern of motor deterioration in PD. They have the potential to help clinicians make more accurate prognoses and help inform treatment strategies.
Data availability statementThe original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethics statementThe studies involving humans were approved by all recruitment and protocols were permitted by the Ethic Review Board (IRB) of Shanghai east hospital (approval number: 2022135). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsCC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. HL: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YB: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. YH: Conceptualization, Data curation, Formal analysis, Writing – original draft. HY: Conceptualization, Methodology, Project administration, Writing – original draft. HS: Investigation, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BZ: Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The grants were awarded by the National Natural Science Foundation of China (82271456 to Bei Zhang; and 82401493 to Can Cui) and the Postdoctoral Fellowship Program of CPSF (GZC20241231 to Yingying Han). The funders had no role in the study design, data collection, data analysis, interpretation, and writing or reviewing of the report.
AcknowledgmentsWe thank all the patients who participated in this study. This manuscript has not been previously published or submitted for publication elsewhere, except as a brief abstract in the proceedings of a scientific meeting or symposium.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that Generative AI was used in the creation of this manuscript. The authors used ChatGPT 4.0 to improve the readability and language of the manuscript. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the published article.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Bloem, BR, Okun, MS, and Klein, C. Parkinson's disease. Lancet. (2021) 397:2284–303. doi: 10.1016/S0140-6736(21)00218-X
Crossref Full Text | Google Scholar
2. Schapira, AHV, Chaudhuri, KR, and Jenner, P. Non-motor features of Parkinson disease. Nat Rev Neurosci. (2017) 18:435–50. doi: 10.1038/nrn.2017.62
Crossref Full Text | Google Scholar
3. Pang, SY, Ho, PW, Liu, HF, Leung, CT, Li, L, Chang, EES, et al. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson's disease. Transl Neurodegener. (2019) 8:23. doi: 10.1186/s40035-019-0165-9
PubMed Abstract | Crossref Full Text | Google Scholar
4. Rizig, M, Bandres-Ciga, S, Makarious, MB, Ojo, OO, Crea, PW, Abiodun, OV, et al. Identification of genetic risk loci and causal insights associated with Parkinson's disease in African and African admixed populations: a genome-wide association study. Lancet Neurol. (2023) 22:1015–25. doi: 10.1016/S1474-4422(23)00283-1
PubMed Abstract | Crossref Full Text | Google Scholar
5. Song, R, Zhang, J, Perszyk, RE, Camp, CR, Tang, W, Kannan, V, et al. Differential responses of disease-related GRIN variants located in pore-forming M2 domain of N-methyl-D-aspartate receptor to FDA-approved inhibitors. J Neurochem. (2023) 168:3936–49. doi: 10.1111/jnc.15942
Crossref Full Text | Google Scholar
7. Endele, S, Rosenberger, G, Geider, K, Popp, B, Tamer, C, Stefanova, I, et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet. (2010) 42:1021–6. doi: 10.1038/ng.677
PubMed Abstract | Crossref Full Text | Google Scholar
8. Carvill, GL, Regan, BM, Yendle, SC, O'Roak, BJ, Lozovaya, N, Bruneau, N, et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet. (2013) 45:1073–6. doi: 10.1038/ng.2727
PubMed Abstract | Crossref Full Text | Google Scholar
9. Paoletti, P, and Neyton, J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. (2007) 7:39–47. doi: 10.1016/j.coph.2006.08.011
Crossref Full Text | Google Scholar
10. Ferreira, DG, Temido-Ferreira, M, Vicente Miranda, H, Batalha, VL, Coelho, JE, Szegö, ÉM, et al. α-synuclein interacts with PrPC to induce cognitive impairment through mGluR5 and NMDAR2B. Nat Neurosci. (2017) 20:1569–79. doi: 10.1038/nn.4648
PubMed Abstract | Crossref Full Text | Google Scholar
11. Wu, SL, Wang, WF, Shyu, HY, Ho, YJ, Shieh, JC, Fu, YP, et al. Association analysis of GRIN1 and GRIN2B polymorphisms and Parkinson's disease in a hospital-based case-control study. Neurosci Lett. (2010) 478:61–5. doi: 10.1016/j.neulet.2010.04.063
PubMed Abstract | Crossref Full Text | Google Scholar
12. Zainal Abidin, S, Tan, EL, Chan, SC, Jaafar, A, Lee, AX, Abd Hamid, MHN, et al. DRD and GRIN2B polymorphisms and their association with the development of impulse control behaviour among Malaysian Parkinson's disease patients. BMC Neurol. (2015) 15:59. doi: 10.1186/s12883-015-0316-2
PubMed Abstract | Crossref Full Text | Google Scholar
13. Tsai, SJ, Liu, HC, Liu, TY, Cheng, CY, and Hong, CJ. Association analysis for genetic variants of the NMDA receptor 2b subunit (GRIN2B) and Parkinson's disease. J Neural Transm (Vienna). (2002) 109:483–8. doi: 10.1007/s007020200039
Crossref Full Text | Google Scholar
14. Li, J, Yi, M, Li, B, Yin, S, Zhang, Y, Huang, Z, et al. Polymorphism of neurodegeneration-related genes associated with Parkinson's disease risk. Neurol Sci. (2022) 43:5301–12. doi: 10.1007/s10072-022-06192-8
PubMed Abstract | Crossref Full Text | Google Scholar
15. Krishnamoorthy, S, Rajan, R, Banerjee, M, Kumar, H, Sarma, G, Krishnan, S, et al. Dopamine D3 receptor Ser9Gly variant is associated with impulse control disorders in Parkinson's disease patients. Parkinsonism Relat Disord. (2016) 30:13–7. doi: 10.1016/j.parkreldis.2016.06.005
PubMed Abstract | Crossref Full Text | Google Scholar
16. Postuma, RB, Berg, D, Stern, M, Poewe, W, Olanow, CW, Oertel, W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
Crossref Full Text | Google Scholar
17. Jost, ST, Kaldenbach, MA, Antonini, A, Martinez-Martin, P, Timmermann, L, Odin, P, et al. Levodopa Dose Equivalency in Parkinson's Disease: Updated Systematic Review and Proposals. Mov Disord. (2023) 38:1236–52. doi: 10.1002/mds.29410
PubMed Abstract | Crossref Full Text | Google Scholar
18. Davis, MY, Johnson, CO, Leverenz, JB, Weintraub, D, Trojanowski, JQ, Chen-Plotkin, A, et al. Association of GBA Mutations and the E326K Polymorphism With Motor and Cognitive Progression in Parkinson Disease. JAMA Neurol. (2016) 73:1217–24. doi: 10.1001/jamaneurol.2016.2245
PubMed Abstract | Crossref Full Text | Google Scholar
19. Stebbins, GT, Goetz, CG, Burn, DJ, Jankovic, J, Khoo, TK, and Tilley, BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord. (2013) 28:668–70. doi: 10.1002/mds.25383
PubMed Abstract | Crossref Full Text | Google Scholar
20. Arpin, DJ, Mitchell, T, Archer, DB, Burciu, RG, Chu, WT, Gao, H, et al. Diffusion Magnetic Resonance Imaging Detects Progression in Parkinson's Disease: A Placebo-Controlled Trial of Rasagiline. Mov Disord. (2022) 37:325–33. doi: 10.1002/mds.28838
PubMed Abstract | Crossref Full Text | Google Scholar
21. Oeth, P., Beaulieu, M., Park, C., Kosman, D., del Mistro, G., van den Boom, D., et al., iPLEX™ assay: increased plexing efficiency and flexibility for MassARRAY system through single base primer extension with mass-modified terminators. (2005). Available at: https://www.researchgate.net/publication/290120569 (Accessed September 27, 2007).
22. Hamza, TH, Zabetian, CP, Tenesa, A, Laederach, A, Montimurro, J, Yearout, D, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet. (2010) 42:781–5. doi: 10.1038/ng.642
PubMed Abstract | Crossref Full Text | Google Scholar
23. Yan, J, Liu, A, Fan, H, Qiao, L, Wu, J, Shen, M, et al. Simvastatin Improves Behavioral Disorders and Hippocampal Inflammatory Reaction by NMDA-Mediated Anti-inflammatory Function in MPTP-Treated Mice. Cell Mol Neurobiol. (2020) 40:1155–64. doi: 10.1007/s10571-020-00804-7
PubMed Abstract | Crossref Full Text | Google Scholar
24. Schirinzi, T, di Lazzaro, G, Sancesario, GM, Summa, S, Petrucci, S, Colona, VL, et al. Young-onset and late-onset Parkinson's disease exhibit a different profile of fluid biomarkers and clinical features. Neurobiol Aging. (2020) 90:119–24. doi: 10.1016/j.neurobiolaging.2020.02.012
PubMed Abstract | Crossref Full Text | Google Scholar
26. Guo, Y, Goetz, CG, Stebbins, GT, Mestre, TA, and Luo, S. Using Movement Disorder Society Unified Parkinson's Disease Rating Scale Parts 2 and 3 Simultaneously: Combining the Patient Voice with Clinician Ratings. Mov Disord. (2023) 38:453–63. doi: 10.1002/mds.29308
PubMed Abstract | Crossref Full Text | Google Scholar
27. Elfil, M, Bahbah, EI, Attia, MM, Eldokmak, M, and Koo, BB. Impact of Obstructive Sleep Apnea on Cognitive and Motor Functions in Parkinson's Disease. Mov Disord. (2021) 36:570–80. doi: 10.1002/mds.28412
PubMed Abstract | Crossref Full Text | Google Scholar
28. Choudhury, P, Zhang, N, Adler, CH, Chen, K, Belden, C, Driver-Dunckley, E, et al. Longitudinal motor decline in dementia with Lewy bodies, Parkinson disease dementia, and Alzheimer's dementia in a community autopsy cohort. Alzheimers Dement. (2023) 19:4377–87. doi: 10.1002/alz.13357
PubMed Abstract | Crossref Full Text | Google Scholar
29. Canerina-Amaro, A, Pereda, D, Diaz, M, Rodriguez-Barreto, D, Casañas-Sánchez, V, Heffer, M, et al. Differential Aggregation and Phosphorylation of Alpha Synuclein in Membrane Compartments Associated With Parkinson Disease. Front Neurosci. (2019) 13:382. doi: 10.3389/fnins.2019.00382
PubMed Abstract | Crossref Full Text | Google Scholar
30. Johnson, KA, Conn, PJ, and Niswender, CM. Glutamate receptors as therapeutic targets for Parkinson's disease. CNS Neurol Disord Drug Targets. (2009) 8:475–91. doi: 10.2174/187152709789824606
PubMed Abstract | Crossref Full Text | Google Scholar
31. Karcz-Kubicha, M, Lorenz, B, and Danysz, W. GlycineB antagonists and partial agonists in rodent models of Parkinson's disease--comparis
留言 (0)