Isolated pontine infarction is the most prevalent type of posterior circulation stroke, with approximately one-third of patients experiencing early neurological deterioration (END) (1), Those with END typically have a poor prognosis (2). The underlying mechanisms of isolated pontine infarction can be categorized into three types: basilar artery branch disease (BABD), small-artery disease (SAD) and large-artery occlusive disease (LAOD) (3). Identifying the predictors of END in these patients is crucial for risk assessment and the development of targeted interventions. Each type can be reflected by some imaging features, such as the presence of basilar artery vulnerable plaque, degree of stenosis, etc.
Cerebral small vessel disease (CSVD) is a complex clinical syndrome characterized by the presence of lacunes, white matter hyperintensities (WMH), enlarged perivascular spaces (EPVS), microbleeds (CMB), and brain atrophy observed on brain imaging (4). The burden of CSVD has been identified as a predictor of stroke outcomes in previous studies (5). One study indicated that a higher CSVD score is associated with poor prognosis and recurrence in acute ischemic stroke (6). Conversely, another study involving patients with mild ischemic stroke found that imaging markers of CSVD did not correlate with END (7). Additionally, a small sample study examined the relationship between WMH and END in patients with isolated pontine infarction, revealing that both periventricular and subcortical WMH were useful in predicting END in this population (8). However, this study focused solely on WMH, neglecting other features of CSVD, including lacunes, CMB, and EPVS. CSVD may adversely affect stroke prognosis by compromising dynamic cerebral autoregulation (9), which is also one of the contributing factors of END.
Therefore, imaging biomarker in predicting END in patients with isolated pontine infarction need further study, especially the correlation between the CSVD burden, along with its individual features and END requires comprehensively investigation. The objective of this study was to examine the association between CSVD burden, along with its individual imaging features, and other imaging biomarkers and END in patients with isolated pontine infarction.
MethodsThis is a retrospective study conducted at a comprehensive stroke center. Ischemic stroke patients with isolated pontine infarction from January 2020 to December 2022 were screened independently by two neurologists. Subjects were included if they met the following criteria: (1) age ≥ 18 years; (2) presentation with focal neurological symptoms and corresponding pontine infarction validated by Diffusion Weighted Imaging (DWI); (3) admission within 24 h of symptom onset; (4) patients with complete imaging evaluations, including MRA/CTA and intracranial vessel wall imaging. Subjects were excluded if they had received intravenous thrombolysis, underwent endovascular thrombectomy, or had missing recordings of neurological changes or NIHSS after admission. The flow-chart of subjects screening was shown in Figure 1.
Figure 1. Flow chart of subjects screening.
Demographic characteristics, atherosclerotic risk factors (such as hypertension, diabetes, hyperlipidemia, previous stroke history, coronary artery disease, and chronic kidney disease), blood test results, blood pressure at admission, and the National Institutes of Health Stroke Scale (NIHSS) scores at admission and after deterioration were all obtained from the medical report. The infarct mechanism was categorized as basilar artery branch disease (BABD), small-artery disease (SAD) and large-artery occlusive disease (LAOD) based on the infarct location and vascular lesions (3). In this study, END was defined as an increase of ≥1 point on the motor NIHSS or ≥2 points on the total NIHSS score within 72 h of admission (8).
This study protocol was approved by Ethics Committee of Beijing Tsinghua Changgung Hospital (22028-0-02), and informed consent was waived due to the retrospective nature of the cohort.
Imaging protocolAll patients were scanned using a 3.0 T MR scanner (Discovery 750, GE Healthcare, Milwaukee, USA) equipped with an 8-channel head coil. The protocol included brain MRI followed by MRA. The detailed sequences and their parameters were as follows: (1) T1-weighted (T1W) fast spin echo (FSE), TR/TE 1750/24 msec, field of view (FOV) 24 cm × 24 cm, matrix 320 × 256, thickness 5 mm; (2) T2-weighted (T2W) FSE, TR/TE 5000/95 msec, FOV 24 cm × 24 cm, matrix 512 × 512, thickness 5 mm; (3) T2 fluid-attenuated inversion recovery (FLAIR), FSE, TR/TE 9000/145 msec, FOV 24 cm × 24 cm, matrix 256 × 256, thickness 5 mm; (4) diffusion-weighted imaging (DWI), echo-planar imaging (EPI), TR/TE 3000/65 msec, FOV 24 cm × 24 cm, matrix 160 × 160, thickness 5 mm; (5) apparent diffusion coefficient (ADC), EPI, TR/TE 3000/65.7–84; (6) susceptibility-weighted imaging (SWI), gradient echo (GRE), TR/TE 80–15,000/45.
MRA included 3D time-of-flight (TOF) MRA and Cube T1-weighted imaging. The 3D TOF MRA was obtained using an axial fast-spin echo (FSE) sequence with the following parameters: TR/TE of 22/2.5 msec, a field of view (FOV) of 22 × 21 cm, a flip angle (FA) of 20°, and a spatial resolution of 0.6 × 1.0 × 1.2 mm3. The 3D Cube T1-weighted imaging utilized a spin echo (SE) sequence with parameters of TR/TE of 800/16 msec, a FOV of 23 × 18 cm, an FA of 90°, and a spatial resolution of 0.7 × 0.6 × 0.6 mm3.
Image analysisAll images were evaluated by two experienced radiologists who were blinded to the clinical information. A new pontine infarct was defined as a hyperintense area on DWI with a corresponding decrease in signal on the ADC.
Cerebral small vessel disease burden was evaluated by two independent neurologists according to previously established criteria, which has been established to have quite good consistency among evaluators and comparability with quantitative study (10). The total CSVD score were calculated based on the following features: 1 point for each of the following: ≥1 lacune, deep WMH (dWMH) ≥ 2, periventricular WMH (pWMH) = 3, ≥ 1 CMB, and moderate to severe (2–4) PVS. Lacunes were defined as rounded or ovoid lesions measuring 3–20 mm in diameter, located in the basal ganglia, internal capsule, centrum semiovale, or brainstem, exhibiting cerebrospinal fluid (CSF) signal intensity on T2-weighted and FLAIR images, typically with a hyperintense rim on FLAIR and no increased signal on DWI. CMBs were evaluated using SWI and defined as small (≤5 mm), homogeneous, round foci of low signal intensity on gradient echo images in the cerebellum, brainstem, basal ganglia, white matter, or cortico-subcortical junction, differentiated from vessel flow voids and mineral deposits in the globus pallidus. Both deep and periventricular WMH were assessed according to the Fazekas scale, which ranges from 0 to 3 (11). PVS was defined as small (≤1 mm) punctate hyperintensities (if perpendicular) and linear hyperintensities (if longitudinal to the plane of the scan) on T2-weighted images in the basal ganglia or centrum semiovale, and they were rated on a previously described, validated semiquantitative scale ranging from 0 to 4.
In addition, intracranial artery stenosis was recorded in each vessel, with the degree of stenosis categorized as <30%, 30–49%, 50–69%. Intraplaque hemorrhage (IPH) on basilar artery was identified on Cube T1-weighted imaging, defined as T1-hyperintense plaque exhibiting signal intensity greater than that of the surrounding brain parenchyma or muscle (12).
Statistical analysisSPSS 19.0 was utilized for statistical analysis. Variables with a normal distribution were presented as mean ± standard deviation (SD), while those with a skewed distribution were presented as median (interquartile range, IQR). T-tests and chi-square tests were employed to compare variables between the END and non-END groups. Multivariate logistic regression was conducted to adjust for confounding factors with a p-value of less than 0.1 in univariate analysis and to identify the association between cerebral small vessel disease burden and END in these patients. A statistical significance level was set at 0.05.
ResultsA total of 107 subjects who met the criteria were included in this study. The mean age of the participants was 67 years, with a male predominance of 77.6% (83 out of 107). Among these subjects, 33.6% (36 out of 107) experienced early neurological deterioration (END) within 72 h of admission, with most cases occurring within 48 h. A comparison of demographic and risk factors between the END and non-END groups is presented in Table 1. Patients with END exhibited a higher prevalence of hyperlipidemia, and a greater proportion of patients in the END group were on dual antiplatelet therapy. The mechanisms of infarction differed significantly between the END and non-END groups; Both groups had a contribution of large artery atherosclerosis (LAA) exceeding 50%, while small artery disease (SAD) was more prevalent in the non-END group. No statistically significant differences were found in other atherosclerotic risk factors between the END and non-END groups.
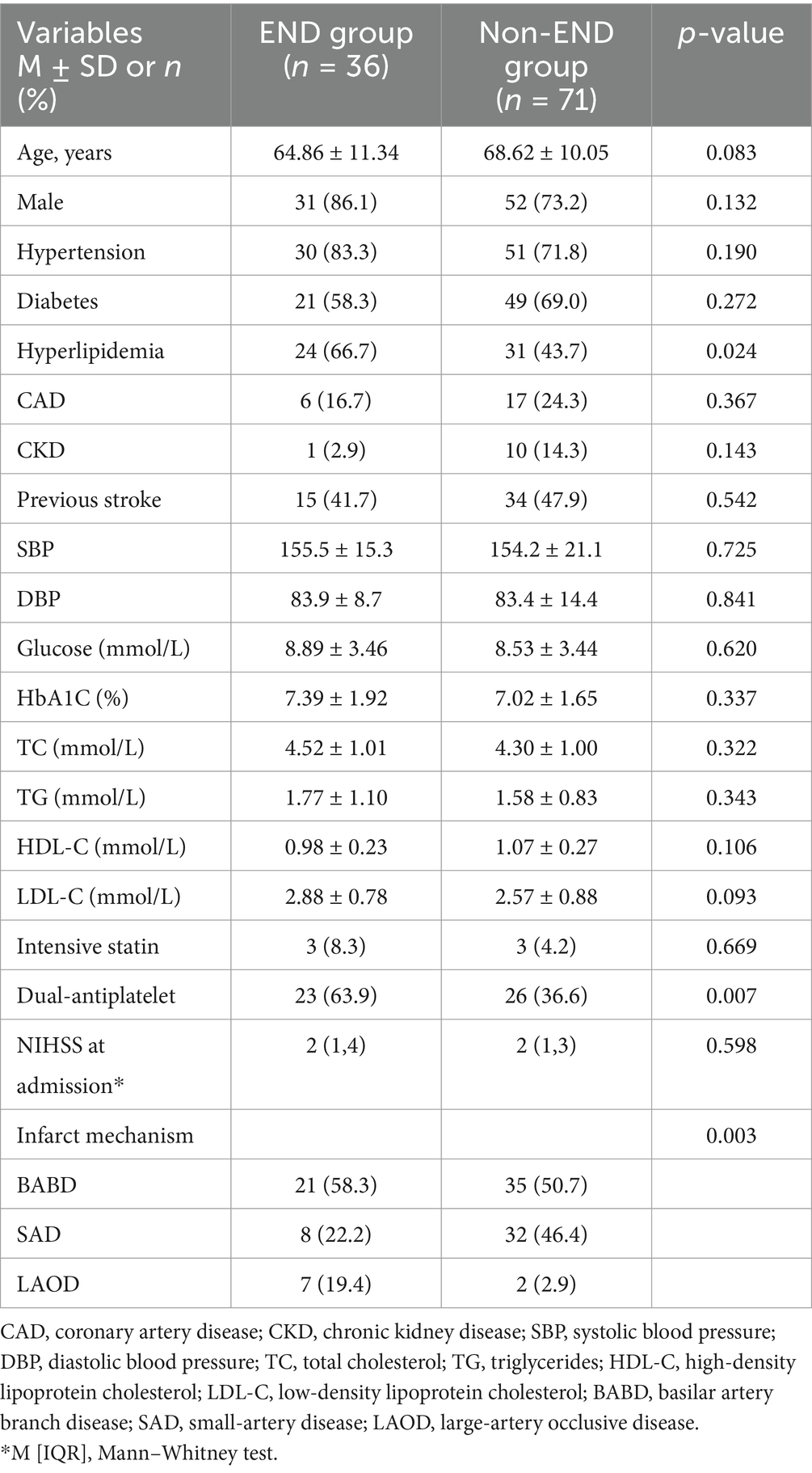
Table 1. Baseline characteristics of subjects between END and non-END group.
As shown in Table 2, the features of cerebral small vessel disease, including lacunes, white matter hyperintensities, microbleeds, and enlarged perivascular spaces (EPVS), were compared between the END and non-END groups. However, neither the total burden of cerebral small vessel disease (CSVD) nor its individual features demonstrated any significant differences between the END and non-END groups (all p > 0.05). Additionally, the prevalence of basilar artery (BA) stenosis, internal carotid artery (ICA) and middle cerebral artery (MCA) stenosis, and basilar artery intraplaque hemorrhage (IPH) were also compared between the two groups. The results indicated that patients with END were more likely to have basilar artery IPH.
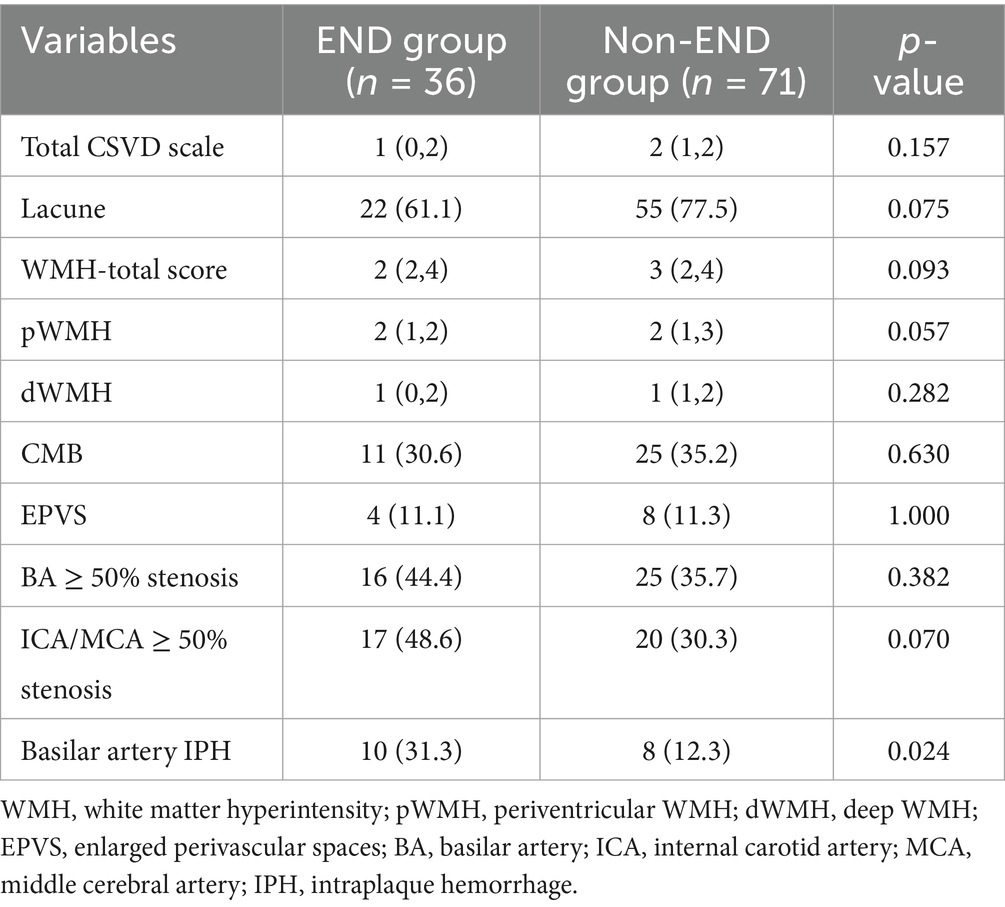
Table 2. Image features comparison between END and non-END group.
In multivariate regression analysis, after adjusting for confounding variables, we did not observe any significant association between CSVD burden and END, nor any relationship between CSVD features and END, with all p-values exceeding 0.05. However, basilar artery IPH was independently associated with END in the multivariate regression analysis, with odds ratio (OR) = 3.233 and a 95% confidence interval (CI) of (1.032, 10.123; Table 3).
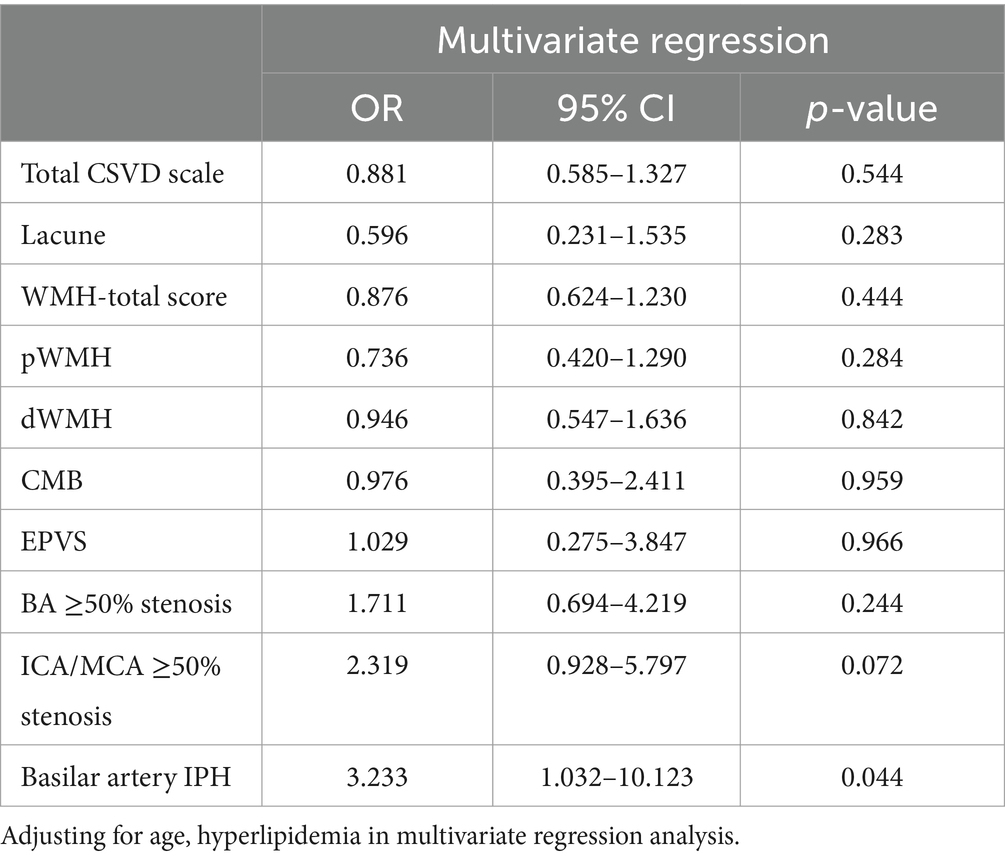
Table 3. Predictors of END in logistic regression analysis.
DiscussionOur study demonstrates negative correlations between the burden of cerebral small vessel disease and early neurological deterioration (END) in patients with isolated pontine infarction. However, intraplaque hemorrhage (IPH) in the basilar artery is independently correlated with END in these patients. The findings of our study could enhance our understanding of END in isolated pontine infarction.
Prevalence of END in isolated pontine infarction and underlying mechanismOne-third of the patients in our study presented with END, and most cases of END occurred within 48 h after the onset of symptoms. Patients with isolated pontine infarction may initially present with dizziness and limb numbness, but they typically have a lower NIHSS score. In many instances, the dizziness is non-specific and challenging to differentiate from other causes. The most common mechanism of isolated pontine infarction identified in our study is basilar artery branch disease, which aligns with findings from previous study. In that study, BABD was the most frequent cause of isolated pontine ischemia, followed by SAD and LAOD (3). These results suggest that patients experiencing sudden dizziness or other mild posterior circulation symptoms warrant increased attention, particularly in the initial days, as they are more likely to experience symptom deterioration. Furthermore, other studies have indicated that patients with pontine infarction induced by branch atheromatous disease are more likely to experience recurrence at the same site and through the same mechanism (13). Therefore, rapid evaluation of the etiology and mechanism is essential, as it will provide valuable evidence for optimal medical management in this patient population.
CSVD and END in isolated pontine infarctionThe association between CSVD and stroke outcomes has been extensively studied, yet the findings remain inconclusive (6, 14–16). While the predictive value of CSVD in early neurological deterioration has not been thoroughly investigated, a study involving 82 patients indicated that severe WMH are associated with END in cases of isolated pontine infarction, both severe pWMH (OR = 6.17; 95% CI 1.93–19.75, p = 0.002) and dWMH (OR = 3.19; 95% CI 1.10–9.23, p = 0.032) were identified as independent predictors of END after adjusting for confounding factors (8). However, our study did not find any significant association between WMH and END. One possible explanation for this discrepancy is that the population in our study is younger than that of the referenced study, as WMH are known to be age-related imaging features; Furthermore, no other CSVD imaging biomarkers were included in their study, and the sample size was not sufficiently large to draw definitive conclusion.
Another study on intracranial branch atheromatous disease demonstrated that the culprit plaques in large parent arteries, rather than cerebral small vessel disease, contribute to early neurological deterioration in stroke patients with this condition (17). This finding is consistent with our study, in which nearly half of the subjects’ infarction mechanisms can be attributed to BABD. Additionally, our results suggest that vulnerable plaques in the basilar artery are independent predictor of END in multivariate regression analysis. Furthermore, a possible explanation for the negative correlation observed between CSVD and END in cases of isolated pontine infarction may be that the CSVD burden was primarily evaluated in the anterior circulation, which does not fully reflect the vascular autoregulatory capability in the posterior circulation.
Other imaging predictors of END in isolated pontine infarctionMost previous studies on imaging biomarkers in isolated pontine infarction have focused on the topological morphological characteristics, including the infarct location, volume, and signal intensity on the Apparent Diffusion Coefficient (ADC). Ventral pontine infarcts (2), infarct volume (calculated as maximum length multiplied by thickness) (18), and the mean relative ADC (rADC) value within the ischemic lesion have been shown to be closely related to early neurological deterioration in patients with isolated pontine infarcts (19). We did not include these features in our study, as not all infarcts visible on imaging can be accurately reflected in the NIHSS score, particularly when dealing with the complex anatomy of the pons.
Another intriguing finding in our study is that anterior circulation vascular stenosis may serve as a potential predictor of END in this population, although it demonstrates only marginal statistical significance in both univariate and multivariate regression analyses. We hypothesize that patients with a greater atherosclerotic burden often experience some degree of dysfunction in cerebral autoregulation, however, this conclusion warrants further investigation.
Advantages and limitationsOur study comprehensively investigated the association between the burden of cerebral small vessel disease, its independent features, and END in cases of isolated pontine infarction, despite the absence of positive findings. This research provides insight into the mechanisms underlying isolated pontine infarction. The results revealed that BABD is the most common mechanism associated with isolated pontine infarction, while basilar artery intraplaque hemorrhage is identified as an independent factor contributing to END in these patients. This study also contributes new knowledge regarding the imaging features that can predict END in the posterior circulation.
Some limitations should also be acknowledged here. First, the sample size in our study is not large enough to draw definitive conclusions, a larger sample study is needed in the future to validate our results. Second, we employed semi-quantitative scoring to evaluate the burden of CSVD, and no quantitative imaging features were utilized in this study, such as infarct volume, white matter hyperintensity volume, and perfusion index, among others. Finally, the cause-and-effect relationship could not be established due to the retrospective nature of the study, and some bias could possibly also have an effect on the conclusion.
ConclusionIn conclusion, early neurological deterioration in isolated pontine infarction is not uncommon, the mechanisms underlying END and the related factors warrant further investigation. Our study demonstrated a negative correlation between cerebral small vessel disease and END in isolated pontine infarction. However, the presence of vulnerable plaques in the basilar artery as a predictor of END in these patients deserve additional attention.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Beijing Tsinghua Changgung Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective case study.
Author contributionsXS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FH: Conceptualization, Data curation, Methodology, Writing – review & editing. DH: Data curation, Project administration, Resources, Writing – original draft. HZ: Investigation, Methodology, Software, Writing – original draft. CW: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. LC: Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft. ZP: Conceptualization, Data curation, Writing – original draft. JW: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study is supported by National Natural Science Foundation of China (82401555), National Key R&D Program of China (2023YFC2506600), Tsinghua Precision Medicine, Tsinghua University (20219990033), and Capital’s Funds for Health Improvement and Research (2020-1-2241).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Yang, H, Liu, H, Zhang, K, Zong, C, Wang, A, Wang, Y, et al. Neuroimaging markers of early neurological deterioration in acute isolated pontine infarction. Neurol Sci. (2023) 44:3607–14. doi: 10.1007/s10072-023-06837-2
PubMed Abstract | Crossref Full Text | Google Scholar
2. Vynckier, J, Maamari, B, Grunder, L, Goeldlin, MB, Meinel, TR, Kaesmacher, J, et al. Early neurologic deterioration in lacunar stroke. Neurology. (2021) 97:2661. doi: 10.1212/WNL.0000000000012661
PubMed Abstract | Crossref Full Text | Google Scholar
3. Vemmos, KN, Spengos, K, Tsivgoulis, G, Manios, E, Zis, V, and Vassilopoulos, D. Aetiopathogenesis and long-term outcome of isolated pontine infarcts. J Neurol. (2005) 252:212–7. doi: 10.1007/s00415-005-0639-9
PubMed Abstract | Crossref Full Text | Google Scholar
4. Wardlaw, JM, Smith, EE, Biessels, GJ, Cordonnier, C, Fazekas, F, Frayne, R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
PubMed Abstract | Crossref Full Text | Google Scholar
5. Song, TJ, Kim, J, Song, D, Yoo, J, Lee, HS, Kim, YJ, et al. Total cerebral small-vessel disease score is associated with mortality during follow-up after acute ischemic stroke. J Clin Neurol. (2017) 13:187–95. doi: 10.3988/jcn.2017.13.2.187
PubMed Abstract | Crossref Full Text | Google Scholar
6. Lau, KK, Li, L, Schulz, U, Simoni, M, Chan, KH, Ho, SL, et al. Total small vessel disease score and risk of recurrent stroke. Neurology. (2017) 88:2260–7. doi: 10.1212/WNL.0000000000004042
PubMed Abstract | Crossref Full Text | Google Scholar
7. Chen, Z, Li, W, Sun, W, Xiao, L, Dai, Q, Cao, Y, et al. Correlation study between small vessel disease and early neurological deterioration in patients with mild/moderate acute ischemic stroke. Int J Neurosci. (2017) 127:579–85. doi: 10.1080/00207454.2016.1214825
PubMed Abstract | Crossref Full Text | Google Scholar
8. Nam, KW, Lim, JS, Kang, DW, Lee, YS, Han, MK, and Kwon, HM. Severe white matter Hyperintensity is associated with early neurological deterioration in patients with isolated pontine infarction. Psychiatr Neurol. (2016) 76:117–22. doi: 10.1159/000448888
PubMed Abstract | Crossref Full Text | Google Scholar
9. Liu, Z, Ma, H, Guo, ZN, Wang, L, Qu, Y, Fan, L, et al. Impaired dynamic cerebral autoregulation is associated with the severity of neuroimaging features of cerebral small vessel disease. CNS Drug Rev. (2022) 28:298–306. doi: 10.1111/cns.13778
PubMed Abstract | Crossref Full Text | Google Scholar
10. Staals, J, Makin, SD, Doubal, FN, Dennis, MS, and Wardlaw, JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurol Genet. (2014) 83:1228–34. doi: 10.1212/WNL.0000000000000837
PubMed Abstract | Crossref Full Text | Google Scholar
11. Fazekas, F, Chawluk, JB, Alavi, A, Hurtig, HI, and Zimmerman, RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. (1987) 149:351–6.
12. Song, X, Zhao, X, Liebeskind, DS, Xu, W, Zhang, J, Wei, C, et al. Associations between systemic blood pressure parameters and intraplaque hemorrhage in symptomatic intracranial atherosclerosis: a high-resolution MRI-based study. Hypertens Res. (2020) 43:688–95. doi: 10.1038/s41440-020-0411-7
PubMed Abstract | Crossref Full Text | Google Scholar
13. Wu, L, Li, Y, Ye, Z, Liu, D, Dai, Z, Zhu, J, et al. Site and mechanism of recurrent pontine infarction: a hospital-based follow-up study. Brain Sci. (2022) 12:520. doi: 10.3390/brainsci12050520
PubMed Abstract | Crossref Full Text | Google Scholar
14. Coutureau, J, Asselineau, J, Perez, P, Kuchcinski, G, Sagnier, S, Renou, P, et al. Cerebral small vessel disease MRI features do not improve the prediction of stroke outcome. Neurol Genet. (2021) 96:e527–37. doi: 10.1212/WNL.0000000000011208
PubMed Abstract | Crossref Full Text | Google Scholar
15. Rensma, SP, van, T, Launer, LJ, and Stehouwer, C. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2018) 90:164–73. doi: 10.1016/j.neubiorev.2018.04.003
PubMed Abstract | Crossref Full Text | Google Scholar
16. Wang, X, Lyu, J, Meng, Z, Wu, X, Chen, W, Wang, G, et al. Small vessel disease burden predicts functional outcomes in patients with acute ischemic stroke using machine learning. CNS Drug Rev. (2023) 29:1024–33. doi: 10.1111/cns.14071
PubMed Abstract | Crossref Full Text | Google Scholar
17. Men, X, Hu, M, Guo, Z, Li, Y, Zheng, L, Wu, R, et al. Culprit plaques of large parent arteries, rather than cerebral small vessel disease, contribute to early neurological deterioration in stroke patients with intracranial branch atheromatous disease. Cerebrovas Dis. (2024) 53:88–97. doi: 10.1159/000530371
PubMed Abstract | Crossref Full Text | Google Scholar
18. Li, H, Dai, Y, Wu, H, Luo, L, Wei, L, Zhou, L, et al. Predictors of early neurologic deterioration in acute pontine infarction. Stroke. (2020) 51:637–40. doi: 10.1161/STROKEAHA.119.027239
PubMed Abstract | Crossref Full Text | Google Scholar
19. Oge, DD, Topcuoglu, MA, and Arsava, EM. Apparent diffusion coefficient signature of ischemic tissue predicts neurological progression in isolated pontine infarcts. Eur Stroke J. (2022) 7:66–70. doi: 10.1177/23969873211072956
留言 (0)