Over 500 million years ago, ancient cold-blooded fishes emerged along with adaptive immunity (1). Together with immunological memory (2), they form the basis of natural immunity, and vaccinology. These phenomena manifest as secretion of microbe-specific agglutinins, memory responses to antigen, accelerated rejection of allografts, and acquired protection from reinfection. They are observable and common to all fish including jawless fish (3, 4), cartilaginous fish (5–7), and bony fish (8, 9), with published reports dating back to over 80 years ago (10). Nowadays, we routinely observe adaptive immune and memory responses in a variety of fish species (11–15). Studying how these responses manifest promises solutions for preventing disease in fish. However, despite fish immunology coming to maturity, driven by comparative immunology and the importance of certain species to aquaculture, we still face difficulties identifying and studying the cells and mechanisms responsible for these phenomena.
It is especially difficult to identify homologous and functional molecules, cells and mechanisms in teleost fishes due to their rich diversity, evolutionary history and distance. For example, although the single-cell RNA sequencing of grass carp B cells identified plasma cells (16), there was a glaring absence of any population that corresponds to memory B cells. Fundamentally, it is phenomena such as longevity and antibody secretion (11–15, 17, 18) that are observed time and time again rather than any specific phenotypic marker or mechanism. Therefore, it is from this angle that we studied the common carp B cell response, and searched for memory B and plasma cell analogues.
We leveraged our laboratory model of Cyprinus carpio with the myxozoan (Cnidaria) parasite Sphaerospora molnari (19, 20). The myxozoans are ancient cnidarians that have co-evolved with and cycle between invertebrate and fish hosts. They cause economically important seasonal outbreaks in both wild and farmed fish stocks. In addition to the historical uncertainty over their phylogeny (21–23), the Myxozoa are also a scientific metazoan oddity due to adaptations such as one species losing the capacity for mitochondrial respiration (24). Generally, B cells respond strongly to myxozoan infections with proliferation and antibody production (25, 26). Myxozoan infections potentially elicit polyreactive antibodies (25); they induce distinct transcriptional signatures that frequently include il10 upregulation (26–28); infections are ultimately chronic and latent (29). These features of both the host response and the pathogenesis of myxozoan infections led us to question whether the B cell response elicits antibodies that mitigate disease and produces differentiated memory B and plasma cells that provide long-term protection. In other words, we investigated whether the B cell response is protective and productive.
In the present study, by removing B cells via immunosuppression (19, 30–32), we provide evidence that B cells and antibodies limit the otherwise severe parasitemia and disease. Although we measured proliferation via incorporation of the thymidine analogue (5-ethynyl-2′-deoxyuridine, EdU) and detected gene signatures that indicate a productive B cell response, there are currently no specific phenotypic markers nor reagents to directly detect memory B cells. Thus, we relied on a single anti-carp IgM monoclonal antibody at our disposal. As described by Shibasaki et al. in a recent report of a germinal center analogue in fish, EdU labels cells engaged in an immune response and germinal center activity (33). It is through tracing the long-term retention of the thymidine analogue EdU that we directly detected resting memory B cells. A corticosteroid-resistant, enlarged, and head/anterior kidney lymphoid organ-resident B cell population expressing high levels of cytosolic IgM may represent plasma cells that constitutively secrete antibodies after resolution of the acute immune response. In this complex teleost host-myxozoan parasite interaction, humoral memory of a past infection, and protection are achievable. As our methodology is based on fundamental phenotypes of memory B and plasma cells rather than any homologous markers, it is applicable towards the study of these subpopulations and these phenomena in other species.
2 Results2.1 The B cell compartment expands and produces S. molnari-specific antibodies that limit parasitemiaTo study the role played by B cells and the antibody response in S. molnari infection, we compared B cell-sufficient and B cell-depleted common carp throughout infection with the parasite. We previously revealed the most severe form of S. molnari infection via immunosuppression with the synthetic corticosteroid triamcinolone acetonide (19, 30–32). Here, we demonstrate that immunosuppression completely depletes the peripheral IgM+ B cell population within 3 days of administration (Figure 1) and allows us to study the outcome of infection in the absence of B lymphocytes.
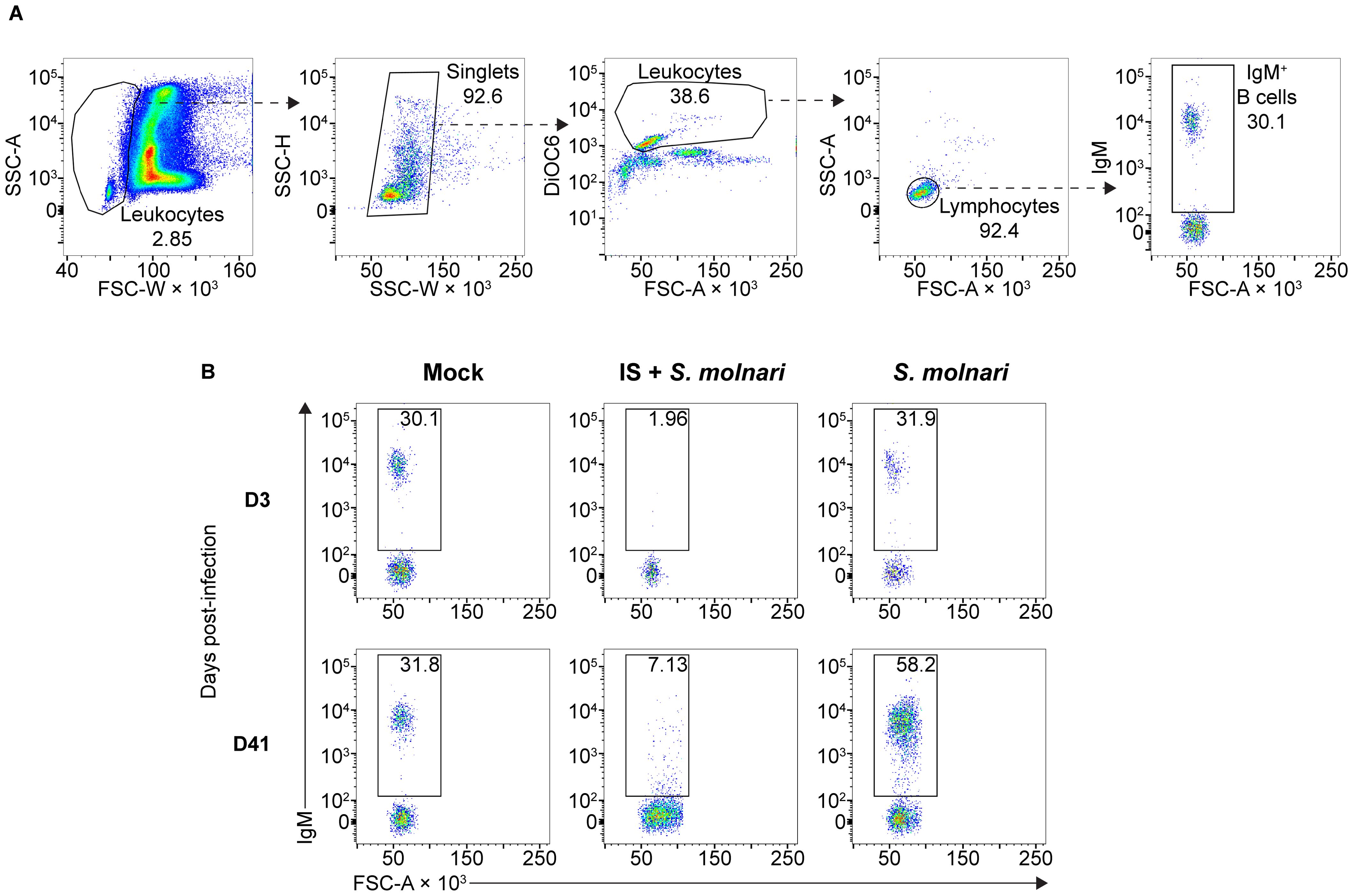
Figure 1. The circulating IgM+ B cell compartment of the common carp expands upon infection with the myxozoan S. molnari or is depleted upon immunosuppression. (A) A representative gating strategy for quantifying the IgM+ B cells in the whole blood of common carp. From the leftmost to the rightmost plots, dashed arrows each point out gated subpopulations (labeled with their proportions) being further analyzed in the plot to their right. The oval morphology of fish red blood cells gives them a heterogeneous scatter profile that overlaps with that of leukocytes. To exclude them, we first broadly gated on cells with low forward scatter width (FSC-W, x-axis), representing all leukocytes. Subsequently, doublets were excluded based on disproportionate side scatter (SSC) width (W) and height (H). Cells stained with the mitochondria- and endoplasmic reticulum-specific DiOC6 (y-axis) can distinguish active cells (leukocytes) from relatively inactive cells (red blood cells) with lower mitochondria or endoplasmic reticulum content. Among the leukocytes, the FSClow SSClow population represents lymphocytes which include IgM+ B cells based on staining with the monoclonal anti-carp IgM antibody WCI12 (mean fluorescence intensity, y-axis of the rightmost plot). Through this strategy, (B) we quantified the number of IgM+ B cells (gate and proportions indicated) throughout the experiment: from an early timepoint (day 3, top row) to a late timepoint (day 41, bottom row). Each column represents separate experimental groups either infected with 2,500,000 parasites (S. molnari), immunosuppressed and infected with an inoculum of 2,500,000 parasites (IS + S. molnari), or mock-infected (Mock). Representative plots are shown.
Fish were either not treated with the corticosteroid (immunosufficient) or immunosuppressed with it (IS). Under each of these two conditions, we further subdivided fish into three groups receiving one of three ten-fold diluted doses of diethylaminoethyl (DEAE) cellulose-purified S. molnari blood-stage parasite cells (BSs) ranging from 2,500,000 to 25,000. Additionally, mock-infected fish were included as a control group.
Following infection, we measured changes in peripheral blood composition, focusing on expansion of the B cell compartment which is a hallmark of the infection and B cell activation (Figures 1, 2A) (26). Control fish that were mock-infected varied little throughout the experiment and little at early timepoints relative to the non-IS groups. When compared to this mock-infected group, the three IS groups were completely depleted of IgM+ B cells (Figures 1B, 2A, Supplementary Figure S1). Lymphopoiesis only recovered and produced homeostatic B cell counts on day 41 post-infection in some groups and some individuals (Figure 2A). In contrast, the immunosufficient group had circulating B cells and this compartment began expanding as early as day 21 post-S. molnari infection. At this point, the number of peripheral blood IgM+ B cells increased gradually starting with the group that received the highest dose of 2,500,000 parasites followed by the group receiving ten times less, until IgM+ B cell counts were four-fold that of the control group on day 41 in the 6th week of infection, the timepoint when we previously observed a peak of B cell expansion (26). The dose-dependent expansion of the B cells may be a sign of B cell activation and an antigen-specific response. Surprisingly, the blood B cell concentration of the group infected with 25,000 BSs was not significantly different from the mock-infected group (Figure 2A, Supplementary Figure S1).
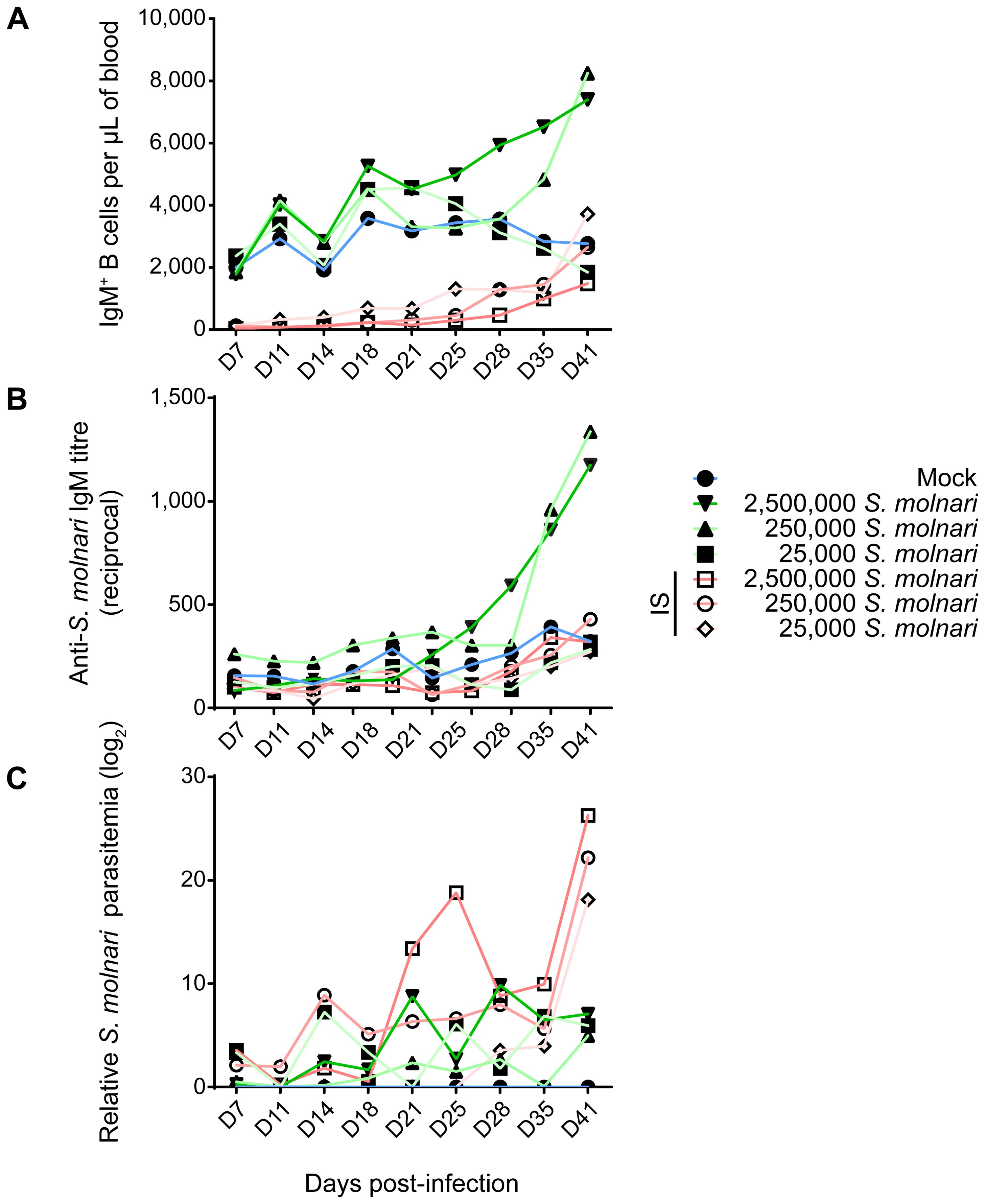
Figure 2. Infected common carp produce S. molnari-specific IgM which may limit parasitemia. By measuring (A) the concentration of peripheral blood IgM+ B cells, (B) anti-S. molnari IgM antibody titres, and (C) parasitemia, we followed the progression of S. molnari infection in each of 6 groups of infected fish and one control group, represented by different line colors and symbols (legend on the right). We collected samples and took measurements at the indicated timepoints ranging from 7 to 41 days post-infection (x-axes, D7 to D41). Filled or hollow symbols represent mean measurements from fish that were healthy or immunosuppressed (IS) respectively, at the time of infection; different shades of green or red as well as symbols represent different doses of S. molnari used in laboratory infection; the control mock-infected group is represented by blue lines. (A) Through the strategy depicted in Figure 1, we quantified the concentration of IgM+ B cells throughout the experiment. (B) The unit of measurement presented on the y-axis is reciprocal antibody titre, such that a higher number reflects a higher anti-S. molnari titre. (C) Parasitemia data was logarithmically transformed and presented in logarithmic units (base 2). n ≥ 5 biological replicates per timepoint per group. Please, refer to Supplementary Figure S1 for the statistical analyses.
To measure the B cell response and the outcome of the infection, we quantified antibody titres and parasitemia throughout the experiment. Only baseline non-significantly different anti-S. molnari IgM antibodies were detected in the mock-infected and the IS fish (Figure 2B, Supplementary Figure S1). In immunocompetent fish, specific antibody titres were dose-dependent. Curiously, in the group that received the fewest parasites, antigen-specific IgM titres remained at baseline (Figures 2A, B), with a titre comparable to that of the mock-infected and all IS groups (Supplementary Figure S1). We measured an increase in anti-S. molnari antibody titres as early as day 28 post-infection in the group receiving the highest dose of parasite (2,500,000 per fish), with the group receiving 10 times fewer parasites following shortly a week later. By the end of the experiment (41 days post-infection), specific antibody titres in these two groups were exponentially higher (<1:1000) than in any other group. Overall, these two groups had comparable anti-S. molnari IgM titres but significantly higher titres than any other group (Supplementary Figure S1).
In IS fish, an exponential escalation of the infective dose (between 25,000 to 2,500,000) was matched by an exponential dose-dependent increase in parasitemia (Figure 2C, Supplementary Figure S1). In contrast, the measurements of parasitemia in immunosufficient fish varied in kinetics (e.g., the timepoints at which parasitemia peaks). In these groups, the level of BSs in the blood of immunocompetent fish was ultimately dose-independent unlike in IS groups—parasitemia was not significantly different among these groups (Supplementary Figure S1), and by day 41, all had over 2900-fold less parasitemia than any IS group.
Taken together, our infection model allows us to study the contribution of the B cells to the anti-parasitic response. The immunocompetent fish receiving the lowest dose of 25,000 S. molnari had neither increased B cell counts nor increased specific antibody titres (Figures 2A, B). Instead, it is possible that they were protected by non-B cells which helped clear the parasite. Overall, we observed that a B cell response requires activation and proliferation, before culminating in antibody secretion. Our data indicate that the immune system of the common carp mounts a protective humoral/B cell response proportional to the parasite inoculum/challenge, with antibodies at least partly suppressing the parasite that would otherwise multiply unchallenged.
2.2 In response to S. molnari infection, IgM+ B cells proliferate predominantly in the lymphoid tissue, and express gene signatures of activation and differentiationWe shifted our attention to the splenic and head/anterior kidney lymphoid tissues where B cell responses are initiated. To characterize the B cell response, we measured proliferation and gene expression as indicators of activation and differentiation.
We detected significantly higher proportions of proliferating IgM+ B cells in the blood and head kidney beginning on week 4 (Figure 3). Proliferation uniformly peaked (reached its highest point) and was significantly different from corresponding control groups at week 5 post-infection in all compartments (Figure 3B). This preceded the peak in both blood B cell numbers and antibody titres by a week (Figures 2A, B). EdU incorporation and proliferation returned to baseline in all compartments two weeks later, indicating resolution of acute S. molnari infection. As for site specificity, the main compartment of proliferation at week 5 was the head kidney lymphoid organ where over 16% of IgM+ B cells had incorporated EdU versus about 7% in the blood and 8% in the spleen (Figure 3B). Proliferation being predominantly in the head kidney indicates that it may be a major site of B cell activation and differentiation in S. molnari infection.
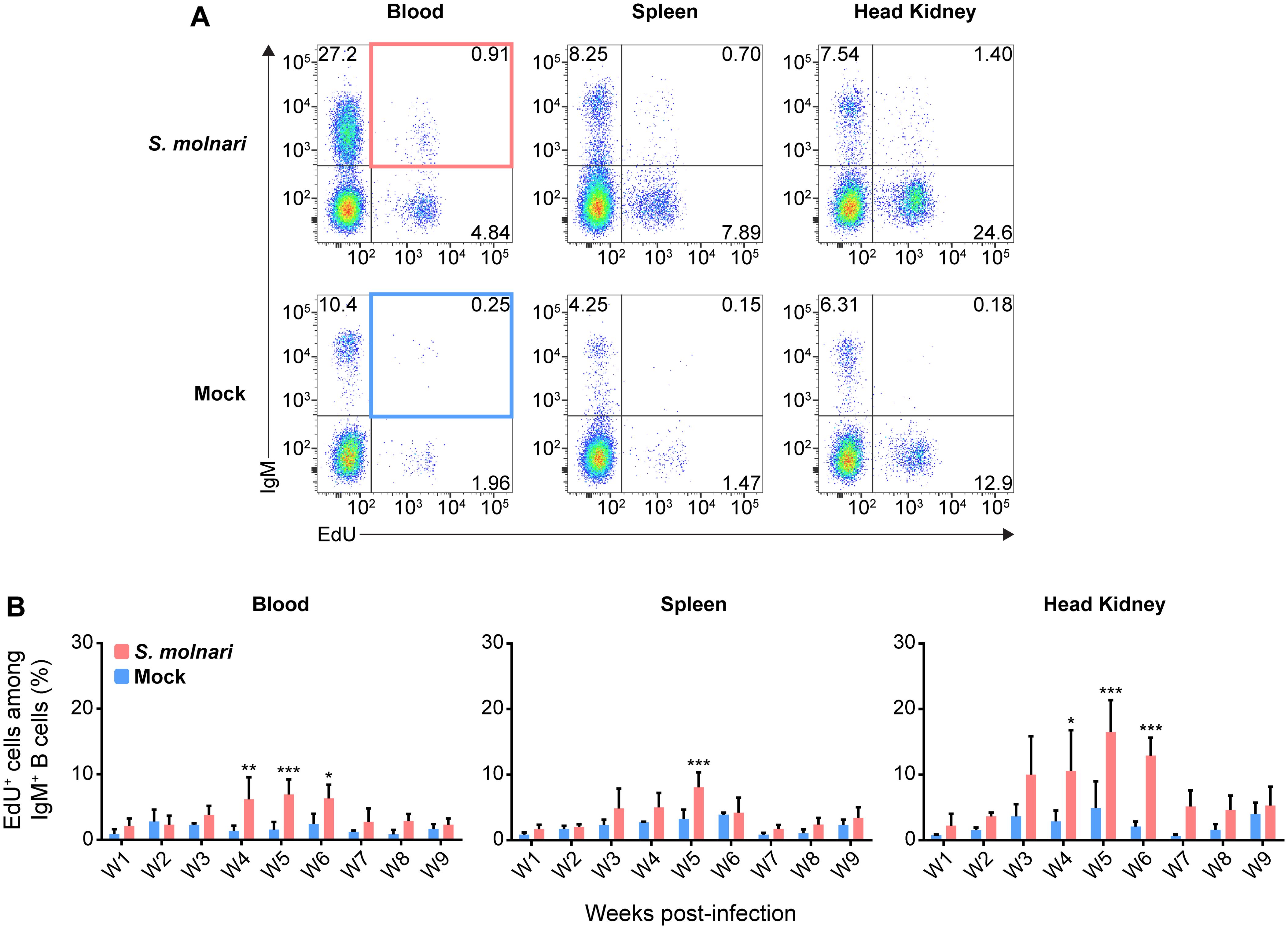
Figure 3. Common carp IgM+ B cells proliferate in the blood, the splenic lymphoid organ, and the head kidney lymphoid organ following S. molnari infection. (A) We exposed fish to the thymidine analogue (EdU) at different time points throughout S. molnari infection or in non-infected fish (Mock). As an indicator of proliferation, IgM+ B cells that incorporated EdU were identified by flow cytometry by adapting the gating strategy presented in Figure 1A to detect EdU+ cells in different tissue compartments. Representative plots from week 5 of the infection are presented here, organized by compartment (columns) and fish status (rows). The top two quadrants in every plot are the IgM+ B cell populations of interest. In the first column, the top right quadrants are marked by red or blue quadrilaterals which are the gates we used to quantify IgM+ EdU+ B cells from S. molnari-infected or control fish, respectively. (B) The proportion of IgM+ EdU+ B cells among total IgM+ B cells was quantified throughout the infection and summarized in bar graphs. Each bar extends to the mean with SD error bars. n = 3 or n = 4 biological replicates per timepoint for the Mock and S. molnari groups, respectively. ns (not significant); * p < 0.05; ** p < 0.01; *** p < 0.001.
To uncover and explain the changes that the B cells are undergoing in the head kidney throughout the infection, we profiled gene expression of magnetic-activated cell sorted (MACS-sorted) IgM+ cells (Figure 4A). We selected several predicted orthologues of mammalian and fish markers of B cell differentiation and/or survival (Figure 4B) (13, 34–39). Among the most differentially expressed genes (Figure 4B, top row, over 2-log change in relative gene expression), the significant downregulation of membIgM (membrane-bound or cell surface IgM), and upregulation of secIgM (secretory IgM), xbp1, and tnfrsf13b may together indicate differentiation of B cells into antibody-secreting and memory cells (Supplementary Figure S2). Specifically, the peak of secIgM and xbp1 expression at week 5 post-infection corresponds with the timepoint when we began measuring an increase in anti-S. molnari IgM titres (Figure 2B) and may be indicative of B cell differentiation into antibody-secreting plasmablasts. The most differentially expressed gene was tnfrsf13b which was a significant 6 log units higher than the control group only at week 9 (Supplementary Figure S2). As a receptor in the BAFF/APRIL axis that promotes B cell survival and is also upregulated in rainbow trout by myxozoan infection (38, 39), expression of tnfrsf13b (alias taci) may indicate the late differentiation of memory B cells. To date, no one has identified an orthologue of the human plasmablast marker syndecan-1 (alias cd138) in teleost fish (40). We measured expression of the related syndecan-3 but only observed that it was significantly downregulated at weeks 3 and 5. pax5 and cxcr5 expression were unchanged but the former trended toward downregulation at late timepoints. Regarding tissue specificity, a two-way ANOVA determined that secIgM, xbp1, and tnfrsf13b expression were significantly higher in the head kidney than in the blood compartment. Post hoc testing determined that secIgM (at weeks 5, 7, and 9) and xbp1 (weeks 7, and 9) were significantly overexpressed in the head kidney but not the blood (Supplementary Figure S2). These late-stage and lymphoid organ-specific changes may indicate that secIgM and xbp1 are markers of B cell differentiation in the head kidney.
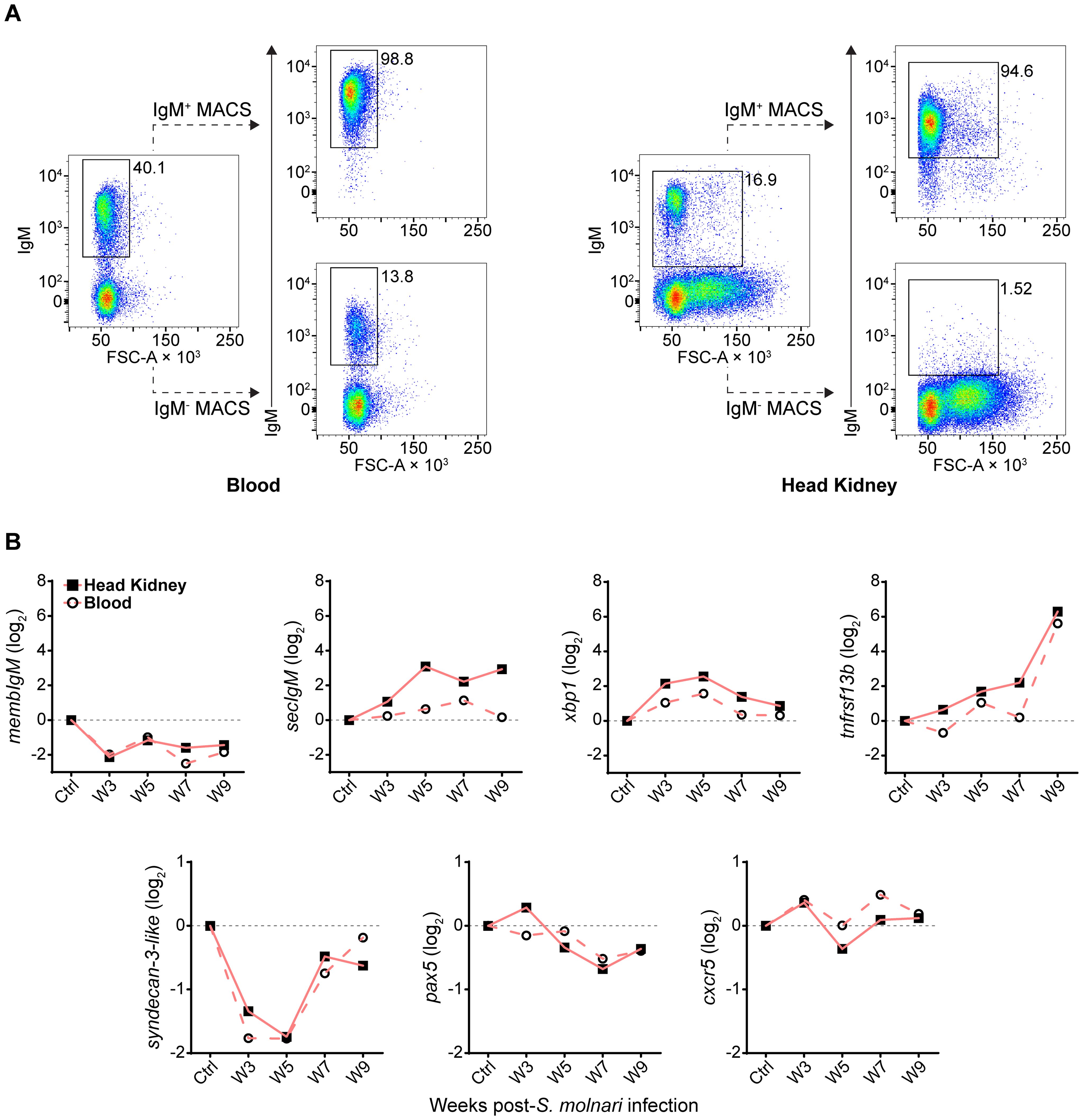
Figure 4. Following S. molnari infection, common carp IgM+ B cells express markers of B cell activation and differentiation in the blood and head kidney. (A) Head kidney or blood IgM+ B cells were MACS-sorted using the monoclonal anti-carp IgM antibody WCI12. Here are two representative MACS results for the blood (left) and the head kidney (right). In each plot, the proportion of IgM+ B cells is adjacent to the corresponding flow cytometry gate. The dashed lines point to the result of MACS: either the eluted IgM+ fraction (IgM+ MACS) or the unbound/washed negative fraction (IgM- MACS). All plots depict the mean fluorescence intensity of WCI12 staining (y-axes) versus forward scatter area (FSC-A, x-axes). (B) RNA from MACS-enriched IgM+ B cells was used to quantify expression of select markers of activation and differentiation throughout S. molnari infection. Logarithmically transformed (base 2) relative expression (2−ΔΔCT method) of each marker (y-axes titles) is presented in each plot and organized by highly or weakly differentially expressed genes: respectively, markers in the top row (over 2-log maximum change) or bottom row (under 2-log change). The control group (Ctrl) includes data collected from fish sampled prior to infection. We plotted the mean of relative expression of each group at each timepoint represented by either filled squares connected by lines (head kidney compartment) or hollow circles connected by dashed lines (blood compartment). n ≥ 4 biological replicates per group per timepoint. Please refer to Supplementary Figure S2 for statistical analyses.
We further characterized these specimens via multiplex qPCR by profiling expression of B cell markers that were identified by Pan et al. in Ctenopharyngodon idella grass carp, another cyprinid species (16). Specifically, they are transcripts the authors identified via single-cell RNA sequencing of sorted head kidney IgM+ B cells. Here we present the expression levels of their potential Cyprinus carpio orthologues in our MACS-sorted IgM+ B cells (Figure 5). Expression of the 18 genes were hierarchically clustered within the three different compartments throughout the 10-week experiment for comparison between the blood, spleen, and head kidney compartments (Supplementary Figure S3).
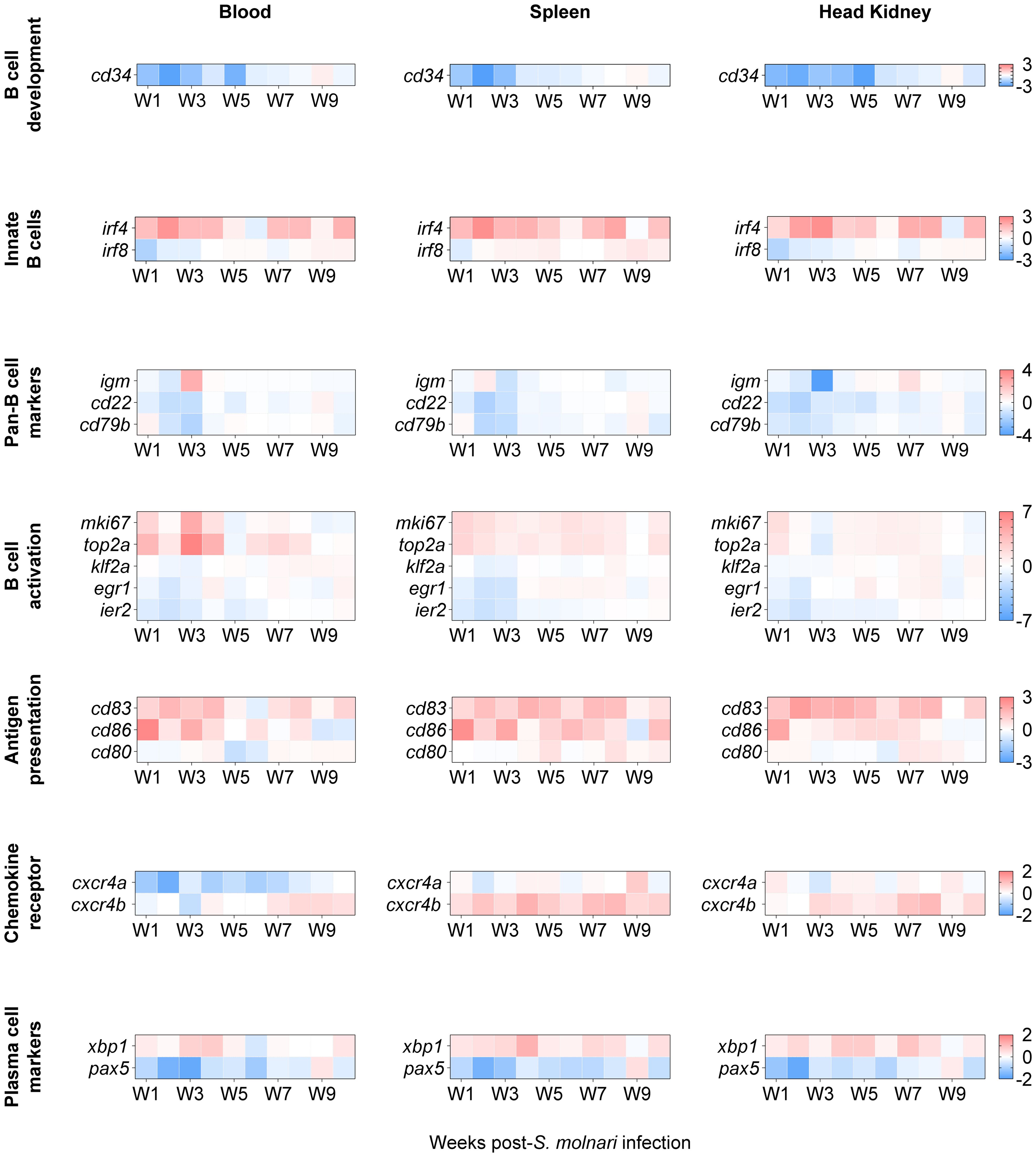
Figure 5. The transcription profile of cyprinid markers of developing, innate, activated/proliferating, antigen-presenting, and differentiated IgM+ B cell subpopulations. We profiled expression of markers defining distinct B cell subpopulations, i.e. genes initially identified in single-cell RNA sequencing analysis of grass carp IgM+ B cells (16). Here, genes were categorized (in separate rows of heat maps) based on the subpopulations they cluster, by developmental stage, and/or by activities such as activation and antigen presentation. The two-color gradient represents either overexpression or downregulation relative to the control group (not shown here). The legends for each gene category are on the rightmost side of each set of heat maps. Data in absolute number of copies of each gene were normalized to the number of copies detected in the control group and then logarithmically transformed (base 2) to better reflect both positive and negative fold changes. n ≥ 4 biological replicates per gene per timepoint. For statistical analyses, please refer to Supplementary Figure S4.
The genes were categorized by their annotation and expected roles according to Pan et al.’s single-cell RNA sequencing data (16), and/or the cell population they help identify (Figure 5). The expression of cd83, cxcr4b, egr1, klf2a, and ier2 were significantly different compared to the non-infected control group and the results of post hoc multiple comparisons tests are summarized in Supplementary Figure S4. Notably, three of them are activation markers. klf2a, and egr1 were downregulated at early timepoints (specifically, week 2 post-infection); egr1 was later upregulated between week 5 and 8 preferentially in the lymphoid organs while upregulation of klf2a was delayed and centered around week 8 (Supplementary Figure S4). Among the co-stimulatory molecules, cd83 was significantly overexpressed relative to the control group in all compartments and throughout the experiment with the exception of the week 5 and week 6 timepoint (Figure 5, Supplementary Figure S4) unlike cd86 which peaked at week 1 in all three compartments. Relative to the non-infected control group, cxcr4b was overexpressed at almost all timepoints between week 2 and week 10 in the splenic and head kidney lymphoid organs (Figure 5, Supplementary Figure S4). cxcr4b may be the functional carp orthologue for retaining or directing select B cells to lymphoid tissues unlike cxcr4a (Figure 5) and cxcr5 (Figure 4).
A notable difference with Pan et al.’s study (16) is that we are studying these markers at the population level (in bulk) rather than at the level of single cells. Therefore, we still expect changes in subpopulations of IgM+ B cells and changes in B cell marker expression even if these are more difficult to detect and not statistically significant. Among the markers whose expression was not significantly different from the control group, cd34 is a marker of mouse and human hematopoietic stem cells. cd34 expression was downregulated relative to the control group in all compartments during the first 5 weeks of infection (Figure 5). Two markers of innate B cells, irf4 and irf8, trended oppositely with the former highly overexpressed and the latter downregulated at early timepoints in all compartments. Among the pan-B cell markers, the most notable change was a 2.5-log upregulation and a 4-log downregulation of igm compared to their controls in the blood and head kidney, respectively, at the same week 3 timepoint. Similarly, the expression patterns of the activation markers mki67 and top2a matched those of igm in the same cellular compartments at the same week 3 timepoint, suggesting that all three may be markers of the same IgM+ cell subpopulation. Finally, we again observed a reverse pattern of xbp1 and pax5 expression which is an indicator of an ongoing immune response and B cell differentiation (Figures 4, 5).
In summary, considering that all these markers define distinct cyprinid IgM+ B cell subpopulations (16), our results suggest that there is: reduced hematopoiesis or lymphopoiesis; activation of an innate B cell population; antigen-presenting cell (APC) activity or costimulation; activation, proliferation, and differentiation of B cells; migration/retention of cells in lymphoid tissues. Together, these changes may be the origin of the humoral response against the parasite (Figure 2).
2.3 Memory B cells are produced throughout the acute response to S. molnari and persist as resting cellsFish that survive a primary myxozoan infection presumably harbor memory lymphocytes that protect them from secondary infections. We gathered evidence that the initial immune response produces a greater breadth of B cells than we could previously appreciate with anti-carp IgM staining alone. Yet, we could not directly identify memory cells as there is so far no marker for let alone a defined subpopulation of teleost memory B cells. Furthermore, we must study these cells on the order or scale of months that are most relevant to immunological memory, vaccine success, and protection from natural seasonal (re-)infections.
We revisited the EdU pulse labeling method, and searched for EdU+ IgM+ B cells months after infection which should theoretically reveal B cells that were i) activated upon encountering S. molnari (antigen), ii) proliferated and incorporated EdU as a result, and iii) differentiated into long-lived quiescent cells that retain the EdU. The latter are selected into the memory pool following the contraction and resolution phases of the primary immune response. To test this, we assigned fish to six labeling windows/groups receiving EdU during a specific week following acute S. molnari infection. Approximately six months after infection and approximately five months following EdU injection, we detected EdU+ cells in the blood, spleen, and head kidney compartments (Figures 6A, B).
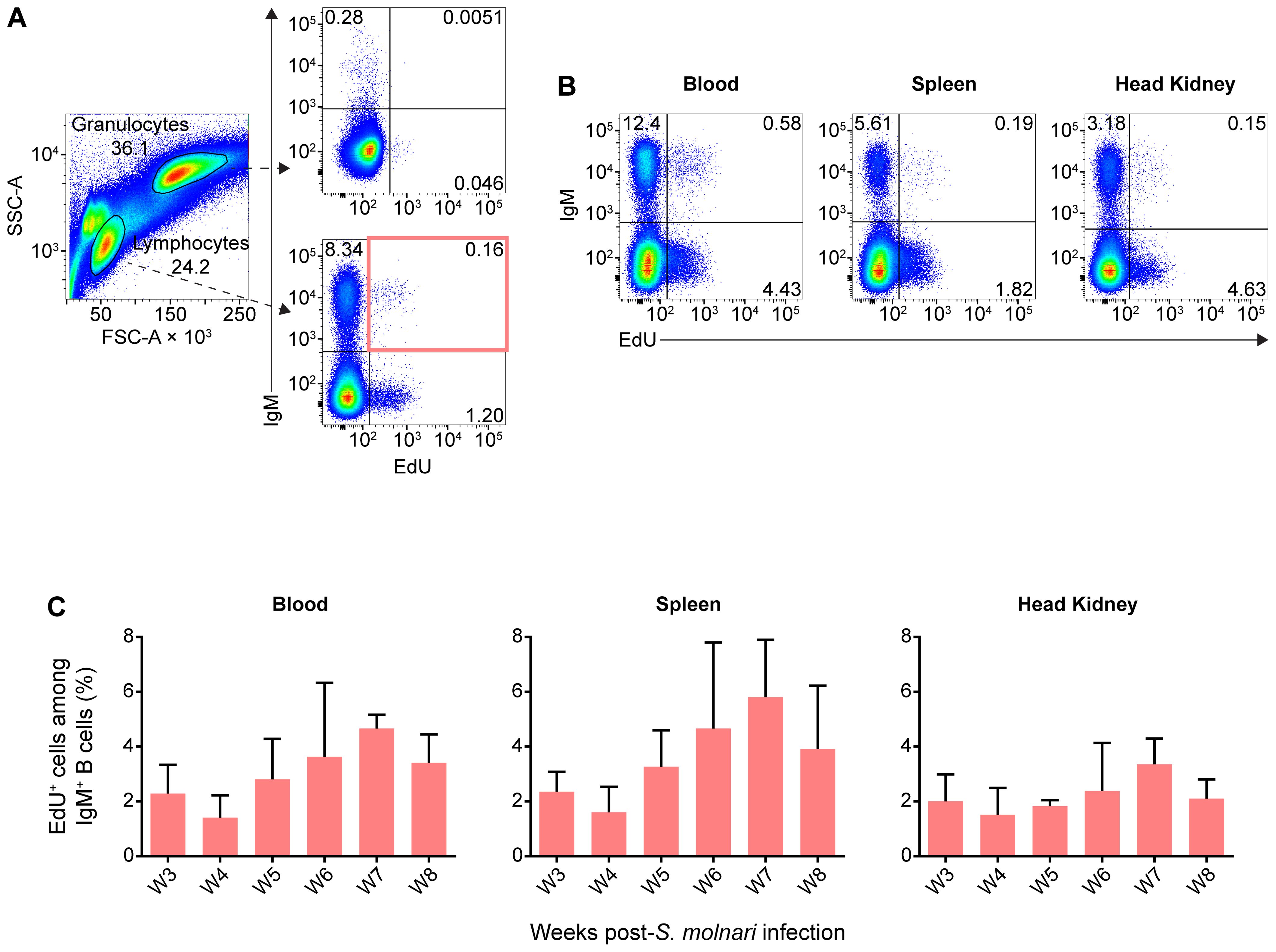
Figure 6. IgM+ B cells proliferating during acute S. molnari infection persist over five months later as resting cells. (A) In flow cytometry analysis of representative common carp head kidney leukocytes (left plot), distinct side scatter area (SSC-A, y-axis) and forward scatter area (FSC-A, x-axis) profiles help distinguish granulocytes (FSC-Ahigh SSC-Ahigh) from lymphocytes (FSC-Alow SSC-Alow). As indicated by dashed arrows, these two leukocyte populations were subsequently analyzed for/categorized by IgM expression (y-axis) and intensity of incorporated EdU (x-axis). Despite the months-long gap between EdU labeling and detection instead of next-day detection (Figure 3), EdU+ subpopulations were readily detectable among lymphocytes (bottom right plot), but rare among granulocytes (top right plot). (B) Additional examples of EdU+ IgM+ lymphocytes from the blood, spleen, and head kidney are presented. Proportions of each quadrant/subpopulation are noted on plots. In (A) we highlighted the main EdU+ IgM+ population of interest via a red rectangle and the proportion of this subpopulation relative to total IgM+ lymphocytes is summarized in bar graphs in (C). These graphs are organized along the x-axes by separate bars (mean + SD), each representing a labeling window: the week post-S. molnari infection in which each group was injected with EdU. Representative data presented from the week 4 and 7 labeling windows in (A, B), respectively. n ≥ 3 biological replicates.
Astonishingly, EdU+ IgM+ B cells were readily detectable in the lymphocyte gate, representing around 2% to 5% of all IgM+ B cells (Figures 6A, B) and potentially include memory cells. In contrast, a negligible amount of weakly EdU+ cells were detected among granulocytes from the same fish (Figure 6A). Compared to proliferation during the acute infection (Figure 3), the distribution of EdU+ cells was mainly in the blood and spleen rather than the head kidney, which matches the location of the resting B cells described by Ma et al. (13); interestingly, they were not labeled during the 5th week of acute infection, and peak proliferation (Figures 3, 6C). The majority of EdU+ IgM+ cells originated from week 7 post-infection and at the time of measurement, represented 6% of all splenic IgM+ cells, and this number was about 4% and 3% in the peripheral blood and head kidney, respectively.
In other words, out of all the proliferating and EdU-incorporating cells during acute infection, a subset survived, having not been turned over, not proliferated further, and not diluted EdU below the threshold of detection. This B cell population may be partly composed of the fish equivalent of the memory B cell, a non-dividing recirculating B cell subset awaiting reactivation.
2.4 A head kidney-exclusive B cell subpopulation with high levels of intracellular IgM may represent plasma cellsPlasma cells and their antibody effector molecules make up another pillar or wall of humoral memory. We were able to detect anti-S. molnari antibodies eight months post-infection, long after resolution of parasite infection and without any reinfection or immunization (Figure 7A). With these antibodies being presumably produced by plasma cells, we devised methods to detect this B cell subset based on the exceptional amounts of Ig they produce, their levels of membrane IgM (memb IgM) (36), their size, their compartmentalization in the head kidney (13), and hypothesized resistance to hormonal stress (41).
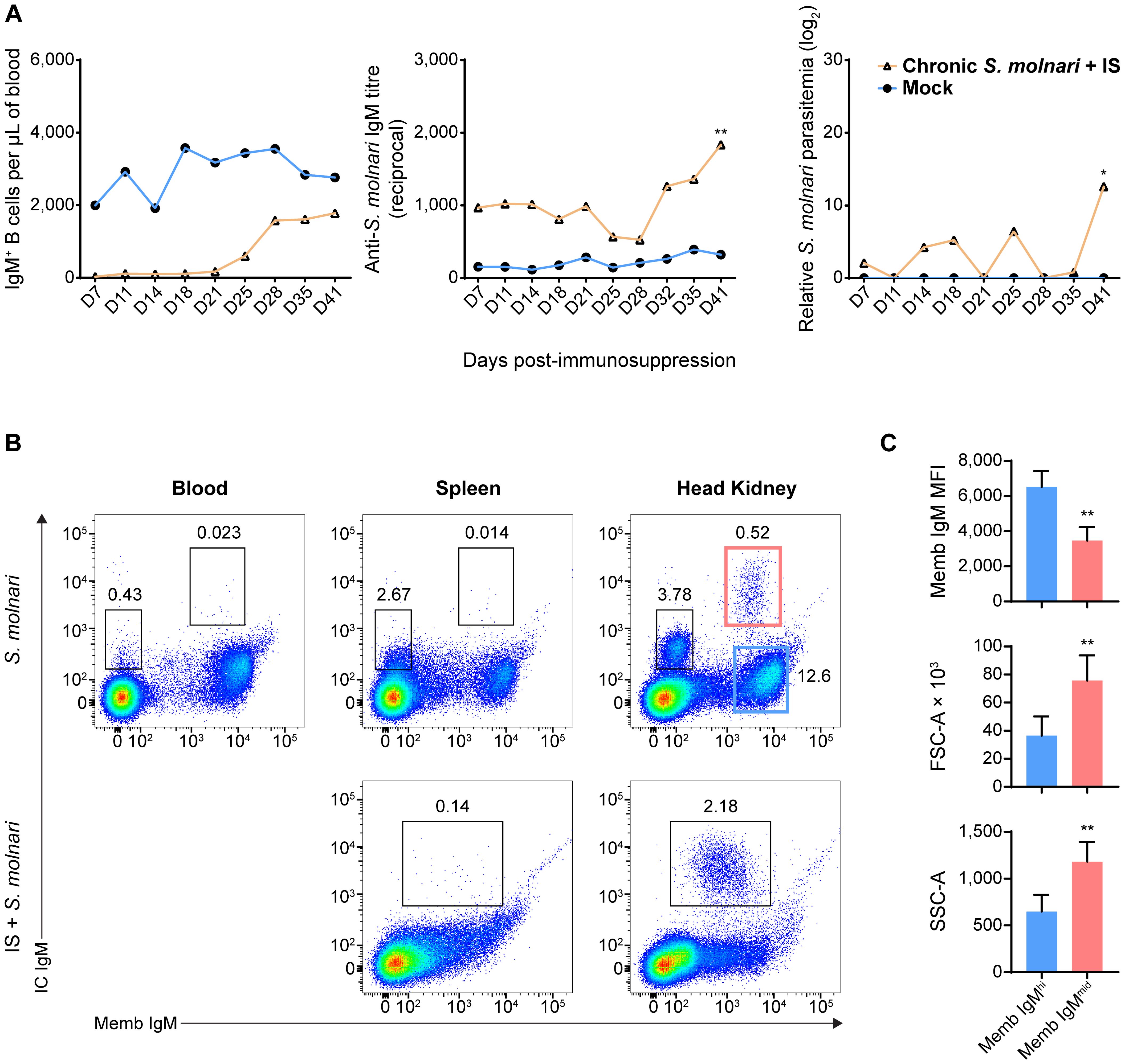
Figure 7. A population of head kidney-specific large B cells with exceptionally high levels of intracellular IgM and resistance to immunosuppression may represent plasma cells of the common carp. (A) We studied a cohort of immunocompetent carp infected eight months prior which have not achieved sterile immunity (labeled ‘Chronic S. molnari’). Unlike fish infected for the first time (Figure 1, the ‘Mock’ control group was sampled simultaneously as the ‘Chronic S. molnari’ group), these carp constitutively produced anti-S. molnari IgM (middle plot) which are conventionally produced by plasma cells. As we hypothesized that plasma cells are resistant to immunosuppression, the ‘Chronic S. molnari’ cohort of fish was treated with triamcinolone acetonide (IS) which did not decrease specific antibody titres throughout the experiment despite completely depleting circulating IgM+ B cells (left plot). Nonetheless, parasitemia (right plot) was measurable in these fish. Orange line and triangle symbols represent the ‘Chronic S. molnari + IS’ group whereas the blue line and circles represent an uninfected control cohort mock-infected (Mock). We performed immunosuppression on day 0 and thus the x-axis represents the days post-immunosuppression or -mock injection. (B) We identified a population of putative plasma cells via costaining for membrane/cell surface IgM (memb IgM, x-axes) and intracellular IgM (IC IgM, y-axes) lymphocytes. This IC IgMhigh population was detectable exclusively in the head kidney (rightmost column). Despite near-complete depletion of IgM+ B cells in the blood (Figures 1B, 2A), and head kidney, triamcinolone acetonide treatment (IS) enriched this IC IgMhigh population in the head kidney (bottom right plot). (C) Each bar indicates the mean of either the mean fluorescence intensity (MFI) of membrane IgM staining, forward scatter area (FSC-A), or side scatter area (SSC-A) + SD. For these metrics, statistically significant differences between the memb IgMhigh and memb IgMmid populations, respectively outlined in blue and red rectangles in (B), were determined by the Wilcoxon signed rank test with the two populations as matched pairs in n = 8 biological replicates. * p < 0.05; ** p < 0.01.
Thus, we costained cells in a step-wise manner to detect the extracellular membrane IgM (Memb IgM) and intracellular IgM (IC IgM) using a single monoclonal anti-carp IgM antibody. In the blood and spleen, the B cells stained minimally for IC IgM with a slight upward shift of the BCRhigh population of cells (Figure 7B, top row). This did not reveal any new population among the cells we have always detected (Figure 1). However, the head kidney revealed two new B cell populations that were previously undetectable through Memb IgM staining alone (Figure 7B, top row): a Memb IgM- IC IgMmid population and a Memb IgMmid IC IgMhigh population. The former is relatively abundant (representing around 3% of all leukocytes) while the latter is exceedingly rare (representing around 0.5% of all leukocytes). Overall, based on their phenotype and tissue-specificity, we hypothesized that one of these populations represents plasma cells.
To test these cells’ resistance to stress, we immunosuppressed fish that had previously been infected with S. molnari. As corticosteroid-induced immunosuppression targets lymphopoiesis (42), dividing cells, and short-lived cells, we hypothesized that plasma cells would be resistant and would not be depleted unlike their naïve B cell counterparts. This treatment eliminates virtually all IgM+ B cells from the blood (Figures 1B, 2A). In the spleen and head kidney, this approach depleted the most abundant B cell populations including the Memb IgM- B cell population (Figure 7B, bottom row). In contrast, the Memb IgMmid IC IgMhigh population became readily detectable in the head kidney (over 2% of all leukocytes in the example). We also hypothesized that they would continue producing antibodies despite the immunosuppression and were responsible for keeping the parasite latent long-term. Thus, we measured antibody titres and parasitemia in the weeks following immunosuppression (Figure 7A). Anti-S. molnari IgM antibody titres doubled and coincided with the reemergence of the parasite and an over 10-log increase in parasitemia at the final timepoint of 41 days post-immunosuppression.
Together, our data indicate that the initial B cell response (Figures 1, 2) elicits constitutive antigen-specific antibody production. The source of these antibodies may be the corticosteroid-resistant, head kidney-resident, IC IgMhigh B cell population, which are significantly larger and denser than their counterparts with high Memb IgM expression (Figure 7C). These cells and antibodies may be one component of constitutive defense keeping S. molnari latent and/or protecting the host from future infections.
3 DiscussionOverall, our data indicate that B cell activation follows a similar course to that in ‘higher’ vertebrates and produces specialized cellular subsets with memory and (constitutive) antibody-secreting phenotypes, together forming ‘two walls of protection’ (43). Our results indicate that the initial B cell response is protective and prevents the worst form of disease caused by S. molnari (the only two deaths recorded in this experiment were from the IS groups).
3.1 The B cell response, memory B and plasma cells in a teleost fishIn mammals, antigen activates B cells and they differentiate in lymphoid tissues. This process can be divided into at least two phases: an initial phase that produces the short-lived plasmablasts and some memory B cells, whereas a second phase produces memory B and long-lived plasma cells that have affinity-matured and have been positively selected for in germinal center reactions (44, 45).
The B cell response we observed was dose-dependent, and antigen-specific. In comparison to the response of rainbow trout to the model hapten-protein antigen TNP-KLH, anti-S. molnari antibody production was accelerated, and titres were exponentially higher at the same timepoint (12). The initial surge in antibody production at the 5-week timepoint (Figure 2B) is likely the product of short-lived plasma cells or plasmablasts and matches the timing of the significant increase in igm transcripts we previously observed in the same model and in this study (Figures 4, 5) (26).
We measured a peak of proliferation at week 5 post-infection and detected a significant proportion of EdU+ IgM+ B cells in both the head kidney and spleen. These cells likely include those described in a recent report by Shibasaki et al. of a germinal center analogue in the rainbow trout spleen (33). By detecting highly proliferating B and T cells adjacent to melanomacrophages, Shibasaki et al. convincingly identified sites that support clonal expansion, antigen receptor affinity maturation, and clonal selection. One expected output is antibody-secreting cells which circulate and contribute to the sharp rise in the number of IgM+ and EdU+ B cells at week 5 and 6 in the blood even though it is not a site of proliferation (Figures 2A, 3). The short-term distribution of antibody-secreting cells throughout the periphery was also observed by Davidson et al. in Limanda limanda following immunization with human gamma globulin (11). The precise identity of the circulating B cells can be confirmed with a strategy like the one we used to detect the kidney-resident IC IgMhigh cells. However, they are only expected to be short-lived and provide short-term protection.
Regardless of which phenotypic markers they express, regardless of species-to-species variations, the purest and most universal definition of what is a memory B cell is their longevity. We traced the proliferating cells of the primary response to determine if they persist long after resolution of the acute response. We hypothesize that the EdU+ cells we detected months after infection/labeling include clones that avoided caspase-mediated apoptosis in the melanomacrophage centers (33). The upregulation of secIgM, xbp1, tnfrsf13b, and downregulation of membIgM, and pax5 may also be byproducts of ongoing selection and differentiation. We detected memory B cells in every compartment, and they formed at every timepoint studied. The memory B cells detected in the spleen and blood matches where Ma et al. observed them in rainbow trout (13). These cells are likely recirculating between the two compartments, explaining why their proportions are very similar in the two compartments. In contrast, the head kidney of carp and other bony fish species appears to be a niche for plasma cells that do not recirculate. These cells likely reside in the head kidney which is a survival niche for plasma cells of other bony fish species as well (13, 14, 16, 46).
The emergence of memory cells and the peak of their formation in week 7 (Figure 6C) was relatively late and did not correlate with the peak of B cell proliferation in week 5 (Figures 2B, 3). Considering the timing and selection mechanisms ongoing in lymphoid tissues, it is likely that early memory B cells express low affinity BCRs and this affinity gradually matures as observed in rainbow trout and channel catfish (12, 13, 15) driven by activation-induced cytidine deaminase-mediated antigen receptor diversification in germinal center analogues (33). In our study, we hypothesize that the late-stage memory cells emerging in week 7 may be successful progenitors of melanomacrophage center reactions, and express affinity-matured BCRs which confer a survival/fitness advantage.
3.2 Humoral memory and the implications for vaccination and natural immunityIn humans, there is evidence that exposure to a pathogen is sufficient to protect an individual for a lifetime: in a 2008 study, survivors of the 1918 H1N1 influenza pandemic continued to harbor memory cells producing strain-specific anti-hemagglutinin neutralizing antibodies, nearly 90 years after exposure to the virus (47). If fish B cells behave in much the same way, there are major implications for vaccination and natural acquired immunity against pathogens. In a study similar to ours in mice, memory B and plasma cell retained BrdU at the 8-week timepoint (48). Here, we continued to detect EdU+, IgM+ B cells well after 6 months post-infection and EdU pulse labeling. A recent Atlantic salmon vaccine trial demonstrated persistent antigen-specific antibody titres over 80 weeks post-immunization, in a full aquaculture production cycle (18). Gilthead seabream that had survived a previous infection with the myxozoan Enteromyxum leei maintained specific antibody titres for over 16 months (17). This progress and such milestones are consequential because we need to know how long memory is maintained post-vaccination or whether fish having already survived an initial infection will be immune during next year’s outbreak. Understanding how this memory is generated and maintained will help us move away from purely empirical vaccines and methods, and towards conferring acquired immunity.
Existing models propose that memory B cells could be maintained by continuous antigen exposure while others propose that their longevity may be intrinsic to ‘tonic’ BCR signaling (44). The aforementioned Atlantic salmon vaccine trial demonstrated retention of select antigens many months post-vaccination in granulomas forming in the pancreas but not in lymphoid organs (18). Alternatively, B cells could also be re-exposed to antigen via booster immunizations to provide survival signals and maintain antibody titres (44). Considering the discovery of a germinal center analogue in fish (33), the fish memory B cells we detected may potentially also re-enter melanomacrophage centers to further diversify their antibody repertoire and/or differentiate into (short-lived) plasma cells, providing a ‘faster’ and ‘stronger’ secondary immune response as occurs in mammals (44).
Finally, we do not know if the survival of memory cells in laboratory settings would be reproducible in natural settings where animals are exposed to environmental stressors. At least in Atlantic salmon in a farm setting, specific antibody titres were maintained over 80 weeks post-immunization despite the seasonal changes in temperature and photoperiod (18). In our study, we mimicked the effects of stress-induced cortisol/glucocorticoids in S. molnari-infected fish. Abnormally high stress-induced plasma cortisol increases the susceptibility of trout to fungal and bacterial infection (49). In our study, after administration of the corticosteroid, antibody titres remained stable (Figure 7A) without de novo B cell lymphopoiesis (44), without circulating naïve B cells, and without de novo antibody production, despite the half-life of fish IgM being reportedly mere days (50). Thus, it suggests that anti-S. molnari plasma cells would survive such a stressor and that their antibody production is not affected (Figure 7B). The resistance of plasma cells may also be non-hormonal as observed in rainbow trout plasma cells resistant to hydroxyurea (14). The overall resilience of plasma cells may make them key to long-term protection that supports, for example, the physiology and life cycle of spawning fish as hypothesized by Zwollo (41). In this ‘immunological imprinting’ scenario, plasma cells are generated in juvenile fish against pathogens in their rearing site. As adults returning to these sites during their ‘spawning journey’, Zwollo hypothesizes that plasma cells may be resistant to cortisol, have a survival advantage over other B cell populations, and continue producing antibodies in anticipation of familiar pathogens (41).
Mechanistically, fish splenic and kidney leukocytes express glucocorticoid receptors (51) and cortisol induces apoptosis of C. carpio peripheral blood leukocytes (52). In addition to higher bacterial disease susceptibility (49), another study using triamcinolone acetonide demonstrated that it can nullify immune protection and perhaps memory of C. carpio against the protozoan parasite Ichthyophthirius multifiliis via an unknown mechanism (31). Interestingly, the same authors measured unchanged specific antibody titres following administration of the drug (30).
3.3 Potential lessons from the S. molnari infection modelChronic infections pose the greatest challenges even in well-studied species. For example, despite decades of concentrated research efforts, there is still no vaccine available against pathogens causing chronic diseases such as human immunodeficiency virus and malaria. Interestingly, in malaria infection, despite robust production of plasmablasts and anti-Plasmodium antibodies, this was ultimately metabolically taxing and impaired production of memory B and plasma cells (53). Our data suggests that we can rule out this possibility to explain chronic S. molnari infection because we measured a persistent constitutive anti-S. molnari response.
In natural settings, could immunosuppression (e.g., via cortisol) be a trigger for parasite activation, spore formation, and perpetuating the life cycle? Sterilizing immunity to myxozoan infections is rare and fish become carriers, suggesting that the parasite can evade the immune system, potentially a general feature of myxozoans that have co-evolved with fish hosts (54, 55). Despite continued secretion of anti-S. molnari antibodies, humoral memory could not provide sterilizing immunity in chronically infected fish (Figure 7A). Even though the immune antisera of infected fish can lyse the parasite (manuscript in preparation), the latent parasite was able to re-emerge 41 days after administration of the corticosteroid. It is possible that plasma cells expressing antibodies specific to an original ‘founder’ variant could no longer recognize an escape variant of the parasite, or that other immune non-B cells are protective and also eliminated by the corticosteroid we administered, i.e., the B cell response is necessary but insufficient alone. Taken together, myxozoan parasite escape and sporulation may be side effects of the ‘immunological imprinting hypothesis’ and hormonal immunosuppression (41). In this model, interseasonal carriers such as the common carp or wild trout may spawn and produce invertebrate-infective spores. Infected invertebrates may produce the fish-infective spores, completing the parasite life cycle and causing the annual outbreaks of myxozoan infections.
3.4 Concluding remarksIn summary, we adapted and devised methods that may be applicable to other vertebrate species in which adaptive immunity and immunological memory can be demonstrated because it relies on a common phenomenon, not a common mechanism or marker. Perhaps what holds true for all vertebrates and the only all-encompassing definition is that memory lymphocytes are antigen-experienced after engaging in a past immune response, and persist after resolution of that response. Immunologists have observed immunological memory for over a century and achieving humoral memory will help us meet the needs of immunologists, parasitologists, and fish husbandry.
4 Materials and methods4.1 Key resources tablePlease refer to Table 1 for the key resources used in this study.
Table 1. Key resources table.
4.2 Experimental model detailsAnimal procedures were performed in accordance with Czech legislation (section 29 of Act No. 246/1992 Coll. on Protection of animals against cruelty, as amended by Act No. 77/2004 Coll.). Animal handling complied with the relevant European guidelines on animal welfare (Directive 2010/63/EU on the protection of animals used for scientific purposes) and the recommendations of th
留言 (0)