Colorectal cancer is a common malignant tumor of the digestive tract. Studies have shown that colorectal cancer is currently the third most prevalent type of cancer worldwide (1, 2). In 2020, over 1.93 million cases of colorectal cancer were diagnosed globally, accounting for 10% of all cancer diagnoses, with more than 930,000 deaths attributed to the disease. In 2022, colorectal cancer ranked second in the estimated incidence of cancer in China and fifth in mortality rates (3). The incidence of colon cancer is increasing, with most patients being diagnosed at an advanced stage. Some scholars predict that by 2025, the number of colorectal cancer cases and deaths in China will reach approximately 6.423 million and 2.211 million, respectively (4). Despite continuous advancements in medical technology and treatment methods, nearly 60% of the cancer patients still fail to achieve a “clinical cure” (5, 6).
Recurrence and metastasis are crucial factors hindering clinical cure of colorectal cancer, with lymph node metastasis being one of the primary causes. Accurate preoperative assessment of lymph node metastasis, thorough intraoperative dissection of potentially metastatic lymph nodes, and maximizing the number of lymph nodes retrieved are all of great significance in accurately assessing pathological staging, guiding postoperative adjuvant treatment plans, and improving survival rates (7–10).
To maximize the number of lymph nodes retrieved, various lymph node tracing techniques have been developed, such as India ink, indocyanine green fluorescence imaging, and dye tracing methods (methylene blue, toluidine blue, etc.). However, each of these methods has its inevitable drawbacks. In recent years, carbon nanoparticle tracing technique has gradually attracted attention from scholars. Carbon nanoparticles (CNs) have a diameter of approximately 150 to 200 nm, which lies between that of capillaries (5 to 6 nm) and lymphatic vessels (about 500 nm). Therefore, after CNs are injected into the intestinal mucosa surrounding tumor tissues, they can only enter the lymphatic vessels and not the capillaries, allowing for accurate localization of lymph nodes. Multiple studies have demonstrated that CN tracing technique can significantly increase the number of lymph nodes detected during radical resection surgeries for colorectal (11–13), breast (14, 15), and gastric cancers (16, 17), and it also exhibits high safety and lymphatic tropism (18, 19). However, there have been no reports on whether CN tracing technique can improve patients’ five-year overall survival and disease-free survival rates. Therefore, data from 1,869 patients who underwent radical resection for colorectal cancer at the First Affiliated Hospital of Chongqing Medical University from March 2013 to February 2017 were collected in this study. Based on follow-up results, survival curves for patients’ five-year overall survival and disease-free survival were plotted, and univariate and multivariate analyses were conducted to explore the effect of CN tracing technique on these outcomes.
Patients and methodsPatientsIn this retrospective cohort study, clinical data from 2,237 patients who underwent laparoscopic radical resection for colorectal cancer at the First Affiliated Hospital of Chongqing Medical University between March 2013 and February 2017 were included. Among them, 368 patients were lost to follow-up by the fifth postoperative year, leaving a total of 1,869 patients for this study. These patients were then divided into two groups based on whether preoperative endoscopic carbon nanoparticle suspension was used for lesion localization: the localization group with 758 patients and the non-localization group with 1,111 patients. Specific information on the patients can be found in Table 1.
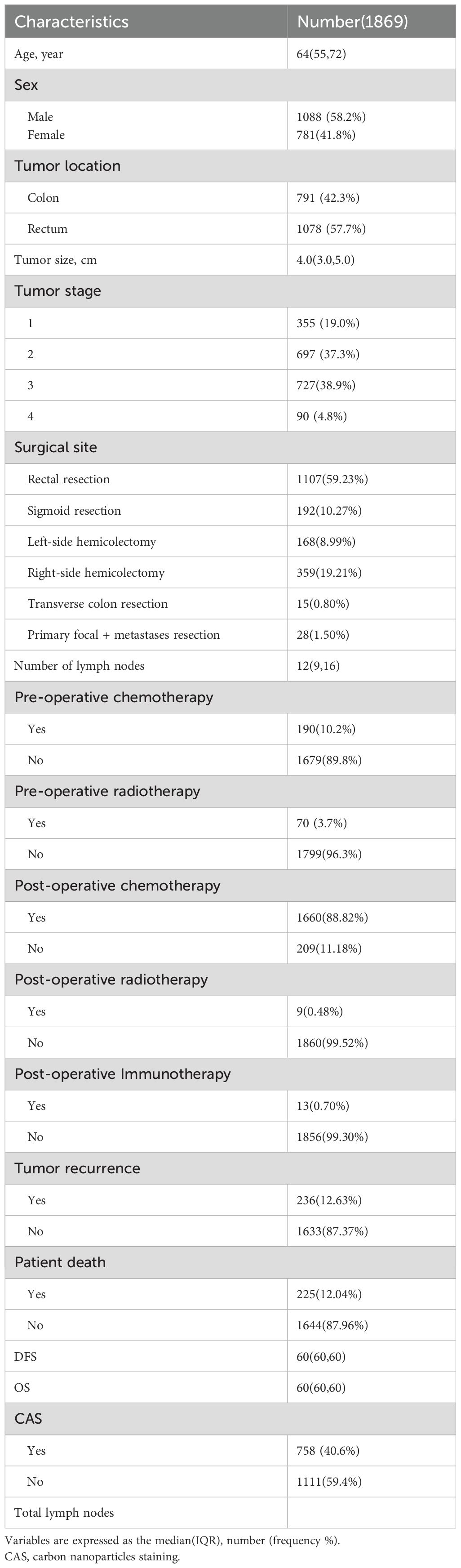
Table 1. Clinical characteristics of patients.
Inclusion and exclusion criteriaInclusion Criteria: 1. Age: over 18 years; 2. Diagnosis: histologically confirmed colorectal cancer; 3. Treatment modality: radical resection surgery for colorectal cancer; 4. Intervention: preoperative carbon nanoparticle marking via colonoscopy.
Exclusion Criteria: 1. Patients who did not receive radical surgery (e.g., those who underwent local excision or received palliative treatment); 2. Multiple primary cancers; 3. Missing clinical data. 4. Complicated with severe cardiopulmonary, hepatic and renal disease or coagulation disorders.
Preoperative carbon nanoparticle injection for lesion localizationOne to seven days before laparoscopic resection for colorectal cancer (excluding neoadjuvant therapy), 4-6 points were marked with carbon nanoparticle suspension around the inner wall of the intestinal lumen at 1-2 cm from the anal side of the lesion, with approximately 1ml at each point. For small lesions, the injection was performed submucosally near the base of the lesion using the same method. If the base was small, an additional injection point was added in the submucosa area on the opposite side of the tumor.
Surgical methodsAll patients strictly underwent surgery in accordance with complete mesocolic excision (CME) and total mesorectal excision (TME). Regional lymph node dissection for colon cancer must encompass paracolic, mesenteric and central lymph nodes. In this study, regional lymph node dissection was performed on all patients using D3 radical surgery, mainly including the resection of black-stained lymph nodes, the removal of lymph nodes based on the course of blood vessels, lymph nodes palpable by hand, and lymph nodes that were black-stained and visible outside the resection range of the radical procedure.
Postoperative evaluation and follow-upPatients were followed up through a combination of telephone calls and outpatient visits. Follow-ups were conducted every 3-6 months within the first two years after surgery and annually thereafter. Tumor recurrence and patient survival status within 5 years were investigated during the follow-up. Based on the follow-up results, overall survival and disease-free survival curves were plotted, and the overall survival and disease-free survival rates for patients who received preoperative carbon nanoparticle tracing technique were calculated.
Statistical analysisContinuous variables that did not follow normal distribution were expressed as the median (Interquartile Range (IQR)) and categorical variables were presented as number and frequency (%). Differences between groups were compared by the Mann-Whitney U test and Kruskal-Wallis test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Univariate and multivariate cox regression models were used to evaluate the association between the number of lymph nodes harvested and the survival rate. Survival curves were obtained using the Kaplan-Meier method and the Log-rank test was used for comparison of the groups. P<0.05 was considered statistically significant. All statistical analyses were performed with SPSS for Windows (version 25.0, SPSS Inc).
ResultsPatient characteristicsFrom March 2013 to February 2017, a total of 2,237 patients were enrolled in this study, of which 368 patients were excluded due to loss to follow-up, leaving 1,869 patients for inclusion. There were 1,088 males and 781 females, with a median age 64 years. Among them, 758 patients received a preoperative carbon nanoparticle injection, while 1,111 patients did not. As shown in Table 1. The number of lymph nodes retrieved in the CAS group (15(11,19)) was significantly higher than that in the non-CAS group (11(7,15))(p< 0.05) as shown in Table 2.
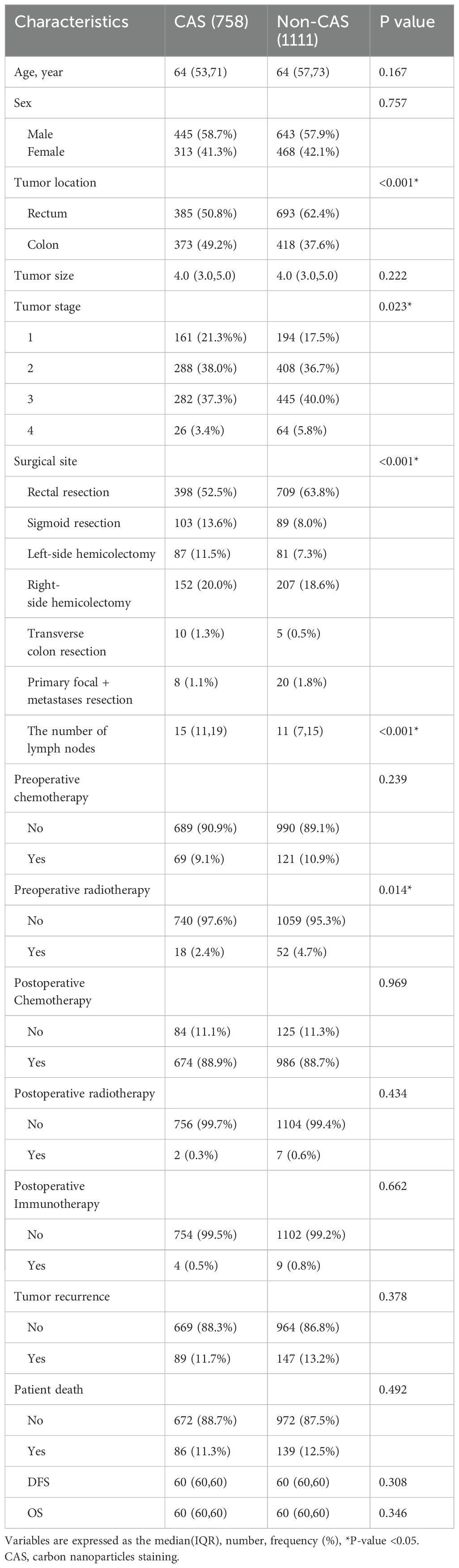
Table 2. Comparison between the CAS group and Non-CAS group.
SurvivalBased on the patient data obtained from follow-ups, survival curves and survival tables for five-year overall survival and disease-free survival rates were plotted for the CAS and non-CAS groups, as shown in Figures 1 and 2. The study results revealed that the five-year overall survival rate were 90.8% for the CAS group and 87.4% for the non-CAS group, while the disease-free survival rate were 88.5% and 83.4% respectively. The Log rank test showed no statistically significant difference in five-year overall survival and disease-free survival rates between the CAS and Non-CAS groups (p=0.42, p=0.41, p> 0.05).
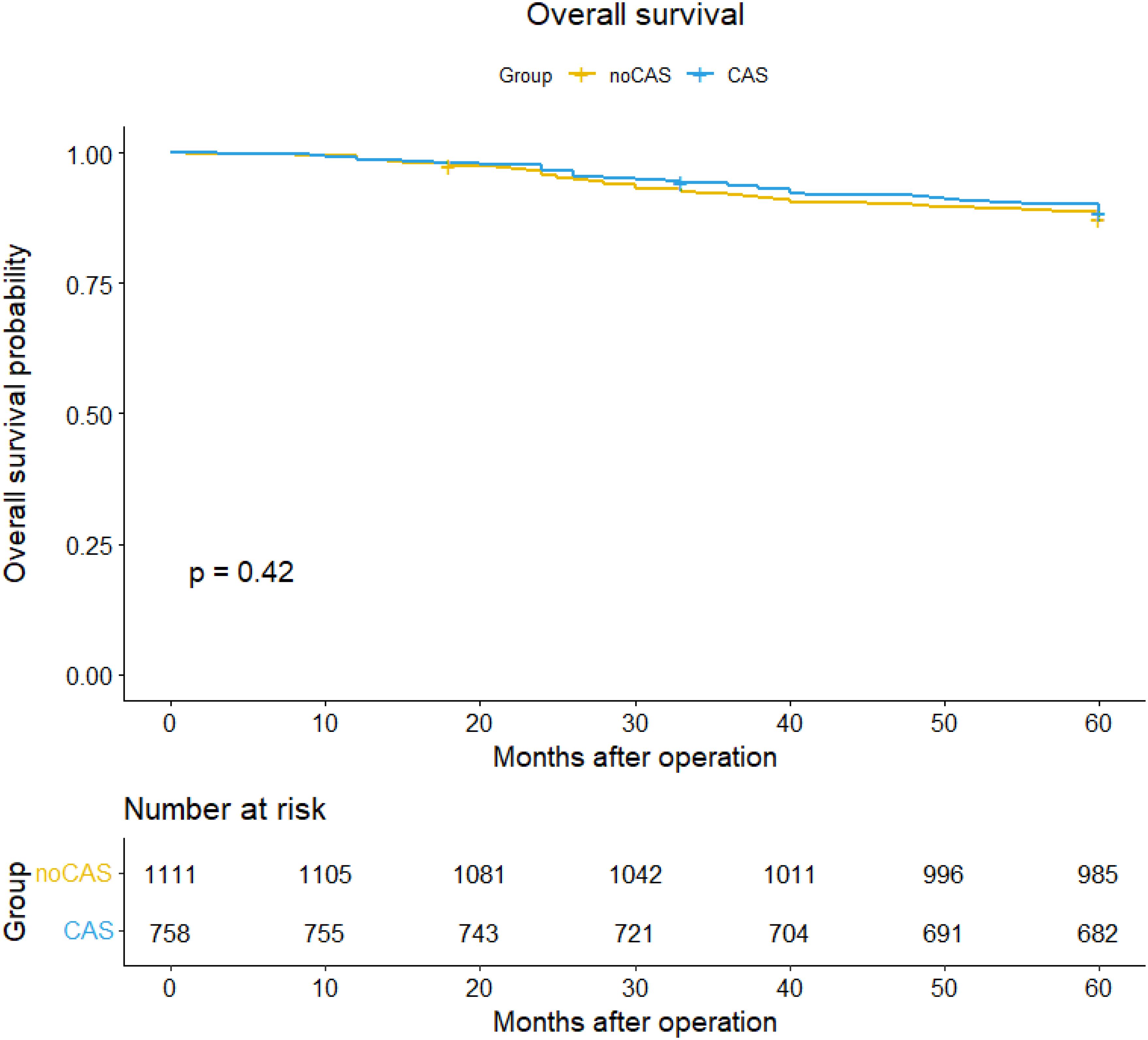
Figure 1. Overall survival curve and survival table.
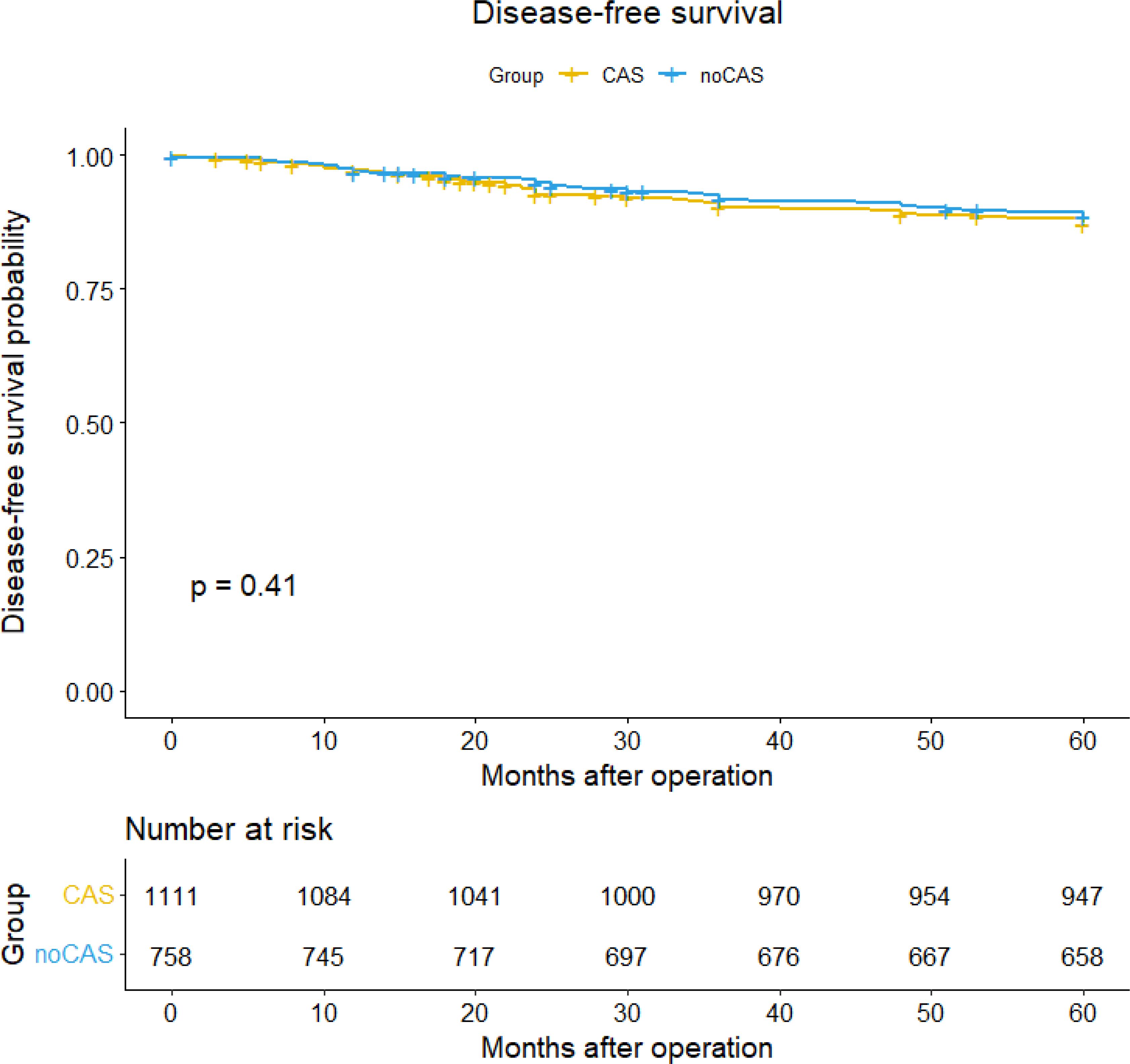
Figure 2. Disease-free survival curve and survival table.
Univariate and multivariate cox regression analysis of overall survivalAs shown in Table 3, the result of univariate cox regression analysis showed that patient age, tumor stage, tumor size, surgical procedures, postoperative chemotherapy, postoperative radiotherapy and postoperative tumor recurrence were significantly influencing the overall survival of colorectal cancer patients (p < 0.05). The result of multivariate cox regression analysis showed that patient age, tumor stage, postoperative chemoradiotherapy, postoperative radiotherapy and postoperative tumor recurrence were independent factors influencing the overall survival rate in colorectal cancer patients (p < 0.05).
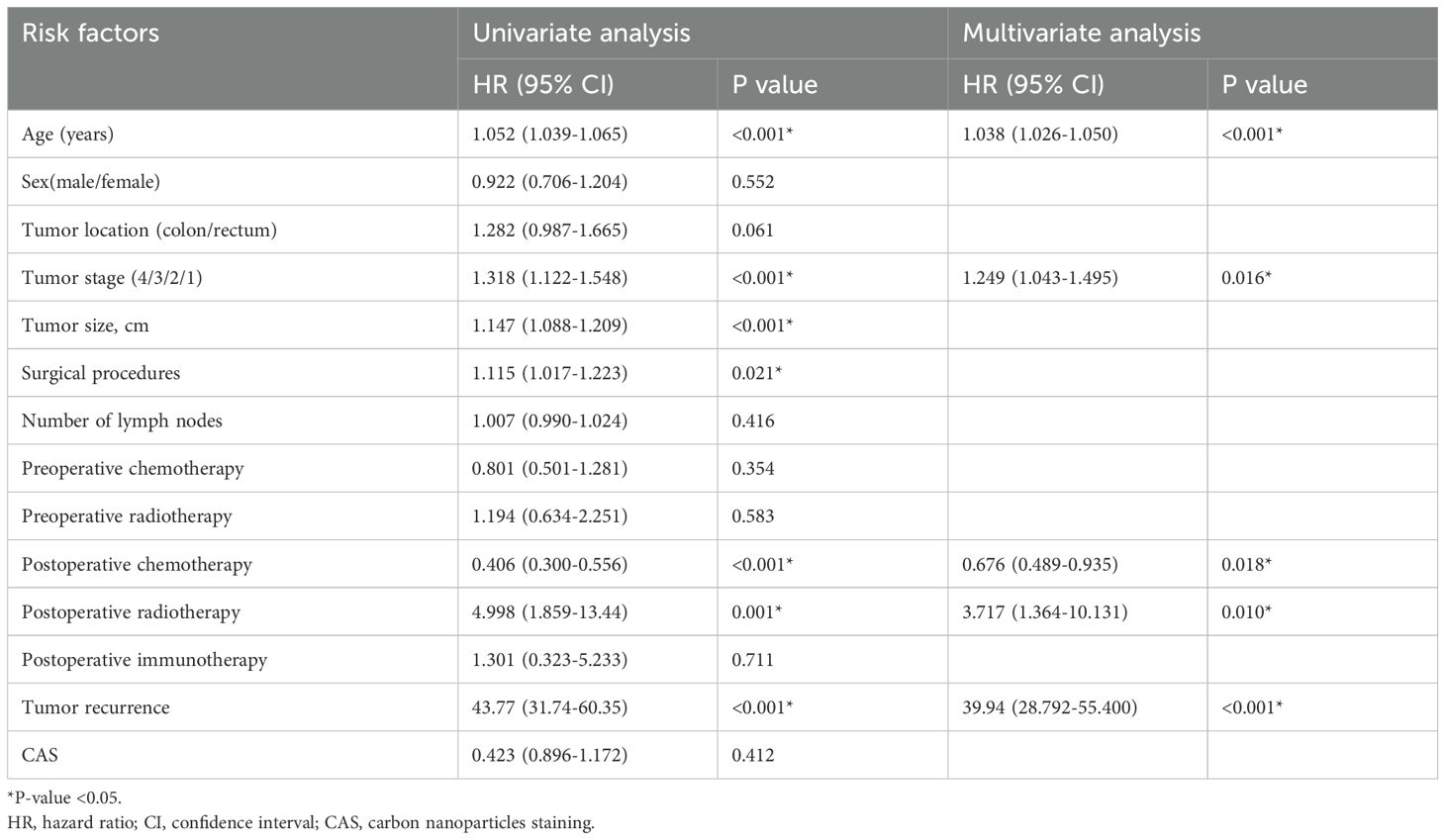
Table 3. Univariate and multivariate cox regression analysis of overall survival.
Univariate and multivariate cox regression analysis of disease-free survivalAs shown in Table 4, the result of univariate cox regression analysis showed that patient age, tumor stage, tumor size, surgical approach, postoperative chemotherapy, postoperative radiotherapy and postoperative tumor recurrence were significantly influencing overall survival of colorectal cancer patients (p < 0.05). The result of multivariate cox regression analysis showed that patient age, tumor stage, postoperative chemoradiotherapy, postoperative radiotherapy and postoperative tumor recurrence were independent factors influencing the disease-free survival rate in colorectal cancer patients (p < 0.05).
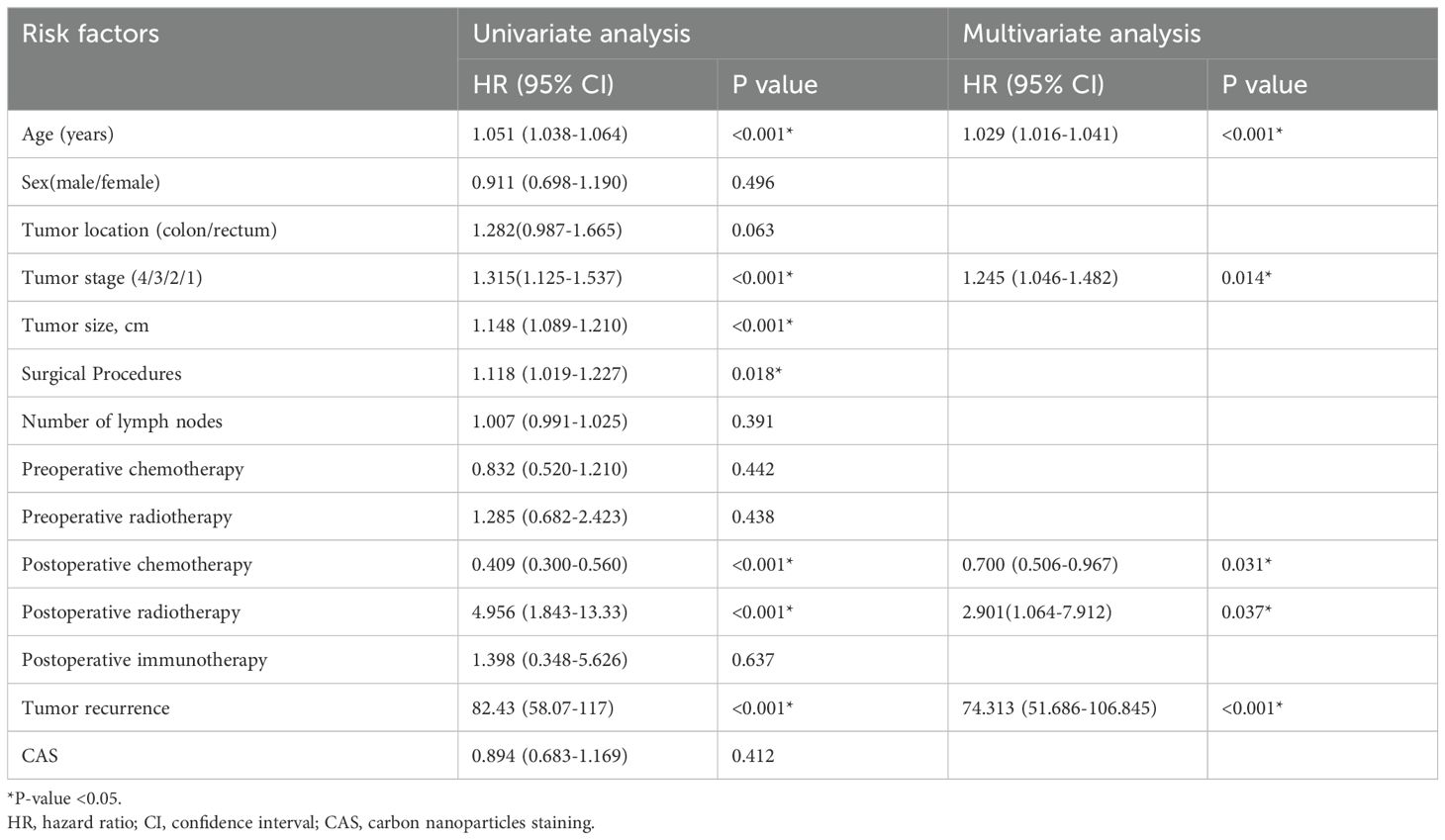
Table 4. Univariate and multivariate cox regression analysis of disease-free survival.
The number of lymph nodes detected between the three groups(stage III) was significantly different (P<0.05), among which stage IIIA was significantly different from stage IIIC (P=0.001) and stage IIIB was significantly different from stage IIIC (P=0.0003). The results were shown in Table 5.
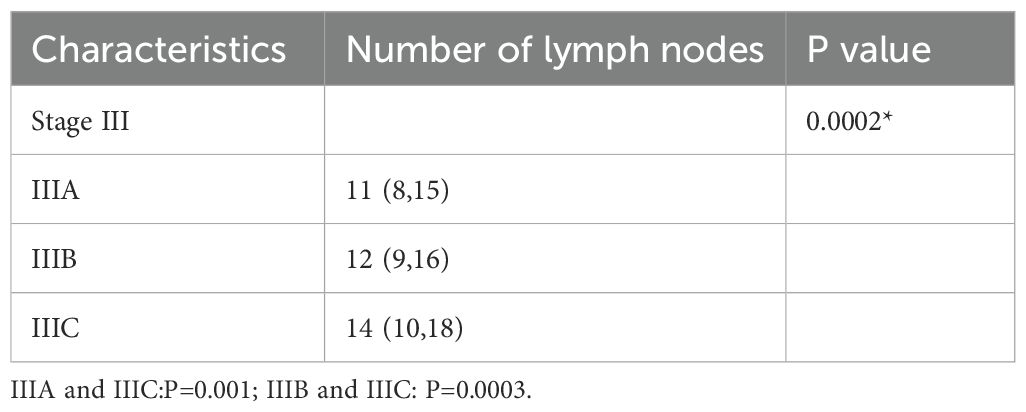
Table 5. Number of lymph nodes detected in patients (stage III).
There were significant differences in the number of lymph nodes of the CAS group among the three stage (P=0.027). Among which stage IIIA was significantly different from stage IIIC (P=0.012).
There were significant differences in the number of lymph nodes of the Non-CAS group among the three stage(P=0.0002).Among which stage IIIA was significantly different from stage IIIB (P=0.0076),IIIA was significantly different from stage IIIC (P=0.0001) and IIIB was significantly different from stage IIIC (P=0.0135).The results were shown in Table 6.
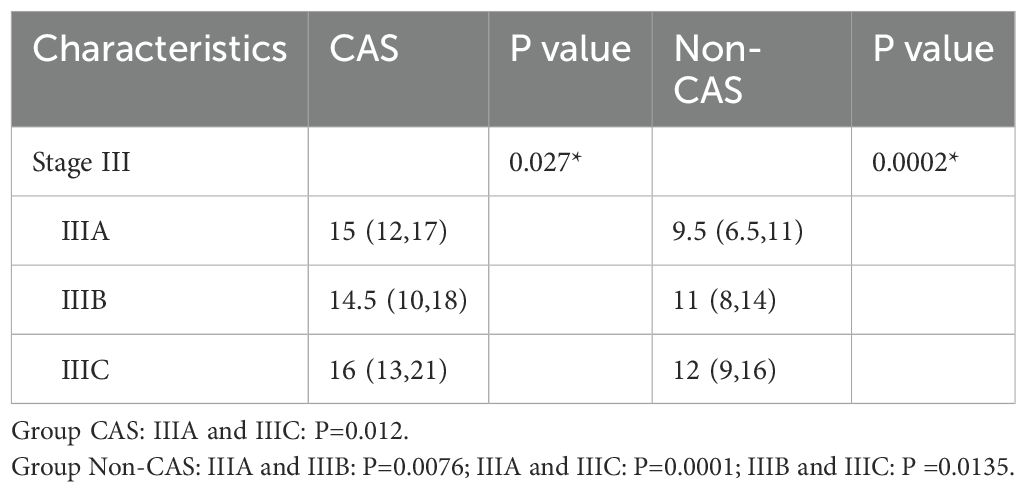
Table 6. Number of lymph node detection in patients between CAS and non-CAS groups (III stage).
The number of lymph nodes detected between the CAS and non-CAS groups of the three stage was significantly different s(P<0.05).The results were shown in Table 7.
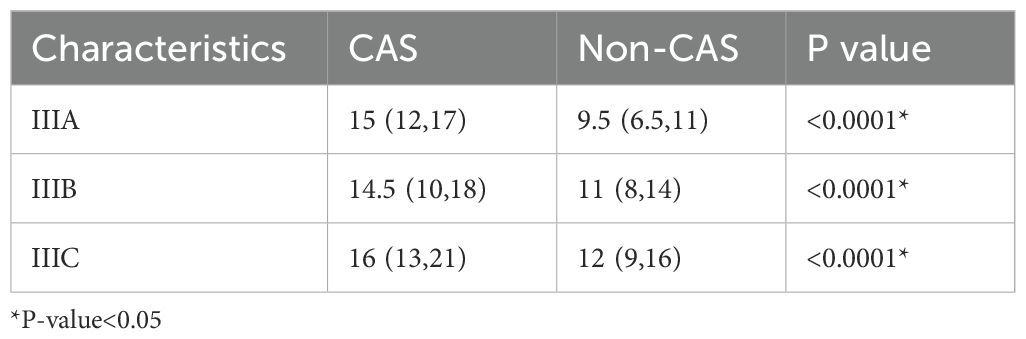
Table 7. Number of total lymph nodes detected between the CAS and non-CAS groups (III stage).
The Figures 3 and 4 showed that the difference of five-year overall survival and disease-free survival between the two groups was not statistically significant (P>0.05).
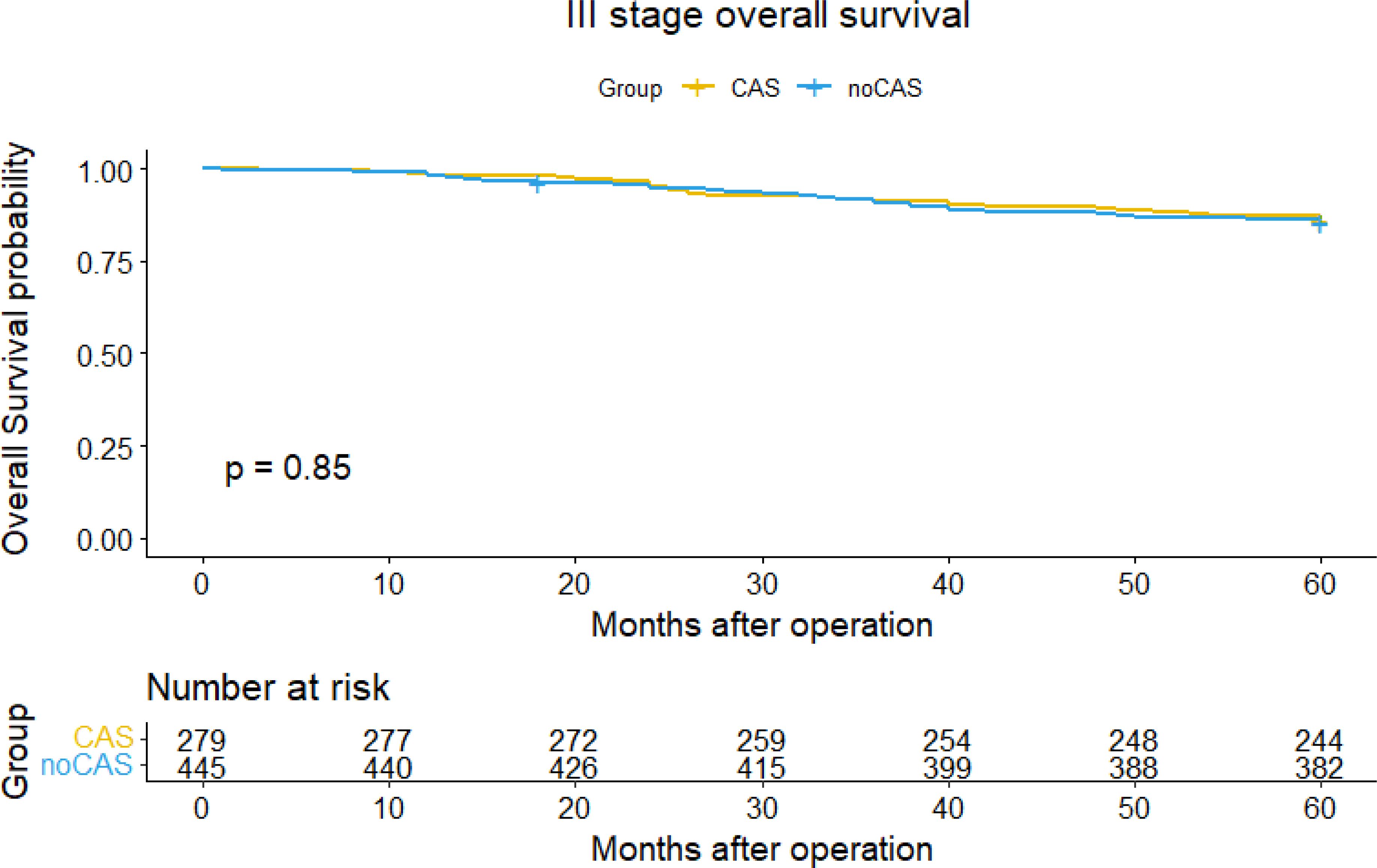
Figure 3. Overall survival curve and survival table (stage III).
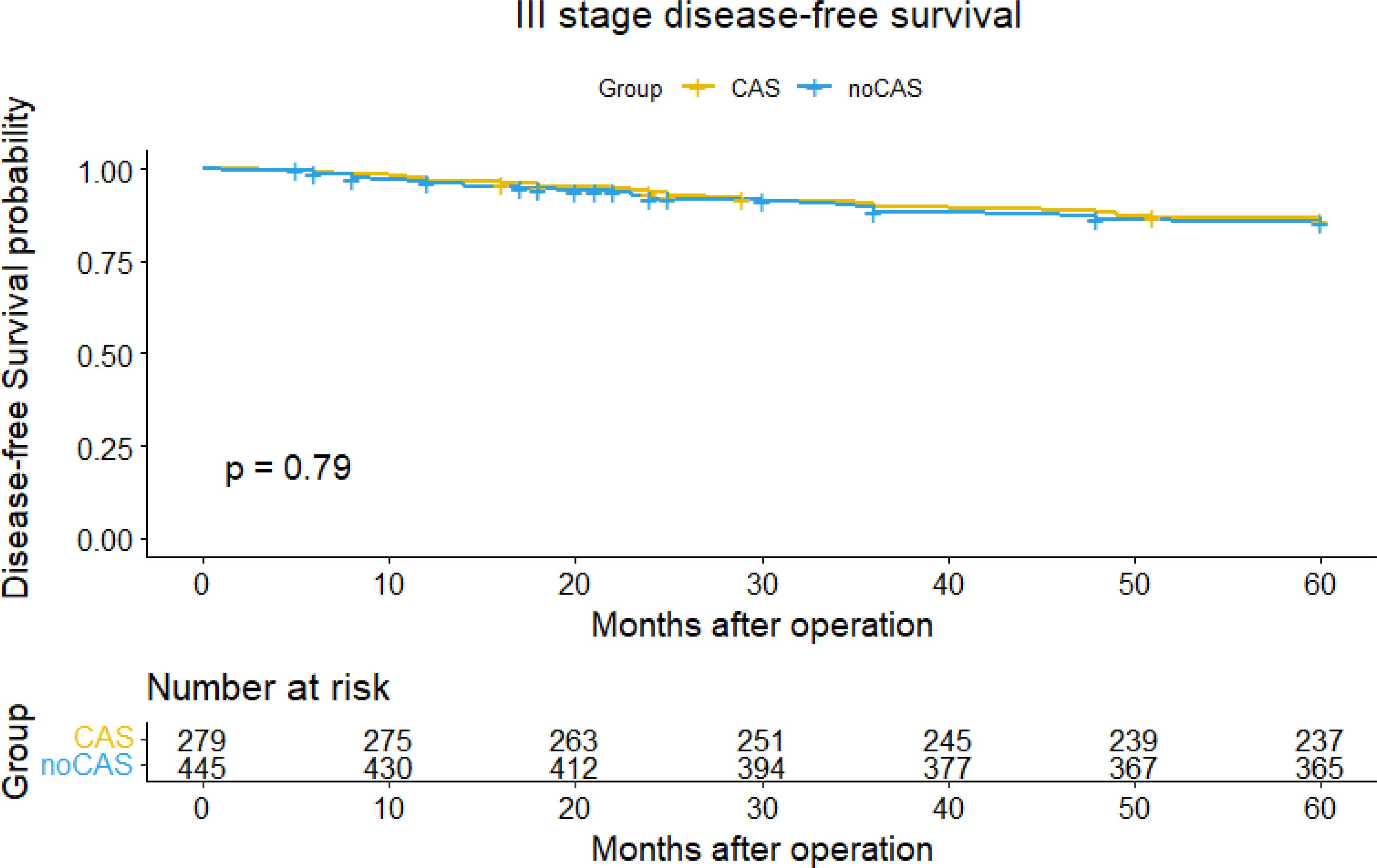
Figure 4. Disease-free survival curve and survival table (stage III).
Univariate and multivariate cox regression analysis of overall survival and disease-free survival of stage IIIThe result of univariate cox regression analysis showed that patient age, tumor size, postoperative chemotherapy, postoperative radiotherapy and postoperative tumor recurrence were significantly influencing overall survival and disease-free survival of colorectal cancer stage III patients (p < 0.05). The result of multivariate cox regression analysis showed that patient age, postoperative chemoradiotherapy, postoperative radiotherapy and postoperative tumor recurrence were independent factors influencing the overall survival rate of colorectal cancer stage III patients (p < 0.05). Multivariate cox regression analysis showed that postoperative chemoradiotherapy and tumor recurrence were independent factors influencing the disease-free survival rate of colorectal cancer stage III patients (p < 0.05). The results were shown in Tables 8 and 9.
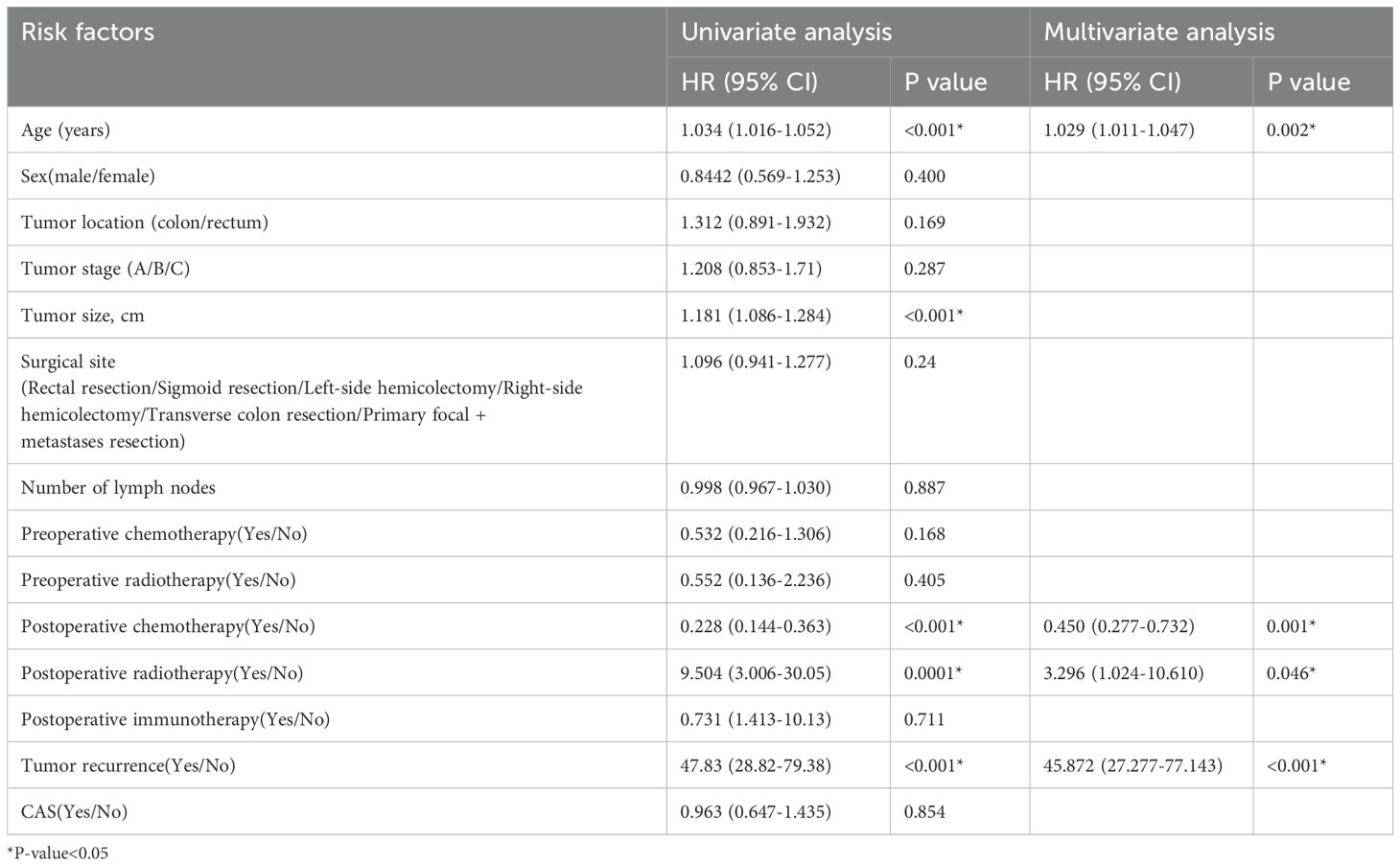
Table 8. Univariate and multivariate cox regression analysis of overall survival rate (III stage).
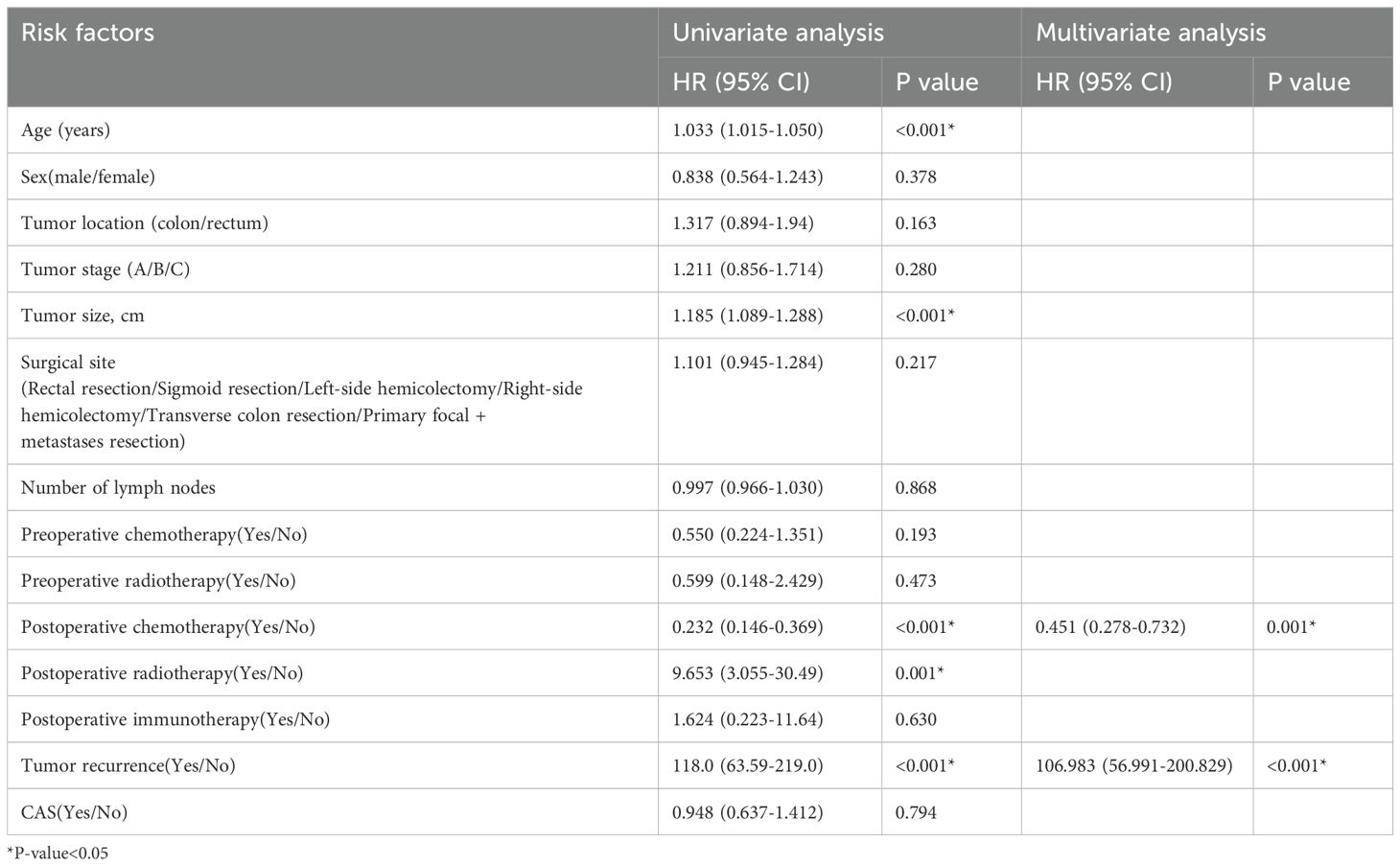
Table 9. Univariate and multivariate cox regression analysis of disease-free survival rate (III stage).
In summary, our study demonstrates that the use of carbon nanoparticle tracing technique significantly increases the total number of lymph nodes retrieved in colorectal cancer patients but has no significant effect on their five-year overall survival or disease-free survival rates. However, patient age, tumor stage, postoperative chemoradiotherapy, and tumor recurrence have significant effects on both overall survival and disease-free survival rates. Patients with stage III colorectal cancer also showed the same results, and postoperative chemotherapy and tumor recurrence had a significant impact on both overall and disease-free survival in stage III patients.
DiscussionLymph node metastasis is a crucial pathway for the spread of colorectal cancer. Lymph node exploration allows for the identification of both the number and location of metastatic lymph nodes, enabling accurate staging of the cancer and providing essential basis for subsequent treatment. Moreover, the extent of lymph node metastasis is closely associated with patient prognosis. By assessing the extent of lymph node metastasis through lymph node exploration, physicians can evaluate patient prognosis and devise appropriate treatment plans. Additionally, the results of lymph node exploration can guide the selection of appropriate adjuvant therapies to reduce the risk of recurrence. Therefore, accurately dissecting lymph nodes and maximizing the number of lymph nodes retrieved are crucial in improving the cure rate in colorectal cancer patients (20–22).
In recent years, numerous lymph node tracing methods, such as India ink (23), methylene blue (24), and indocyanine green (ICG) fluorescence (25), have been applied in surgical procedures, each with their respective drawbacks. India ink suffers from poor diffusion properties, long retention time, and potential adverse reactions during marking, including inflammatory responses, local ulcers, and even obstructive necrosis (26). Methylene blue, on the other hand, has a small particle diameter and rapid diffusion which results in a short marking duration and can hinder surgical visibility. ICG fluorescence, which requires near-infrared light for visualization, significantly limits its widespread application (27). Recently, a novel carbon nanoparticle tracing technique has garnered substantial attention. Carbon nanoparticles (28), with diameters precisely between those of capillaries and capillary lymphatics, can easily enter capillary lymphatics but not capillaries, imparting excellent lymphatic system tropism and safety (29). Additionally, after local injection, carbon nanoparticles are phagocytosed by macrophages, allowing their entry into the lymphatic system and in vivo staining of lymph nodes in tumor regions. In a study by Renjie Wang et al. (19) involving 239 patients with stage I-III colorectal cancer who received the preoperative carbon nanoparticle tracing technique, no allergic reactions, drug-related complications, acute or chronic toxicity, or other adverse effects were observed. According to relevant studies, the carbon nanoparticle tracing technique has been extensively applied in radical resection for colorectal cancer. J W Cai et al. (31) found that among 1,421 patients undergoing radical resection for colorectal cancer, the total number of lymph nodes detected in the carbon nanoparticle tracing group was significantly higher than that in the control group (22.2 ± 11.2 vs. 19.0 ± 9.5, t=3.025, p=0.003), consistent with the conclusions of this study. A study by Rong Wang et al. (32) involving 113 patients with advanced colorectal cancer revealed that the carbon nanoparticle marking group reduced intraoperative lesion exploration time and total surgery duration, with less intraoperative blood loss and a relatively higher anus-preserving rate. However, the effect of the carbon nanoparticle tracing technique on the five-year overall survival rate and disease-free survival rate in colorectal cancer patients remains unreported.
This study aims to collect a substantial sample of clinical data from patients to more accurately and reliably analyze the effect of carbon nanoparticle tracing technique on the five-year overall survival rate and disease-free survival rate in patients undergoing radical resection for colorectal cancer. Our findings revealed that the number of lymph nodes detected in the carbon nanoparticle tracing group was significantly higher than that in the control group (localized group: 15.6 ± 7.4, non-localized group: 11.5 ± 6.5). This result is consistent with the results in a previous report by Yang Bin et al., who found that the lymph node staining rate after carbon nanoparticle injection was 56.8% (412/725), and that carbon nanoparticle lymphatic tracing could increase the number of lymph nodes dissected, particularly lymph nodes with diameters <5mm [4.6% (33/725)], which was significantly higher than in the non-tracing group [2.0% (10/478), p=0.025]. Similarly, Xiangchun Zhang et al. studied the medical records of 53 patients undergoing laparoscopic radical resection for colorectal cancer and found that the average number of lymph nodes detected and the number of micro-lymph nodes (<5mm) detected in the carbon nanoparticle injection group were significantly higher than those in the unstained control group [(16.7 ± 3.2) vs. (12.6 ± 2.3), p<0.01; (5.3 ± 2.4) vs. (2.1 ± 1.2), p<0.01].
Furthermore, by calculating the five-year overall survival rate, disease-free survival rate, and their survival curves for the localization group and the non-localization group, we found that carbon nanoparticle tracing technique had no significant effect on the five-year overall survival rate or disease-free survival rate of patients. This is consistent with the findings of a report by Liyu Wang et al. (30) who observed no significant difference in the 3-year survival rate between the carbon nanoparticle tracing group and the control group across stages I-IV of colorectal cancer. We attribute this to the multitude of factors influencing the long-term prognosis of colorectal cancer, such as patient age, clinical symptoms and complications, primary tumor location, postoperative adjuvant therapy, histological type and differentiation of the tumor, tumor recurrence, lymph node metastasis, and depth of invasion. The application of carbon nanoparticle tracing technique alone can only effectively increase the number of lymph nodes detected to a certain extent and improve the reliability of postoperative pathological N staging, but its effect on the five-year survival rate is limited.
To further identify the factors influencing the five-year overall survival rate and disease-free survival rate of patients, we conducted both univariate and multivariate analyses. The results indicated that patient age, tumor stage, postoperative radiotherapy and chemotherapy, as well as the postoperative recurrence status of the patients, were independent factors affecting the overall survival rate of colorectal cancer patients. Similarly, patient age, tumor stage, postoperative radiotherapy and chemotherapy, and tumor recurrence status were identified as independent factors influencing the disease-free survival rate. However, the use of preoperative carbon nanoparticle tracing technique did not affect the five-year overall survival rate or disease-free survival rate in the patients.
Finally, focusing on data from patients with stage III colorectal cancer, we found that nanocarbon tracer technology increased the number of lymph nodes detected in patients with stage III colorectal cancer, but did not affect the patients’ 5-year overall survival or disease-free survival, and postoperative chemotherapy and tumor recurrence had a significant impact on both overall and disease-free survival in stage III patients.
There are, however, some limitations in this study. Firstly, it provides a general analysis of colorectal cancer patients without a detailed analysis of each tumor stage. Secondly, this study does not analyze data regarding the degree of black staining of lymph nodes or the detected number of lymph nodes with a diameter of less than 5 mm, indicating some deficiencies in the analysis of specific indicators. Lastly, we does not count the number of lymph nodes detected outside of the locoregional spread., and we will add data on this aspect in a subsequent study.
In summary, the carbon nanoparticle tracing technique can effectively increase the number of lymph nodes detected during radical resection for colorectal cancer and enhance the accuracy of postoperative pathological N staging, which may assist in guiding adjuvant therapy. However, it has no significant effect on the five-year overall survival rate or disease-free survival rate in patients.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the ethics committee of Chongqing Medical University (Chongqing, China. protocol code: 2021-233). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsGW: Writing – original draft. ZJ: Writing – original draft. YW: Data curation, Supervision, Writing – review & editing. QK: Writing – review & editing. DH: Writing – review & editing. ZW: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Research and application demonstration of colorectal cancer prevention and treatment technology (2019ZX003).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsHR, hazard ratio; CI, confidence interval; CAS, carbon nanoparticles staining; OS, overall survival; DFS, disease-free survival; ICG, indocyanine green.
References3. Zhang L, Cao FC, Zhang GY, Shi LS, Chen SC, Zhang ZH, et al. Trends in and predictions of colorectal cancer incidence and mortality in China from 1990 to 2025. Front Oncol. (2019) 9. doi: 10.3389/fonc.2019.00098
PubMed Abstract | Crossref Full Text | Google Scholar
4. Sung H, Ferlay J, Siegel RL, Laversanne ML, Soerjomataram IS, Jemal AJ, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
6. Xu L, Zhao JH, Li ZL, Sun J, Lu Y, Zhang RQ, et al. National and subnational incidence, mortality and associated factors of colorectal cancer in China: A systematic analysis and modelling study. J Global Health. (2023) 13. doi: 10.7189/jogh.13.04096
PubMed Abstract | Crossref Full Text | Google Scholar
8. Mounika RN, Ananthamurthy A. Lymph node yield in colorectal cancer specimens and its impact on pathological staging: Does number matter? J Cancer Res Ther. (2023) 19:671–4. doi: 10.4103/jcrt.jcrt_980_21
PubMed Abstract | Crossref Full Text | Google Scholar
10. Betge J, Harbaum LH, Pollheimer MJP, Lindtner RAL, Kornprat PK, Ebert MPE, et al. Lymph node retrieval in colorectal cancer: determining factors and prognostic significance. Int J Colorectal Dis. (2017) 32:991–8. doi: 10.1007/s00384-017-2778-8
PubMed Abstract | Crossref Full Text | Google Scholar
11. Liu F, Peng D, Liu X-Y, Liu X-R, Li Z-W, Wei Z-Q, et al. The effect of carbon nanoparticles staining on lymph node tracking in colorectal cancer: A propensity score matching analysis. Front Surg. (2023) 10. doi: 10.3389/fsurg.2023.1113659
PubMed Abstract | Crossref Full Text | Google Scholar
12. Koimtzis G, Geropoulos G, Stefanopoulos L, Chalklin CG, Karniadakis I, Alexandrou V, et al. The role of carbon nanoparticles as lymph node tracers in colorectal cancer: A systematic review and meta-analysis. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms242015293
PubMed Abstract | Crossref Full Text | Google Scholar
13. Maffioli A, Danelli P. Carbon nanoparticles application during colorectal cancer surgery: Updates from China. Digestive Liver Dis. (2020) 52:1443–4. doi: 10.1016/j.dld.2020.09.023
PubMed Abstract | Crossref Full Text | Google Scholar
14. Wang Z-H, Gang T-R, Wu S-S, Lu C, Gao G-X, Xu W, et al. Single-port endoscopic-sentinel lymph node biopsy combined with indocyanine green and carbon nanoparticles in breast cancer. Surg Endoscopy. (2023) 37:7591–9. doi: 10.1007/s00464-023-10018-9
PubMed Abstract | Crossref Full Text | Google Scholar
15. Zhang L, Huang Y, Yang C, Zhu T, Lin Y, Gao H, et al. Application of a carbon nanoparticle suspension for sentinel lymph node mapping in patients with early breast cancer: a retrospective cohort study. World J Surg Oncol. (2018) 16. doi: 10.1186/s12957-018-1414-6
PubMed Abstract | Crossref Full Text | Google Scholar
16. Lei Y, Zhao Z-M, Li Y-S. Assessment of the efficacy and safety of carbon nanoparticles-guided lymph node dissection in gastric cancer surgery: a systematic review and meta-analysis. Int J Clin Oncol. (2023) 28:764–76. doi: 10.1007/s10147-023-02333-x
PubMed Abstract | Crossref Full Text | Google Scholar
17. Feng Y, Yang K, Sun H-H, Liu Y-P, Zhang D, Zhao Y, et al. Value of preoperative gastroscopic carbon nanoparticles labeling in patients undergoing laparoscopic radical gastric cancer surgery. Surg Oncol. (2021) 38. doi: 10.1016/j.suronc.2021.101628
PubMed Abstract | Crossref Full Text | Google Scholar
18. Liu P, Tan J, Tan Q, Xu L, He T, Lv Q. Application of carbon nanoparticles in tracing lymph nodes and locating tumors in colorectal cancer: A concise review. Int J Nanomed. (2020) 15:9671–81. doi: 10.2147/IJN.S281914
PubMed Abstract | Crossref Full Text | Google Scholar
19. Li X, et al. The safety and effectiveness of carbon nanoparticles suspension in tracking lymph node metastases of colorectal cancer: a prospective randomized controlled trial. Japanese J Clin Oncol. (2020) 50:535–42. doi: 10.1093/jjco/hyaa011
PubMed Abstract | Crossref Full Text | Google Scholar
20. Saha S, Phelimon B, Efeson M, Helina A, Elgamal M, Kiya G, et al. The role of sentinel lymph node mapping in colon cancer: detection of micro-metastasis, effect on survival, and driver of a paradigm shift in extent of colon resection. Clin Exp Metastasis. (2021) 39:109–15. doi: 10.1007/s10585-021-10121-y
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ichimasa K, Kudo S-E, Miyachi H, Kouyama Y, Mochizuki K, Takashina Y, et al. Current problems and perspectives of pathological risk factors for lymph node metastasis in T1 colorectal cancer: Systematic review. Digestive Endoscopy. (2022) 34:901–12. doi: 10.1111/den.14220
PubMed Abstract | Crossref Full Text | Google Scholar
22. Chang GJ, Rodrigas-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. JNCI J Natl Cancer Institute. (2007) 99:433–41. doi: 10.1093/jnci/djk092
PubMed Abstract | Crossref Full Text | Google Scholar
23. Sato K, Shimoda H, Miura T, Sakamoto Y, Morohashi H, Watanabe S, et al. Widespread anorectal lymphovascular networks and tissue drainage: analyses from submucosal India ink injection and indocyanine green fluorescence imaging. Colorectal Dis. (2021) 23:1334–45. doi: 10.1111/codi.15582
PubMed Abstract | Crossref Full Text | Google Scholar
24. Carvalho A, Gonçalves N, Teixeira P, Goulart A, Leão P. The impact of methylene blue in colorectal cancer: Systematic review and meta-analysis study. Surg Oncol. (2024) 53. doi: 10.1016/j.suronc.2024.102046
PubMed Abstract | Crossref Full Text | Google Scholar
25. Emile SH, Elfeki H, Shalaby M, Sakr A, Sileri P, Laurberg S, et al. Sensitivity and specificity of indocyanine green near-infrared fluorescence imaging in detection of metastatic lymph nodes in colorectal cancer: Systematic review and meta-analysis. J Surg Oncol. (2017) 116:730–40. doi: 10.1002/jso.v116.6
留言 (0)