Sickle cell disease (SCD) refers to a group of inherited disorders affecting red blood cells, driven by a specific mutation in the beta-globin gene. This mutation leads to the substitution of valine for glutamic acid at the sixth position in the beta-globin chain, promoting the formation of hemoglobin S (HbS) and the characteristic sickling of red blood cells, which underlies the clinical complications of the disease (Kato et al., 2018; Driss et al., 2009; Bunn, 1997; Pauling and Itano, 1949). The mutation results in abnormal, sickle-shaped red blood cells that obstruct blood flow, causing vaso-occlusion, organ damage, and systemic inflammation (Abdulmalik et al., 2020) as well as reduced life expectancy (Akinsheye and Klings, 2010). Sickle Cell Anemia (SCA), the most severe form, affects individuals homozygous for the HbS allele (HbSS) and is associated with acute complications such as anemia, infections and sepsis, as well as chronic issues including renal dysfunction, neurological decline, and impaired quality of life (Ware et al., 2017; Elmariah et al., 2014; Fitzhugh et al., 2010). Acute chest syndrome (ACS) and stroke present potentially life-threatening conditions that necessitate immediate and well-coordinated efforts for early detection as well as urgent and specialized care. Moreover, SCD significantly impacts cognitive and psychosocial functioning (Prussien et al., 2020). Individuals with the HbAS genotype (carriers of one mutated allele) are asymptomatic and are generally protected from severe manifestations, while those with HbSS experience a full spectrum of symptoms and complications (Ware et al., 2017; Eltzschig and Eckle, 2011; Lee et al., 2013).
One of the harmful by-products of SCD is cell-free heme, released during hemolysis, which triggers inflammation, oxidative stress, and vascular complications, including brain damage (Kato et al., 2017). Understanding the role of heme is essential for developing effective therapies. In 2021, an estimated 7.74 million individuals globally were affected by SCD (Thomson et al., 2023), with approximately 75% residing in sub-Saharan Africa (Wastnedge et al., 2018; Makani et al., 2011). In the United States, around 100,000 individuals, predominantly of African descent, are affected (CDC, 2024). Worldwide, SCA affects over 300, 000 newborns annually (WHO, 2024; Piel et al., 2013). A genetic survey estimates indicate SCD may account for approximately 50%–90% of child mortality in children under the age of five (Grosse et al., 2011). With reduced infectious disease mortality, SCD has become a significant contributor to childhood deaths from non-communicable diseases, making it a global health priority for achieving sustainable development goals (Ware et al., 2017; McGann, 2016). In Ghana, where SCD is highly prevalent, approximately 2% of newborns are diagnosed annually, with many carrying HbAS, HbSS, or HbSC genotypes (Oppong et al., 2020; Ohene-Frempong et al., 2008). The HbC variant, common in West Africa (Weatherall, 2008) is associated with milder forms of SCD complications and provides some protection against Plasmodium falciparum infections (Weatherall, 2008; Rihet et al., 2004; Kreuels et al., 2010; Driss et al., 2011; Iqbal et al., 2016). A review in Ghana’s largest teaching hospital revealed that SCD cases constituted a substantial proportion of the patient population, with many presenting for management of complications (Asare et al., 2018). Children with SCD in malaria-endemic regions are at high risk of complications, including severe anemia, chronic intravascular hemolysis, and painful vaso-occlusive crises (VOC), which can lead to silent cerebral infarcts (Platt, 2005; Hulbert et al., 2011) and Cerebral Malaria (CM) (Santaterra et al., 2020; Schiess et al., 2020).
Recent studies have underscored the high risk of stroke in children with SCA, especially without early intervention and treatment (Hulbert et al., 2011; Verduzco and Nathan, 2009; Chambliss et al., 2021). Limited healthcare access and financial constraints contribute to the high mortality rate among children with SCA in sub-Saharan Africa (Tshilolo et al., 2008). Early diagnosis of SCD is typically achieved through symptomatic presentation or neonatal screening. Thus, there is an urgent need to identify predictive markers for severe and potential risk of complications in these patients.
Recent research has highlighted the need for predictive biomarkers to improve early diagnosis, detection of complications and intervention in SCD (Rees and Gibson, 2012).
Brain-derived Neurotrophic Factor (BDNF), a nerve growth factor, plays a crucial role in neuronal survival, adaptation, and response to ischemic brain injury, with implications for endothelial cells (ECs) survival and neo-angiogenesis in ischemic tissues (Hyacinth et al., 2012; Chen et al., 2013; Bathina and Das, 2015; Kim and Winstein, 2017; Ramaswamy et al., 2009). Proinflammatory cytokines and chemokines, and angiogenic markers like Placental Growth Factor (PIGF) have been linked to SCD severity and complications (Zhang and An, 2007; Hasanvand, 2022; Korobova et al., 2023; Perelman et al., 2003; Gu et al., 2018). These biomarkers are crucial in understanding pathological mechanisms like hemolysis, inflammation, and endothelial dysfunction in SCD (Rees and Gibson, 2012). A detailed role of biomarkers associated with SCD is presented in Table 2. Currently, there is no reliable diagnostic test to measure plasma-free heme levels limiting clinicians' ability to assess heme-induced inflammation and its harmful effects (Immenschuh et al., 2017). The scavenger proteins help reduce the accumulation of cell-free Hb and free heme during excessive hemolysis by sequestering and facilitating their clearance (Balla et al., 2005; Schaer et al., 2013). Identifying robust biomarkers could improve disease outcome prediction, support personalized treatment strategies, and prevent irreversible complications. Early identification of these biomarkers could facilitate the development of personalized treatment strategies and improve patient outcomes (Conran and Belcher, 2018). Therefore, we investigated specific circulatory markers associated with inflammation and brain injury that might serve as indicators of life-threatening complications in children with heme-induced inflammation, such as in SCD. By analyzing these biomarkers across different genotypes, we seek to identify prognostic indicators that can inform clinical management and improve patient outcomes (Brousse et al., 2014). Additionally, this strategy could enhance differentiation between mild and severe SCD, deepen our understanding of its mechanisms, and improve clinical management. To our knowledge, this study is the first to explore circulating factors as both indicators and predictors of SCD progression and complications.
MethodsEthical considerationsThe present study received approval from the ethics boards of the Morehouse School of Medicine (approval number 1404521-9) and the University of Ghana College of Health Sciences (CHS-Et/M.6-P4.8/2021). Parents/guardians of participating children read and signed parental informed consent.
Study subjectsThis study enrolled volunteer children diagnosed with SCD, including HbSS and HbSC genotypes, Sickle cell trait (SCT) with HbAS and HbAC genotypes, and HbCC, along with a control group with normal HbAA. Eligible participants were children aged 3–8 years, confirmed through Hb Electrophoresis testing. Participants were recruited from the Child Health SCD Clinic at the Korle-Bu Teaching Hospital (KBTH), Accra, Ghana, between 2021 and 2022. Control participants were drawn from neighboring communities.
Inclusion criteriaEligible participants were children diagnosed with SCD (HbSS, HbSC), SCT (HbAS, HbAC), HbCC, or HbAA as a control group. All children aged 3–8 years old had their SCD status confirmed through hemoglobin electrophoresis. To ensure that inflammatory markers studied were solely attributed to SCD, only participants who were HIV-negative and not infected with P. falciparum were included in the study.
Exclusion criteriaChildren diagnosed with thalassemia syndromes, other hemoglobinopathies, leukemia, or other cancers were excluded from the study. Participants with no history of sickle cell crises or blood transfusions within the past 3 months were also excluded, as well as those with acute bacterial, viral, or parasitic infections, including P. falciparum. Additionally, individuals undergoing hydroxyurea therapy, children under the age of 2, and those who tested positive for HIV were excluded.
Data collection, site and clinical assessmentA voluntary, in-person questionnaire was administered to gather health information, with a focus on SCD. The questionnaire, available in English and translated into local dialects (Ga, Twi, Ewe, and Hausa) as needed, collected data on recent infections requiring treatment, current pain status, age at diagnosis, frequency of pain episodes, ongoing treatments, and other SCD severity related information. Clinical data from children within the specified age group presenting with SCD, SCT, and other Hb genotypes at the Child Health Department (CHD) of KBTH were extracted using a standardized data abstraction method. This instrument documented clinic attendance/visits, phenotypes, Plasmodium parasitemia, hematological parameters, and complications. KBTH serves as a major pediatric referral center in Greater Accra and includes facilities such as La General Hospital, Princess Marie Louise Children Hospital, Kaneshie Polyclinic, Ussher Polyclinic, and Mamprobi Polyclinic. Additionally, the CHD houses the Sickle Cell Unit, a specialized unit catering to approximately 200 children biweekly, and an emergency unit for children experiencing crisis episodes.
Sample size and selectionA total of 377 samples were collected between July 2021 and July 2022 as part of NIH/NINDS R01NS091616 (Stiles, PI) and NIH/FIC UJMT Fogarty Global Health Fellows Program #D43TW009340 (Chi PI; Lekpor, Fellow), and 1K01TW010282 (Driss, PI)-funded projects focusing on severe malaria at Morehouse School of Medicine (MSM), Atlanta, GA, USA, in collaboration with the Department of Pathology, University of Ghana Medical School. From this pool, we randomly selected 80 age- and sex-matched participants representing all hemoglobin (Hb) genotype groups. This subset consisted of 16 individuals, each with HbAA, HbSS, and HbSC, 14 individuals, each with HbAS and HbAC, and 4 with HbCC, all drawn from the Greater Accra region. The participants were selected using convenience sampling, which may introduce selection bias and may limit the representativeness of the larger population.
Blood sample collection and processingBlood samples were collected into sodium citrate tubes (Cat # 454322, Bio-ONE, United States), and plasma was isolated using SepMate™ tubes (STEM Cell Technologies, Cat# 854115) and processed within 4 h of collection. The isolated plasma samples were stored at −80°C and later thawed gradually at room temperature before analysis, as recommended by other groups (Grievink et al., 2016).
Hemoglobin status determinationHemoglobin status was determined using cellulose acetate membrane electrophoresis, following method described by Ngwengi et al. (Ngwengi et al., 2020). This analysis was conducted in the Hematology Department of Korle-Bu Teaching Hospital. Complete blood count (CBC) was performed on whole blood samples using an ABX Micros ES 60, an 18-parameter hematology analyzer, also within the Department of Hematology of the Korle Bu Teaching Hospital.
Malaria and HIV testingMalaria infection status was assessed using Rapid Diagnostic Test (RDTs) Kits (First Response® Malaria Ag. pLDH/HRP2 Combo Card Test kit (WHO reference number: PQDx0285-010-00, PI16FRC25), which detects Plasmodium falciparum-specific HRP2 and Pan lactate dehydrogenase (LDH) to identify multiple malaria species. This test was done to exclude malaria-positive participants. HIV status was determined using First Response® HIV-1–2 rapid diagnostic test (RDT) kits (Cat# PI05FRC30) to exclude HIV-positive participants.
Multiplexed immunoassay measurementThe plasma levels of biomarkers were quantified using the MSD Multi-Spot Assay System MESO Scale QuickPlex® (SQ 120, MSD, Maryland, United States). This system was chosen for its sensitivity and specificity in measuring multiple cytokines simultaneously. The cytokine analysis was performed on customized U-plex plates and V-plex with undiluted plasma samples, adhering strictly to the manufacturer’s protocol. A comprehensive range of cytokines was targeted using the U-PLEX Development Pack (MSD® k15231N) and V-PLEX Plus Neuroinflammation Panel 1 (Meso-Scale Diagnostics Cat#: K151ACM-1). The U-PLEX enabled the complexing of selected biomarkers of interest, including Angiopoietin 1 (Ang 1), Angiopoietin 2 (Ang 2), Brain-Derived Neurotrophic Factor (BDNF), C-X-C motif chemokine ligand 10, CXCL10. It is also known as Interferon Gamma-Induced Protein 10 (IP-10), Interleukin-1 alpha (IL-1α), Interleukin-6 (IL-6), Heme Oxygenase-1, Haptoglobin, Hemopexin (HO-1, Hp, Hpx). The V-PLEX panel allowed for the simultaneous measurement of 37 cytokines, including C-Reactive Protein (CRP), Eotaxin, Eotaxin-3, Fibroblast Growth Factor (basic FGF), Intercellular Adhesion Molecule 1 (ICAM-1), Interferon Gamma (IFN-γ), a variety of interleukins (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12/IL-23p40, IL-13, IL-15, IL-16, and IL-17A), CXCL10, Monocyte Chemoattractant Protein-1 (MCP-1), MCP-4, Macrophage-Derived Chemokine (MDC), Macrophage Inflammatory Protein-1 alpha (MIP-1α), MIP-1β, Placental Growth Factor (PlGF), Serum Amyloid A (SAA), Thymus and Activation-Regulated Chemokine (TARC), Tie-2, Tumor Necrosis Factor Alpha (TNF-α), TNF-β, Vascular Cell Adhesion Molecule 1 (VCAM-1), Vascular Endothelial Growth Factor A (VEGF-A), VEGF-C, VEGF-D, and VEGF Receptor 1 (VEGFR-1/Flt-1). Plasma heme levels were quantified in triplicates using 25 μL aliquots of each sample. A colorimetric assay (QuantiChrom™ Heme Assay Kit Cat# DIHM-250, BioAssay Systems, USA) was employed, and the assays were conducted at the MSM Core Lab. This approach was selected to represent a diverse range of high and low heme levels.
Statistical analysisThe D'Agostino & Pearson normality test determined the data’s distribution. For normally distributed data, differences between two groups were evaluated using an unpaired two-tailed Student’s t-test. For data not following a normal distribution, the nonparametric Mann-Whitney U test was used for two-group comparisons (as detailed in Table S3). Statistical analyses were conducted using GraphPad PRISM version 9.5.1 for Windows 10 (GraphPad Software, La Jolla, California). Plasma biomarker concentrations were presented as mean ± standard deviation (SD). One-way ANOVA tests, supplemented by Tukey’s multiple comparison tests, were used to compare biomarker levels across different Hb subgroups and complete blood count parameters. Student's t-tests were employed to analyze differences in mean values for two-group comparisons. This approach allowed for the assessment of specific differences between groups. The study was powered based on preliminary multiplex immunoassay data from a subset of samples. Assuming a minimum detectable difference of 2 standard deviations between group means, a sample size of 10 per group was calculated to provide a minimum of 90% power at a 95% confidence level. Correlation analyses were performed using Pearson or Spearman coefficients, as appropriate. A p-value threshold of <0.05 was set for statistical significance, and exact p-values were reported. Cytokines, chemokines, growth factors and heme scavenger analyte concentrations outside the linear detection range of the immunoassays were excluded from the analysis.
Predictive analysis using cytokine ratio calculationsThe ratios of circulating biomarker levels across different Hb variants were calculated to identify distinct biomarker profile changes associated with Hb sickle status. Heme levels were measured and divided by the cytokine of interest for the heme-to-cytokine ratio to assess whether cell-free heme levels impact inflammation. Similarly, the circulating biomarker-to-scavenger ratio was used to explore how cytoprotective proteins might modulate inflammation. Additionally, ratios of two distinct inflammatory markers were analyzed to evaluate how specific interactions vary across different genotypes. By integrating both heme and other biomarkers, this approach facilitates the development of predictive algorithms to estimate the risk of severe complications due to hemolysis based on the cumulative effects of diverse inflammatory mediators associated with different genotypes.
Receiver operating characteristics (ROC)ROC curves were generated to assess the discriminative power of biomarker tests between different study groups. The Area Under the ROC Curve (AUC) was used to evaluate the performance of the diagnostic tests, focusing on their specificity and sensitivity in distinguishing between the groups. An AUC value closer to one indicates a higher discriminatory ability, implying that the test results more effectively separate the distributions of the analyzed groups. The ROC analysis determined cutoff values that optimized sensitivity and specificity. The curves and the associated AUC metrics quantified the overall accuracy and validity of the experimental tests in categorizing individuals based on their biomarker profiles, which indicate their specific disease or Hb sickle state (Harp et al., 2020). A p-value of <0.05 was set as the threshold for statistical significance in all tests.
Principal component analysis (PCA)PCA was conducted using Past 4.1.0 software (Hammer and Harper, 2001). This analysis covered biomarkers targets, scavengers, and their respective concentrations, including Ang 1, Ang 2, BDNF, TNF-α, CXCL10, IL-1α, IL-6, CXCL10, CCL11, IFN-γ, IL-10, TNF-α, MDC, MIP-1β, MCP-1, IL-1α, IL-6, IL-16, IL-12p40, VEGFA, VEGF-C, VEGF-D, PlGF, bFGF, Flt-1, Tie 2, HO-1, Hpx Hp, and complete blood count parameters [White Blood Cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hb), and Platelets (PLT)]. For the PCA, although no data was missing, one low value due to measurement units was present and was excluded to ensure a consistent and comprehensive analysis. A correlation matrix analysis evaluated the relationships between the variables across different groups.
ResultsDemographics and hematological findingsSignificant variations in White Blood Cell (WBC) counts were observed among the groups, with the highest levels found in the HbSS group, followed by HbSC, HbAS, HbAC, and HbAA. Specifically, WBC counts were significantly higher in HbSS compared to HbAS (p = 0.0001), HbAA (p = 0.0107), HbAC (p = 0.0178), and HbSC (p = 0.0145). For Red Blood Cell (RBC) levels, the HbSS group had significantly lower levels compared to all other groups, with HbSS being lower than HbAA, HbAS, HbAC, HbSC, and HbCC (p < 0.0001 for all comparisons). Additionally, significant differences in RBC counts were observed between HbAA and HbSC (p = 0.0375), and between HbSC and HbCC (p = 0.0156) and Platelet (PLT) counts differed significantly in HbSS compared to other Hb genotypes (p < 0.0001) (Table 1; Supplementary Table S1).
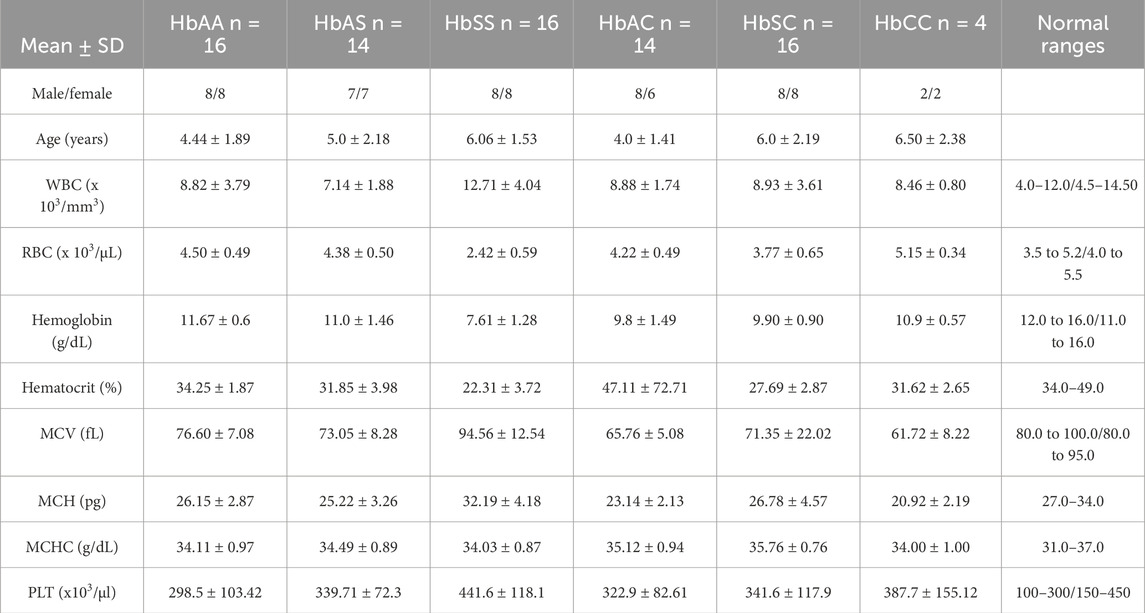
Table 1. Mean and standard deviation of clinical characteristics of all sickle Hb genotypes groups of individuals based defined. There was significant difference in age and gender distribution among the groups.
As expected, there was a significant reduction in Hb levels in the sickle cell group compared with other Hb genotypes. Hb levels significantly differed between HbSS versus the following: HbAA (p < 0.0001), HbAS (p < 0.0001), and HbCC (p = 0.0001). Furthermore, MCV (p < 0.001), levels were higher in the HbSS group compared to the other groups, whilst MCH (p = 0.0045), and MCHC (p < 0.0001) were generally normal across the other Hb groups (Table 1). MCV may be slightly elevated due to the presence of larger reticulocytes but remains within the normal range. This elevation is not related to hydroxyurea use as patients on hydroxyurea were excluded from this study. Supplementary Table S1 provides all other statistically significant hematologic comparisons between Hb subgroups.
Variations of circulating plasma inflammatory markers across different Hb genotypesMean plasma concentrations of various inflammatory markers were analyzed across different subgroups of sickle Hb genotypes to examine potential differences in their profiles associated with specific genotypes (Figure 1; Supplementary Table S2).
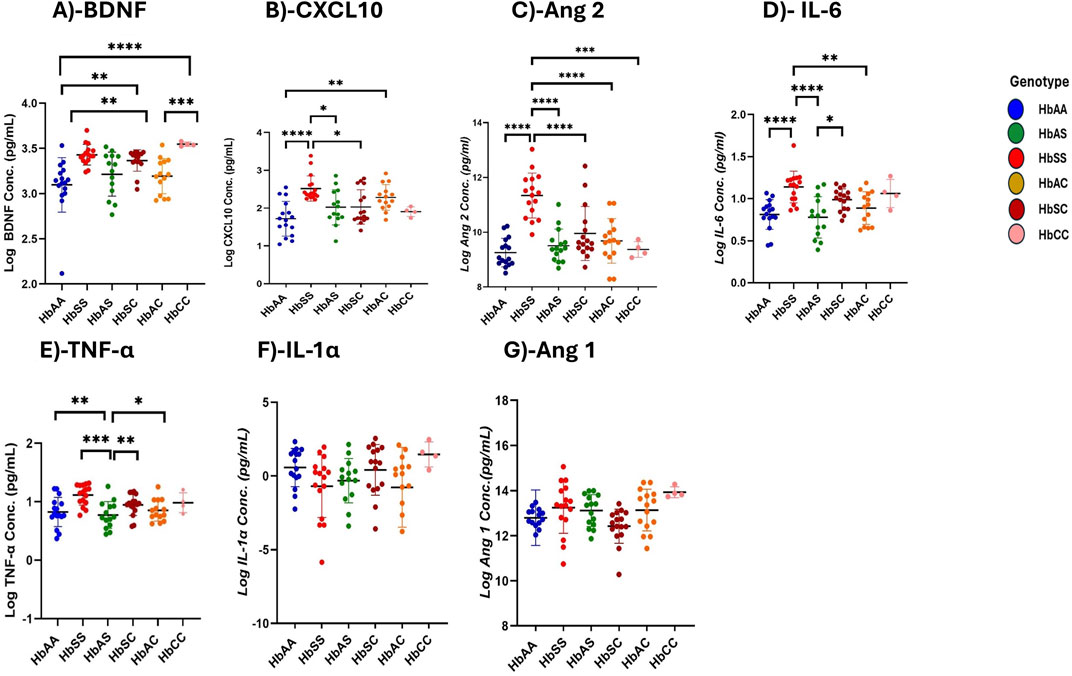
Figure 1. Differential Expression of Inflammatory, Vascular, and Neurotrophic Markers Across Sickle Hemoglobin Genotypes. Multiple comparisons for each inflammatory marker using one-way ANOVA with Turkey’s comparison tests. Scatter plots indicate minimum and maximum mean plasma values observed. The following significant variations were observed across different sickle Hb genotypes: (A) BDNF: HbAA vs. HbSS (p < 0.0001), HbAA vs. HbCC (p < 0.0001), HbSS vs. HbAC (p = 0.0017), HbAC vs. HbCC (p = 0.0005) and HbAA vs. HbSC (p = 0.0069). (B) CXCL10: HbAA vs HbSS (P< 0.0001), HbAA vs HbAC (P = 0.0040) and HbSS vs HbSC (P = 0.0116) and HbSS vs HbAS (P = 0.0142). (C) Ang 2: HbSS vs. HbAA (p < 0.0001), HbSS vs. HbAS (p < 0.0001), HbSS vs. HbAC (p < 0.0001), HbSS vs. HbSC (p < 0.0001) and HbSS vs. HbCC (p = 0.0002). (D) IL-6: HbAA vs. HbSS (p < 0.0001) HbAS vs. HbSS (p < 0.0001), HbSS vs. HbAC (p = 0.0062) and HbAS vs. HbSC (p = 0.0349). (E) TNF-α: HbAA vs. HbSS (p = 0.0011), HbAS vs. HbSS (p = 0.0002) and HbSS vs. HbAC (P0.0017), HbSS vs. HbSC (p = 0.003). (F) Assessment of IL-1α in different sickle Hb genotypes: no significant differences observed. (G) Assessment of Ang 1 in different sickle Hb genotypes: no significant differences observed. Statistical significance are indicated as *p < 0.03–0.01; **p < 0.009–0.001; ***p < 0.0002–0.00019; ****p < 0.0001 ≤ 0.00001.
BDNF levels were significantly higher in individuals with SCD genotypes following the order HbSS > HbCC > HbSC > HbAS > HbAC > HbAA. Significant plasma mean differences were observed between HbAA and HbSS (1,460 ± 716.5 pg/mL vs. 2,816 ± 190.3 pg/mL, p < 0.0001), HbSS and HbAC (2,816 ± 190.3 pg/mL vs. 1707 ± 762.0 pg/mL, p = 0.0017), HbAA and HbCC (1,460 ± 716.5 pg/mL vs. 2,784 ± 795.6 pg/mL, p < 0.0001), HbAC and HbCC (1707 ± 762.0 pg/mL vs. 1,460 ± 716.5 pg/mL, p = 0.0005), and HbAA and HbSC (1,460 ± 716.5 pg/mL vs. 2,390 ± 523.4 pg/mL, p = 0.0069) (Figure 1A). CXCL10 concentrations were highest in the order HbSS > HbAS > HbSC > HbAC > HbAA > HbCC with significant mean plasma levels observed between HbAA and HbSS (1719 ± 458.5 pg/mL vs. 2,518 ± 334.1 pg/mL, p = 0.002), HbAA and HbSC (1719 ± 458.5 pg/mL vs. 2030 ± 454.8 pg/mL, p = 0.0089), HbSS and HbAC (2,518 ± 334.1 pg/mL vs. 2,282 ± 337.3 pg/mL, P0.0361) (Figure 1B). Plasma mean Ang 2 levels were significantly elevated in HbSS than HbAA (1,134 ± 82.1 pg/mL vs. 925.2 ± 52.0 pg/mL, p = 0.002), HbSS and HbAS (1,134 ± 82.1 pg/mL vs. 950.6 ± 60.7 pg/mL, p < 0.0001), HbSS and HbAC (1,134 ± 82.1 pg/mL vs. 968.2 ± 81.3 pg/mL, p < 0.0001), HbSS and HbSC (1,134 ± 82.1 pg/mL vs. 995.2 ± 98.2 pg/mL, p = 0.0089), HbSS and HbCC (1,134 ± 82.1 pg/mL vs. 937.0 ± 28.6 pg/mL, p = 0.0002), showing that HbSS had the highest concentrations across the groups (Figure 1C). IL-6 concentrations varied significantly, with higher levels in HbSS > HbSC > HbAS > HbAC > HbCC > HbAA. Significant plasma mean differences were observed between HbAA and HbSS (811.6 ± 176.3 pg/mL vs. 1,138 ± 188.3 pg/mL, p < 0.0001), HbAS and HbSS (777.3 ± 245.7 pg/mL vs. 1,138 ± 188.3 pg/mL, p < 0.0001), HbSS and HbAC (1,138 ± 188.3 pg/mL vs. 887.3 ± 193.8 pg/mL, p = 0.0062), and HbAS and HbSC (777.3 ± 245.7 pg/mL vs. 987.6 ± 129.3 pg/mL, p = 0.0349), (Figure 1D). Similarly, mean plasma TNF-α levels were highest in the order HbSS > HbSC > HbCC > HbAC > HbAS > HbAA with significant plasma mean levels between HbAA and HbSS (775.0 ± 444.8 pg/mL vs1397 ± 499.0 pg/mL, p = .0011), HbAS and HbSS (679.0 ± 409.4 pg/mL vs. 1,397 ± 499.0 pg/mL, p = .0002), HbSS and HbAC (1,397 ± 499.0 pg/mL vs. 774.6 ± 374.0 pg/mL, p = 0.0017), and HbSS and HbCC (1,397 ± 499.0 pg/mL vs. 979.0 ± 348.8 pg/mL, p = 0.003) (Figure 1E). No significant differences were found in IL-1α and Ang 1 across the different Hb genotypes, indicating comparable levels among all groups (Figures 1F, G). Additionally, there were no significant differences between males and females for all biomarkers.
Children with SCD exhibit elevated heme and HO-1 levels but reduced Hp and Hpx compared to other sickle Hb genotypesHeme and heme scavenger (Hp, Hpx, HO-1) levels were compared across different Hb subgroups. Plasma-free heme levels were highest in the order HbSC > HbSS > HbAS > HbAS > HbCC > HbAA, with significantly higher levels between HbSS and HbAA (1,160 ± 243.9 vs. 523.4 ± 249.0 pg/mL, p < 0.0001), HbSS and HbAS (1,160 ± 243.9 vs. 513.0 ± 230.7 pg/mL, p < 0.0001), HbSS and HbAC (1,160 ± 243.9 vs. 513.9 ± 231.9 pg/mL, p < 0.0001), HbSS and HbCC (1,160 ± 243.9 vs. 406.7 ± 203.3 pg/mL, p = 0.0002) and also higher in HbSC (1,169 ± 171.5 pg/mL) compared to HbAA, HbAS, HbAC, and HbCC (all p < 0.0001) (Figure 2A; Supplementary Table S3). Hp levels showed significant differences across the subgroups, with the highest levels in HbAA > HbAS > HbAS > HbAC > HbCC > HbSC > HbSS. Significant plasma mean differences were observed between HbAA and HbSS (839.4 ± 64.8 vs. 614.6 ± 32.8 pg/mL, p < 0.0001), HbAA and HbSC (839.4 ± 64.8 vs. 489.9 ± 17.5 pg/mL, p < 0.0001), HbAS and HbSC (797.2 ± 90.6 vs489.9 ± 17.5 pg/mL, p < 0.0001), HbAC and HbCC (805.5 ± 86.6 vs. 714.8 ± 74.6 pg/mL, p < 0.0001), HbSS and HbAC (614.6 ± 32.8 vs. 805.5 ± 86.6 pg/mL, p = 0.0007) and HbAS and HbSS (797.2 ± 90.6 vs. 614.6 ± 32.8 pg/mL, p = 0.0013) (Figure 2B). Plasma mean Hpx levels were significantly different only between HbAA and HbSS (796.6 ± 18.6 vs. 728.7 ± 60.6 pg/mL, p = 0.0416) (Figure 2C). HO-1 levels were highest in the order HbSS > HbSC > HbAC > HbAS > HbAA > HbCC, with HbSS (336.1 ± 34.6 pg/mL) showing significantly higher levels compared to all other subgroups (HbAA, HbAS, HbAC, HbSC) (all p < 0.0001) and (HbCC, p = 0.0005) (Figure 2D).
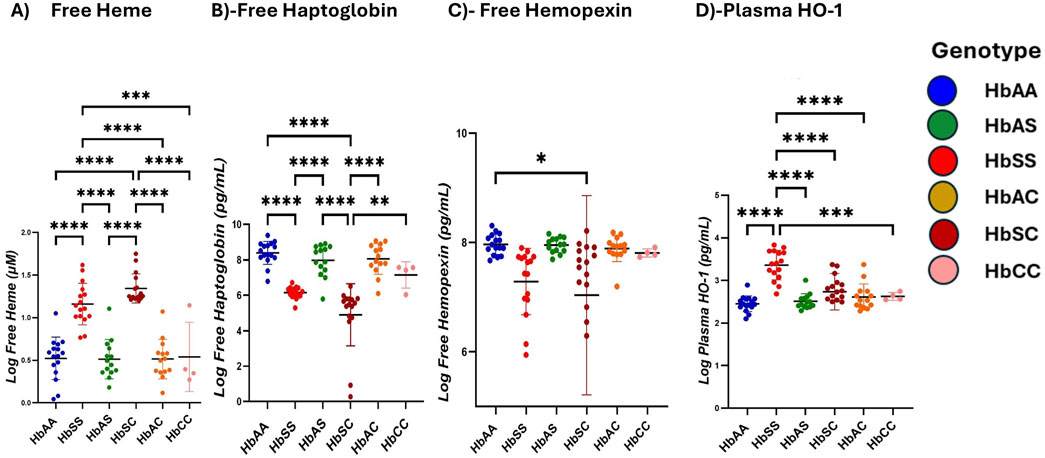
Figure 2. Scavenger proteins differ based on different sickle hemoglobin genotypes. Multiple comparison tests between Heme, heme scavengers, and sickle Hb genotypes using a one-way ANOVA test show significant differences in heme and scavenger protein expression across all sickle Hb genotypes. Scatter plots indicate minimum and maximum mean plasma values. The following assessments were made in: (A) Free heme levels (µM) between HbSS vs. HbAA (p < 0.0001), HbSS vs. HbAS (p < 0.0001), HbSS vs. HbAC (p < 0.0001) and HbSS vs. HbCC (p = 0.0002). Also, in HbAA vs. HbSC (p < 0.0001), (HbAS vs. HbSC (p < 0.0001), HbAS vs. HbAC (p < 0.0001), HbAC vs. HbSC (p < 0.0001) and HbSC vs. HbCC (p < 0.0001). (B) Haptoglobin levels (Pg/mL) in all genotypes: HbSS vs. HbAA (p < 0.0001), HbSC vs. HbAA (p < 0.0001), HbAS vs. HBSC (p < 0.0001), HbAC vs. HbAC (p < 0.0001), HbSS vs. HbAC (p = 0.0007) and HbAS vs. HbSS (p = 0.0013). (C) Hemopexin levels (Pg/mL) in HbAA vs. HbSC (p = 0.0416). (D) Plasma HO-1 levels (Pg/mL): HbSS vs. HbAA (p < 0.0001), HbSS vs. HbAS (p < 0.0001), HbSS vs. HbAC (p < 0.0001), HbSS vs. HbSC (p < 0.0001) and HbSS vs. HbCC (p = 0.0005). Statistical significances are indicated as *p < 0.03–0.01; **p < 0.009–0.001; ***p < 0.0002–0.00019; ****p < 0.0001 ≤ 0.00001.
Heme increased inflammatory marker expression across sickle Hb genotypesRatios of heme, heme scavengers, and biomarker levels were compared across sickle Hb genotypes, including ratios of biomarkers to scavenger proteins. Heme and its scavengers significantly influenced the levels of Ang 1, CXCL10, Ang 2, TNF-α, IL-6, and BDNF across different Hb genotypes, with multiple significant differences observed. Comparisons of biomarker-to-scavenger ratios indicate that scavenger proteins play a critical role in modulating circulatory biomarker expression (Figure 3). Heme: Ang 1 ratio was increased in HbSC > HbSS > HbAA > HbAS > HbAC > HbCC, with significant differences between HbSS and all other groups, and also between HbSC and the other genotypes (Figure 3A). Also, Heme: CXCL10 levels followed the order HbSC > HbSS > HbAS > HbCC > HbAA > HbAC, with significant differences between HbAA and HbSS, HbAA and HbSC, HbSS and HbSC, HbSS and HbAC, HbAS and HbSC, HbSC and HbAC, and HbSC vs. HbCC (Figure 3B).
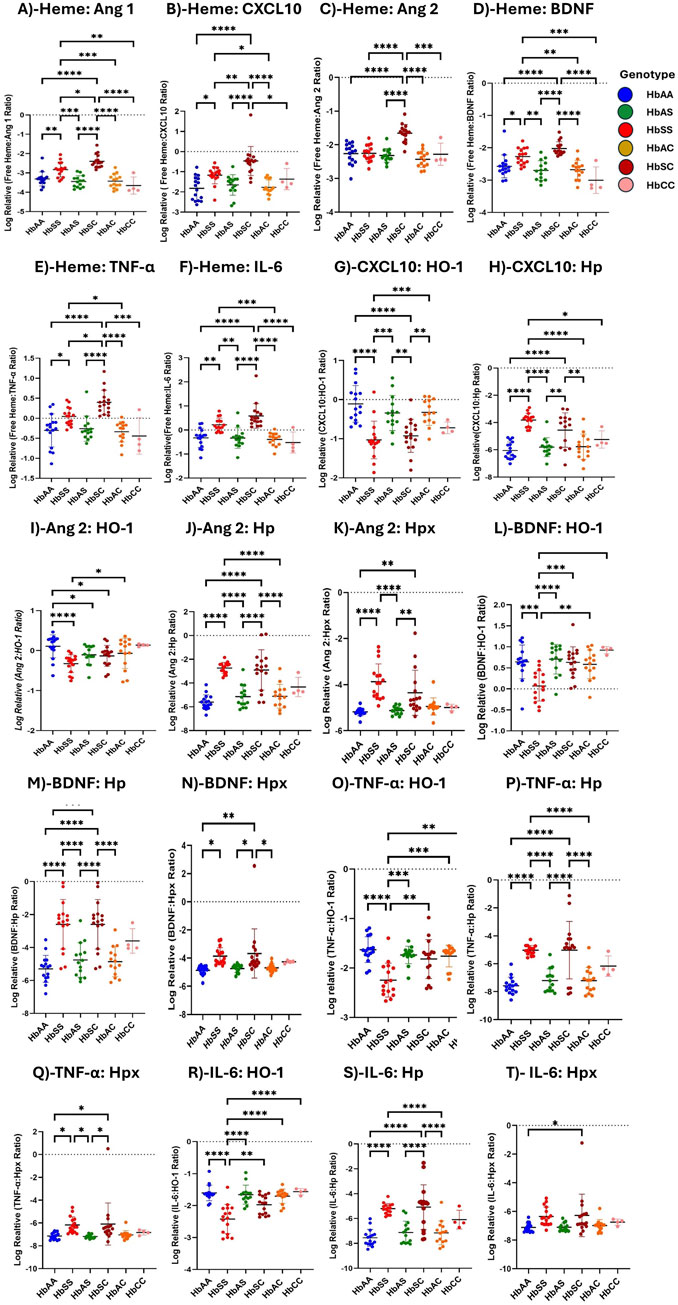
Figure 3. Assessment of Heme, Heme scavengers, and Inflammatory marker ratios across all sickle Hb genotypes using multiple comparison tests. Log-relative heme-to-biomarker ratios were assessed to evaluate heme levels related to inflammatory markers and scavenger protein-to-biomarker ratios were used to evaluate inflammatory markers relative to scavenger proteins. Mean plasma ratios are represented by scatter plots showing maximum and minimum values. Specific mean plasma differences were observed in the following groups: (A) Heme:Ang 1: HbAA vs. HbSS (−3.310 ± 0.363 vs. −2.828 ± 0.380, p = 0.0037); HbAA vs. HbSC (−3.310 ± 0.363 vs. −2.397 ± 0.342, p < 0.0001); HbSS vs. HbAS (−2.828 ± 0.380 vs. −3.447 ± 0.305, p = 0.0001); HbSS vs. HbSC (−2.397 ± 0.342, p = 0.0131); HbSS vs. HbAC (−342.8 ± 36.4, p = 0.0003); HbSS vs. HbCC (−3.653 ± 0.444, p = 0.0013); HbAS vs. HbSC (−3.447 ± 0.305 vs. −2.397 ± 0.342, p < 0.0001); HbSC vs. HbAC (µ-2.397 ± 0.342 vs-342.8 ± 36.4, p < 0.0001); HbSC vs. HbCC (−3.447 ± 0.305 vs-3.653 ± 0.444, p < 0.0001). (B) Heme:CXCL10: HbAA vs. HbSS (−1.8210 ± 5.879 vs. −1.1680 ± 4.243, p = 0.0127); HbAA vs. HbSC (−1.8210 ± 5.879 vs. −0.4640 ± 7.215, p < 0.0001); HbSS vs. HbSC (−1.1680 ± 4.243 vs. −0.4640 ± 7.215, p = 0.0056), HbSS vs. HbAC (−1.1680 ± 4.243 vs. −1.7690 ± 3.629, p = 0.0372); HbAS vs. HbSC (−2.3160 ± 2.032 vs. −0.4640 ± 7.215, p < 0.0001); HbSC vs. HbAC (−0.4640 ± 7.215 vs. −1.7690 ± 3.629, p < 0.0001); HbSC vs. HbCC (−0.4640 ± 7.215 vs. −1.3640 ± 5.272, p = 0.0434). (C) Heme:Ang 2: HbAA vs. HbSC (−2.2620 ± 3.088 vs. 2.023 ± 210.6, p < 0.0001); HbSS vs. HbSC (−2.2530 ± 2.356 vs. −1.6530 ± 2.574, p < 0.0001); HbAS vs. HbSC (−2.702 ± 3.044 vs. −1.6530 ± 2.574, p < 0.0001); HbSC vs. HbAC (−1.6530 ± 2.574 vs. −2.6780 ± 2.899, p < 0.0001); HbSC vs. HbCC (−1.6530 ± 2.574 vs. −2.2810 ± 3.306, p = 0.0006). (D) Heme:BDNF: HbAA vs. HbSS (−2.5730 ± 3.555 vs. −2.2700 ± 2.503, p = 0.0489); HbAA vs. HbSC (−2.5730 ± 3.555 vs. −2.023 ± 210.6, p < 0.0001); HbSS vs. HbAS (−2.2700 ± 2.503 vs. −2.702 ± 3.044, p = 0.0017); HbSS vs. HbAC (−2.2700 ± 2.503 vs. −2.6780 ± 2.899, p = 0.0036); HbAS vs. HbSC (−2.702 ± 3.044 vs. −2.023 ± 210.6, p < 0.0001); HbSC vs. HbAC (−2.023 ± 210.6 vs. −2.6780 ± 2.899 p < 0.0001); HbSC vs. HbCC (−2.023 ± 210.6 vs. −3.0060 ± 4.114, p < 0.0001). (E) Heme:TNF-α: HbAA vs. HbSS (−0.300 ± 0.414 vs. 0.2208 ± 0.273, p = 0.0334); HbAA vs. HbSC (−0.300 ± 0.414 vs. 0.3986 ± 0.304, p > 0.0001); HbSS vs. HbSC (0.2208 ± 0.273 vs. 0.3986 ± 0.304, p = .00276); HbSS vs. HbAC (0.2208 ± 0.273 vs. −0.3890 ± 0.271, p = 0.0184); HbAS vs. HbSC (−0.3365 ± 0.424 vs. 0.3986 ± 0.304, p < 0.0001); HbSC vs. HbAC (0.3986 ± 0.304 vs-0.3890 ± 0.271, p < 0.0001); HbSC vs. HbCC (0.3986 ± 0.304 vs. µ = −0.4419 ± 0.311, p = 0.0001). (F) Heme:IL-6: HbAA vs. HbSS (−0.3256 ± 0.394 vs. 0.2208 ± 0.273, p = 0.0023); HbAA vs. HbSC (−0.3256 ± 0.394 vs. 0.5846 ± 0.515, p < 0.0001); HbSS vs. HbAS (0.2208 ± 0.273 vs. −0.3365 ± 0.424, p = 0.0028); HbSS vs. HbAC (0.2208 ± 0.273 vs. 0.2208 ± 0.273, p = 0.0008); HbAS vs. HbSC (−0.3365 ± 0.424 vs. 0.5846 ± 0.515, p < 0.0001); HbSC vs. HbAC (0.5846 ± 0.515 vs. 0.2208 ± 0.273, p < 0.0001); HbSC vs. HbCC (0.5846 ± 0.515 vs. −0.5202 ± 0.435, p < 0.0001). (G) CXCL10:HO-1: HbAA vs. HbSS (0.1090 ± 0.467 vs. 1.032 ± 0.480, p < 0.0001); HbAA vs. HbSC (0.1090 ± 0.467 vs. −0.9271 ± 0.421, p < 0.0001); HbSS vs. HbAS (1.032 ± 0.480 vs. −0.3424 ± 0.449, p = 0.0005); HbSS vs. HbAC (1.032 ± 0.480 vs. −0.3271 ± 0.333, p = 0.0003); HbAS vs. HbSC (−0.3424 ± 0.449 vs. −0.9271 ± 0.421, p = 0.0048); HbSC vs. HbAC (−0.9271 ± 0.421 vs. −0.3271 ± 0.333, p = 0.0035). (H) CXCL10:Hp: HbAA vs. HbSS (−6.050 ± 0.622 vs. −3.818 ± 0.502, P= <0.0001); HbAA vs. HbSC (−6.050 ± 0.622 vs. −4.551 ± 1.262, p < 0.0001); HbSS vs. HbAS (−3.818 ± 0.502 vs. −5.806 ± 0.695, p = 0.0006); HbSS vs. HbAC (−3.818 ± 0.502 vs. −5.772 ± 0.927, p = 0.0008); HbAS vs. HbSC (−5.806 ± 0.695 vs. −4.551 ± 1.262, p = 0.0004); HbSC vs. HbAC (−4.551 ± 1.262 vs. −5.772 ± 0.927, p = 0.0006). (I) Ang 2: HO-1: HbAA vs. HbSS (0.332 ± 0.219 vs. 0.052 ± 0.371, p < 0.0001); HbAA vs. HbAS (0.332 ± 0.219 vs. 0.321 ± 0.157, p = 0.0153); HbAA vs. HbSC (0.332 ± 0.219 vs. 0.261 ± 0.547, p = 0.0101); and HbSS vs. HbAC (0.052 ± 0.371 vs. 0.334 ± 0.301, p = 0.0101). (J) Ang 2:Hp: HbAA vs. HbSS (−5.609 ± 0.617 vs. −2.733 ± 0.469, p < 0.0001); HbAA vs. HbSC (−5.609 ± 0.617 vs. −2.901 ± 1.700 p < 0.0001); HbSS vs. HbAS (−2.733 ± 0.469 vs. −5.143 ± 0.946, p < 0.0001); HbSS vs. HbAC (−2.733 ± 0.469 vs. −5.111 ± 1.004, p < 0.0001); HbAS vs. HbSC (−5.143 ± 0.946 vs. −2.901 ± 1.700, p < 0.0001); HbSC vs. HbAC (−2.901 ± 1.700 vs. −5.111 ± 1.004, p < 0.0001). (K) Ang 2:Hpx: HbAA vs. HbSS (−5.180 ± 0.168 vs. −3.874 ± 0.778, p = 0.0061); HbAA vs. HbSC (−5.180 ± 0.168 vs. −4.354 ± 0.977, p = 0.0244); HbSS vs. HbAS (−3.874 ± 0.778 vs. −5.12 ± 0.171, p = 0.0141); HbAS vs. HbSC (−5.12 ± 0.171 vs. −4.354 ± 0.977, p = 0.0493). (L) BDNF:HO-1: HbAA vs. HbSS (0.643 ± 0.399 vs. 0.069 ± 0.359, p = 0.0003); HbSS vs. HbAS (0.069 ± 0.359 vs. 0.706 ± 0.352, p < 0.0001); HbSS vs. HbSC (0.069 ± 0.359 vs. 0.631 ± 0.370, p = 0.0004); HbSS vs. HbAC (0.069 ± 0.359 vs. 0.582 ± 0.339, p = 0.0027); HbSS vs. HbCC (0.069 ± 0.359 vs. 0.920 ± 0.084, p = 0.0009). (M) BDNF:Hp: HbAA vs. HbSS (5.298 ± 0.818 vs. −2.594 ± 1.503, p < 0.0001); HbAA vs. HbSC (−5.298 ± 0.818 vs. −2.594 ± 1.503, p < 0.0001); HbSS vs. HbAS (−2.594 ± 1.503 vs. −4.757 ± 1.048, p = 0.0007); HbSS vs. HbAC (−2.594 ± 1.503 vs. = -4.862 ± 0.905, p = 0.0004); HbAS vs. HbSC (−4.757 ± 1.048 vs. −2.594 ± 1.503, p = 0.0007); HbSC vs. HbAC (−2.594 ± 1.503 vs. −4.862 ± 0.905, p = 0.0004). (N) BDNF:Hpx: HbAA vs. HbSS (−4.870 ± 0.323 vs. −3.857 ± 0.599, p = 0.0163); HbAA vs. HbSC (−4.870 ± 0.323 vs. −3.671 ± 1.742, p = 0.0024); HbAS vs. HbSC (−1.736 ± 0.177 vs. −3.671 ± 1.742, p = 0.0136); HbSC vs. HbAC (−3.671 ± 1.742 vs. −4.697 ± 0.298, p = 0.0200). (O) TNF-α:HO-1: HbAA vs. HbSS (−1.630 ± 0.260 vs. −2.246 ± 0.342, p < 0.0001); HbSS vs. HbAS (−2.246 ± 0.342 vs. −1.736 ± 0.177, p = 0.0004); HbSS vs. HbSC (−2.246 ± 0.342 vs. −1.819 ± 0.392p = 0.0012); HbSS vs. HbAC (−2.246 ± 0.342 vs. −1.759 ± 0.219, p = 0.0007); HbSS vs. HbCC (−2.246 ± 0.342 vs. −1.643 ± 0.094, p = 0.0107). (P) TNF-α:Hp: HbAA vs. HbSS (−7.570 ± 0.610 vs. −5.031 ± 0.345, p < 0.0001); HbAA vs. HbSC (−7.570 ± 0.610 vs. −5.024 ± 2.058, P < p < 0.0001); HbSS vs. HbAS (−5.031 ± 0.345 vs. −7.200 ± 0.830, p < 0.0001); HbSS vs. HbAC (−5.031 ± 0.345 vs. −7.204 ± 0.942, p < 0.0001); HbAS vs. HbSC (−7.200 ± 0.830 vs. −5.024 ± 2.058, p < 0.0001); HbSC vs. HbAC (−5.024 ± 2.058 vs. −7.204 ± 0.942, p < 0.0001); (Q) TNF-α;Hpx: HbAA vs. HbSS (−7.142 ± 0.298 vs. −6.172 ± 0.711, p < 0.042); HbAA vs. HbSC (−7.142 ± 0.298 vs. −6.192 ± 1.843, p < 0.021); HbSS vs. HbAS (−6.172 ± 0.711 vs-7.181 ± 0.160, p < 0.021); HbAS vs. HbSC (−7.181 ± 0.160 vs. −6.192 ± 1.843, p < 0.021). (R) IL-6:HO-1: HbAA vs. HbSS (=−1.604 ± 0.244 vs. −2.421 ± 0.455, p < 0.0001); HbSS vs. HbAS (−2.421 ± 0.455 vs. −1.659 ± 0.297, p < 0.0001); HbSS vs. HbSC (−2.421 ± 0.455 vs. −1.976 ± 0.295, p = 0.0017); HbSS vs. HbAC (−2.421 ± 0.455 vs. −1.707 ± 0.223, p < 0.0001); HbSS vs. HbCC (−2.421 ± 0.455 vs. −1.565 ± 0.097, p < 0.0001); (S) IL-6:Hp: HbAA vs. HbSS (−7.545 ± 0.674 vs. −5.207 ± 0.426, p < 0.0001); HbAA vs. HbSC (−7.545 ± 0.674 vs. −4.828 ± 1.792, p < 0.0001); HbSS vs. HbAC (−5.207 ± 0.426 vs. −7.152 ± 0.963, p = 0.0009); HbAS vs. HbSC (−7.122 ± 0.907 vs. −4.828 ± 1.792, p < 0.0001); HbSC vs. HbAC (−4.828 ± 1.792 vs. −7.152 ± 0.963, p < 0.0001); (T) IL-6:Hpx: HbAA vs. HbSC (−7.117 ± 0.303 vs. −6.278 ± 1, p = 0.035). Statistical significance are indicated as *: p < 0.03–0.01; **: p < 0.009–0.001; ***: p < 0.0002–0.00019; ****: p < 0.0001 ≤ 0.00001.
Heme: Ang 2 showed significant variations, with higher levels in HbSC > HbSS > HbAS > HbAC > HbAA > HbAC. Significant differences were observed between HbAA vs. HbSC, HbSS vs. HbSC, HbAS vs. HbSC, HbSC vs. HbAC, and HbSC vs. HbCC (Figure 3C). Furthermore, Heme: BDNF levels were highest in the order HbSC > HbSS > HbAS > HbAC > HbAA > HbCC, with significant differences between several groups, including HbAA vs. HbSS, HbAA vs. HbSC, HbSS vs. HbAS, HbSS vs. HbAC, HbAS vs. HbSC, HbSC vs. HbAC, and HbSC vs. HbCC (Figure 3D).
Heme: TNF-α and Heme: IL-6 ratios showed significant differences across multiple comparisons, with HbSC consistently having the highest levels, followed by HbSS, both significantly higher than those of other groups (Figures 3E, F).
Consequently, with biomarker-scavenger-ratio, CXCL10: HO-1 and CXL10: Hp ratios significantly differed in HbSS, HbSC, HbAA, HbAS, HbAC, and HbCC. In the CXCL10: HO-1 ratio, HbAA showed a higher ratio compared to all other genotypes in the order HbAA > HbAS > HbAC > HbSC > HbSS > HbCC, whereas the CXL10: Hp ratio was significantly higher in HbSS > HbSC > HbAS > HbAC > HbCC > HbAA (Figures 3G, H). Ang 2: HO-1 ratio was altered following the order HbAA > HbAC > HbAS > HbSC > HbCC > HbSS. However, Ang 2:Hp and Ang 2:Hpx ratios indicated elevated levels in HbSC and HbSS, respectively, compared to the other Hb genotypes, with significant differences across groups, including HbAA vs. HbSS, HbAA vs. HbSC, and HbSS vs. HbAC (Figures 3I–K). Further, BDNF: Hp and BDNF: Hpx ratios showed variations across genotypes, with the highest ratios in HbSS and HbSC and lower levels in HbAA, HbAS, HbAC, and HbCC, with significant differences between several comparisons. However, BDNF: HO-1 showed higher levels in the order HbAA > HbAS > HbAC > HbCC > HbSC > HbSS (Figures 3L–N). Additionally, TNF-α: HO-1, TNF-α: Hp, and TNF-α:Hpx ratios were significantly higher in HbSC and HbSS compared to HbAS, HbSC and HbAA groups in all comparisons, indicating a strong inflammatory response (Figures 3O–Q). Furthermore, IL-6: Hp and IL-6: Hpx levels showed a pattern of HbSC > HbSS > HbAS > HbAA, with significant alterations observed between several groups. IL-6: HO-1, however, showed a decreased ratio in HbSS and HbSC compared to HbAA, HbAS, HbAC, and HbCC (Figures 3R–T).
Ratios of circulating inflammatory markers differ among children with different sickle Hb genotypesUsing multiple comparison tests, individual biomarker ratios between each Hb genotype were evaluated. Log-transformed relative inflammatory marker ratio values were analyzed to assess the altered interactions between inflammatory markers across sickle Hb genotypes, specifically evaluating the potential of CXCL10, BDNF, Ang 2, and IL-6 to predict the risk of complications in HbSS individuals compared to healthy controls. The ratios were compared across different Hb genotypes (Figure 4). For the CXCL10:Ang 2 ratio, significant differences were observed between HbAA and HbSC, with HbAA > HbSC (Figure 4A). CXCL10:TNF-α ratio shows significant differences between HbAA and HbSC, and between HbAS and HbSC, in the order HbAA > HbAS > HbAC > HbSC > HbSS > HbCC (Figure 4B). Additionally, for the CXCL10:BDNF ratio, significant differences were noted between HbAA and HbSC, as well as between HbSC and HbAC, with the ratios following the order HbAA > HbAS > HbAC > HbSS > HbSC > HbCC (Figure 4C). In the Ang 2:Ang 1 ratio, significant differences were observed in HbSS compared to HbAA, HbAS, HbAC, and HbCC, with significantly higher ratios following the order HbSC > HbSS > HbAC > HbAS > HbAA > HbCC (Figure 4D). Further, the IL-6:BDNF ratio showed significant differences between HbAA and HbSC, with higher ratios in the order HbAA > HbAS > HbAC > HbSS > HbSC > HbCC (Figure 4E). BDNF:Ang 1 ratio showed significant differences between HbAA and HbSC, HbAS and HbSC, and HbSC and HbAC, with higher levels in the order HbSS > HbSC > HbAC > HbAS > HbCC > HbAA (Figure 4F). For the Ang 2:BDNF ratio, significant differences were found between HbSS and HbAS, as well as between HbSS and both HbSC and HbCC, with higher levels in the order HbSS > HbSC > HbA > HbAC > HbA > HbCC (Figure 4G). The observed differences in biomarker ratios such as Ang:2 Ang 1, BNDF:Ang 1 and Ang 2:BDNF across the sickle Hb genotypes with HbSC often displaying higher biomarker ratios than HbSS, HbAA, HbAS, HbAC and HbCC suggests that HbSC patients may also experience significant vascular issues despite their overall clinical presentation being less severe than HbSS individuals. This may imply that monitoring these biomarker ratios could provide valuable insight into the risk of complications, enabling early intervention to prevent severe inflammatory responses.
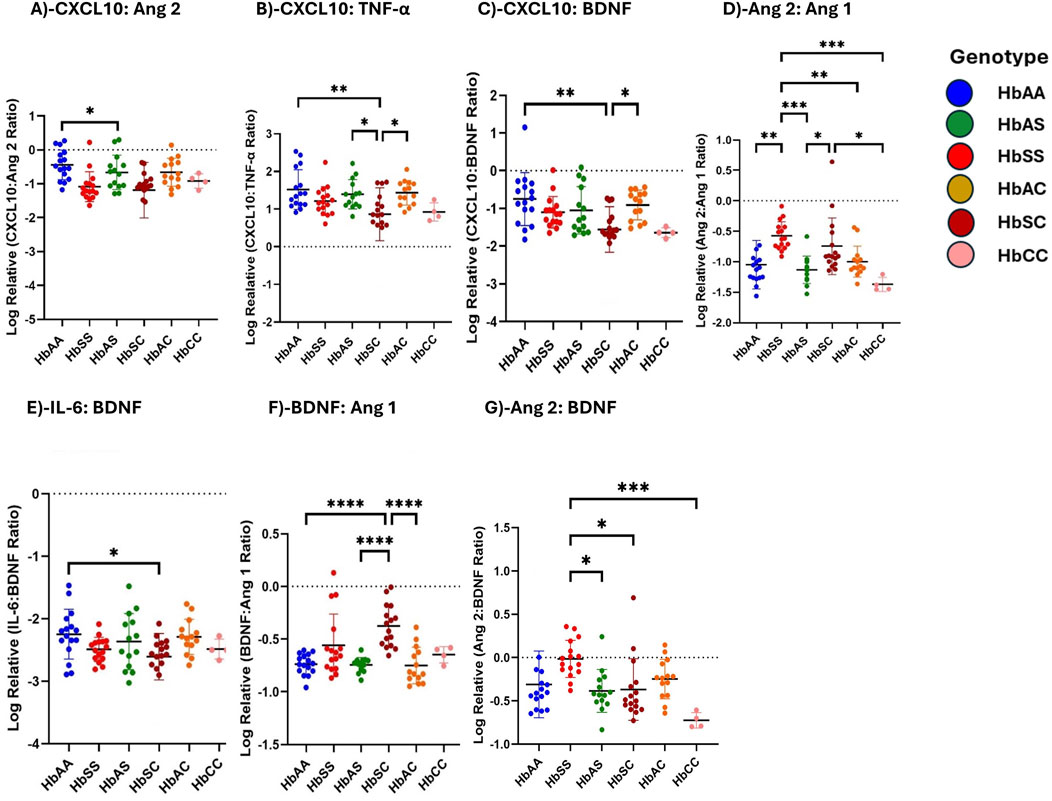
Figure 4. Ratios of Circulating Inflammatory Markers Differ Across Different Sickle Hb Genotypes. Assessment of inflammatory marker concentration ratios across different sickle Hb genotypes. Multiple comparisons test was used to evaluate individual biomarker ratios between each Hb genotype. The mean plasma levels of log-transformed relative individual biomarker ratios were analyzed to determine varied interactions between inflammatory markers across the sickle Hb genotypes. The following mean plasma ratios differ significantly across sickle Hb genotypes: (A) CXCL10:Ang 2: HbAA vs. HbSC (−0.441 ± 0.443 vs. −1.189 ± 0.817 p = 0.0461). (B) CXCL10:TNF-α: HbAA vs. HbSC (1.521 ± 0.524 vs. 0.863 ± 0.704, p = 0.0033), HbAS vs. HbSC (1.394 ± 0.391vs 0.863 ± 0.704, p = 0.0414), HbSC vs. HbAC (0.863 ± 0.704 vs. 1.432 ± 0.323, p = 0.0230). (C) CXCL10:BDNF: HbAA vs. HbSC (−0.752 ± 0.704 vs. −1.558 ± 0.603, p = 0.0014), HbSC vs. HbAC (−1.558 ± 0.603 vs. −0.909 ± 0.393, p = 0.0240). (D) Ang 2:Ang 1: HbAA vs. HbSS (−1.048 ± 0.400 vs. −0.5747 ± 0.230, p = 0.0016), HbSS vs. HbAS, (−0.5747 ± 0.230 vs. −1.130 ± 0.224, p = 0.0002), HbSS vs. HbAC (−0.5747 ± 0.230 vs. −0.998 ± 0.256, p = 0.0093), HbSS vs. HbCC (−0.5747 ± 0.230 vs. −1.372 ± 0.116, p = 0.0006), HbAS vs. HbSC (−1.130 ± 0.224 vs. −0.744 ± 0.465, p = 0.023), HbSC vs. HbCC (−0.744 ± 0.465 vs. −1.372 ± 0.116, p = 0.0127). (E) IL-6:BDNF: HbAA vs. HbSC (−2.247 ± 0.397 vs. −2.607 ± 0.372, p = 0.0465). (F) BDNF:Ang 1: HbAA vs. HbSC (−0.737 ± 0.098 vs. −0.375 ± 0.199, p < 0.0001) HbAS vs. HbSC (−0.744 ± 0.069 vs. −0.375 ± 0.199, p < 0.0001) HbSC vs. HbAC (−0.375 ± 0.199 vs. −0.749 ± 0.173, p < 0.0001). (G) Ang 2:BDNF: HbSS vs. HbAS (−0.016 ± 0.214 vs. −0.385 ± 0.246, p = 0.0115) HbSS vs. HbSC (−0.016 ± 0.214 vs. −0.369 ± 0.355, p = 0.0127), HbSS vs. HbCC (−0.016 ± 0.214 vs-0.725 ± 0.089, p = 0.0006). Statistical significances are indicated as *p < 0.03–0.01; **p < 0.009–0.001; ***p < 0.0002–0.00019; ****p < 0.0001 ≤ 0.00001.
Heme and scavenger ratios differ across children with different sickle Hb genotypesThe ratios of heme and scavenger (HO-1, Hp, Hpx) plasma concentrations were analyzed to evaluate the interactions between heme and its scavengers across different sickle Hb genotypes. Supplementary Table S4; Supplementary Figure S1 illustrate the heme-to-scavenger ratios, showing significant variations across the Hb genotypes. Heme: HO-1 ratio showed significant differences between HbAA and HbSC (p = 0.0003), HbSS and HbSC (p < 0.0001), HbAS and HbSC (p = 0.0004), as well as HbSC and HbAC (p < 0.0001), HbSC and HbCC (p = 0.0062). HbSC showed higher heme relative to HO-1 compared to the other genotypes (Supplementary Figure S1A). For Heme: Hp ratio, significant differences were found between HbSS and HbAA, HbSS and HbAS, HbSS and HbAC, as well as between HbAA and HbSC, HbSC and HbAC (all p < 0.0001). HbSS and HbSC exhibited higher ratios, indicating reduced scavenging capacity of Hp in these genotypes (Supplementary Figure S1B). Additionally, in the Heme: Hpx ratio, significant differences occurred between HbAA and HbSS (p = 0.0031), HbAA and HbSC (p < 0.0001), HbSS and HbAS (p = 0.0048), HbSS and HbAC (p = 0.0086), HbAS and HbSC (p < 0.0001), HbSC and HbAC (p = 0.0001), and HbSC and HbCC (p = 0.0049) (Supplementary Figure S1C). HbSS and HbSC had higher heme relative to Hpx, reflecting the increased heme burden in these genotypes. The HO-1: Hpx and HO-1: Hp ratios showed significant differences between HbSS and HbAA (p < 0.0001), HbSC and HbAA (p = 0.0019), HbSC and HbAS (p = 0.0060), HbSC and HbAC (p = 0.0289), and between HbSS and both HbAA and HbCC, HbSC and both HbAS and HbAC (all p < 0.0001) respectively. HbSC and HbSS had higher ratios than other genotypes, indicating an increased oxidative stress response with lower Hp and Hpx availability (Supplementary Figure S1D–E). Hp:Hpx ratio showed increases in the following order: HbAA > HbAC > HbAS > HbSC > HbSS > HbCC, with significant increases in HbAA and HbSS (p = 0.0031), HbAA and HbAC (p = 0.0231) and HbSC and HbCC (p = 0.0219) also indicating reduced scavenging proteins in the SCD group compared to the other genotypes (Supplementary Figure S1F).
ROC curve analysis of circulating CXCL10, BDNF, Ang 1, Ang 2, IL-6, and TNF-α levels across different sickle Hb genotypesReceiver Operating Characteristic (ROC) curve analysis was conducted to assess the effectiveness of circulating CXCL10, BDNF, Ang 1, Ang 2, IL-6, and TNF-α as potential biomarkers for distinguishing between different sickle Hb genotypes. The Area Under the Curve (AUC) values quantified each biomarker’s ability to differentiate between sickle Hb subgroups based on distinct biomarker profiles, with AUC values between 0.8 and 1.0 indicating strong discriminatory power. CXCL10 effectively discriminated between HbAA vs. HbSC (AUC = 0.98), HbAA vs. HbSS (AUC = 1.0), and HbAA vs. HbAS (AUC = 0.96) (Figures 5A, B). BDNF showed good discriminatory ability between HbAA vs. HbSS (AUC = 0.93), HbAA vs. HbSC (AUC = 0.86), and HbSC vs. HbCC (AUC = 1.0) (Figures 5C–E). Ang 2 also demonstrated a strong separation between HbAA vs. HbSS (AUC = 0.99) (Figure 5F). IL-6 discriminated between HbAA vs. HbSS with an AUC of 0.92 (Figure 5G), while TNF-α showed moderate discriminatory power between HbAA vs. HbSS (AUC = 0.84) (Figure 5H). CXCL10, BDNF, Ang-2, and IL-6 showed high AUC values (≥0.90), demonstrating strong discriminatory power between Hb genotypes, especially for HbSS, HbSC, and HbAA. These markers could serve as effective biomarkers for assessing disease complications and inflammatory status in SCD. The high AUC for CXCL10 and IL-6 suggests their potential to identify inflammatory states and distinguish SCD patients from healthy individuals. For example, the ROC curve analysis showed that a plasma BDNF level of 2,100 pg/mL had 87.5% sensitivity and specificity in predicting complications in SCA, while Ang-2 levels of 8,845 pg/mL were 90% sensitive and 80% specific for predicting vascular complications. However, relying on a single marker may not be sufficient to fully predict the risk of SCD complications.Elevated levels of these markers may correlate with increased risks of complications, such as vaso-occlusive crises or chronic inflammation. Similarly, high AUC values for BDNF and Ang-2 indicate their role in detecting vascular instability and neuroinflammatory responses, which are common in SCD.
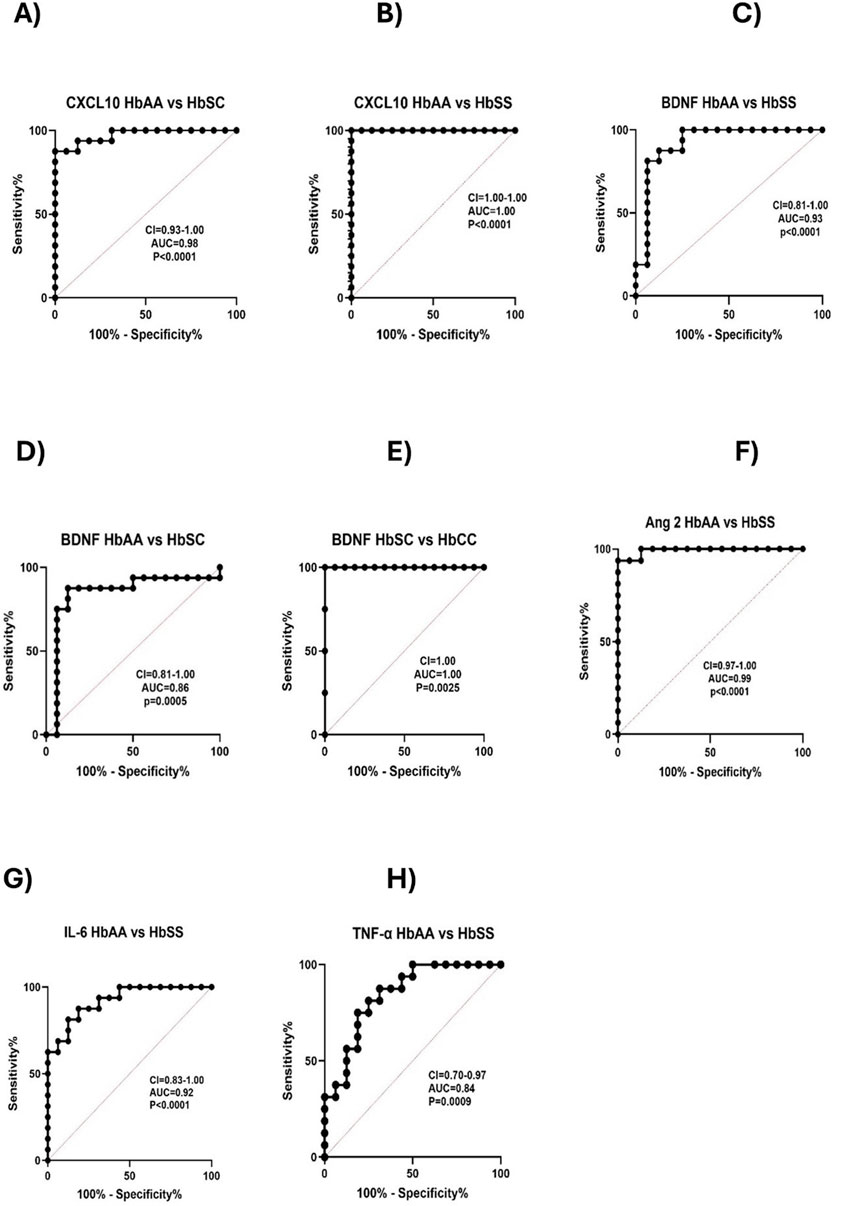
Figure 5. Predictive value of Circulating CXCL10, BDNF, Ang 1, Ang 2, IL-6, and TNF-α across Different Sickle Hb Genotypes. ROC analysis and its AUC were constructed to explore the usefulness of CXCL10, BDNF, Ang 1, Ang 2, IL-6, and TNF-α as potential biomarkers for predicting or as indicators of disease complications based on sickle status. Individual biomarkers independently discriminated between different Hb genotypes. The ROC plot indicated that these markers were good biomarkers: (A) CXCL10 between HbAA vs. HbSC (p < 0.0001, AUC = 0.98). (B) CXCL10: HbAA vs. HbSS (p
留言 (0)