Multiple sclerosis (MS) is the most common autoimmune disorder affecting the central nervous system (CNS), characterized by both neuroinflammation and neurodegeneration (Compston and Coles, 2008). Despite genetic and environmental risk factors, including Epstein–Barr virus infection and lack of vitamin D, the etiology of MS remains unknown (Olsson et al., 2016). MS can be classified into three main forms: relapsing–remitting MS (RRMS), primary progressive MS (PPMS), and secondary progressive MS (SPMS). Typically, the course of MS starts with RRMS, usually diagnosed in younger ages (20s-30s), during which symptoms appear suddenly and are followed by a period of recovery. With age, this course usually evolves into SPMS marked by a progressive decline in neurological function. In contrast, approximately 10–15% of patients present with PPMS, where symptoms progressively worsen from onset without distinct relapses or recovery periods (Ghasemi et al., 2017).
Pathologically, MS is characterized by the disruption of the blood–brain barrier (BBB), allowing peripheral immune cells to infiltrate the CNS. These infiltrating immune cells target, and attack myelin sheaths produced by oligodendrocytes, leading to inflammation and demyelination. The release of pro-inflammatory cytokines, such as interleukins (ILs), interferons (IFNs), and tumor necrosis factors (TNFs), exacerbates this inflammation and damages both nerve fibers and oligodendrocytes, resulting in lesion formation (Ortiz et al., 2014; Weissert, 2017). As MS progresses, persistent inflammation activates astrocytes and microglia contributing to smoldering inflammation and neurodegeneration (Giovannoni et al., 2022). This sustained low-grade inflammation, driven by continuously activated microglia and astrocytes, leads to the release of pro-inflammatory cytokines and reactive oxygen species, creating a neurotoxic environment that disrupts neuronal homeostasis (Giovannoni et al., 2022). In the early stages of MS, remyelination can occur due to surviving oligodendrocytes and oligodendrocyte progenitor cells (OPCs). However, as the disease advances, the depletion of oligodendrocytes and impaired OPC function, compounded by persistent inflammation, result in remyelination failure (Goldschmidt et al., 2009; Klotz et al., 2023). This reduction in regenerative capacity further aggravates the neurotoxic condition of the CNS. Additionally, mitochondrial dysfunction, oxidative stress, and excitotoxicity contribute to synaptic loss, axonal injury, and eventually neuronal death (Lassmann et al., 2012; Figure 1). This ongoing neurodegenerative process underlies the progressive neurological decline observed in progressive MS (pMS; Woo et al., 2024). Despite significant advances in understanding MS, the precise mechanisms driving disease progression remain poorly understood. It is believed that aging plays a critical role in the transition from the inflammatory-dominant phase of RRMS to the neurodegenerative phase of SPMS, being a primary risk factor for the development of progressive MS forms, like other neurodegenerative diseases (Sanai et al., 2016; Musella et al., 2018; Hou et al., 2019).
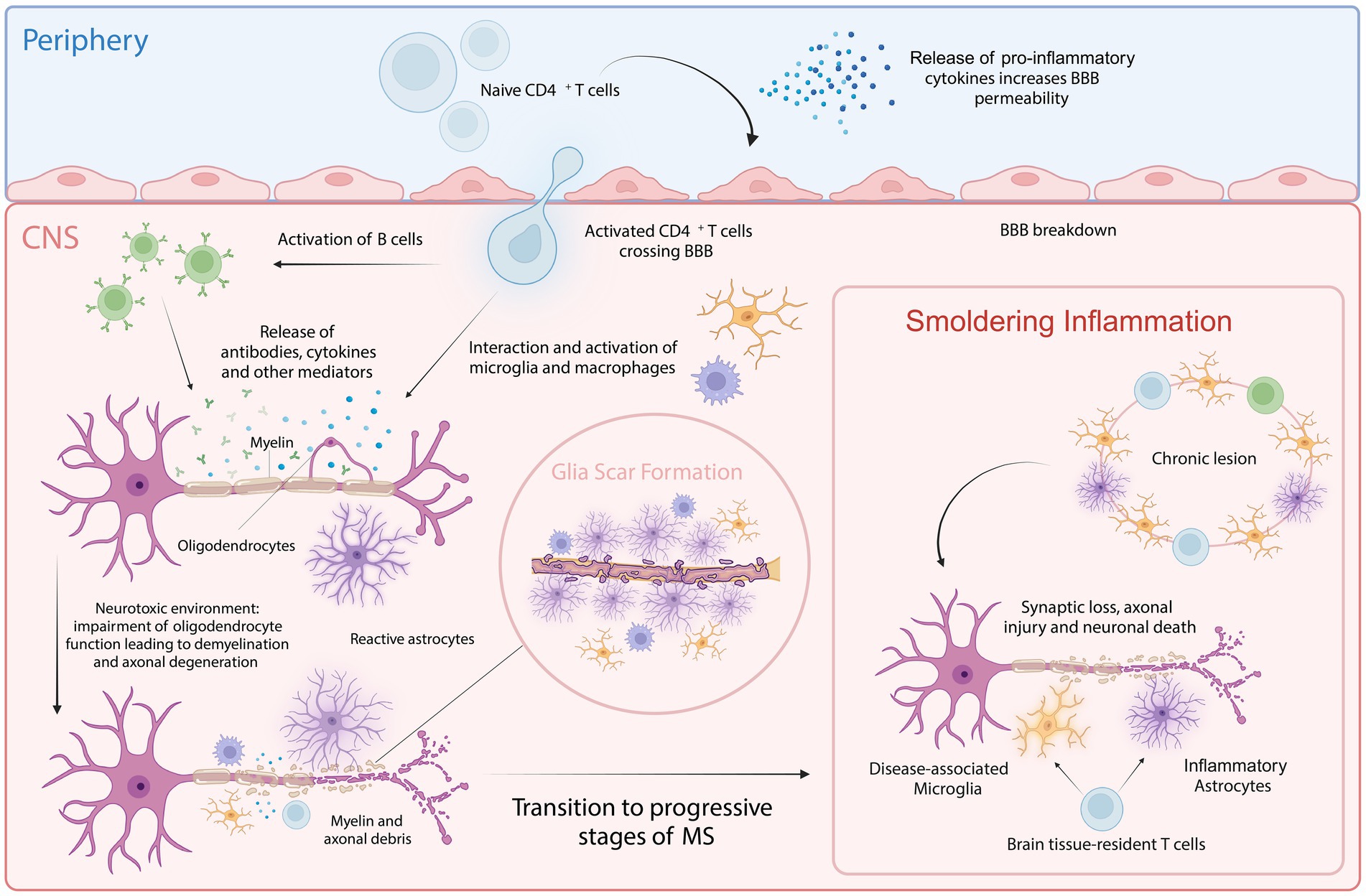
Figure 1. Cellular key players involved in MS pathology. In the periphery, upon activation of naive CD4+ T cells, pro-inflammatory cytokines are released that increase BBB permeability, allowing the autoreactive CD4+ T cells to cross into the CNS. Within the CNS, these T cells interact with and activate resident microglia, infiltrating macrophages and B cells. This interaction amplifies inflammatory response, leading to further recruitment of immune cells and the activation of astrocytes. This process is facilitated by both cytotoxic mechanisms and the release of autoantibodies and cytokines. Chronic inflammation and astrocyte reactivity contribute to glial scar formation, a hallmark of progressive MS. Smoldering inflammation persists within the CNS, marked by ongoing low-level immune activation and the gradual expansion of lesions, further driving neurodegeneration and characterizing progressive stages of MS. Created with BioRender.com.
To better understand the complexity of MS pathology and explore potential therapeutic strategies, various animal models have been developed (Dedoni et al., 2023). The most widely utilized model is experimental autoimmune encephalomyelitis (EAE), which replicates the autoimmune aspects of MS. Initially characterized in monkeys, EAE is induced by immunization with myelin-derived self-antigens such as myelin basic protein (MBP) or myelin proteolipid protein (PLP; Rivers et al., 1933). Presently, murine models are predominantly used to study both the relapsing–remitting and chronic progressive forms of EAE (Tuohy et al., 1989; Tompkins et al., 2002). Although EAE models have shed light on immune system activation and neuroinflammation, they provide a limited understanding of the CNS-intrinsic mechanisms underlying disease progression (Kamma et al., 2022). Other models, like toxin-induced demyelination using cuprizone or lysolecithin, enable investigation of demyelination and remyelination processes (Bénardais et al., 2013; Praet et al., 2014). Nevertheless, some studies using MS animal models have yielded disappointing clinical trial results, likely due to differences between rodent and human physiology (Mestas and Hughes, 2004). Currently, no effective treatments are available targeting repair and regeneration in pMS, highlighting the need for improved disease modeling approaches (Hollen et al., 2020).
Alongside animal disease models, in vitro human cellular methodologies have been developed to model and investigate MS pathology. Traditional 2D cell cultures offer a controlled environment for studying cellular interactions and disease mechanisms, overcoming some of the complexities associated with animal models, but they are limited in replicating the complexity of 3D tissue architecture (Dedoni et al., 2023). The development of 3D in vitro cultures, particularly human organoids, represents a significant advancement. Organoids can be generated from human pluripotent stem cells (hPSCs), including both embryonic stem cells (ESCs) and induced PSCs (iPSCs), as well as organ-specific adult stem cells. These cells self-organize into complex structures that mimic the cellular heterogeneity and organization of human organs (Gazerani, 2023). Patient- and disease-specific iPSCs can be used to generate specific cell types of interest allowing for the modeling and elucidation of specific cellular pathways implicated in disease. Human iPSC (hiPSC) technology is extensively employed to create human “disease-is-a-dish” models (Tang et al., 2022). The development of the first iPSC line, to our knowledge, from a patient with RRMS was in 2012, which marked a significant milestone (Song et al., 2012). Since then, advancements in generating MS-specific iPSCs have greatly enhanced the ability to study this complex neurological condition. Refined protocols now allow for the differentiation of iPSCs into neural progenitor cells (NPCs), neurons, oligodendrocytes, astrocytes, and microglia, offering promising avenues for understanding MS pathogenesis and accelerating the discovery of new therapies (Fortune et al., 2022b).
Brain organoids offer a novel platform for modeling MS pathology at a cellular level, with the potential to uncover disease mechanisms and develop new therapeutic strategies (Jusop et al., 2023). However, challenges remain, as brain organoids often exhibit cell immaturity, which limits their ability to accurately simulate intricate cellular interactions. Additionally, significant cell types like vascular cells, microglia, and immune cells, necessary for replicating neuroinflammatory mechanisms, are often lacking. Furthermore, incomplete maturation, underdeveloped neural circuits, and the inability to replicate aging processes further constrain their effectiveness in modeling neurodegenerative diseases. These limitations underscore the ongoing challenges in refining human brain organoid models for studying immune-mediated neurological diseases like MS (Kim and Chang, 2023).
This review will explore recent advances in brain organoid technologies and their application to MS research, aiming to enhance our understanding of disease mechanisms and guide the development of novel therapeutic approaches.
2 Current state and limitations of brain organoidsTraditional 2D cell culture models face significant limitations in accurately replicating in vivo cell environments, making them unreliable for disease modeling. In 2D cultures, cells adopt a flat and elongated morphology that contrasts with the natural 3D structure of tissues. This disparity is primarily due to reduced spatial organization and a lack of physiological conditions. The absence of crucial cell-to-cell and cell-to-extracellular matrix (ECM) interactions in 2D environments leads to altered gene and protein expression profiles, affecting the complex behavior of diseases (Sun et al., 2021). Additionally, these uniform conditions fail to replicate the inherent gradients of nutrients, oxygen, and waste essential for regulating cell behavior in living tissues.
Over the past few decades, a variety of 3D culture models have been developed, with spheroids and organoids becoming the most widely used. In a 3D culturing environment, cells can proliferate and interact with their surroundings, better mimicking the complex cellular interactions and tissue-specific architecture found in living organisms. This is typically achieved using multicellular aggregates, with or without a structural scaffold (Jensen and Teng, 2020). The shift from conventional 2D to 3D cell culture methodologies has accelerated in recent years, showing significant progress in generating spheroids, organoids, and assembloids. This advancement holds great promise for bridging the gap between 2D cell culture technologies and experiments with animals (Paiva et al., 2023).
2.1 SpheroidsSpheroids were initially developed in the 1970s and are simple clusters that can be created from different cell lines including primary cultures and stem cells. They possess the ability to spontaneously self-assemble into 3D aggregates that arrange into sphere-like formations. The generation of both single-cell and multicellular spheroid models relies on homotypic cell-to-cell adhesion in an environment that inhibits cell attachment to the culture surface (Sakalem et al., 2021). There are various methods available for creating spheroid cultures including the hanging drop and magnetic levitation technique, microfluidic systems, non-adhesive hydrogels and bioprinting. This 3D culture system finds key applications in drug testing and development, disease modeling and regenerative medicine (Białkowska et al., 2020; Griffin et al., 2022). However, due to the relative simplicity of spheroids, they lack polarity and the ability to self-organize into more complex tissue architectures, thus making this model less representative of accurate 3D in vivo conditions (Duval et al., 2017).
2.2 Brain organoids3D brain organoids can exhibit complex architecture encompassing several neural and glial cell types, synaptic connections, and myelination. This 3D culture system mimics the intricacy of human neurodevelopment and brain diseases within a human setting, providing an unprecedented opportunity for in vitro human brain research. First described by Lancaster et al. in 2013 (Lancaster et al., 2013), the protocol outlines the natural self-organizing ability of stem cells into complex organ structures within a 3D ECM. By integrating specific growth factors, the protocol facilitates the neuroectodermal lineage differentiation of iPSCs. During 1 to 9 months, brain organoids mature into different brain areas and cell types, mimicking the development of the human brain in vivo (Lancaster and Knoblich, 2014). Since this initial discovery, many varying protocols have been established to mimic either specific brain areas (guided protocols) or the entire brain (unguided protocols). In the context of unguided differentiation, stem cells autonomously differentiate into organoids with distinct cell types and different brain regions, independent of external stimuli (Lancaster et al., 2013; Lancaster and Knoblich, 2014). In contrast, guided protocols entail the addition of extrinsic factors to imitate the morphogen gradient seen during the development of the embryonic brain, thereby conferring the organoid identities exclusive to certain regions (Eiraku et al., 2008; Kadoshima et al., 2013; Paşca et al., 2015). Moreover, the specific pattern development is also highly reliant on the strength and length of exposure to diffusible morphogens and the intricate interplay between various signaling pathways (Abdel Fattah et al., 2023). A diverse array of morphogen combinations from distinct signaling pathways has been employed to induce the formation of region-specific organoids, such as dorsal (Sebastian et al., 2023) and ventral forebrain (Zayat et al., 2023), hindbrain (Valiulahi et al., 2021), cerebellum (Atamian et al., 2024), spinal cord (Zhou et al., 2024) and choroid plexus (Pellegrini et al., 2020; Chew et al., 2023; Zhao and Haddad, 2024).
2.3 Advantages and limitations of brain organoidsThe ability of brain organoids to self-organize during growth creates a 3D human brain environment with patient-derived genomes and evolutionarily conserved pathways, which are key advantages (Smirnova and Hartung, 2024). This 3D cell culture technology captures unique hallmarks of human brain development, including progenitor zone structures and neural migration and differentiation (Pamies, 2017). Alongside the existence of diverse mature brain cell types such as neuronal and glial cell varieties that replicate neuronal-glial communication and its connectivity, organoids show great promise for their application in disease modeling and precision medicine (Lancaster et al., 2017).
Over time, this methodology has become a new tool to model and investigate underlying mechanisms in neurodegenerative diseases such as pMS (Daviaud et al., 2023). Mutations can be precisely introduced into brain organoids via viral vectors or electroporation (Qian et al., 2019) to study the risk variants significance to disease in human tissue while pairing this approach with high-throughput screening techniques and in vitro single-cell profiling (Eichmüller and Knoblich, 2022). Moreover, brain organoids are proven advantageous in determining the neurotoxicity of medications and environmental contaminants. Conventional models such as 2D cell cultures are often unsuccessful in accurately predicting the effects of neurotoxins in humans because their uniform environment leads to altered cellular responses upon introduction of the neurotoxins (Bal-Price et al., 2010; Semple et al., 2013). In the context of precision medicine, brain organoids could potentially facilitate personalized therapy testing on patient-derived models, enabling patient-specific drug screening before clinical intervention, thus creating a new approach akin to “clinical trials in a dish” (Smirnova and Hartung, 2024). This approach could enable regular assessment of treatments on patient-derived models, helping to determine the most effective individualized course of action. Although organoid research is still in its early stages, it presents unprecedented possibilities for tailoring treatments and improving therapeutic outcomes beyond conventional methods (Yuan et al., 2023; Acharya et al., 2024).
However, it is essential to acknowledge the limitations of 3D brain organoids in studying and modeling diseases for the investigation of their underlying mechanisms. The capacity of this human experimental system to precisely mimic brain function and disease is limited by the lack of all cell types present in the human brain (Andrews and Kriegstein, 2022). Brain organoids predominantly comprise cells originating from the neuroectodermal lineage, constraining non-neuronal differentiation such as the mesodermal-derived microglia and vascular cells. This limits their significance and suitability as microglia play a crucial role in immune defense during disease and homeostasis (Ormel et al., 2018). The absence of vascularization impairs the efficient delivery of nutrients and oxygen to cells within the organoids, resulting in cellular stress, oxygen deprivation and necrosis (Kim and Chang, 2023). In contrast, the inclusion of endothelial cells (ECs) and vascular-like structures in brain organoids reduces cellular apoptosis and enhances neurogenesis (Andrews and Kriegstein, 2022). The organoids’ ability to expand is significantly restricted by the absence of the surrounding meninges and their associated vasculature. This deficiency results in a stochastic growth model driven by the presence of nutrients and the lack of complete spatial organization necessary for proper neural tissue patterning (Lancaster and Knoblich, 2014). Although these models closely resemble various essential aspects of early human brain development, they lack advanced brain activity due to the absence of sensory input and functional neuronal connectivity. Modeling age-dependent neurodegenerative diseases remains challenging because organoids do not naturally age or experience the same long-term environmental and genetic factors that contribute to these diseases in vivo. The ability to artificially induce aging in vitro could improve brain organoids’ capacity to exhibit phenotypes relevant to disease. The variability and reproducibility of organoid models present significant challenges due to differences in culture conditions, developmental trajectories, and the lack of standardized protocols, which can result in inconsistent outcomes. To overcome these issues, it is crucial to use multiple stem cell lines to minimize genetic variability, clearly differentiate between technical and biological replicates, and establish standardized reporting procedures. Additionally, adopting advanced technologies, such as high-resolution microscopy and single-cell sequencing, is recommended to enhance repeatability and transparency in the documentation of experimental conditions. Implementing these strategies can improve the robustness and consistency of organoid models, thereby advancing our understanding of human brain development and disease (Velasco et al., 2019; Hong et al., 2024; Sandoval et al., 2024). Despite these challenges, organoids remarkably resemble the in vitro development of the human brain and have shown widespread application across various fields of neuroscience research (Qian et al., 2019; Smirnova and Hartung, 2024).
2.4 AssembloidsAssembloids are generated by combining multiple spheroids or organoids derived from different types of cells or tissue areas. Human cortical spheroids have been assembled with human subpallium spheroids to potentially mimic more intricate elements of brain connectivity and development, using a co-culture technique. This has provided significant insights into the development of human cortical circuits (Birey et al., 2017). Other advanced techniques to generate assembloids include the fusion of organoids, referring to the physical combination of several organoid types to investigate inter-organ communication (Kanton and Paşca, 2022) and microfluidics. Brain region-specific organoids can be efficiently created by encasing hiPSCs within microcapsules using microfluidic electrospray methods. These organoids are then incorporated into a microfluidic chip, which includes an adjustable upper layer with a paired microhole array and a lower layer with a micropillar array. In these microholes, organoids fuse together and form intricate brain assemblies (Zhu et al., 2023). The unique advantage of these methods lies in their ability to elucidate how various tissue regions interact to exhibit novel biological characteristics. Assembloids hold great potential for simulating complex cell–cell interactions within and between tissues, helping to uncover the underlying pathophysiological mechanisms in disease and development (Kanton and Paşca, 2022). The assembly of brain and blood vessel organoids has shown significant promise for disease modeling; the inclusion of vascular-like structures enhances the organoid’s ability to simulate the nutrient and oxygen dynamics found in vivo. This advancement improves the organoid’s potential to replicate disease-relevant conditions, particularly for modeling complex neurological disorders (Dao et al., 2024).
3 Modelling MS in vitro with stem cell technologyThe development of reliable in vitro or in vivo preclinical disease models is currently hampered by an incomplete understanding of the underlying mechanisms of MS. While the etiology of MS is not fully understood, it is believed to involve a combination of environmental factors, lifestyle, and genetic predispositions (Daviaud et al., 2023). The polygenic complexity of MS is underscored by genome-wide association studies (GWAS), which have identified 233 genetic variations frequently linked to the disease, collectively contributing to 39% of the genetic component of MS risk. This complexity complicates the development of exclusively genetic MS models (Barrie et al., 2024).
However, advances in human stem cell technologies have enabled the generation of patient-derived iPSCs, offering a novel model system to study this complex disease. MS iPSC lines consistently express pluripotency markers and have the capacity to differentiate into the three germ layers (Miquel-Serra et al., 2017; Lopez-Caraballo et al., 2020; Begentas et al., 2021; Fortune et al., 2022a; Lotila et al., 2022). Researchers have further differentiated these MS-derived cells into various cell types, including NPCs/neural stem cells (NSCs), neurons, OPCs, oligodendrocytes, astrocytes, brain microvascular ECs (BMECs), and brain organoids (Tables 1, 2).
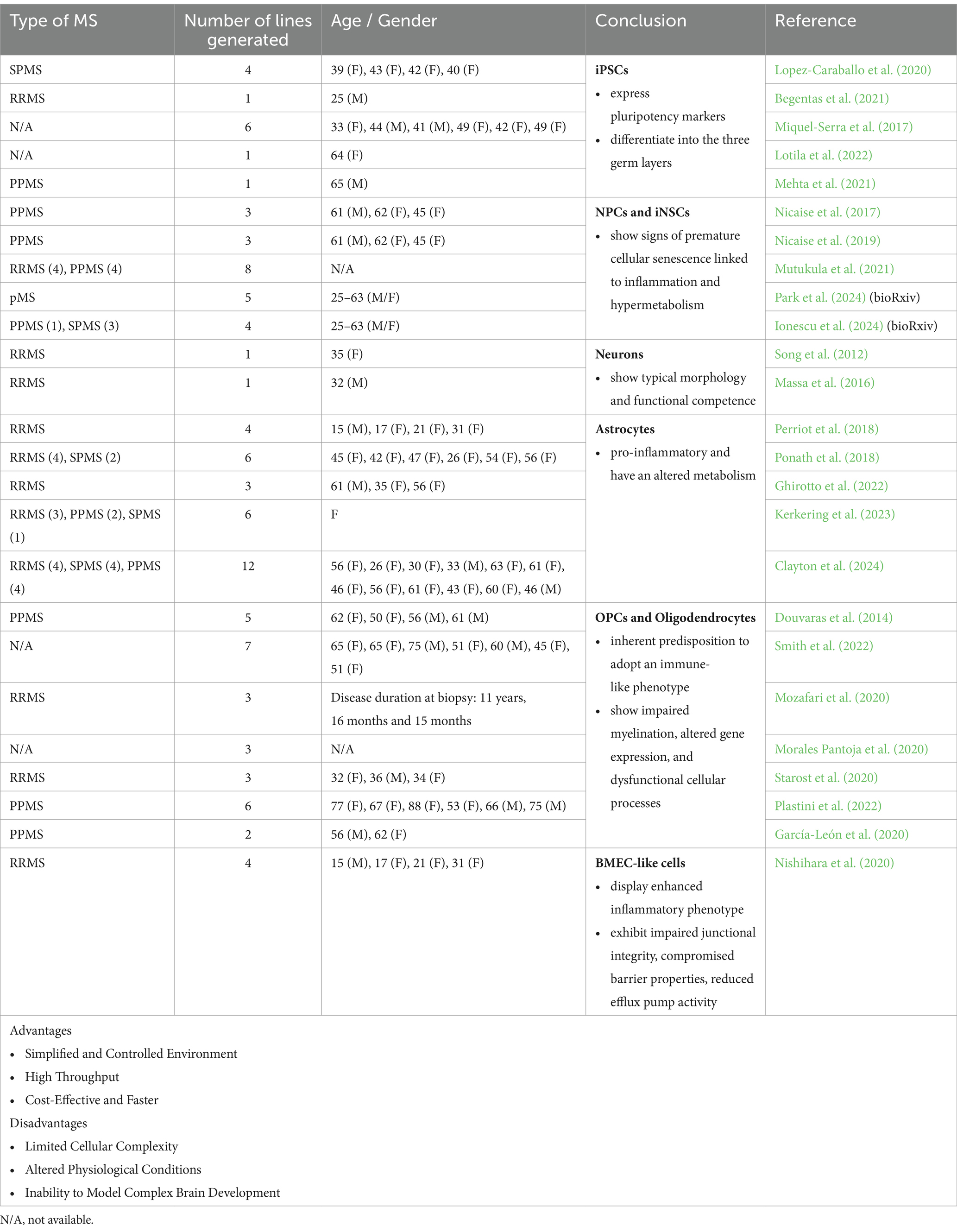
Table 1. Different applications, advantages and disadvantages of 2D cultures (MS patient-derived iPSCs) to model and study MS pathology.
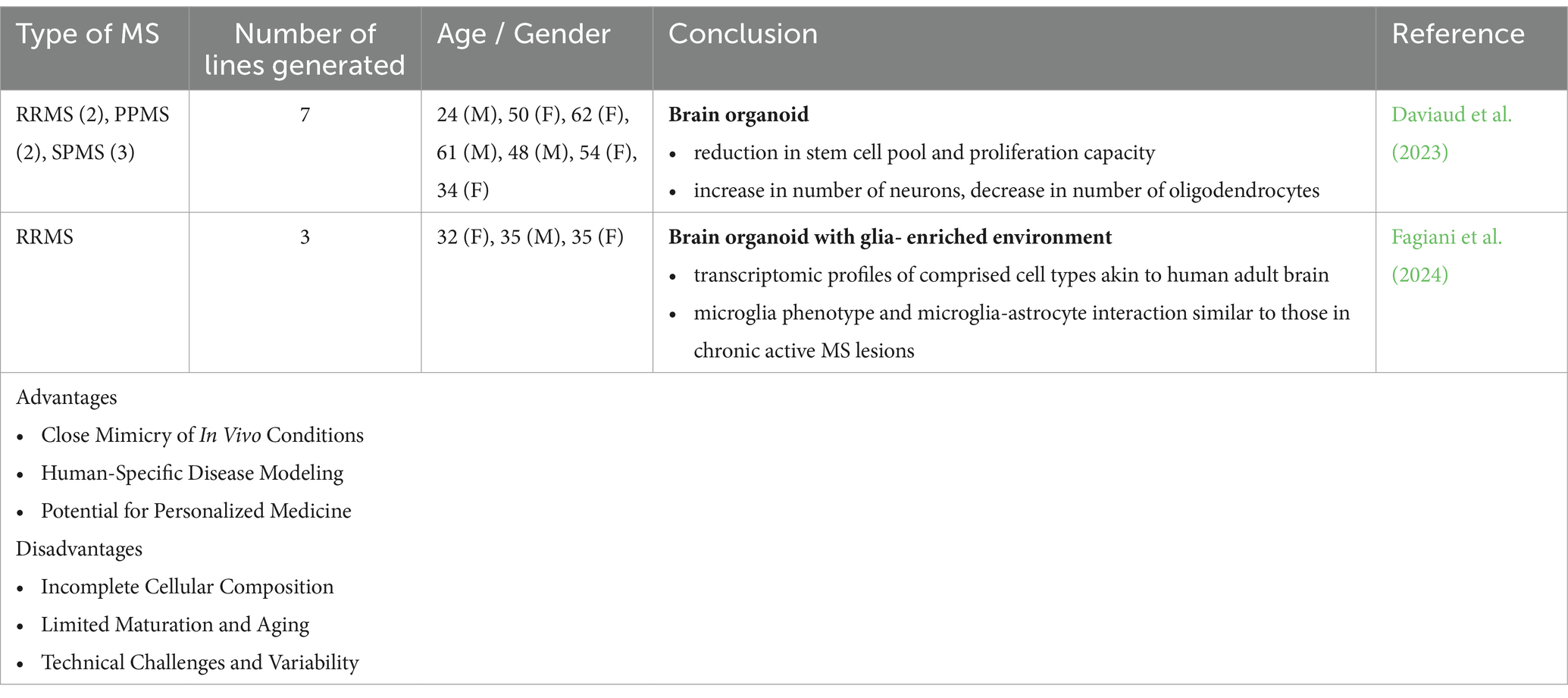
Table 2. Different applications, advantages and disadvantages of 3D cultures (MS patient-derived brain organoids) to model and study MS pathology.
3.1 MS iPSC-derived NPCs and iNSCsNPCs are characterized by their tripotency, ability to self-renew and notable properties for neuroprotection and anti-inflammatory responses within areas of myelin damage after transplantation. However, NPCs derived from patients with pMS exhibit impaired stemness, fail to provide neuroprotection upon transplantation in cuprizone-lesioned mice, and show increased cellular senescence compared to controls (Nicaise et al., 2017, 2019; Mutukula et al., 2021; Daviaud et al., 2023). Recent advances in technology have enabled the investigation of NSCs in pMS through direct reprogramming, bypassing the pluripotent state when epigenetic markers of aging are erased, generating induced NSCs (iNSCs). These pMS iNSCs display senescent features associated with hypermetabolism, inflammatory signaling, increased cholesterol and long-chain fatty acid metabolism, accumulation of lipid droplets, and the secretion of a neurotoxic senescence-associated secretory phenotype (SASP; Ionescu et al., 2024). Single-cell transcriptomic and epigenetic profiling of these iNSC lines identified an IFN-responsive, highly senescent cell cluster in pMS iNSCs, also present in chronic lesions in the pMS brain (Park et al., 2024). Epigenetic dysregulation of IFN-response genes was found in both the original pMS fibroblasts and the iNSCs, suggesting that direct reprogramming preserves significant disease-related features. This method may serve as a valuable model for studying and understanding pMS mechanisms. Senescent and IFN-responsive NSCs may impact neighboring cells within pMS-affected brains and contribute to neurodegenerative processes.
3.2 MS iPSC-derived neuronsPrimary neurons derived from people with RRMS were subjected to detailed electrophysiological analysis to assess the critical role of electrical signaling in neuronal function, particularly within the context of neurodegenerative conditions like MS. These differentiated neurons exhibited key characteristics of mature neurons, including stable resting membrane potentials and substantial action potentials in response to depolarization, as recorded using patch-clamp techniques. Morphologically, they displayed typical neuronal structures and expressed transcription factors and cytoskeletal markers indicative of neuronal differentiation (Song et al., 2012; Massa et al., 2016). These findings provide valuable insights into the functional integrity of neurons in MS and present a relevant approach to study and model disease-affected cell types.
3.3 MS iPSC-derived astrocytesA few studies have generated and analyzed MS iPSCs-derived astrocytes with a focus on their phenotype, functionality and cell intrinsic inflammatory properties (Perriot et al., 2018; Ponath et al., 2018; Ghirotto et al., 2022; Kerkering et al., 2023; Clayton et al., 2024). These studies have identified differences in gene and protein expression linked to signaling pathways that govern neurodegeneration, mitochondrial dysfunction, inflammation, and cell death. These findings suggest intrinsic differences between MS iPSC-derived astrocytes and controls (Ghirotto et al., 2022). Metabolomic analysis further revealed abnormalities in amino acid synthesis and sphingolipid metabolism, indicating potential metabolic dysregulation in MS iPSC-derived astrocytes. Collectively, these findings suggest that MS-specific astrocytes assume a pro-inflammatory phenotype when exposed to cytokines expressed by microglia (Ghirotto et al., 2022; Kerkering et al., 2023). Recently, a study explored the properties of MS iPSC-derived astrocytes independent of inflammatory stimuli or the presence of other immune cells across all MS types. This research suggests that iPSC-derived astrocytes from patients across all MS subtypes acquire a pathological pro-inflammatory profile even in the absence of external inflammatory signals. This phenotype undermines their ability to support a neuroprotective environment, contributing to the progression of MS (Clayton et al., 2024).
3.4 MS iPSC-derived oligodendrocytesMany protocols have been established to generate OPCs and oligodendrocytes from MS patients to investigate the mechanisms underlying remyelination failure in MS (Douvaras et al., 2014; García-León et al., 2020; Lopez-Caraballo et al., 2020; Morales Pantoja et al., 2020; Mozafari et al., 2020; Starost et al., 2020; Plastini et al., 2022; Smith et al., 2022; Clayton et al., 2024). In MS iPSC-derived oligodendrocytes and control subjects, equivalent outcomes were observed regarding OPC proliferation frequency, oligodendrocyte differentiation potential and myelin production in a 2D system (Starost et al., 2020; Clayton et al., 2024). However, when microglia-expressed cytokines (IFNγ and TNFα) were introduced to mimic an inflammatory environment, both control and MS-derived iPSC-OPCs exhibited impaired oligodendrocyte differentiation (Starost et al., 2020). Another study found that long-term exposure to low-dose IFNγ reduced oligodendrocyte formation in both MS-specific oligodendrocytes and controls (Morales Pantoja et al., 2020). This suggests that OPCs generated from MS iPSCs exhibit a differentiation block after exposure to inflammatory factors, similar to those derived from controls. However, proteome analysis of SPMS iPSC-derived OPCs revealed reduced production of specific proteins linked to cellular motility and cell-to-cell communication compared to the controls. Migration of MS-specific OPCs was found to be compromised, suggesting that the migration of OPCs in MS patients may be impaired (Lopez-Caraballo et al., 2020). Transcriptome analysis on PPMS-derived oligodendrocytes and controls identified numerous differentially expressed genes associated with inflammation, apoptosis, and cell adhesion. These findings highlight that while both MS- and control-derived oligodendrocyte lineage cells respond similarly to inflammatory factors, MS-derived cells have an inherent predisposition to adopt an immune-like phenotype and exhibit additional dysfunctions, such as impaired migration, which may contribute to MS-specific pathology (Plastini et al., 2022; Clayton et al., 2024).
3.5 MS iPSC-derived BMECsBrain ECs constitute the BBB, a critical structure that regulates molecular trafficking and constrains the entry of immune cells into the CNS. ECs of the BBB undergo phenotypic alterations characterized by the upregulation of the cell adhesion and signaling molecules intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM)-1, and atypical chemokine receptor 1 (ACKR1) in people with MS (Zierfuss et al., 2024). This upregulation facilitates the increased infiltration of pathogenic immune cells into the CNS causing inflammatory damage to the BBB. The exact mechanisms underlying BBB failure are not fully understood, however, it is generally believed that neuroinflammation plays a significant role. A novel study generated MS iPSC-derived BMEC-like cells to mimic the BBB in vitro and to investigate the impact of BBB dysfunction in MS. BMEC-like cells derived from MS patients exhibited impaired junctional integrity, compromised barrier properties, reduced efflux pump activity, and an enhanced inflammatory phenotype, characterized by elevated expression of adhesion molecules and increased interactions with immune cells (Nishihara et al., 2020). These findings strongly suggest that MS patients possess intrinsic alterations in brain endothelium that contribute to BBB dysfunction, therefore iPSC-derived BMEC-like cells represent a valuable model for studying the underlying BBB dysfunction in MS. These models also hold the potential for identifying novel therapeutic targets aimed at stabilizing the BBB, which may be advantageous for treating both early and progressive stages of MS.
3.6 MS iPSC-derived brain organoidsBrain organoids were generated from iPSC lines derived from patients with RRMS, PPMS, and SPMS to better model and understand the pathological basis underlying the diverse clinical phenotypic expressions of MS. In the organoids generated from the progressive types of MS, a notable decrease in proliferation capacity was observed, accompanied by a reduction in the progenitor pool. This was also associated with increased neurogenesis and a decline in the number of oligodendrocytes (Daviaud et al., 2023). The absence of essential immune cells and vascularization poses limitations in accurately modeling the inflammatory demyelination and axonal degeneration associated with MS.
Another approach to modeling neuroinflammation in MS involves using MS and control NPC-derived forebrain-like organoids with forced expression of SOX10 to accelerate oligodendrocyte differentiation. Microglia are later transplanted into these 3D organoids. This setup results in the formation of neurons, astrocytes, oligodendrocytes, and microglia, with transcriptomic profiles similar to those of the adult brain. When exposed to cerebrospinal fluid (CSF) from MS patients, the microglia exhibit phenotypes resembling those found in chronic active MS lesions and show increased intercellular interactions with astrocytes (Fagiani et al., 2024). Despite not being strictly a brain organoid model, this relatively novel technology provides a scalable platform for drug screening, which can facilitate the discovery of novel therapeutics targeting the mechanisms underlying chronic inflammation and neurodegeneration in MS.
4 Emerging techniques to improve brain organoids for MS researchBrain organoids have emerged as a valuable tool for disease modeling, offering a potential solution to the limitations of animal models in clinical translation. However, these 3D in vitro systems still face significant challenges, particularly in replicating the complex tissue microenvironment of the human brain. Recently, innovative bioengineering techniques have been developed to address these limitations, providing promising advancements in brain organoid models (Jeong et al., 2023). Additionally, these techniques introduce new approaches for modeling MS in vitro, potentially becoming valuable tools for studying pathological mechanisms and drug development (Figure 2).
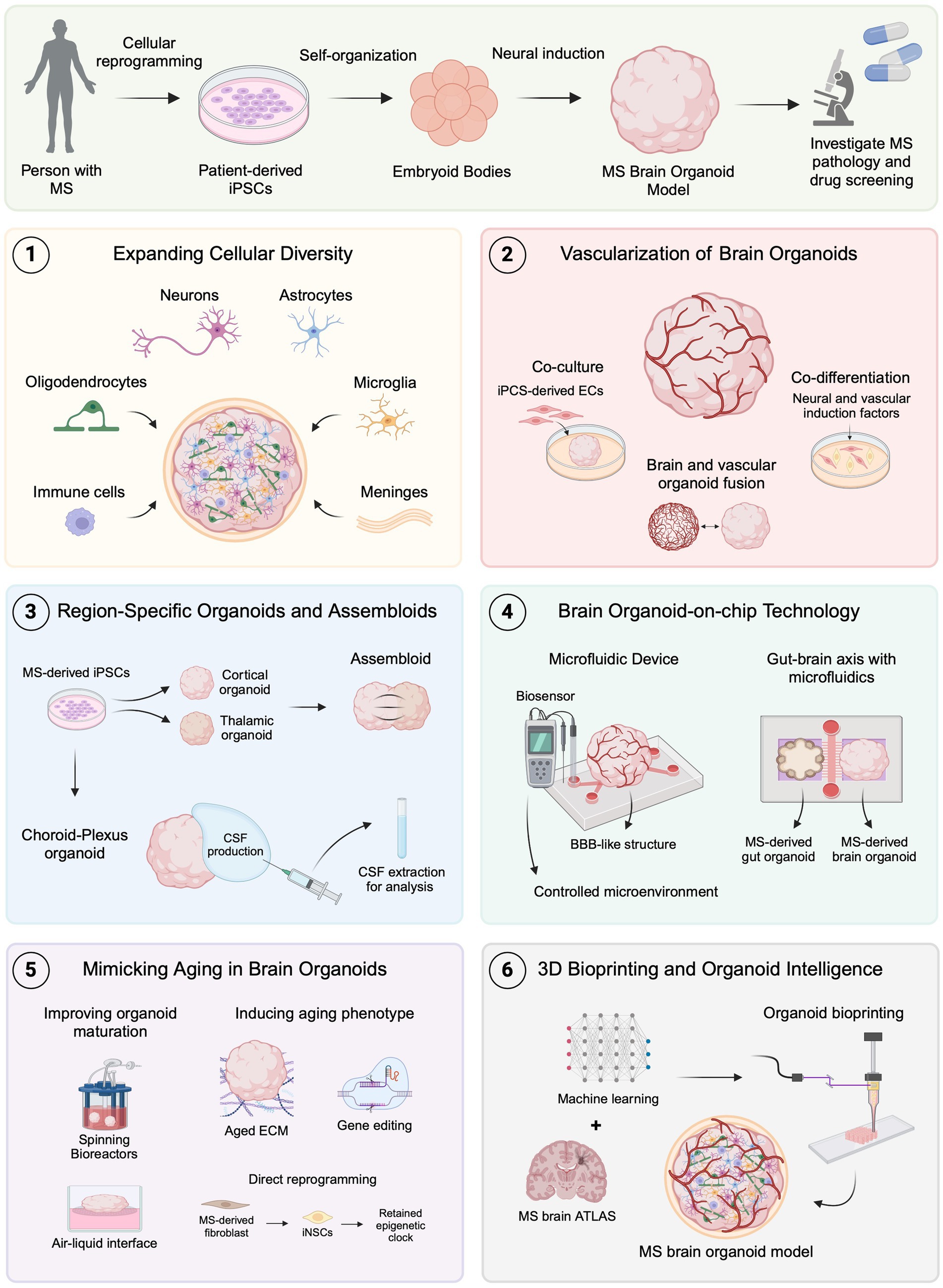
Figure 2. Emerging brain organoid techniques for modeling MS. MS brain organoids generated from patient-derived iPSCs offer a promising platform for studying MS pathology and drug development. (1) Expanding cellular diversity by incorporating oligodendrocytes, microglia, immune cells, and meningeal cells allows for more accurate modeling of MS-specific pathologies. (2) Advances in vascularization, including co-culture, co-differentiation, and organoid fusion, facilitate the formation of BBB-like structures to investigate MS-related BBB dysfunctions. (3) Region-specific organoids, such as choroid plexus, and assembloids, provide new avenues for studying CSF dynamics and neural circuit impairments in MS. (4) Organoid-on-chip technology using microfluidics creates controlled environments and can be used to replicate neuroinflammatory mechanisms and gut-brain axis interactions relevant to MS. (5) Techniques to mimic aging in organoids, including improved maturation protocols, show promise for studying age-related MS progression. (6) The combination of 3D bioprinting and machine learning with brain atlas data could lead to even more precise MS models, advancing the scope of in vitro disease modeling and therapeutic development. Created with BioRender.com.
4.1 Expanding cellular diversity in brain organoidsMost brain organoid models predominantly consist of NPCs, mature neurons, and astrocytes, yet they generally lack crucial cells such as oligodendrocytes, non-neuronal microglia, and peripheral immune cells (Del Dosso et al., 2020). This deficiency compromises the fidelity of brain organoids as accurate representations of the human brain. Incorporating these cell types is essential for developing accurate models of MS since infiltration of peripheral immune cells and oligodendrocyte death leading to demyelination are critical components of MS pathology (Compston and Coles, 2008). Additionally, in pMS, CNS-specific inflammatory mechanisms are believed to drive neurodegeneration (Lassmann et al., 2012), which underscores the significance of integrating microglia, the brain-resident macrophages, into brain organoids for modeling pMS and to better understand mechanisms driving disease progression.
Incorporating oligodendrocytes into brain organoids has been challenging due to the requirement for specific differentiation factors. However, protocols have been developed to promote oligodendrocyte maturation and myelin formation using targeted growth factors and hormones in human iPSC-derived neural models. For example, tailored differentiation protocols have successfully induced the formation of compact myelin sheets (Marton et al., 2019) and enhanced the survival, proliferation, and differentiation of OPCs in embedding-free brain organoids (Al-mhanawi et al., 2023; Shaker et al., 2021). More recently, a SOX10-based approach has generated brain spheroids that contain a stable pool of OPCs and MBP-positive oligodendrocytes exhibiting gene expression profiles resembling those of primary human oligodendrocytes (Fagiani et al., 2024). These advancements enable more accurate in vitro brain models by facilitating the formation of myelin sheaths, which are crucial for studying demyelination and remyelination mechanisms in MS. Myelin-enriched brain organoid models derived from patient iPSCs have been used to study myelin-related disorders and successfully recapitulated key pathological features (James et al., 2021; Feng et al., 2023). This highlights the potential for employing similar methodologies to investigate myelin pathology in MS. Notably, a study generated MS iPSC-derived brain organoids containing oligodendrocytes that showed decreased differentiation and myelination (Daviaud et al., 2023). This model offers a valuable tool for future research on drug efficacy in promoting remyelination, a process known to be impaired in the progressive stages of MS. Despite its innovation as a 3D in vitro model for MS, it lacks other important components of MS pathology, such as neuroinflammation.
In MS, activated microglia release various pro-inflammatory cytokines. These cytokines can be beneficial in the early stages of the disease but become detrimental as they contribute to persistent inflammation and neurodegeneration as the disease progresses (Zrzavy et al., 2017; Yong, 2022; Distéfano-Gagné et al., 2023; Peruzzotti-Jametti et al., 2024). The causes of chronic microglial activity in MS are not yet fully understood, and incorporating microglia into brain organoid models of MS could offer new insights into their role in pMS. Microglia originate from the yolk sac and migrate to the mesoderm during development, differing from neural and glial cells that arise from the neuroectodermal lineage. This developmental distinction has impeded the generation of microglia within neuroectoderm-derived brain organoids (Mosser et al., 2017). Recent advances have successfully integrated microglia into brain organoids using various techniques, creating new opportunities to study their role in MS (Mrza et al., 2024). Primary human microglia can incorporate into five-week-old brain organoids, maintaining homeostatic functions such as synapse remodeling without altering the overall structure of the organoid (Popova et al., 2021). Additionally, iPSC-derived microglia have been co-cultured with brain organoids, demonstrating activation in response to neuronal injury, a process relevant to MS pathology (Abud et al., 2017). Methods to generate microglia within organoids have also been developed. For instance, mesodermal progenitor cells can be differentiated into microglia by optimizing neuroectodermal stimulants (Ormel et al., 2018), and co-culturing hiPSC-derived neural and macrophage progenitors has shown promise in this area (Xu et al., 2021). These advancements underscore the potential for using MS-derived microglia in brain organoids to model disease mechanisms within a brain-like environment, providing insights into chronic neuroinflammation in MS. Recent work has further explored the role of microglia during neuroinflammation in a cortical organoid model of Alzheimer’s disease (AD; Cakir et al., 2022). The overexpression of the myeloid-specific transcription factor PU.1 successfully induced the differentiation of microglia-like cells within the organoids. This approach offers a valuable tool for understanding the role of chronically activated microglia in persistent inflammation related to MS and for studying disease-associated genes expressed in microglia. Genetic mapping has revealed numerous MS susceptibility genes linked to microglia (De Jager, 2019).
Until now, no studies have incorporated immune cells other than microglia into brain organoids (Sabate-Soler et al., 2024). However, this has been reported in other organoids such as liver, gut, and lung by seeding cells together in a scaffold, co-culturing of organoid and immune cells and direct injection of immune cells into the lumen of organoids (Bogoslowski et al., 2023). These techniques show potential ways for integrating peripheral immune cells into brain organoids that could be used to study immune cell activation and infiltration into the MS brain. Even though the field is still far from accurately mimicking the complex immune microenvironment of the MS brain in vitro, recent advances in developing immunized brain organoids represent valuable, translatable platforms for MS research. Future studies could generate iPSC-derived lymphocytes, such as T-like-cells (Iriguchi et al., 2021), from MS patients or directly collect T-cells from blood samples (Fransson et al., 2009) and incorporate them into brain organoids to investigate the adaptive immune mechanisms in MS brain microenvironment, particularly in RRMS, where immune cell infiltration across the BBB is predominant. Additionally, these models could serve as valuable platforms for identifying potential therapeutic targets and developing more effective treatments for MS.
The meninges are membranes covering the CNS that act as protective barriers and have been shown to play a critical role in corticogenesis and brain development by releasing essential signaling factors that influence neuronal differentiation, migration, and the formation of cortical layers (Dasgupta and Jeong, 2019). In pMS, the meninges may facilitate immune cell infiltration and chronic inflammation is frequently observed in this region, which is thought to play a role in neuroinflammatory processes and disease progression, especially cortical grey matter pathology (Serafini et al., 2004; Choi et al., 2012; Magliozzi et al., 2023). The integration of the meningeal layers into brain organoids would not only enhance their development and maturation but could also serve as a potential in vitro model to study meningeal inflammation in pMS. A recent study successfully mimicked the meningeal environment by co-culturing human meningeal cells with cortical organoids (Jalilian and Shin, 2023). Within the first 24 h of co-culture, meningeal cells adhered to and encapsulated the organoids, closely mimicking the architecture of in vivo meningeal membranes. This integration significantly enhanced cortical layer organization and promoted functional maturation, resulting in a more physiologically relevant model of human brain development. This protocol provides a valuable platform for investigating the role of meningeal inflammation and neuroinflammatory processes associated with pMS.
4.2 Advances in brain organoid vascularizationThe neurovascular system is crucial for brain development and function. Therefore, vascularizing brain organoids is important to enhance their fidelity and maturation, as, without an effective vascular system, oxygen and nutrient exchange become suboptimal, leading to cell death (Yu, 2020). Establishing a vascular network in brain organoids is particularly relevant for studying neurological diseases, including MS, where the integrity of the BBB is compromised, allowing immune cells to infiltrate the CNS and contribute to neuroinflammation and demyelination. In vivo, vascular system development relies on key transcription factors and signaling cues that facilitate the self-organization and vessel formation of ECs and perivascular cells such as pericytes (Risau, 1997). Replicating this system in vitro is challenging due to its complexity, but recent bioengineering approaches have shown promising results.
Co-culturing patient-specific iPSC-derived ECs with brain organoids during early developmental stages has been shown to generate robust vascularization, with ECs forming tubular structures that penetrate the organoids (Pham et al., 2018). This approach demonstrates the feasibility of creating patient-specific vascularized organoids, making them particularly relevant for MS research. For instance, vascularized organoids derived from MS patients could be developed to assess BBB properties, such as permeability, and to gain insights into disease mechanisms associated with BBB dysfunction. Another promising method is co-differentiation, where neural and vascular lineages are simultaneously induced, leading to integrated neurovascular networks. Vascular endothelial growth factor (VEGF) has been utilized to promote the formation of blood vessel-like structures in brain organoids while maintaining neuronal differentiation, resulting in BBB-like vascular structures (Ham et al., 2020). Subsequent treatment with Wnt7a has been shown to enhance the formation of pericyte-like cells around the vascular tubes, better mimicking the in vivo BBB structure.
Emerging genetic engineering approaches, such as orthogonal induction (OID), further allow for the simultaneous differentiation of hiPSC-derived ECs and neurons within a single culture system, resulting in patterned, vascularized cortical organoids (Skylar-Scott et al., 2022). In the future, OID could be further explored to induce the generation of a diverse array of cell types from the same cell line through different transcription factors, creating a 3D model that more accurately mimics the human brain. Specifically, applying OID with MS patient-derived iPSCs could significantly enhance in vitro disease modeling by facilitating the generation of diverse cell types relevant to MS pathology.
Finally, vascularized brain assembloids, created by fusing brain organoids with vascular organoids, offer a complete vascular structure containing ECs, smooth muscle cells, and pericytes, demonstrating functional BBB characteristics and promoting neural development (Ahn et al., 2021; Sun et al., 2022). These innovative methods can be further leveraged using MS patient-derived iPSCs to create vascularized organoids, providing valuable tools for studying MS-associated BBB dysfunction and disease mechanisms.
In 2020, a study generated a 3D neurovascular unit organoid comprising neurons, oligodendrocytes, astrocytes, microglia, human brain microvascular ECs, and pericytes to study the effects of neuroinflammation on BBB function (Nzou et al., 2020). Exogenous inflammatory cytokines were found to increase BBB permeability, replicating characteristics of BBB dysfunction associated with neuroinflammation in pathophysiological conditions. For MS research, this approach could be adapted to explore BBB dysfunction driven by neuroinflammation seen in MS by using patient-derived organoids. Additionally, exposing these robust brain organoid models to the CSF from MS patients, which contains elevated levels of inflammatory mediators (Deisenhammer et al., 2019), would allow for a direct evaluation of how disease-specific inflammatory factors impact BBB permeability and function, revealing new insights into MS pathology. Furthermore, such models could be crucial for high-throughput drug screening to identify compounds that restore BBB integrity, like previous studies done in cell-based BBB models for AD (Qosa et al., 2016; Elfakhri et al., 2019). By more accurately replicating the neurovascular environment observed in MS, these advancements will contribute to the development of refined in vitro models, thereby accelerating the preclinical evaluation and discovery of targeted therapeutic strategies. Emerging methods such as incorporating brain organoids into microfluidic devices and employing 3D bioprinting offer promising avenues for creating more robust vascularized organoids, which will be explored further in the following sections (Nwokoye and Abilez, 2024; Rizzuti et al., 2024).
4.3 Region-specific brain organoids and assembloidsPrevious studies have demonstrated the potential for generating region-specific brain organoids through the application of defined extrinsic signals (Qian et al., 2018), in contrast to conventional cerebral organoids that represent broad brain structures (Lancaster et al., 2013). These refined induction protocols can be applied in MS research to model specific brain regions and elucidate region-specific disease mechanisms. Although immune cell infiltration through the disrupted BBB is reduced in pMS, other structures, such as the choroid plexus (ChP), may continue to contribute to peripheral immune cell infiltration and neuroinflammation at this disease stage (Lassmann et al., 2012). Utilizing region-specific brain organoids to study the ChP could provide valuable insights into its role in pMS pathology.
In 2020, a study described a method for generating ChP organoids from hPSCs, which demonstrated the secretion of CSF-like fluid in self-contained compartments (Pellegrini et al., 2020). These organoids exhibited transcriptomic and proteomic profiles closely resembling those of the in vivo ChP structure, highlighting their potential for studying ChP-related disease mechanisms in pMS by generating MS-derived ChP organoids. Additionally, a recent study developed techniques for extracting and analyzing fluid from hPSC-derived ChP organoids using either syringe or centrifugation methods (Chew et al., 2023). These approaches could be utilized to analyze cellular response mechanisms to CSF-like fluid from ChP organoids derived from pMS patients, potentially offering valuable insights into ChP-CSF dynamics and their role in MS pathology.
Recent progress in assembloid technology has enabled the fusion of organoids from distinct brain regions, offering enhanced models to investigate brain function and disease mechanisms (Marton and Pașca, 2020). This approach is particularly relevant for studying diseases like MS, where interregional communication and connectivity are often disrupted. For instance, by fusing cortical and thalamic organoids, researchers generated thalamocortical organoids to explore thalamocortical circuitry dysfunction in psychiatric disorders (Kim et al., 2024). Given that MS is characterized by altered connectivity between the thalamus and cortex, and that thalamic atrophy has been linked to cognitive impairment in MS patients (Tona et al., 2014), generating patient-derived thalamocortical assembloids could provide critical insights into how MS-related disruptions in this circuitry contribute to cognitive deficits.
Such assembloids, when coupled with microelectrode array (MEA) technology, could also facilitate detailed analyses of connectivity dysfunction in MS, as discussed later. Recent technical advancements, such as acoustofluidics and 3D bioprinting, have improved the precision of organoid fusion. Acoustofluidic methods allow for the dynamic alignment and fusion of organoids with minimal disruption (Ao et al., 2021), while 3D bioprinting platforms like Spatially Patterned Organoid Transfer (SPOT) enable precise organoid assembly without compromising their structure (Roth et al., 2023). These techniques could be leveraged to develop more reproducible thalamocortical assembloids, furthering our understanding of MS-related functional deficits and facilitating the discovery of targeted therapeutic strategies.
4.4 Organoid-on-chip technologyMicrofluidic chips are micro-engineered systems that provide precise control over fluid flow through spatially and temporally defined microchannels, creating controlled microenvironments that closely mimic in vivo conditions (Kang, 2022). These devices typically consist of multiple compartments or chambers with integrated pumps and valves that can be easily manipulated, allowing for the integration of various functions within a single chip (Kim et al., 2012; Leung et al., 2022). The appearance of organ-on-chip platforms has allowed in situ monitoring of the extracellular microenvironment using multisensor systems integrated into automated microfluidic controls (Zhang Y. S. et al., 2017). Recently, the development of brain organoids-on-chips has significantly improved brain organoid models by enhancing nutrient and oxygen exchange, which helps reduce necrosis and supports better maturation (Song et al., 2022). Additionally, advances in biosensors and optical imaging have significantly improved the monitoring of biochemical properties and cellular organization in organoid-on-chip models. Integrating enzyme-based biosensors allows continuous tracking of glucose and lactate levels (Misun et al., 2016), while optical techniques such as 3D tissue clearing and high-resolution imaging enable structural analysis of thicker tissues (Chen et al., 2016; Salman et al., 2020). These innovations could facilitate real-time monitoring of disease-specific changes in MS brain organoid models.
Recent studies have highlighted the potential of microfluidic systems to create perfusable vascular networks within brain organoids (Tan et al., 2023). Additionally, these devices can replicate the physiological conditions of the BBB, including the shear stress exerted by blood flow on ECs (Chaulagain et al., 2023). This shear stress is essential for enhancing EC maturation and promoting the formation of vessels with BBB properties (DeStefano et al., 2017). As a result, microfluidic systems offer a more realistic in vitro representation of the human BBB compared to static culture environments. Brain organoids were co-cultured with human umbilical vein ECs (HUVECs) within a microfluidic device, resulting in the formation of perfusable vascular networks that significantly enhanced neuronal differentiation and organoid maturation (Isshiki et al., 2020). In 2022, neurovascular organoids were developed using 3D-printed microfluidic chips, allowing for precise spatial interactions between organoid and vascular cells, which are essential for the establishment of an in vivo-like vascular network (Salmon et al., 2022). Furthermore, multiple studies have utilized microfluidic devices to construct vascular systems that exhibit BBB properties and functionality, facilitating their use in drug permeability screenings (Griep et al., 2013; Wang et al., 2017; Papademetriou et al., 2018; Wevers et al., 2018). This could be applied to test potential drugs for MS, and their efficiency in crossing the BBB, especially for pMS, in which pathological mechanisms are CNS-exclusive (Absinta et al., 2020).
Microfluidic devices have si
留言 (0)