Cardiovascular disease (CVD) is the primary cause of premature death and disability and the leading global burden of disease today (1). The majority of CVDs, including myocardial infarction, stroke, arrhythmia, and sudden cardiac death, have been found to have a distinct circadian rhythm, with the incidence generally peaking in the morning (6:00–12:00 AM) (2–6). This is related to periodic changes in sympathetic tone, blood pressure, endothelial function, platelet aggregation and plasma fibrinolytic activity during morning rise (7–9). A small number of studies additionally suggested that the afternoon or evening might be the second peak of CVD onset (6, 10, 11). Whether this bimodal distribution of rhythms is also present in Chinese STEMI patients remains unclear. The impact of STEMI onset time on the future adverse cardiac events is controversial. Some studies showed a worse prognosis for patients with night or early morning onset, while others showed that the onset time of STEMI is not associated with adverse events (3, 12–14). Therefore, the present study aimed to assess the circadian rhythm pattern of STEMI onset in the Chinese population, and to compare the impact of different onset times on the clinical outcomes of STEMI patients receiving standardized treatment.
2 Methods 2.1 Study design and participantsPatients were recruited from a multi-center, prospective, observational cohort study of 12,043 patients with AMI hospitalized at 20 tertiary hospitals from China between December 2017 and December 2019 (NCT03297164). Of those patients, 4,229 cases were excluded for the following reasons: patients with non-ST-segment elevation acute myocardial infarction (NSTEMI) who rarely present with typical chest pain (n = 3,609), patients with STEMI who have an uncertain time of onset of chest pain (n = 629). Finally, 7,805 patients with STEMI with a clear time of onset were included in the present study.
STEMI onset time was defined as the time of occurrence of the patient's perceived persistent chest pain, which was obtained from patients or their guardians. All enrolled patients were further divided into four groups according to the time of STEMI onset (6 h interval) based on previous reports (3): Group A, 12:00 AM-5:59 AM (n = 1,591); Group B, 6:00 AM-11:59 AM (n = 2,618); Group C, 12:00 PM-5:59 PM (n = 1,957); Group D, 6:00 PM-11:59 PM (n = 1,591). The study flow chart is shown in Figure 1.
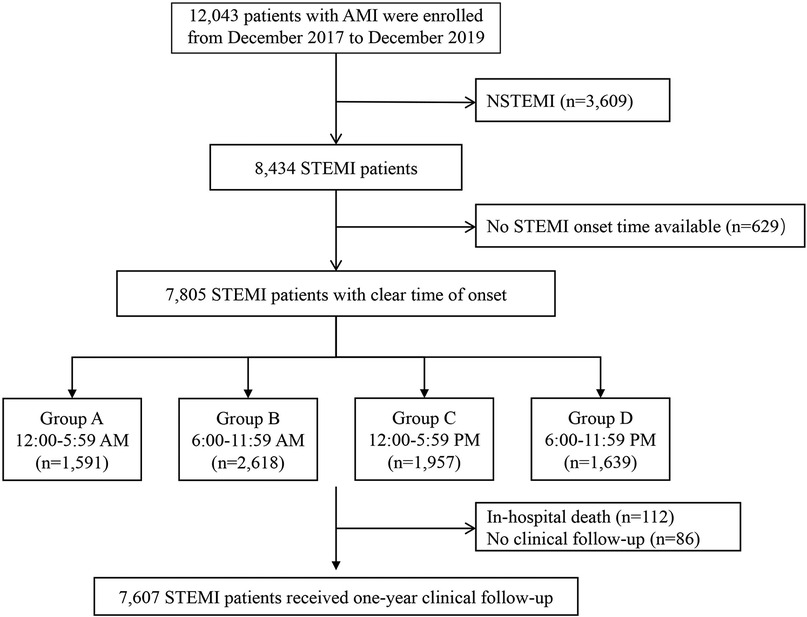
Figure 1. Study flow chart. AMI, acute myocardial infarction; NSTEMI, ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
AMI was diagnosed according to the Fourth Universal Definition of Myocardial Infarction, including STEMI and NSTEMI (15). STEMI was defined as continuous chest pain that lasted > 30 min, arrival at the hospital within 12 h of the symptom onset, ST-segment elevation > 0.1 mV in at least 2 contiguous leads, or new-onset left bundle-branch block on the 12-lead electrocardiogram (ECG), and elevated of cardiac markers (creatine kinase-myocardial band or troponin T/I) (16). NSTEMI was defined as ischemic symptoms in the absence of ST-segment elevation on the ECG, with elevated cardiac markers levels (17). Definitions of traditional coronary risk factors are included in the Supplementary Material.
The study was approved by the Ethics Committee of The Second Affiliated Hospital of Harbin Medical University (Harbin, China), complied with the ethical guidelines of the Declaration of Helsinki. All patients provided written informed consent before the start of the study.
2.2 Endpoints and clinical follow-upPatients received scheduled follow-up at 1, 6, and 12 months after discharge via clinical visit or telephone interview. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE) within one year after discharge, which were defined as the composite of all-cause death, nonfatal recurrent myocardial infarction, coronary revascularization, and nonfatal stroke. Detailed definitions of individual events are provided in the Supplementary Material.
2.3 Statistical analysisAll statistical analysis was performed using SAS V.9.4 (SAS Institute, Inc, Cary, North Carolina) and R V.4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P-value < 0.05 indicates that the difference is statistically significant. The nonparametric one-sample Kolmogorov–Smirnov test was used to assess the normality of continuous variables. Normally distributed variables are described as mean ± standard deviation (SD) and compared by using the Analysis of Variance (ANOVA), whereas non-normally distributed variables are described as median [interquartile range (IQR)] and compared by using the Kruskal Wallis H test. Categorical variables were presented as number (percentage), and compared using either a Chi-square test or Fisher exact test, as appropriate. Multivariable Cox proportional hazards model was performed to evaluate the association between onset time and clinical outcomes. The results are reported as hazard ratio (HR) and 95% CI, using Group B as reference.
For the periodic onset density of STEMI at different time periods, the optimal parameters fit the von Mises distribution using maximum likelihood estimation, and peak shape was analyzed using the Kolmogorov-Smirnov test to assess whether the circadian pattern of STEMI onset had uniform (no peak), unimodal (one peak) or bimodal distribution (two peaks). We performed stratified analysis by day of the week, age, sex, and coronary risk factors, evaluated the heterogeneity in circadian rhythm distribution between subgroups.
3 Results 3.1 Baseline characteristicsFrom December 2017 to December 2019, a total of 7,805 patients with STEMI were included in the final analysis. The median age of patients was 61.7 years, 5,574 (74%) were male, and 2,618 (33.4%) presented with symptoms between 12:00 AM and 5:59 AM. The baseline clinical characteristics of patients in different groups are summarized in Table 1. Compared to the other three time periods, patients in Group A were more likely to be older (P < 0.001), and had a longer time from symptom onset to hospital admission (P < 0.001). There were no statistically significant differences in receiving revascularization therapy among the four groups (P > 0.05).
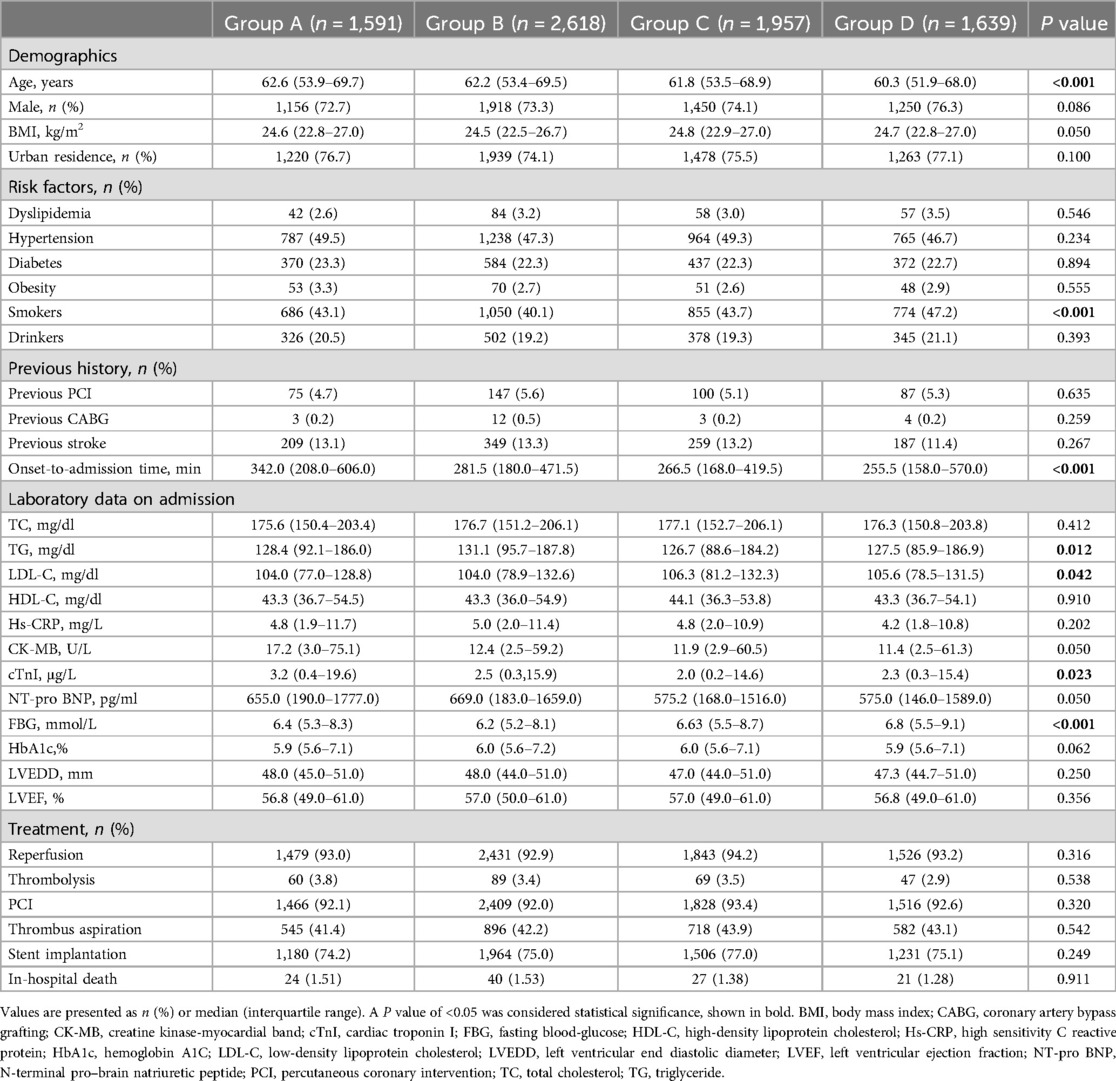
Table 1. Baseline characteristics among groups based on time of STEMI onset.
3.2 Bimodal circadian rhythm pattern of STEMI onsetFigure 2 shows the best-fit curve for the temporal distribution of STEMI onset. In the overall study cohort, the onset of STEMI exhibited a bimodal distribution over 24 h, with a significant primary peak at 8:38 AM [95% confidence interval (CI): 7:49 to 9:28 AM] and a small second peak at 12:55 PM (95% CI: 7:39 AM to 18:10 PM) (bimodal: P < 0.001).
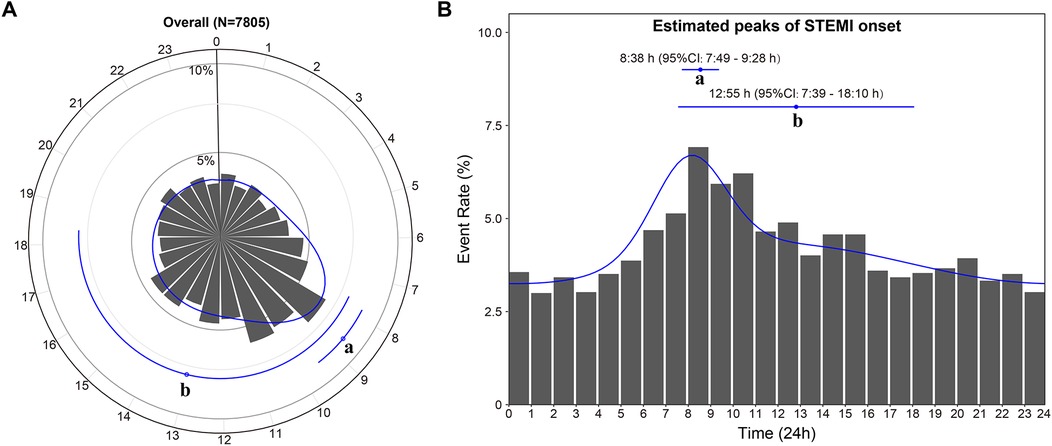
Figure 2. Circadian rhythm pattern of STEMI onset in the overall study cohort. The primary peak (a) and secondary peak (b) of the onset of STEMI within 24 h are depicted in the circle plot (A) and histogram (B). The estimated peak onset times and 95% CIs are represented by the dots with error lines, while the solid line corresponds to the fitted von Mises distribution. CI, confidence interval; STEMI, ST-segment elevation myocardial infarction.
3.3 Circadian rhythm variations of the day of the week and the quarters of the yearThe onset of STEMI still had two peaks on each day of the week (bimodal, all P < 0.001), with timing varying depending on the day (Figure 3). On weekdays, the time of the first peak remained consistent, at around 8:30 AM. And the morning peak became sharper on Monday and Tuesday (Figures 3A,B). Compared with other dates, the Sunday delayed the occurrence times of first peak [10:12 AM (95% CI: 7:04 AM to 13:20 PM)] and second peak [22:30 PM (95% CI: 21:47 to 23:13 PM)], and exhibited a highly concentrated evening peak time (Figure 3G). Interestingly, there is still a bimodal distribution of STEMI onset in quarter 1 (January-March), quarter 2 (April-June), and quarter 3 (July-September) (bimodal, all P < 0.001) (Supplementary Figures S1A–C), whereas the unimodal distribution of STEMI onset can be found in quarter 4, with only one morning peak at 8:59 AM (bimodal: P > 0.05) (Supplementary Figure S1D).

Figure 3. Circadian rhythm pattern of STEMI onset based on the day of the week. Panel (A–G) shows circular plots fitting the von Mise distribution based on the day of the week. The estimated peak onset times and 95% CIs are shown below each circular plot. P values are used to assess whether the circadian rhythm pattern of STEMI onset was uniform, unimodal or bimodal. CI, confidence interval; STEMI, ST-segment elevation myocardial infarction.
3.4 Bimodal circadian rhythms in different subgroupsEach subgroup's STEMI distribution still showed a similar bimodal pattern, with the morning peaks showing relatively fixed time and being much clearer (all P < 0.001) (Figure 4). We noted some differences in the secondary peaks among the three STEMI subgroups. The second peak's time was shifted forward and close to the primary peak in STEMI patients aged ≥ 65 years (Figure 4B). Both peaks tend to occur in the morning (primary peak at 8:32 AM and secondary peak at 10:33 AM). There was no difference between the male and the female. In patients with dyslipidemia or blood glucose ≥ 140 mg/dl, the second peak of STEMI onset both appeared somewhat late (Figures 4G,J).
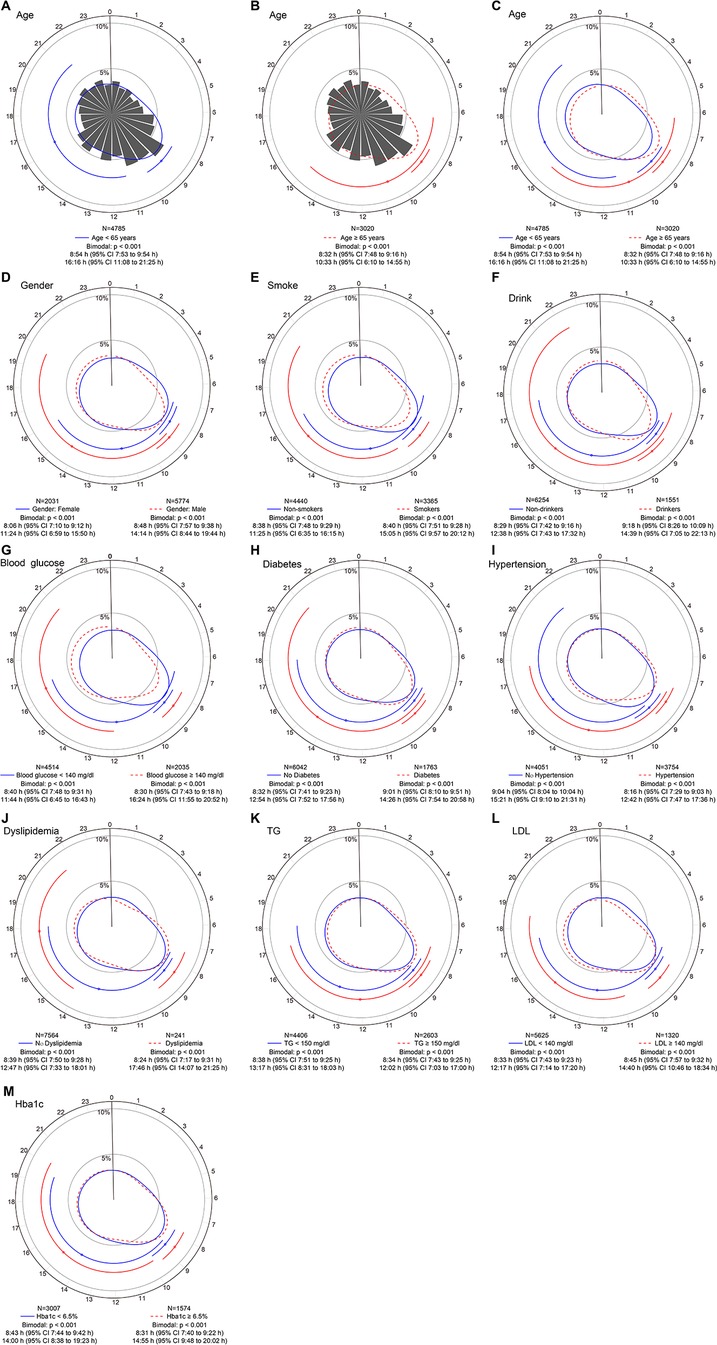
Figure 4. Circadian rhythm pattern of STEMI onset based on baseline factors. Panel (A–C) shows circular plots fitting the von Mise distribution in subgroups aged ≥65 years and <65 years. Panel (D–M) show circular plots fitting the von Mise distribution in subgroups stratified by sex, smoking habits, drinking habits, blood glucose levels, diabetes, hypertension, dyslipidemia, triglyceride (TG) levels, low-density lipoprotein (LDL) levels and glycated hemoglobin (HbA1c) levels. The estimated peak onset times and 95% CIs are shown below each circular plot. p values are used to determine whether the circadian rhythm pattern of STEMI onset was uniform, unimodal or bimodal. CI, confidence interval; STEMI, ST-segment elevation myocardial infarction.
3.5 Clinical outcomesThe median follow-up of 1-year was completed in 97.5% (7,607/7,805) of patients. There were 112 patients (1.4%) who died prior to discharge, and there was no significant difference in in-hospital mortality among the four groups (P = 0.911) (Table 1). There was no significant difference in the incidence of periprocedural complications (all P > 0.05) (Supplementary Table S1) and bleeding during hospitalization (P > 0.05) (Supplementary Table S2) among the four groups. The medication at discharge was also comparable (all P > 0.05) (Supplementary Table S3). No difference was also observed in angiographic and PCI-related results (Supplementary Table S4).
During the one-year follow-up period, 400 patients (5.2%) experienced MACCE after discharge, including 186 deaths (2.4%), 45 recurrent myocardial infarctions (0.65%), 169 revascularizations (2.1%), and 52 strokes (0.5%). There were no significant differences in the primary endpoint at one year among the four groups (P = 0.905) (Table 2). Multivariate Cox regression models (Supplementary Table S5) showed that the independent predictors of 1-year MACE were age (HR: 1.03; 95% CI: 1.02–1.05) and cardiogenic shock at admission (HR: 129.78; 95% CI: 22.45–613.66). Age (HR: 1.04; 95% CI: 1.01–1.07), cardiogenic shock at admission (HR: 57.99; 95% CI: 5.66–594.12), and the femoral approach (HR: 1.98; 95% CI: 1.13–3.49, radial approach as reference) can independently predict all-cause death. The timing of STEMI onset still cannot independently predict MACCE and all-cause death after adjustment for these variables. In addition, when we conducted the analysis only with patients admitted before 2019, the result was similar to those in the main analyses (Supplementary Table S6).
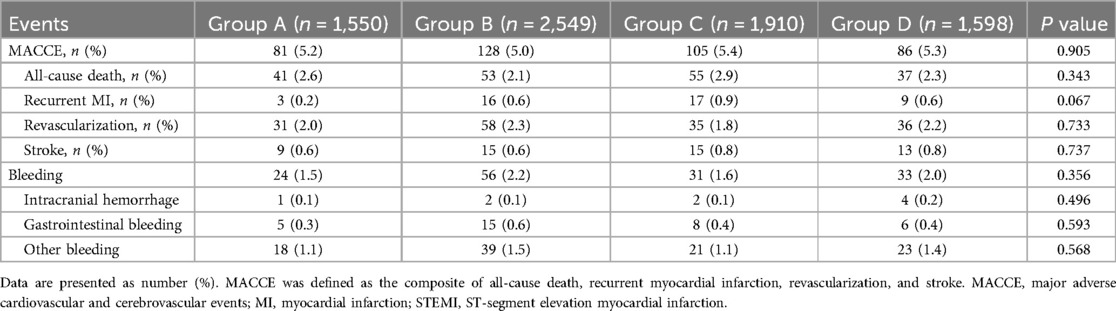
Table 2. Clinical outcomes at one year according to time of STEMI onset.
4 DiscussionIn this large multi-center, prospective study, circadian rhythm variation in symptom onset was assessed in 7,805 hospitalized STEMI patients, expanding previous clinical understanding. The main findings of this study were as follows: (1) In the Chinese population, the onset of STEMI exhibited a bimodal circadian rhythm pattern with a primary peak at 8:38 AM and a secondary peak at 12:55 PM. (2) For the onset of STEMI on every day of the week, the best-fit distribution is all shown as a bimodal curve. The onset time of the two peaks was delayed on Sunday, and the secondary peak became clearer. (3) The bimodal distribution pattern of STEMI onset persisted in subgroups of age, sex, and other traditional coronary risk factors. (4) There was no significant correlation between symptom onset time and MACCE.
4.1 Circadian rhythm of STEMI onsetWe discovered a bimodal distribution of STEMI case density in the Chinese population, with an early peak of symptom onset at 8:38 AM, a subsequent spike at 12:55 PM, and a progressive decline in ischemia in the evening and at night. This finding was in line with previous studies that reported acute cardiovascular events had a circadian pattern, with symptoms most commonly occurring between 6:00 AM and 12:00 AM (2–6). Secondary peaks in the afternoon or evening have also been reported in minority studies (10). On the contrary, the investigation of Masiewicz and Sari et al. showed that the afternoon was the most common time for the circadian rhythm variant of myocardial infarction (18, 19). Some controversies remain regarding the peak distribution of the onset time of myocardial infarction. Most of the above results convert continuous-time variables into quadruple-categorical data, which could lead to information loss and thus reduce statistical power (20). Consequently, we remedied these shortcomings by assessing circadian rhythm variations in STEMI incidents among a large prospective myocardial infarction cohort using a more sensitive and robust statistical approach (i.e., maximum likelihood estimation fitting the von Mises distribution). In summary, our findings indicated a characteristic bimodal pattern of STEMI onset across 24 h, albeit with a less prominent and earlier peak in the afternoon.
The primary peak of STEMI onset may be correlated with changes in some physiological parameters. In the morning, sympathetic nerve activity, catecholamine and cortisol levels, blood pressure, heart rate, and coronary artery tone are all increased (7). Furthermore, there is an elevated risk of thrombosis due to increased platelet aggregation and adhesion, decreased plasma fibrinolytic activity (9), and decreased vascular endothelial function after morning rise (8). These physiological changes may lead to an increased likelihood of unstable plaque rupture and myocardial infarction in the morning. As confirmed by the OCT study, atherosclerotic plaque rupture is more frequent in the morning (21).
A novel finding of this study is the observation of a second peak in STEMI onset at 12:55 PM. Perhaps phenomenon correlates with the traditional Chinese eating habits. In China, the lunch is generally considered the largest meal of the day. Lipovetzky's study showed a significantly increased risk of acute coronary syndrome after intake of large meals (22). Furthermore, increased physical activity in the afternoon can also trigger a small degree of myocardial ischemia (23).
4.2 Heterogeneity of bimodal circadian rhythm distributionThe bimodal distribution pattern of STEMI onset was unaffected by pre-determined stratification variables, with only the minority of subgroups exhibiting slightly altered times of the second peak. A regular sleep-wake schedule may cause similar primary peak times on weekdays. The sudden increase in physical activity and mental stress from a rest day to a weekday encourages leukocyte aggregation in atherosclerosis, contributing to vascular inflammation and plaque instability and raising the risk of myocardial infarction (24), which explains the possible mechanism for the sharpest primary peaks on Monday and Tuesday. On Sunday, both peaks were delayed, with the primary peak late by close to 1.5 h, and the second peak occurred at night (22:30 PM) and was more apparent. Two factors may explain this phenomenon: Firstly, on work-free days, people wake up about one hour forty minutes later than on work days (25), whereas the peak of myocardial infarction incidence is mostly around one to two hours after the person wakes up (26). Secondly, weekend evenings are typically used for social activities and family get-together. During these times, people are prone to mood swings and overeating, which puts additional strain on their hearts (27). These findings may have potential implications for medical resource allocation to weekends, especially weekends night, when hospitals face shortages of doctors and interventional cardiologists during these times. Females may account for a significant portion of the second peak, according to a small sample study that included 522 STEMI patients (28). Nevertheless, our results agree with those of Xu's (3), indicating that male and female circadian rhythm variations are consistent. The results about age subgroups align with earlier data that indicated elderly AMI patients (>65 years) have nearly no late peak and a very pronounced early peak (29).
4.3 Timing of STEMI onset and clinical prognosisThe effect of the timing of STEMI onset on prognosis remains controversial. A recent study showed nocturnal onset was independently linked to an increased risk of long-term adverse cardiovascular events (12). However, the study by Sager et al. demonstrated that the timing of onset was not significantly associated with infarct size or 5-year all-cause mortality (13). Our large-sample, multi-center study found no association between the timing of STEMI onset and mortality or the incidence of MACCE, even after adjusting for cardiovascular risk factors and information related to coronary intervention. In the present study, cardiogenic shock was an independent predictor of 1-year MACE or mortality in patients with STEMI, supporting the previous studies that cardiogenic shock is the primary cause of poor prognosis after myocardial infarction (30, 31). Tokarek et al. found that the femoral approach was independently associated with higher periprocedural mortality compared with radial approach in patients with STEMI (32), along with our results.
In general, longer symptom-to-admission times were associated with larger myocardial infarct size (33) and worse prognosis (34). Previous studies have shown that the severity of coronary lesions and the door-to-balloon time balloon time were important factors affecting the prognosis of STEMI (35, 36). In addition, previous studies have also found that STEMI patients presenting during off-hours have longer door-to-balloon times and poorer clinical outcomes (37, 38). Although patients with onset time at 0:00–5:59 AM have longer prehospital delays, the time of STEMI onset usually did not significantly impact our treatment strategy, as door-to-balloon time, data related to PCI, and the medication at discharge were comparable among the four groups. Notably, onset-to-admission time and door-to-balloon time did not significantly affect the MACCE or mortality in our study. STEMI is an acute cardiovascular event, and timely medical intervention in the hospital may weaken the correlation between circadian rhythm and poor prognosis. This suggests the importance of raising awareness of STMEI symptoms and activating the medical system as early as possible. Notedly, further confirmation of these findings is necessary through a larger prospective study.
5 LimitationsThe limitations of the present study are as follows. Firstly, this was a secondary analysis of multi-center research, with a few patients (7.5%) not having a clear time of onset of persistent chest pain. Secondly, the time of symptom onset was self-reported by the patients and dependent on subjective perceptions. Thirdly, this study did not consider the level of physical activity prior to symptom onset, as well as the patients' working hours and work status. Disruptions in physiological rhythms may affect our results. Finally, all patients were enrolled in China. So, our findings might not be generalizable to patients in other countries. Although the present study was based on a relatively large STEMI cohort, the sample size was still relatively low. Further prospective studies with larger sample sizes are warranted to investigate the circadian rhythm of myocardial infarction to confirm the generalizability of our results.
6 ConclusionIn the Chinese population, the onset of STEMI followed a specific circadian rhythm pattern with a bimodal distribution, including a clear primary peak at 8:38 AM and a less clear secondary peak at 12:55 PM. The timing of STEMI onset did not affect our treatment strategy and 1-year clinical outcomes.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statementThe studies involving humans were approved by The study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (Harbin, China), and complied with the ethical guidelines of the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsYG: Data curation, Writing – original draft. LC: Writing – original draft. LL: Data curation, Writing – original draft. ZW: Data curation, Writing – original draft. CF: Supervision, Writing – review & editing. BY: Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key Research and Development Program of China (grant number 2016YFC1301100 to Dr. Yu), National Natural Science Foundation of China (grant nos. 62135002 to Dr. Yu and 82202286 to Dr. Fang), the Natural Science Foundation of Heilongjiang Province (YQ2023H014 to Dr. Fang), Heilongjiang Postdoctoral Fund (LBH-Z21186 to Dr. Fang), and Innovation Foundation of Harbin Medical University (2022-KYYWF-0282 to Dr. Fang).
AcknowledgmentsThe authors thank all the investigators and all supporting staff.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1393390/full#supplementary-material
References1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
PubMed Abstract | Crossref Full Text | Google Scholar
3. Xu C, Dong M, Sun L, Deng Y, Zhou J, Yuan Z, et al. Sex differences in the impact of day/night distribution of ST-segment elevation myocardial infarction onset on in-hospital outcomes: findings from the improving care for cardiovascular disease in China-acute coronary syndrome project. Sleep Med. (2022) 95:112–9. doi: 10.1016/j.sleep.2022.04.011
PubMed Abstract | Crossref Full Text | Google Scholar
4. Turin TC, Kita Y, Rumana N, Takashima N, Ichikawa M, Sugihara H, et al. Morning surge in circadian periodicity of ischaemic stroke is independent of conventional risk factor status: findings from the takashima stroke registry 1990–2003. Eur J Neurol. (2009) 16(7):843–51. doi: 10.1111/j.1468-1331.2009.02605.x
PubMed Abstract | Crossref Full Text | Google Scholar
5. Portaluppi F, Hermida RC. Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy. Adv Drug Deliv Rev. (2007) 59(9–10):940–51. doi: 10.1016/j.addr.2006.10.011
PubMed Abstract | Crossref Full Text | Google Scholar
6. Delisle BP, George AL Jr., Nerbonne JM, Bass JT, Ripplinger CM, Jain MK, et al. Understanding circadian mechanisms of sudden cardiac death: a report from the national heart, lung, and blood institute workshop, part 2: population and clinical considerations. Circ Arrhythm Electrophysiol. (2021) 14(11):e010190. doi: 10.1161/CIRCEP.121.010190
PubMed Abstract | Crossref Full Text | Google Scholar
8. Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, et al. Early morning attenuation of endothelial function in healthy humans. Circulation. (2004) 109(21):2507–10. doi: 10.1161/01.CIR.0000128207.26863.C4
PubMed Abstract | Crossref Full Text | Google Scholar
9. Budkowska M, Lebiecka A, Marcinowska Z, Wozniak J, Jastrzebska M, Dolegowska B. The circadian rhythm of selected parameters of the hemostasis system in healthy people. Thromb Res. (2019) 182:79–88. doi: 10.1016/j.thromres.2019.08.015
PubMed Abstract | Crossref Full Text | Google Scholar
10. Edahiro R, Sakata Y, Nakatani D, Suna S, Usami M, Matsumoto S, et al. Association of lifestyle-related factors with circadian onset patterns of acute myocardial infarction: a prospective observational study in Japan. BMJ Open. (2014) 4(6):e005067. doi: 10.1136/bmjopen-2014-005067
PubMed Abstract | Crossref Full Text | Google Scholar
11. Casetta I, Granieri E, Fallica E, la Cecilia O, Paolino E, Manfredini R. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol. (2002) 59(1):48–53. doi: 10.1001/archneur.59.1.48
PubMed Abstract | Crossref Full Text | Google Scholar
12. Peng H, Sun Z, Di B, Ding X, Chen H, Li H. Contemporary impact of circadian symptom-onset patterns of acute ST-segment elevation myocardial infarction on long-term outcomes after primary percutaneous coronary intervention. Ann Med. (2020) 53(1):247–56. doi: 10.1080/07853890.2020.1863457
PubMed Abstract | Crossref Full Text | Google Scholar
13. Sager HB, Husser O, Steffens S, Laugwitz KL, Schunkert H, Kastrati A, et al. Time-of-day at symptom onset was not associated with infarct size and long-term prognosis in patients with ST-segment elevation myocardial infarction. J Transl Med. (2019) 17(1):180. doi: 10.1186/s12967-019-1934-z
PubMed Abstract | Crossref Full Text | Google Scholar
14. Holmes DR J, Aguirre FV, Aplin R, Lennon RJ, Nestler DM, Bell MR, et al. Circadian rhythms in patients with ST-elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. (2010) 3(4):382–9. doi: 10.1161/CIRCOUTCOMES.109.913343
PubMed Abstract | Crossref Full Text | Google Scholar
15. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. (2018) 72(18):2231–64. doi: 10.1016/j.jacc.2018.08.1038
PubMed Abstract | Crossref Full Text | Google Scholar
16. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr., Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. (2013) 127(4):e362–425. doi: 10.1161/CIR.0b013e3182742cf6
PubMed Abstract | Crossref Full Text | Google Scholar
17. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr., Ganiats TG, Holmes DR Jr., et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. (2014) 64(24):e139–28. doi: 10.1016/j.jacc.2014.09.017
PubMed Abstract | Crossref Full Text | Google Scholar
18. Masiewicz S, Gutovitz S, Hart L, Leaman SM, Jehle D. Presentation times of myocardial infarctions to the emergency department: disappearance of the morning predominance. J Emerg Med. (2020) 58(5):741–8. doi: 10.1016/j.jemermed.2020.01.002
PubMed Abstract | Crossref Full Text | Google Scholar
19. Sari I, Davutoglu V, Erer B, Tekbas E, Ucer E, Ozer O, et al. Analysis of circadian variation of acute myocardial infarction: afternoon predominance in Turkish population. Int J Clin Pract. (2009) 63(1):82–6. doi: 10.1111/j.1742-1241.2008.01717.x
PubMed Abstract | Crossref Full Text | Google Scholar
20. Naggara O, Raymond J, Guilbert F, Roy D, Weill A, Altman DG. Analysis by categorizing or dichotomizing continuous variables is inadvisable: an example from the natural history of unruptured aneurysms. AJNR Am J Neuroradiol. (2011) 32(3):437–40. doi: 10.3174/ajnr.A2425
PubMed Abstract | Crossref Full Text | Google Scholar
21. Araki M, Yonetsu T, Kurihara O, Nakajima A, Lee H, Soeda T, et al. Circadian variations in pathogenesis of ST-segment elevation myocardial infarction: an optical coherence tomography study. J Thromb Thrombolysis. (2021) 51(2):379–87. doi: 10.1007/s11239-020-02220-6
PubMed Abstract | Crossref Full Text | Google Scholar
22. Lipovetzky N, Hod H, Roth A, Kishon Y, Sclarovsky S, Green MS. Heavy meals as a trigger for a first event of the acute coronary syndrome: a case-crossover study. Isr Med Assoc J. (2004) 6(12):728–31.15609883
PubMed Abstract | Google Scholar
23. Krantz DS, Kop WJ, Gabbay FH, Rozanski A, Barnard M, Klein J, et al. Circadian variation of ambulatory myocardial ischemia. Triggering by daily activities and evidence for an endogenous circadian component. Circulation. (1996) 93(7):1364–71. doi: 10.1161/01.cir.93.7.1364
PubMed Abstract | Crossref Full Text | Google Scholar
24. Hinterdobler J, Schott S, Jin H, Meesmann A, Steinsiek AL, Zimmermann AS, et al. Acute mental stress drives vascular inflammation and promotes plaque destabilization in mouse atherosclerosis. Eur Heart J. (2021) 42(39):4077–88. doi: 10.1093/eurheartj/ehab371
PubMed Abstract | Crossref Full Text | Google Scholar
25. Skeldon AC, Dijk DJ. Weekly and seasonal variation in the circadian melatonin rhythm in humans: entrained to local clock time, social time, light exposure or sun time? J Pineal Res. (2021) 71(1):e12746. doi: 10.1111/jpi.12746
PubMed Abstract | Crossref Full Text | Google Scholar
26. Goldberg RJ, Brady P, Muller JE, Chen ZY, de Groot M, Zonneveld P, et al. Time of onset of symptoms of acute myocardial infarction. Am J Cardiol. (1990) 66(2):140–4. doi: 10.1016/0002-9149(90)90577-N
PubMed Abstract | Crossref Full Text | Google Scholar
28. Itaya H, Takagi T, Sugi K, Nakamura M. Contents of second peak in the circadian variation of acute myocardial infarction in the Japanese population. J Cardiol. (2012) 59(2):147–53. doi: 10.1016/j.jjcc.2011.11.011
PubMed Abstract | Crossref Full Text | Google Scholar
29. Yamasaki F, Seo H, Furuno T, Hamashige N, Kawai K, Owaki T, et al. Effect of age on chronological variation of acute myocardial infarction onset: study in Japan. Clin Exp Hypertens. (2002) 24(1–2):1–9. doi: 10.1081/ceh-100108710
PubMed Abstract | Crossref Full Text | Google Scholar
30. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. (2012) 367(14):1287–96. doi: 10.1056/NEJMoa1208410
PubMed Abstract | Crossref Full Text | Google Scholar
31. McNamara RL, Kennedy KF, Cohen DJ, Diercks DB, Moscucci M, Ramee S, et al. Predicting in-hospital mortality in patients with acute myocardial infarction. J Am Coll Cardiol. (2016) 68(6):626–35. doi: 10.1016/j.jacc.2016.05.049
PubMed Abstract | Crossref Full Text | Google Scholar
32. Tokarek T, Dziewierz A, Plens K, Rakowski T, Dudek D, Siudak Z. Radial approach reduces mortality in patients with ST-segment elevation myocardial infarction and cardiogenic shock. Pol Arch Intern Med. (2021) 131(5):421–8. doi: 10.20452/pamw.15886
PubMed Abstract | Crossref Full Text | Google Scholar
33. Redfors B, Mohebi R, Giustino G, Chen S, Selker HP, Thiele H, et al. Time delay, infarct size, and microvascular obstruction after primary percutaneous coronary intervention for ST-segment-elevation myocardial infa
留言 (0)