Prior studies suggest lymphopenia following radiation therapy may impact toxicity and cancer control. Lymphocytes are highly radiosensitive, and exposure even to low doses of radiation therapy (RT) (<2 Gy) poses a significant risk of lymphocyte death (1). Given their role in mediating the immune response to cancer, radiation-related lymphopenia (RRL) has the potential to impact disease-specific survival in patient cohorts treated with radiation regimens (1). In a study of solid tumors of the brain, head and neck, thorax, and abdomen, lymphopenia cases categorized as Grade 3 or higher by the CTCAEv.4 following radiation therapy were common and shown to negatively impact overall survival rates (2–4).
In prostate cancer, chronic RRL has been noted in patients treated with conventionally fractionated pelvic radiation therapy (5). More specifically, pelvic nodal irradiation (PNI) was found to be correlated with RRL in patients with prostate cancer, and increased treatment volume was positively associated with a higher RRL burden (6, 7). The absolute lymphocyte count (ALC) nadir typically occurs during or immediately after radiation. The effect of utilizing hypofractionated high integral dose therapies in prostate cancer, such as stereotactic body radiation therapy (SBRT), on RRL incidence and severity is unknown.
SBRT precisely targets tumors with intrafractional image guidance in up to five fractions (8). When compared to chemotherapy, patients treated for pancreatic cancer with SBRT experienced a lower incidence of lymphopenia overall, suggesting that differences in treatment regimen can have a significant effect on lymphocyte levels (9). When compared to conventionally fractioned radiation therapy (CFRT) for pancreatic cancer, SBRT was again found to result in a lower lymphopenia incidence, with acute Grade 3/4 lymphopenia of 6% for SBRT versus 38% for CFRT (10, 11).
As shown in the literature, the occurrence of more severe lymphopenia can potentially impact the efficacy of current and future courses of treatment. In a patient cohort treated with SBRT for lung cancer, lymphopenia following SBRT was found to be associated with a poorer prognosis for survival (12). This study identified low pre-treatment lymphocyte counts, longer duration of treatment, and higher mean dose as predictors for the possibility of developing lymphopenia post-SBRT (12). Given this, delivering doses of radiation to avoid lymphocyte-rich structures has been thought to improve outcomes in solid malignancies (9).
Radiation therapy has been increasingly utilized in combination with immunotherapy, and lymphopenia has been found to be an indicator of poor prognosis post-treatment with immunotherapy (13). Patients who developed lymphopenia after radiation therapy and later received immunotherapy treatment, had worse outcomes when compared to patients who did not experience RRL (13). Further characterizing the impacts of RRL from varied radiation treatment types could be used to improve outcomes when utilizing RT in combination with other classes of agents. This prospective study sought to evaluate the impact of prostate SBRT plus or minus supplemental pelvic nodal radiation (PNI) on chronic RRL incidence and severity.
MethodsBetween 2012 and 2023, serial serum ALCs were measured in 226 men treated at MedStar Georgetown with robotic SBRT using the CyberKnife® (CK) (36.25 Gy in 5 fractions) alone or CK (19.5 Gy in 3 fractions) followed by supplemental PNI using VMAT (37.5–45.0 Gy in 15–25 fractions) per an institutional protocol (IRB#: 2012-1175) (14, 15). Patients were not randomized to treatment groups.
Four to six gold fiducials were placed into the prostate. Seven days after fiducial placement, patients underwent magnetic resonance (MR) imaging followed shortly thereafter by a thin-cut computed tomography (CT) scan. Fused CT and MR images were used for treatment planning. The clinical target volume (CTV) included the prostate and the proximal seminal vesicles (to the point where the seminal vesicles separate). The planning target volume (PTV) equaled the CTV expanded 3 mm posteriorly and 5 mm in all other dimensions. The prescription dose was 36.25 Gy to the PTV delivered in five fractions of 7.25 Gy, corresponding to a tumor equivalent dose in 2Gy fractions (EQD2) of approximately 85–90 Gy assuming an α/β ratio of 1.5.
Treatment plans were composed of hundreds of pencil beams using variable-sized circular collimators (Iris, Accuray) to generate highly conformal plans using Multiplan® or Volo® (Accuray Inc., Sunnyvale, CA) inverse treatment planning. Plans were inhomogeneous by design to minimize dose to adjacent critical structures. Radiation was delivered every other day to a mean prescription isodose line of 83% in 5 approximately 35-min treatments. On average, 100–200 beams were employed. Target position was verified every 30–60 s during treatment using paired, orthogonal kV images (16).
For the SBRT + PNI patients, the CTV1 included the prostate, areas of radiographic extracapsular extension, and seminal vesicles proximal to the point of separation. The SBRT PTV1 was equal to the CTV1 expanded 3 mm posteriorly and 5 mm in all other dimensions. The prescription dose was 19.5 Gy to PTV1 delivered in 3 fractions of 6.5 Gy over 3–5 days. Following SBRT, intensity-modulated radiation treatment (IMRT) treatment was initiated the following week. PTV2 included the prostate, entire seminal vesicles, and RTOG consensus pelvic nodes with a margin of 1.0 cm around CTV1, except at the rectal interface where a margin of 0.5 cm was added. The superior extent of disease was the sacrum-L5 junction. Daily doses of 1.8 to 2.5 Gy were delivered to PTV2 5 days a week to a total dose of 37.5–45 Gy in 15–25 fractions. One hundred percent of PTV2 received at least 95% of the prescription dose, and 5% of the volume received no more than 105% of the prescription dose.
Baseline ALC (k/μl) was measured on a complete blood count (CBC) panel 1–2 hr prior to the first fraction of robotic SBRT and at each follow-up appointment (1, 3, 6, 9, 12, 18, and 24 months). Lymphopenia was graded using the CTCAEv.4: Grade 1 (0.8–1.0 k/μl), Grade 2 (0.5–0.8 k/μl), Grade 3 (0.2–0.5 k/μl), and Grade 4 (<0.2 k/μl). To compare two different treatment groups, the Wilcoxon signed-rank test was used. A p-value of <0.05 determined statistical significance.
ResultsDemographic and clinical patient characteristics are presented in Table 1. Of 226 patients (SBRT alone: n = 169, SBRT + PNI: n = 57), the median age was 72 years, and 45% of patients were non-White. High-risk prostate cancer and utilization of androgen deprivation therapy (ADT) were more common in those who received PNI (p < 0.01) (Table 1). The median pelvic nodal PTV volume was 697.4 ± 129.8 cc.
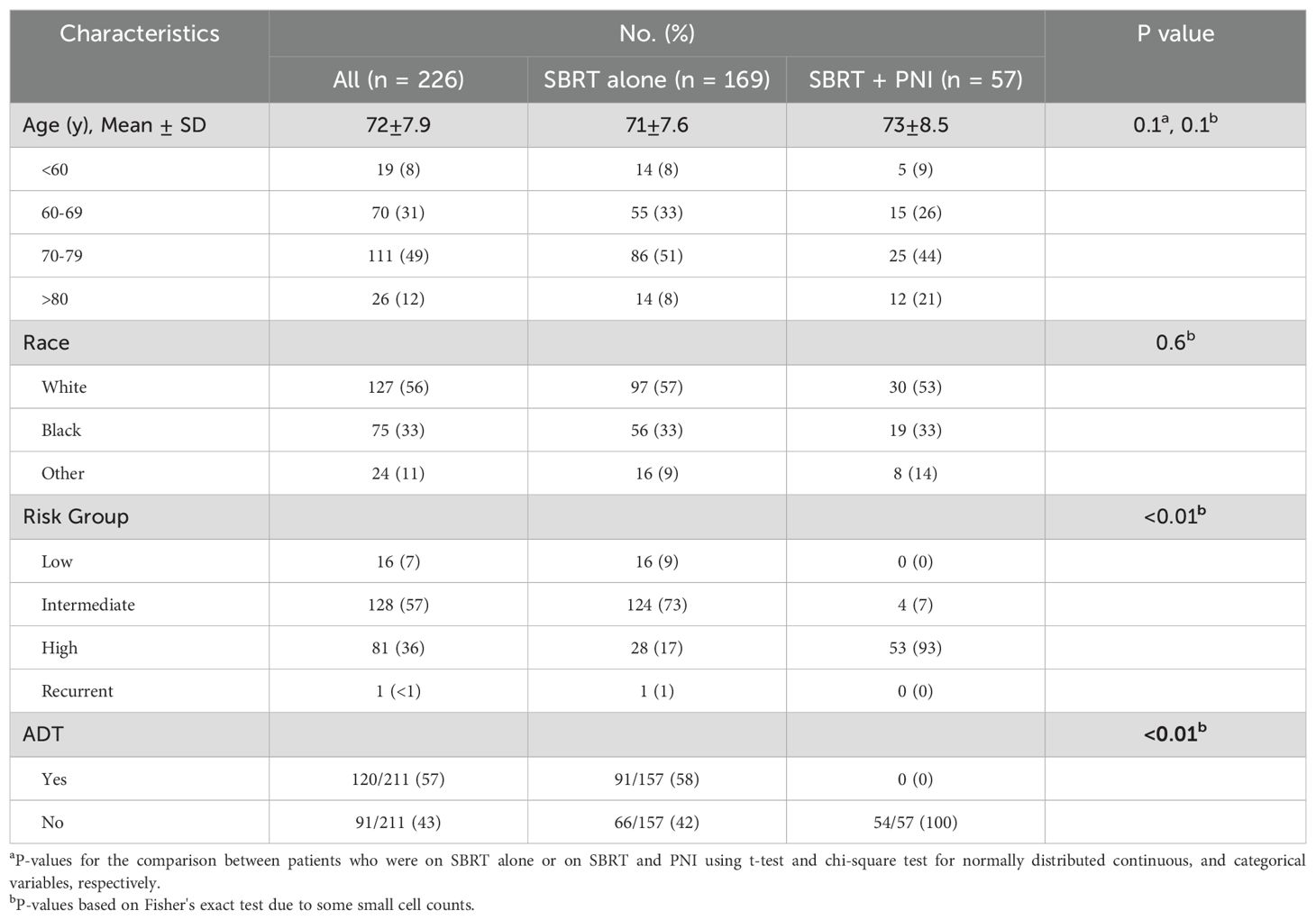
Table 1. Demographic and clinical characteristics.
Baseline lymphopenia was uncommon and of low grade (Table 2). For the SBRT alone group, the baseline ALC of 1.7 k/μl decreased by 21% to 1.4 k/μl at 3 months and then stabilized (Table 3A; Figure 1). In the two years following SBRT, 23% (n = 38) of these men experienced lymphopenia (Grade 1: n = 25; Grade 2: n = 13) (Table 2). No SBRT alone patient presented with a Grade 3 or Grade 4 lymphopenia over the 24-month time course studied (Table 2).

Table 2. Lymphopenia (CTCAEv.4) categories: toxicities by grade.
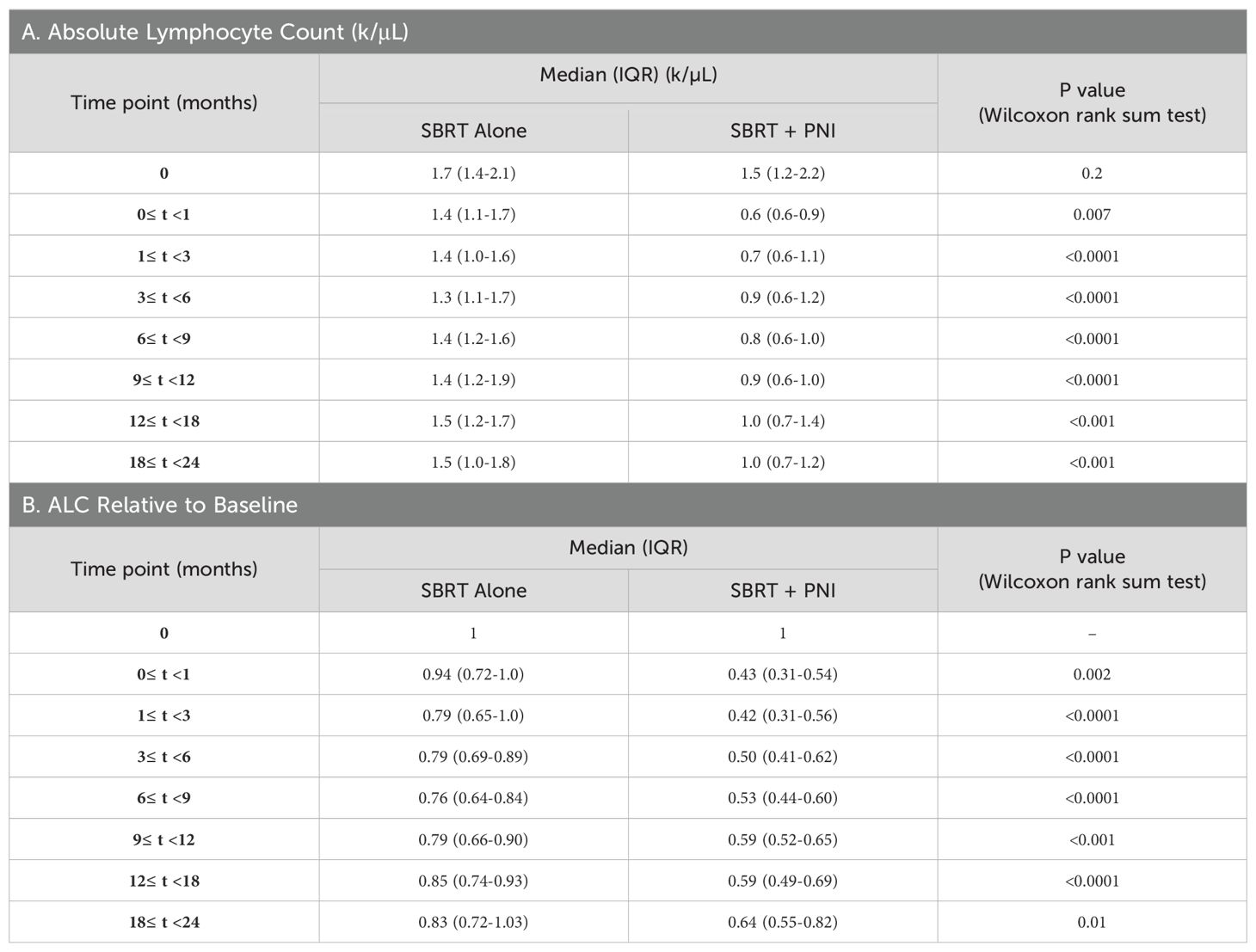
Table 3. Median (IQR) plots.
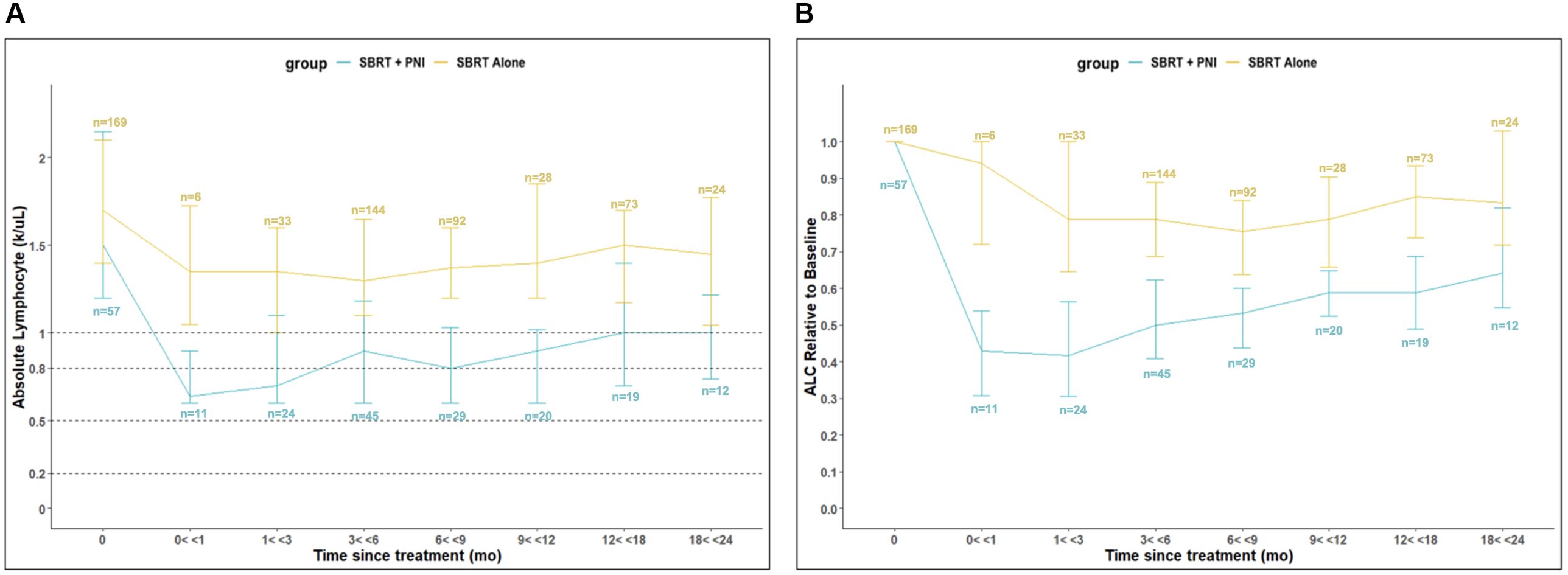
Figure 1. Absolute Lymphocyte Count (ALC) Relative to Baseline in SBRT vs. SBRT + PNI Patients. (A) Abosolute Lymphocyte Count (ALC) (k/uL). (B) ALC Relative to Baseline. An absolute number (n) of patients is shown above or below respective timepoints, also shown in Supplementary Table 1.
SBRT + PNI had significantly lower ALC and a greater decrease in ALC relative to individual baseline values throughout the 2-year follow-up period. The baseline ALC of 1.5 k/µl decreased by 57% to 0.6 k/µl at 3 months and recovered to a 36% decrease from baseline (1.0 k/µl) at 24 months (Tables 3A, B; Figure 1). In the 2 years following SBRT, 82% (n = 47) of these men experienced lymphopenia (Grade 1: n = 13; Grade 2: n = 27; Grade 3: n = 7) (Table 2). Notably, 12% of the men treated with SBRT + PNI experienced Grade 3 lymphopenia. No patient experienced Grade 4 RRL over the 24-month follow-up period (Table 2).
DiscussionThis study demonstrates that within our elderly patient cohort, the low incidence of high-grade lymphopenia further supports the safety of prostate SBRT, plus or minus PNI, for the treatment of prostate cancer. However, RRL was more severe when PNI was utilized. When compared to SBRT alone, patients who received SBRT + PNI experienced a significantly greater decrease in ALC relative to baseline throughout the follow-up period. This result suggests that in combination with SBRT, supplemental PNI increases the severity of lymphopenia post-RT.
Patients treated with SBRT alone experienced a maximum decline to the lowest median ALC by 6 months post-treatment, after which recovering to a median of 1.5 k/µl (88% of time point 0 value) at 12 months and plateauing through 24 months. This reflects a nearly full recovery to baseline lymphocyte populations after 12 months. Additionally, median ALC values in this group are graded as “No Toxicity” across the entire 24-month recovery period. Patients treated with SBRT + PNI experienced a maximum decline in ALC by 1 month post-treatment, falling to the lowest median value of 0.6 k/µl, which is considered Grade 2 lymphopenia. Throughout the recovery period, median ALC values were significantly lower than those of the SBRT alone group, and by the 24-month time point, they had only reached 1.0 k/µl, 67% of the time point 0 value. This suggests a slower and less robust recovery of lymphocyte populations in those treated with supplemental PNI.
The low rate of high-grade lymphopenia with prostate SBRT alone is not unexpected. RRL is believed to be partially due to irradiation of lymphocytes as they transverse the irradiated field, even if it does not include lymphoid tissues such as the bone marrow and/or lymph nodes (3). There is significant prostatic blood flow, and during a single 30- to 40-min SBRT treatment session, the entire blood volume could transverse the prostate. However, only a small percentage (approximately 2%) of overall lymphocytes are circulating in the blood at a given time, while approximately 50% reside in the lymph nodes (17).
Lymphocytes originate from hematopoietic stem cells in the bone marrow (BM), after which they travel to lymph nodes, between nodes, and enter the blood. Homeostasis is the mechanism that prevents lymphocyte depletion in the blood, via the recruitment of lymphocytes from lymphatic organs (18). The pelvic bone is the primary site of hematopoiesis in adults, harboring the majority of proliferating BM and posing as a potential organ at risk when utilizing pelvic IMRT (19).
Given the large treatment volume utilized by pelvic IMRT, identifying optimal dose constraints for pelvic IMRT is necessary to minimize hematologic toxicities and subsequent loss of activity in the bone marrow (19). Circulating lymphocytes may also be irradiated at a higher rate due to the large pelvic treatment volumes that incorporate lymph nodes and large blood vessels. Differences in radiosensitivity between lymphocyte subpopulations impact their relative contributions, likely altering the dynamics of the immune response via changes in lymphocyte diversity and activity (20).
In addition, supplemental PNI is highly fractionated to limit gastrointestinal (GI) toxicities, however, due to the high radiosensitivity of lymphocytes, repeatedly dosing likely increases the risk of high-grade lymphopenia (21–23). Hypofractionation might be an approach to limit both lymphocyte lethality and reduced lymphocyte recruitment to circulating blood following RT (17).
The interpretations of comparable results on RRL post-SBRT in other disease states can be applied to the treatment of prostate cancer in minimizing lymphopenia incidence and grade during and after the course of treatment. In a prospective study, marrow-sparing IMRT was effectively utilized to reduce radiation dose to functional BM in patients with other pelvic malignancies, such as cervical and endometrial cancers (24). This approach was not utilized in this study but could be employed in the future to minimize unintentional dosing of the bone marrow3.
Limitations of our study include a smaller number of patients in the SBRT + PNI group and variation in risk group and utilization of ADT between groups, however, disease risk and ADT are not known to cause lymphopenia (25). Additionally, patients were not randomized to treatment groups, and the SBRT + PNI group had lower ALCs at baseline, potentially suggesting prior damage. The authors believe that these confounding variables are unlikely to be responsible for the difference in lymphopenia between the two groups. This study also lacks a measure of the acute effects of SBRT and PNI on ALC shown during or immediately following the treatment course. If we had assessed earlier time points, the nadir would have likely been lower. Finally, we did not have information on how RRL specifically impacted specific lymphocyte subsets. Further characterization of the effects of irradiating lymphoid tissues could provide mechanistic insights into the role of RRL in reducing response to treatment.
Abdominal and pelvic nodal oligometastatic recurrences are common after previous treatment for prostate cancer. Nodal radiation therapy utilizing wider treatment volumes is considered tolerable and effective in disease control; however, the increased risk of lymphopenia associated with this treatment course suggests that smaller treatment volumes could reduce toxicities. SBRT aiming to treat sites with macroscopic evidence of disease as suggested by PSMA-PET has been suggested to be a viable salvage treatment option for both pelvic and para-aortic recurrences (26, 27). The benefit-to-risk ratio should be considered when electing to utilize wide prophylactic volumes such as with PNI, given disease control observed with smaller volumes and decreased risk of toxicities.
Lymphopenia risk can be integrated into radiotherapy treatment planning by utilizing computational tools, such as the HEDOS framework, to estimate the dose to circulating blood cells based on time structure of the treatment and circulation of blood cells through irradiated organs (28). In addition, pelvic bone marrow dose-volume predictors of late lymphopenia following pelvic lymph node RT for prostate cancer, including baseline ALC, could be considered during treatment planning to minimize toxicities proactively (29).
Prostate cancer has been conventionally recognized to be an immunologically “cold” solid tumor, due to its strongly immunosuppressive tumor microenvironment (TME) and low levels of T-cell infiltration and driver mutations (30). Given this phenotype, lymphopenia may not significantly impact prostate cancer progression as it does in other solid tumors. Additionally, immunotherapy has shown reduced efficacy in prostate malignancies due to this tumor profile (31). However, our patient cohort could serve as a potential model system for studying RRL, as patients are generally healthy and have not received previous treatment with chemotherapy or immunotherapy agents. Previously, it has been shown that in patients treated with PD-(L)1 checkpoint inhibitors, prior radiation therapy was strongly associated with lymphopenia at 3 months post-treatment, and patients with lymphopenia had a shorter time to progression (32). Across multiple cancer sites and treatment types, lymphopenia correlates with decreased overall survival, highlighting the importance of minimizing and managing this toxicity in our treatment approaches (33).
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Georgetown MedStar Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsKG: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. MJuK: Formal analysis, Validation, Visualization, Writing – original draft. MJiK: Formal analysis, Investigation, Validation, Visualization, Writing – original draft. IK: Writing – original draft. DiK: Writing – original draft. PJ: Writing – review & editing. ZL: Writing – original draft. RC: Writing – original draft. ZZ: Writing – original draft. MD: Writing – original draft. AZ: Writing – original draft. DeK: Writing – original draft. MA: Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing. SC: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1459732/full#supplementary-material
References1. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with Malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. (2013) 31:140–4. doi: 10.3109/07357907.2012.762780
PubMed Abstract | Crossref Full Text | Google Scholar
2. Venkatesulu B, Giridhar P, Pujari L, Chou B, Lee JH, Block AM, et al. Lymphocyte sparing normal tissue effects in the clinic (LymphoTEC): A systematic review of dose constraint considerations to mitigate radiation-related lymphopenia in the era of immunotherapy. Radiother Oncol. (2022) 177:81–94. doi: 10.1016/j.radonc.2022.10.019
PubMed Abstract | Crossref Full Text | Google Scholar
3. Damen PJJ, Kroese TE, van Hillegersberg R, Schuit E, Peters M, Verhoeff JJC, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. (2021) 111:936–48. doi: 10.1016/j.ijrobp.2021.07.1695
PubMed Abstract | Crossref Full Text | Google Scholar
4. Dai D, Tian Q, Shui Y, Li J, Wei Q. The impact of radiation induced lymphopenia in the prognosis of head and neck cancer: A systematic review and meta-analysis. Radiother Oncol. (2022) 168:28–36. doi: 10.1016/j.radonc.2022.01.003
PubMed Abstract | Crossref Full Text | Google Scholar
5. Sini C, Fiorino C, Perna L, Noris Chiorda B, Deantoni CL, Bianchi M, et al. Dose-volume effects for pelvic bone marrow in predicting hematological toxicity in prostate cancer radiotherapy with pelvic node irradiation. Radiother Oncol. (2016) 118:79–84. doi: 10.1016/j.radonc.2015.11.020
PubMed Abstract | Crossref Full Text | Google Scholar
6. Cozzarini C, Noris Chiorda B, Sini C, Fiorino C, Briganti A, Montorsi F, et al. Hematologic toxicity in patients treated with postprostatectomy whole-pelvis irradiation with different intensity modulated radiation therapy techniques is not negligible and is prolonged: preliminary results of a longitudinal, observational study. Int J Radiat Oncol Biol Phys. (2016) 95:690–5. doi: 10.1016/j.ijrobp.2016.01.022
PubMed Abstract | Crossref Full Text | Google Scholar
7. SChad MD, Dutta SW, Muller DM, Wijesooriya K, Showalter TN. Radiation-related lymphopenia after pelvic nodal irradiation for prostate cancer. Adv Radiat Oncol. (2019) 4:323–30. doi: 10.1016/j.adro.2019.01.005
PubMed Abstract | Crossref Full Text | Google Scholar
8. Das IJ, Dawes SL, Dominello MM, Kavanagh B, Miyamoto CT, Pawlicki T, et al. Quality and safety considerations in stereotactic radiosurgery and stereotactic body radiation therapy: an ASTRO safety white paper update. Pract Radiat Oncol. (2022) 12:e253–68. doi: 10.1016/j.prro.2022.03.001
PubMed Abstract | Crossref Full Text | Google Scholar
9. Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. (2016) 94:571–9. doi: 10.1016/j.ijrobp.2015.11.026
PubMed Abstract | Crossref Full Text | Google Scholar
10. Zhong J, Patel K, Switchenko J, Cassidy RJ, Hall WA, Gillespie T, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. (2017) 123:3486–93. doi: 10.1002/cncr.30706
PubMed Abstract | Crossref Full Text | Google Scholar
11. Tchelebi LT, Lehrer EJ, Trifiletti DM, Sharma NK, Gusani NJ, Crane CH, et al. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): An international systematic review and meta-analysis. Cancer. (2020) 126:2120–31. doi: 10.1002/cncr.32756
PubMed Abstract | Crossref Full Text | Google Scholar
12. Zhao Q, Li T, Chen G, Zeng Z, He J. Prognosis and risk factors of radiation-induced lymphopenia in early-stage lung cancer treated with stereotactic body radiation therapy. Front Oncol. (2019) 9:1488. doi: 10.3389/fonc.2019.01488
PubMed Abstract | Crossref Full Text | Google Scholar
13. Cho Y, Park S, Byun HK, Lee CG, Cho J, Hong MH, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. (2019) 105:1065–73. doi: 10.1016/j.ijrobp.2019.08.047
PubMed Abstract | Crossref Full Text | Google Scholar
14. Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol. (2013) 8:58. doi: 10.1186/1748-717X-8-58
PubMed Abstract | Crossref Full Text | Google Scholar
15. Carrasquilla M, Sholklapper T, Pepin AN, Hodgins N, Lei S, Rashid A, et al. Intensity modulated radiation therapy with stereotactic body radiation therapy boost for unfavorable prostate cancer: five-year outcomes. Front Oncol. (2023) 13:1240939. doi: 10.3389/fonc.2023.1240939
PubMed Abstract | Crossref Full Text | Google Scholar
16. Lei S, Piel N, Oermann EK, Chen V, Ju AW, Dahal KN, et al. Six-dimensional correction of intra-fractional prostate motion with cyberKnife stereotactic body radiation therapy. Front Oncol. (2011) 1:48. doi: 10.3389/fonc.2011.00048
PubMed Abstract | Crossref Full Text | Google Scholar
17. Blum KS, Pabst R. Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs? Immunol Lett. (2007) 108:45–51. doi: 10.1016/j.imlet.2006.10.009
PubMed Abstract | Crossref Full Text | Google Scholar
18. Pham TN, Coupey J, Candeias SM, Ivanova V, Valable S, Thariat J. Beyond lymphopenia, unraveling radiation-induced leucocyte subpopulation kinetics and mechanisms through modeling approaches. J Exp Clin Cancer Res. (2023) 42:50. doi: 10.1186/s13046-023-02621-4
PubMed Abstract | Crossref Full Text | Google Scholar
19. Kumar T, Schernberg A, Busato F, Laurans M, Fumagalli I, Dumas I, et al. Correlation between pelvic bone marrow radiation dose and acute hematological toxicity in cervical cancer patients treated with concurrent chemoradiation. Cancer Manag Res. (2019) 11:6285–97. doi: 10.2147/CMAR.S195989
PubMed Abstract | Crossref Full Text | Google Scholar
21. MacLennan IC, Kay HE. Analysis of treatment in childhood leukemia. IV. The critical association between dose fractionation and immunosuppression induced by cranial irradiation. Cancer. (1978) 41:108–11.
PubMed Abstract | Google Scholar
22. Saito T, Toya R, Matsuyama T, Semba A, Oya N. Dosimetric Predictors of Treatment-related Lymphopenia induced by Palliative Radiotherapy: Predictive Ability of Dose-volume Parameters based on Body Surface Contour. Radiol Oncol. (2017) 51:228–34. doi: 10.1515/raon-2016-0050
PubMed Abstract | Crossref Full Text | Google Scholar
23. Yuan C, Wang Q. Comparative analysis of the effect of different radiotherapy regimes on lymphocyte and its subpopulations in breast cancer patients. Clin Transl Oncol. (2018) 20:1219–25. doi: 10.1007/s12094-018-1851-2
PubMed Abstract | Crossref Full Text | Google Scholar
24. Liang Y, Bydder M, Yashar CM, Rose BS, Cornell M, Hoh CK, et al. Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic Malignancies. Int J Radiat Oncol Biol Phys. (2013) 85:406–14. doi: 10.1016/j.ijrobp.2012.04.044
PubMed Abstract | Crossref Full Text | Google Scholar
25. Lin Y, Kim J, Metter EJ, Nguyen H, Truong T, Lustig A, et al. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immun Ageing. (2016) 13:24. doi: 10.1186/s12979-016-0079-7
PubMed Abstract | Crossref Full Text | Google Scholar
26. Francolini G, Garlatti P, Di Cataldo V, Triggiani L, Simoni N, Detti B, et al. Pattern of recurrence after stereotactic body radiotherapy for para-aortic oligo-recurrent prostate cancer, a multicentric analysis. Radiol Med. (2023) 128:1423–8. doi: 10.1007/s11547-023-01701-x
PubMed Abstract | Crossref Full Text | Google Scholar
27. Francolini G, Bellini C, Di Cataldo V, Detti B, Bruni A, Alicino G, et al. Pattern of recurrence after stereotactic radiotherapy in prostate cancer patients with nodal pelvic relapse. A multi-institutional retrospective analysis. Clin Oncol (R Coll Radiol). (2022) 34:57–62. doi: 10.1016/j.clon.2021.09.014
PubMed Abstract | Crossref Full Text | Google Scholar
28. Shin J, Xing S, McCullum L, Hammi A, Pursley J, Correa CA, et al. HEDOS-a computational tool to assess radiation dose to circulating blood cells during external beam radiotherapy based on whole-body blood flow simulations. Phys Med Biol. (2021) 66. doi: 10.1088/1361-6560/ac16ea
PubMed Abstract | Crossref Full Text | Google Scholar
29. Pavarini M, Alborghetti L, Aimonetto S, Maggio A, Landoni V, Ferrari P, et al. Pelvic bone marrow dose-volume predictors of late lymphopenia following pelvic lymph node radiation therapy for prostate cancer. Radiother Oncol. (2024) 195:110230. doi: 10.1016/j.radonc.2024.110230
PubMed Abstract | Crossref Full Text | Google Scholar
30. Wang I, Song L, Wang BY, Rezazadeh Kalebasty A, Uchio E, Zi X. Prostate cancer immunotherapy: a review of recent advancements with novel treatment methods and efficacy. Am J Clin Exp Urol. (2022) 10:210–33.
32. Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. (2017) 8:114268–80. doi: 10.18632/oncotarget.23217
PubMed Abstract | Crossref Full Text | Google Scholar
33. Lambin P, Lieverse RIY, Eckert F, Marcus D, Oberije C, van der Wiel AMA, et al. Lymphocyte-sparing radiotherapy: the rationale for protecting lymphocyte-rich organs when combining radiotherapy with immunotherapy. Semin Radiat Oncol. (2020) 30:187–93. doi: 10.1016/j.semradonc.2019.12.003
留言 (0)