Bladder cancer is the sixth most common genitourinary malignant disease in the United States with 81,180 estimated new cases and 17,100 estimated deaths in 2022 (1). A radical cystectomy with pelvic lymph node dissection (PLND) is the gold standard for muscle-invasive bladder cancer and recurrent high-grade non-muscle-invasive bladder cancer (2). However, its morbidity from complications was as high as 31.5%-64% because of the surgical trauma (3–11). Minimally invasive surgery can achieve comparable oncological control while reducing the incidence of complications and shortening hospital stays (12–15). Notably, studies have demonstrated that the adoption of a completely minimally invasive approach in the surgical treatment of bladder cancer has led to an overall reduction in transfusion rates of 50% (14, 16). It is still a highly comorbid operation, with a risk of major complications in at least one-third of patients in contemporary practice (4).
Previous studies have identified several factors, including urinary diversion (3, 11), the American Society of Anesthesiologists (ASA) score (6), the Charlson comorbidity index, and preoperative coagulation functions (10), that influence complications post-radical cystectomy. Interestingly, the presence of preoperative renal insufficiency was associated with major complications in elderly patients in our previous study (17, 18). However, it is not clear whether preoperative renal insufficiency increases the incidence of complications after a radical cystectomy.
The aim of this study was to compare the incidence of complications among patients with different preoperative renal functions and explore the factors affecting major complications in radical cystectomy procedures.
2 Methods2.1 Patient populationA retrospective analysis of morbidity data from chart review was performed in this study. We defined the following inclusion criteria (1): patients who underwent a radical cystectomy from January 2006 to April 2017 at Peking University First Hospital; and (2) postoperative pathology indicated urothelial carcinoma of the bladder. The exclusion criteria were (1) distant metastasis (n=14); (2) PLND not performed (n=130); and (3) lack of preoperative creatinine data (n=3). Finally, 705 patients who had received a radical cystectomy and PLND were enrolled (Figure 1). Among them, 60 (8.5%) patients had preoperative renal insufficiency (serum creatinine > 133umol/L) (19) and 645 (91.5%) had a normal preoperative renal function. Imaging and pathological examinations were performed for all patients before the operation. The indications for a radical cystectomy included a T2-4aNxM0 tumor, high-risk and recurrent non-muscleinvasive bladder cancer (NMIBC), and BCG-resistant Tis, as well as extensive papillary disease that could not be controlled with a transurethral resection of the bladder tumor and intravesical therapy alone. Patients with a cT2-4aN0-xM0 tumor were recommended for neoadjuvant chemotherapy, whereas adjuvant chemotherapy was recommended for patients with pT3/4 or pN+ disease if no neoadjuvant chemotherapy had been given. The final treatment plans were determined according to the patient’s tolerance and preference. This study was approved by the clinical research ethics committee of Peking University First Hospital, Beijing, China.
Figure 1. Flowchart of all eligible patients. PLND, pelvic lymph node dissection.
2.2 Outcomes measures2.2.1 Clinical and pathological dataThe preoperative serum creatinine (sCr) was collected for all patients, and sCr ≥ 133 umol/L was defined as preoperative renal insufficiency. According to the preoperative renal function, the patients were categorized into two groups: the normal renal function group and the preoperative renal insufficiency group. Clinical characteristics including age, sex, operation method (open radical cystectomy or laparoscopic radical cystectomy), the ASA score (20), time of operation, postoperative stay, estimated blood loss (EBL), body mass index (BMI), and urinary diversion type were collected in this study. Histological type, surgical margin status, lymph node yield, and number of positive lymph nodes were collected in pathological data. Pathological T stage and pathological nodal stage were classified by using the TNM staging system of bladder cancer of the American Joint Committee on Cancer Staging Manual 8th edition. The K/DOQI (clinical practice guidelines for chronic kidney disease) grades specific disease and conditions into four grades: grade 1—compensatory stage of renal insufficiency (177umol/L ≥ sCr > 133umol/L); grade 2—decompensatory stage of renal insufficiency (443umol/L ≥ sCr > 177umol/L); grade 3— renal failure stage (707umol/L ≥ sCr > 443umol/L); grade 4— uremia stage (sCr > 707umol/L) (19).
2.2.2 Surgical approachThe surgical approach was conducted as previously described in the literature (13). The choice of operative method—open radical cystectomy (ORC) or laparoscopic radical cystectomy (LRC)—was determined by the patient’s condition and the surgeon’s preference. The entire procedure involved an en-bloc resection of the bladder, a bilateral PLND, and extracorporeal urinary diversion. PLND encompassed the removal of internal iliac, presacral, obturator, and external iliac lymph nodes, and urinary diversion included options such as a ureterocutaneostomy, ileal conduit, or orthotopic neobladder. The selection of the urinary diversion type was based on the tumor stage and the patient’s physical condition and was decided upon after thorough discussions between the medical team and the patient. However, the option of an orthotopic neobladder was excluded in cases where there was suspicion of urethral invasion.
2.2.3 Assessment of complicationsThe collection and grading of complications were conducted by two clinical doctors. Definitions of complications after radical cystectomy were from the catalog of studies by Vetterlein et al. (21), which employed the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. The 90-day complications after a radical cystectomy were collected from digitalized charts and outpatient information was included. Each complication was graded using the Clavien–Dindo classification (CDC) (22). A major complication was characterized as CDC III-V (10).
2.3 Statistical analysisStatistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 25.0 software (IBM Corporation, Armonk, NY, USA). All p-values were two-sided and p < 0.05 was considered to be statistically significant. For the continuous variables, the Kolmogorov–Smirnov test was used to identify the normality. Continuous variables were presented as means ± standard deviation for normal distributions, or as medians and interquartile ranges (IQR) for skewed distributions. The independent t-test was used to compare the variables following a normal distribution, and variables following a non-normal distribution were analyzed using the Mann–Whitney U test. The x2 test or Fisher’s exact test was used to compare the disordered categorical variables, while the ordered categorical variables were analyzed by using the Mann–Whitney U test. A logistic regression analysis of all patients was used to identify the risk factors associated with the major complications. Spearman’s correlation analysis was used to examine the relationship between the classification of renal insufficiency and the CDC. Concurrently, for the sensitivity analysis, a one-to-one matching was conducted using propensity scores. In estimating the propensity scores, a logistic regression model was employed, incorporating the following factors: age, ASA score, type of urinary diversion, the pathological T stage, and the pathological nodal stage. A caliper width of 0.02 was applied for the propensity score matching (PSM). Post-matching, the CDC of the patients and the incidence of major complications were compared.
3 Results3.1 Patient characteristicsThere were 705 patients enrolled in the present study. Among them, 645 (91.5%) patients had a normal preoperative renal function and 60 (8.5%) had an elevated sCr level higher than the normal value. There were 501 (71%) patients who experienced at least one complication within 90 days of the surgery and 34 (4.8%) patients had major complications.
The perioperative and pathological outcomes are presented in Table 1. Compared with the normal renal function group, the patients with preoperative renal insufficiency had higher ASA scores (p < 0.001), higher clinical T stages (p < 0.001), higher pathological T stages (p < 0.001), and higher pathological nodal stages (25% vs 14.3%, p = 0.027). Furthermore, more patients with preoperative renal insufficiency underwent a ureterocutaneostomy (43.3% vs 25.7%, p = 0.014) and had a higher rate of positive margins (6.7% vs 1.6%, p = 0.026) after a radical cystectomy.
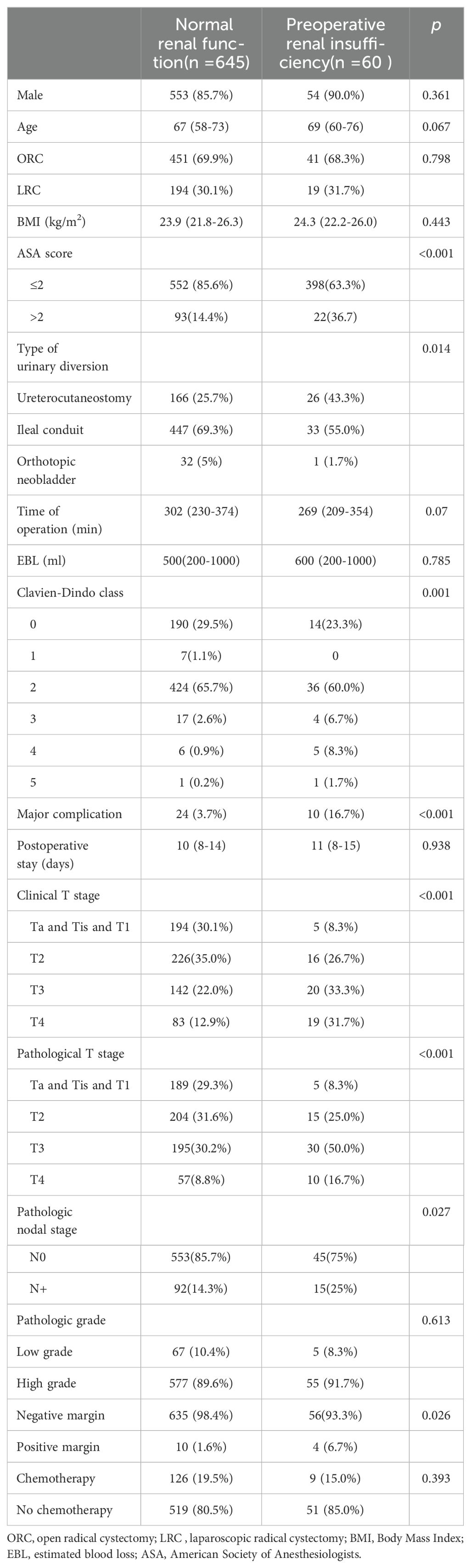
Table 1. The clinicopathologic characteristics of all patients.
3.2 Postoperative complicationsA total of 501 (71.16%) patients suffered from complications after the operation. Among them, 34 patients had more than one major complication. Compared with the group with normal renal function, the patients with preoperative renal insufficiency also had higher CDCs and had higher rate of major complications (16.7% vs 3.7%, p < 0.001). Spearman’s correlation analysis showed a slightly positive correlation between the classification of renal insufficiency and the CDC (r=0.094, p = 0.013).
To further identify the risk of the postoperative major complications, multivariate logistic regression modeling was performed. We found preoperative renal insufficiency (OR = 6.805 [95%CI: 2.706-17.112]; p < 0.001) was associated with a higher risk of major complications (Table 2).
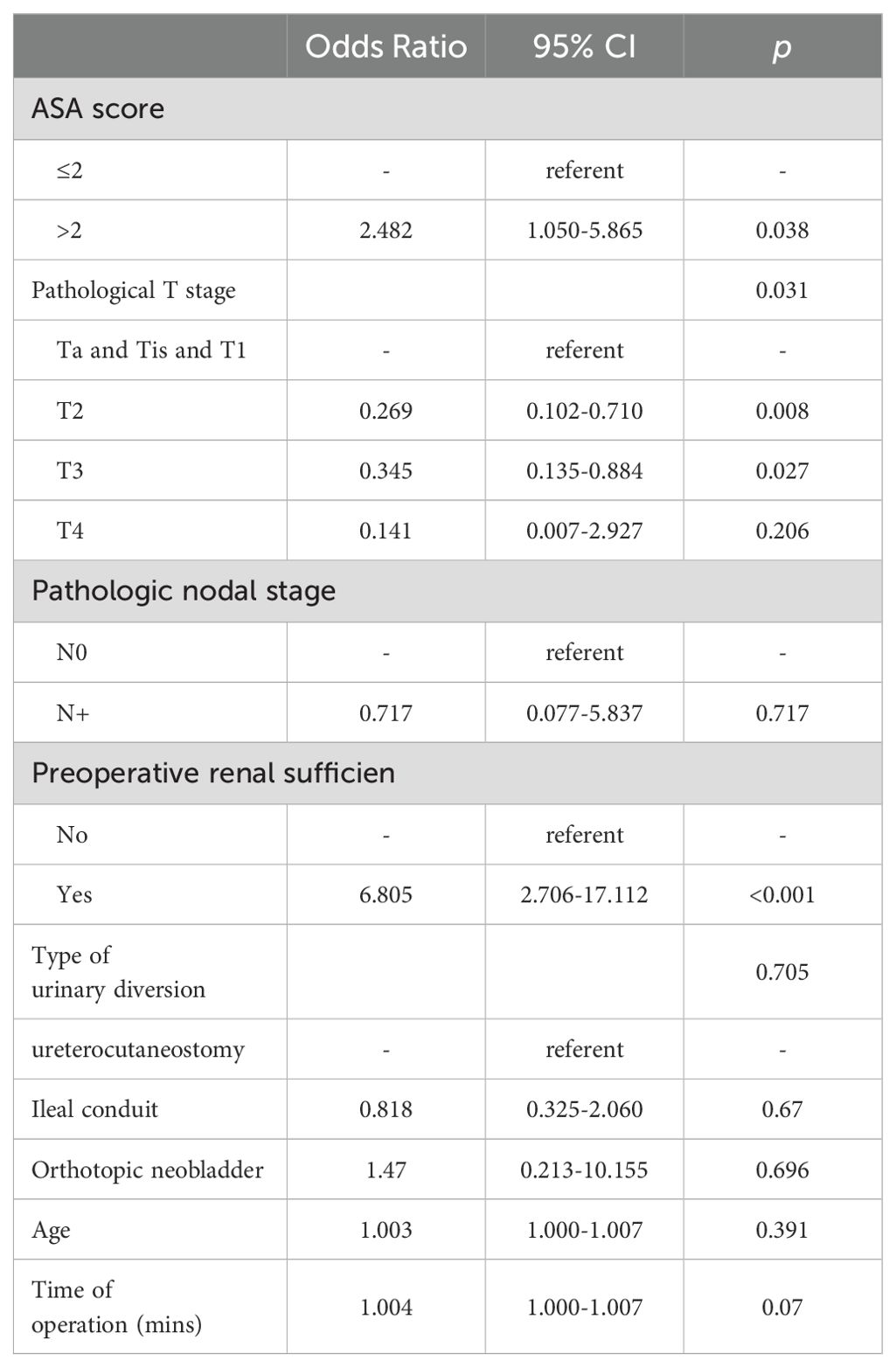
Table 2. Logistic regression analysis of variables associated with major complication.
Ultimately, we conducted a comparison of the types of major complications between patients with normal preoperative renal function and those with preoperative renal insufficiency (Table 3). Most major complications occurred within 30 days postoperatively, with only two patients in the normal preoperative renal function group and one patient in the preoperative renal insufficiency group experiencing major complications beyond 30 days. Our findings revealed that among the patients with preoperative renal insufficiency, three (5%) experienced cardiovascular complications, whereas only one (0.16%) patient with normal renal function developed such major complications.
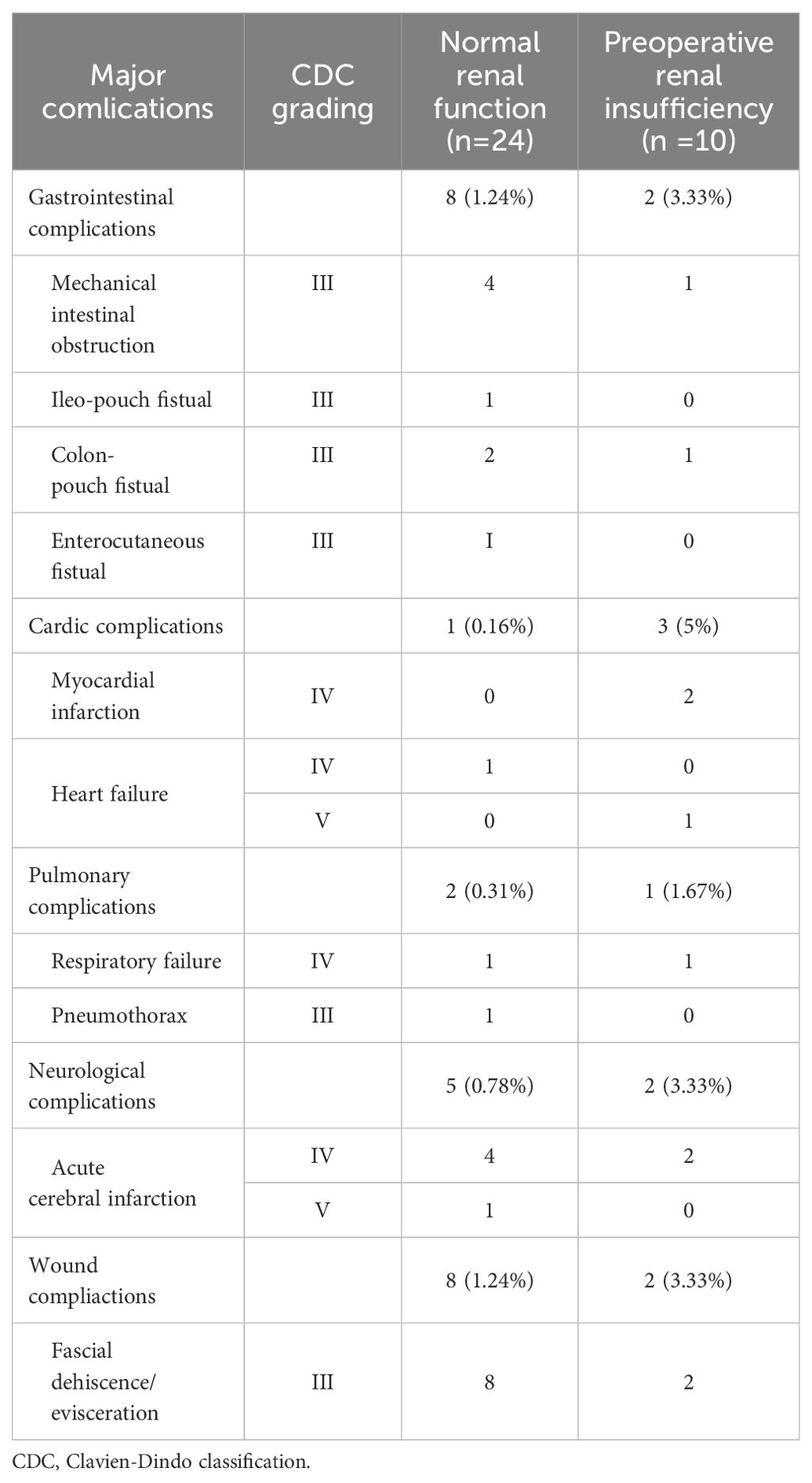
Table 3. Frequencies and grading of perioperative 90-d major complications in patients who underwent radical cystectomy and pelvic lymph node dissection.
3.3 Propensity score matchingIn order to reduce the selection bias, a one-to-one PSM was performed (Table 4). In total, 118 patients (59 with normal preoperative renal function and 59 with preoperative renal insufficiency) were successfully matched. After matching, the two groups had no significant difference in terms of age, ASA score, pathological T stage, and pathological N stage. A comparison of complications after matching was carried out for the sensitivity analysis (Table 5). After matching, the patients in the preoperative renal insufficiency group had higher CDCs and a higher incidence of major complications (16.9% vs 1.7%, p = 0.004).
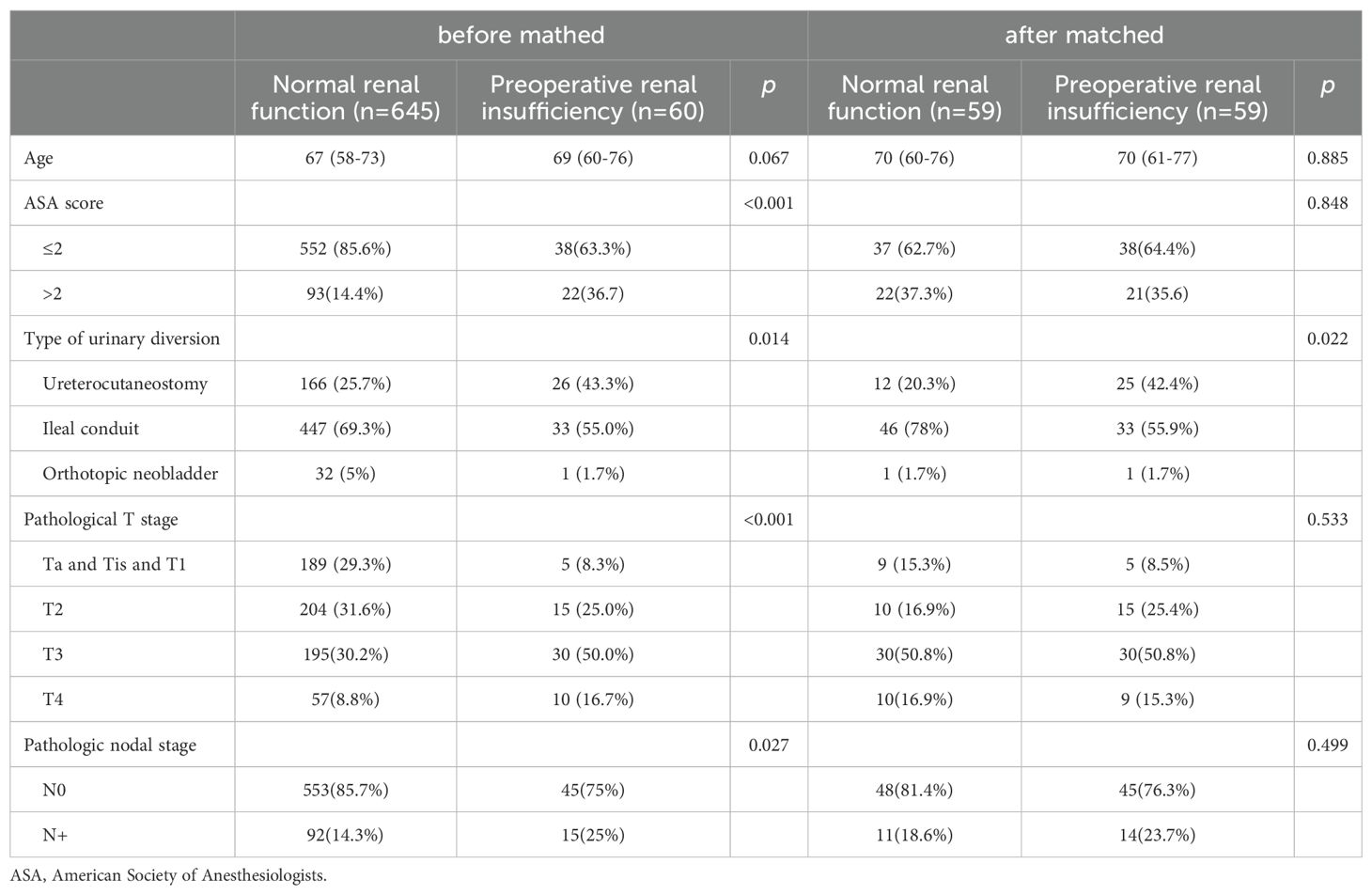
Table 4. The clinicopathologic characteristics of the patients before matched and after matched.
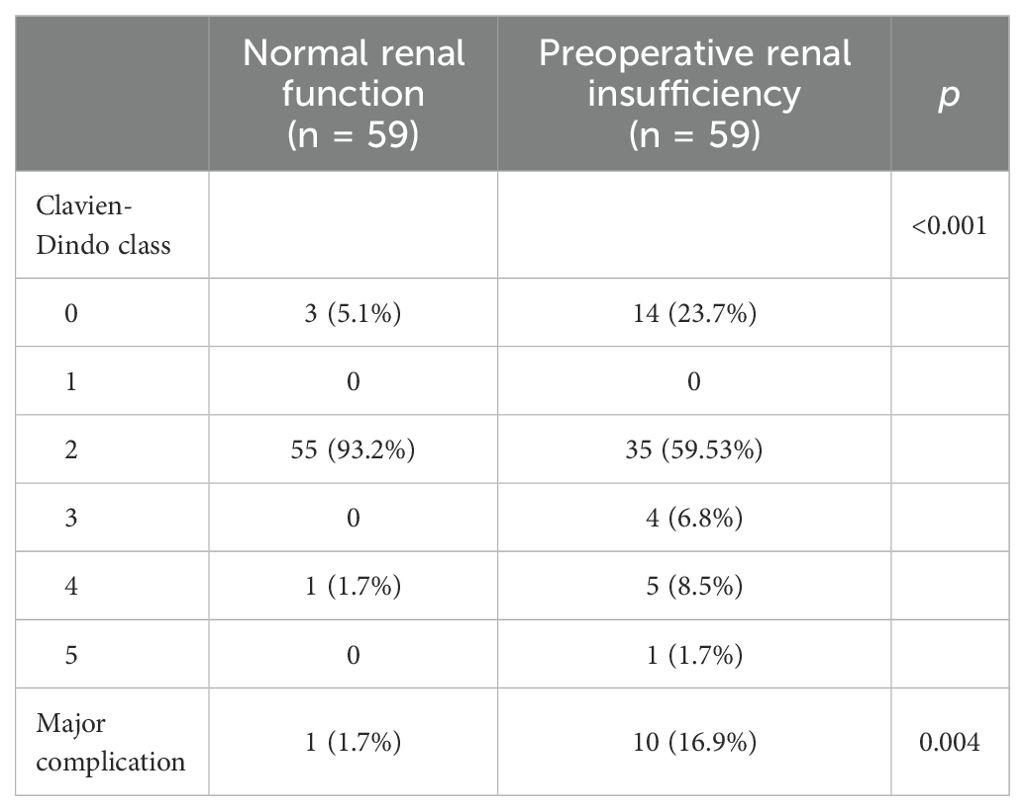
Table 5. Comparasion of complications for patients after matching.
4 DiscussionIn our analysis of the radical cystectomy database, we investigated the correlation between preoperative renal insufficiency and the occurrence of major complications following a radical cystectomy for bladder cancer. The study yielded three principal findings. First, patients with renal insufficiency exhibited a higher CDC grade for postoperative complications, with a particularly elevated incidence of major complications. Second, there was a significant positive correlation between preoperative renal function and the incidence of major complications. Third, preoperative renal insufficiency was identified as a risk factor for the development of major complications.
A radical cystectomy has a high morbidity due to its difficulty and surgical trauma. According to the data of previous studies, the incidence of postoperative complications ranges from 31.5% to 64% and 13% to 35% of patients suffer from more than one major complication after a radical cystectomy (3–11). In this study, the incidence of 90-day complication rates was 71%, which was higher than the data reported in the literature. This variation of postoperative complications was caused by the interobserver variability in defining and grading complications, and, through a rigorous evaluation of clinical data, a greater number of complications would be detected, especially grade 1 and 2 complications. Furthermore, the 90-day major complication rate was 4.8% in our center, which was lower than the data reported (23). This could be due to an improvement in perioperative management in our institution.
Different surgical access methods can influence the incidence of postoperative complications. Existing literature has confirmed that a LRC was associated with a lower rate of postoperative complications compared to a ORC (24). However, there were no statistically significant differences in surgical access methods between the patients with normal preoperative renal function and those with preoperative renal insufficiency (Table 1). We conducted subgroup analyses to compare the incidence of postoperative complications between the patients with normal renal function and those with preoperative renal insufficiency across different surgical access methods (Supplementary Table 1). For patients who underwent a ORC, the incidences of postoperative complications (p = 0.002) and major complications (19.5% vs. 4.4%, p = 0.001) were significantly higher in the preoperative renal insufficiency group. In contrast, for patients who underwent a LRC, the incidence of major postoperative complications was higher in the preoperative renal insufficiency group (10.5% vs. 2.1%, p = 0.091), although this difference was not statistically significant, which may be attributed to the small sample size. Therefore, the surgical access method may not mitigate the increased risk of postoperative complications associated with preoperative renal insufficiency.
We used serum creatinine levels instead of glomerular filtration rate to assess renal function primarily because serum creatinine testing is convenient and the clinical data was readily available. Furthermore, although creatinine levels are influenced by various factors, studies have shown that serum creatinine correlates well with glomerular filtration rate, particularly in patients with renal impairment (25). A large proportion of the patients who undergo a radical cystectomy have renal dysfunction. Some of these patients have a history of chronic kidney disease, while most of them have an invasion of the distal ureter due to an advanced tumor, leading to postrenal obstruction (26). In our cohort, among the patients with renal insufficiency, 38 (63.3%) patients had an invasion of the distal ureter, and these patients all had posterior renal insufficiency. From the results given in Table 1, patients with preoperative renal insufficiency had higher pathological T stages (p < 0.001), and higher pathological nodal stages (25% vs 14.3%, p = 0.027), which indicated that patients with preoperative renal insufficiency group had more advanced tumors.
Previous studies indicated that patients with preoperative renal insufficiency who undergo a radical cystectomy were at a higher risk of renal function deterioration (27), and preoperative renal insufficiency was associated with worse oncological outcomes postoperatively (28, 29). In this study, we found that patients with preoperative renal insufficiency had a higher incidence of major complications, and preoperative renal insufficiency was a risk factor for major complications in patients after a radical cystectomy. This finding was consistent with the results of our previous study, although this was analyzed in a different population (17). In addition, preoperative renal insufficiency was associated with major complications for elderly patients after a radical cystectomy, when the endpoint was a comprehensive complication index (CCI) ≥33.7 (18). Wuethrich et al. reviewed the predictors for 90-day postoperative major complications and also found preoperative renal insufficiency to be an independent predictor (11). Not only for radical cystectomy, a previous study identified renal dysfunction to be an important risk factor for death after non-cardiac surgery, and the risk increases for patients with moderate to severe kidney dysfunction (30). We also compared the major complications between the two groups and the incidence of cardiac complication in the preoperative renal insufficiency group was higher than in the normal renal function group. This result indicated that the presence of impaired kidney function before a radical cystectomy may be associated with an increased risk of cardiac complications, which was proved in the previous study on non-cardiac surgery (31).
Effectively reducing postoperative complications can be achieved through enhanced perioperative management, such as the application of enhanced recovery after surgery (ERAS) protocols. Studies have confirmed that ERAS contributes to improve complication rates, decrease hospital length-of-stay, and/or time to bowel recovery (32–35). Additionally, identifying and correcting patient risk factors preoperatively are also effective (23, 36). The etiology of renal insufficiency can be categorized into pre-renal, renal, and post-renal causes. In the context of bladder cancer patients, the majority of renal impairments are attributed to post-renal causes, specifically due to the invasion of the ureteral orifices by advanced-stage tumors. Preoperative evaluation of the cause of preoperative renal insufficiency is necessary. For patients with preoperative post-renal renal insufficiency, a percutaneous nephrostomy is a viable method to ameliorate renal function prior to the surgery. The restoration of renal function may mitigate the risk of major complications. Furthermore, improved renal function could potentially enhance the tolerability of neoadjuvant chemotherapy, a treatment modality that has been shown to offer oncological benefits in terms of downstaging and improving survival outcomes (37).
With the advancement of minimally invasive techniques, robot-assisted radical cystectomy (RARC) has been increasingly adopted in various medical centers. According to the literature, the incidence of postoperative complications following RARC ranges from 62% to 67% (15, 38, 39). RARC offers comparable oncological control to open radical cystectomy while reducing the incidence of complications and shortening hospital stays (12, 15). Consequently, there is a need for further clinical research to explore the relationship between preoperative renal insufficiency and the incidence of postoperative complications in patients undergoing RARC for bladder cancer.
This study has some limitations. First, its retrospective nature introduces inherent risks of selection bias. To mitigate this, we employed a multivariate regression analysis, which allowed us to control for potential confounding factors as rigorously as possible. Second, the absence of a large cohort of patients with preoperative renal insufficiency who underwent a nephrostomy to ameliorate renal function prior to a radical cystectomy is a significant constraint. This limitation affects the generalizability of our findings. Therefore, we advocate for future multi-institutional studies to provide external validation of our results. Third, intraoperative and long-term complications were not described because of a lack of data. It would be interesting to compare intraoperative complications and postoperative complications at different terms in a further study.
5 ConclusionsIn our cohort, patients with preoperative renal insufficiency exhibited a higher incidence of complications following a radical cystectomy and renal insufficiency was a significant risk factor for major complications.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by the clinical research ethics committee of Peking University First Hospital (Protocol number: 2015[977]). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because The informed consent requirement was exempted by the ethics committee, because of the retrospective design.
Author contributionsHW: Formal analysis, Writing – original draft, Writing – review & editing. HWH: Data curation, Formal analysis, Methodology, Writing – review & editing. HH: Data curation, Writing – review & editing. ZX: Funding acquisition, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This paper was supported by the National Natural Science Foundation of China (grant numbers:81272829).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1453346/full#supplementary-material
AbbreviationsPLND, pelvic lymph node dissection; CDC, Clavien–Dindo classification; EBL, estimated blood loss; sCr, serum creatinine; ASA, American Society of Anesthesiologists; BMI, Body Mass Index.
References1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
Crossref Full Text | Google Scholar
2. Witjes JA, Bruins HM, Cathomas R, Compérat E, Cowan NC, Efstathiou JA, et al. EAU guidelines on muscle invasive and metastatic bladder cancer. EAU Guidelines. (2021). doi: 10.1016/j.eururo.2020.03.055
Crossref Full Text | Google Scholar
3. Hautmann RE, de Petriconi RC, Volkmer BG. Lessons learned from 1,000 neobladders: the 90-day complication rate. J Urol. (2010) 184:990–4; quiz 1235. doi: 10.1016/j.juro.2010.05.037
Crossref Full Text | Google Scholar
4. Johnson SC, Smith ZL, Golan S, Rodriguez JF 3rd, Smith ND, Steinberg GD. Temporal trends in perioperative morbidity for radical cystectomy using the National Surgical Quality Improvement Program database. Urologic Oncol. (2017) 35:659.e13–.e19. doi: 10.1016/j.urolonc.2017.07.013
Crossref Full Text | Google Scholar
5. Morgan TM, Keegan KA, Barocas DA, Ruhotina N, Phillips SE, Chang SS, et al. Predicting the probability of 90-day survival of elderly patients with bladder cancer treated with radical cystectomy. J Urol. (2011) 186:829–34. doi: 10.1016/j.juro.2011.04.089
Crossref Full Text | Google Scholar
6. Novara G, De Marco V, Aragona M, Boscolo-Berto R, Cavalleri S, Artibani W, et al. Complications and mortality after radical cystectomy for bladder transitional cell cancer. J Urol. (2009) 182:914–21. doi: 10.1016/j.juro.2009.05.032
Crossref Full Text | Google Scholar
7. Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel). (2020) 8:15. doi: 10.3390/medsci8010015
Crossref Full Text | Google Scholar
8. Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. (2009) 55:164–74. doi: 10.1016/j.eururo.2008.07.031
Crossref Full Text | Google Scholar
9. Williams SB, Kamat AM, Chamie K, Froehner M, Wirth MP, Wiklund PN, et al. Systematic review of comorbidity and competing-risks assessments for bladder cancer patients. Eur Urol Oncol. (2018) 1:91–100. doi: 10.1016/j.euo.2018.03.005
Crossref Full Text | Google Scholar
10. Yuh BE, Nazmy M, Ruel NH, Jankowski JT, Menchaca AR, Torrey RR, et al. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur Urol. (2012) 62:806–13. doi: 10.1016/j.eururo.2012.06.007
Crossref Full Text | Google Scholar
11. Wuethrich PY, Vidal A, Burkhard FC. There is a place for radical cystectomy and urinary diversion, including orthotopic bladder substitution, in patients aged 75 and older: Results of a retrospective observational analysis from a high-volume center. Urologic Oncol. (2016) 34:58.e19–27. doi: 10.1016/j.urolonc.2015.08.011
Crossref Full Text | Google Scholar
12. Bochner BH, Dalbagni G, Marzouk KH, Sjoberg DD, Lee J, Donat SM, et al. Randomized trial comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: oncologic outcomes. Eur Urol. (2018) 74:465–71. doi: 10.1016/j.eururo.2018.04.030
Crossref Full Text | Google Scholar
13. Huang H, Yan B, Hao H, Shang M, He Q, Liu L, et al. Laparoscopic versus open radical cystectomy in 607 patients with bladder cancer: Comparative survival analysis. Int J urology: Off J Japanese Urological Assoc. (2021) 28:673–80. doi: 10.1111/iju.14537
Crossref Full Text | Google Scholar
14. Mastroianni R, Tuderti G, Ferriero M, Anceschi U, Bove AM, Brassetti A, et al. Robot-assisted radical cystectomy with totally intracorporeal urinary diversion versus open radical cystectomy: 3-year outcomes from a randomised controlled trial. Eur Urol. (2024) 85:422–30. doi: 10.1016/j.eururo.2024.01.018
Crossref Full Text | Google Scholar
15. Parekh DJ, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet (London England). (2018) 391:2525–36. doi: 10.1016/S0140-6736(18)30996-6
Crossref Full Text | Google Scholar
16. Mastroianni R, Ferriero M, Tuderti G, Anceschi U, Bove AM, Brassetti A, et al. Open radical cystectomy versus robot-assisted radical cystectomy with intracorporeal urinary diversion: early outcomes of a single-center randomized controlled trial. J Urol. (2022) 207:982–92. doi: 10.1097/JU.0000000000002422
Crossref Full Text | Google Scholar
17. Wang H, Huang H, Shang M, Hao H, Xi Z. Comparative study of perioperative and oncological outcomes between elderly patients and younger patients who received radical cystectomy and pelvic lymph node dissection: A single-center retrospective study. Cancer Manage Res. (2022) 14:603–13. doi: 10.2147/CMAR.S350587
Crossref Full Text | Google Scholar
18. Huang H, Zhang Z, Hao H, Wang H, Shang M, Xi Z. The comprehensive complication index is more sensitive than the Clavien-Dindo classification for grading complications in elderly patients after radical cystectomy and pelvic lymph node dissection: Implementing the European Association of Urology guideline. Front Oncol. (2022) 12:1002110. doi: 10.3389/fonc.2022.1002110
Crossref Full Text | Google Scholar
19. Levey A. S., de Jong P. E., Coresh J, El Nahas M, Astor B. C., Matsushita K., et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney international. (2011) 80:17–28. doi: 10.1038/ki.2010.483
Crossref Full Text | Google Scholar
20. Hocevar LA, Fitzgerald BM. American Society of Anesthesiologists Staging. Treasure Island (FL: StatPearls (2023).
21. Vetterlein MW, Klemm J, Gild P, Bradtke M, Soave A, Dahlem R, et al. Improving estimates of perioperative morbidity after radical cystectomy using the european association of urology quality criteria for standardized reporting and introducing the comprehensive complication index. Eur Urol. (2020) 77:55–65. doi: 10.1016/j.eururo.2019.08.011
Crossref Full Text | Google Scholar
22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surgery. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
Crossref Full Text | Google Scholar
23. Zhang H, Li A, Wang W, Xu S, Li C, Teng L. Efficacy and safety of radical cystectomy with ileal conduit for muscle-invasive bladder cancer in the elderly: a multicenter retrospective study. Front Oncol. (2024) 14:1402360. doi: 10.3389/fonc.2024.1402360
Crossref Full Text | Google Scholar
24. Palazzetti A, Sanchez-Salas R, Capogrosso P, Barret E, Cathala N, Mombet A, et al. Revisión sistemática de resultados perioperatorios y complicaciones después de cistectomía radical abierta, laparoscópica y asistida por robot. Actas Urologicas espanolas. (2017) 41:416–25. doi: 10.1016/j.acuro.2016.05.009
Crossref Full Text | Google Scholar
25. Kashani K, Rosner MH, Ostermann M. Creatinine: From physiology to clinical application. Eur J Internal Med. (2020) 72:9–14. doi: 10.1016/j.ejim.2019.10.025
Crossref Full Text | Google Scholar
26. Suh J, Yuk HD, Jeong CW, Kwak C, Kim HH, Ku JH. Prognostic impact of preoperative renal insufficiency on metastasis-free survival after radical cystectomy. J Cancer. (2021) 12:7320–5. doi: 10.7150/jca.61847
Crossref Full Text | Google Scholar
27. Tilala YM, Panda S, Tripathi A, Sharma S, Paul AS, Choudhuri S, et al. Long term outcomes and impact on renal function following radical cystectomy. Urologia. (2024) 91:505–11. doi: 10.1177/03915603241249231
Crossref Full Text | Google Scholar
28. Sari Motlagh R, Ghoreifi A, Yanagisawa T, Kawada T, Kikic Z, Gill I, et al. Survival of patients with chronic kidney disease treated with radical cystectomy and risk factors of glomerular filtration rate loss following radical cystectomy: two systematic reviews and meta-analyses of interplay between radical cystectomy and renal function. Eur Urol Focus. (2024) 10:169–81. doi: 10.1016/j.euf.2023.06.014
Crossref Full Text | Google Scholar
29. Kim D, Nam W, Kyung YS, You D, Jeong IG, Hong B, et al. Effect of decreased renal function on poor oncological outcome after radical cystectomy. Invest Clin Urol. (2023) 64:346–52. doi: 10.4111/icu.20230063
Crossref Full Text | Google Scholar
30. Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surgery. (2016) 103:1316–25. doi: 10.1002/bjs.10186
Crossref Full Text | Google Scholar
31. Mathew A, Devereaux PJ, O'Hare A, Tonelli M, Thiessen-Philbrook H, Nevis IF, et al. Chronic kidney disease and postoperative mortality: a systematic review and meta-analysis. Kidney Int. (2008) 73:1069–81. doi: 10.1038/ki.2008.29
Crossref Full Text | Google Scholar
32. Lee G, Patel HV, Srivastava A, Ghodoussipour S. Updates on enhanced recovery after surgery for radical cystectomy. Ther Adv Urol. (2022) 14:17562872221109022. doi: 10.1177/17562872221109022
Crossref Full Text | Google Scholar
33. Llorente C, Guijarro A, Hernández V, Fernández-Conejo G, Passas J, Aguilar L, et al. Outcomes of an enhanced recovery after radical cystectomy program in a prospective multicenter study: compliance and key components for success. World J Urol. (2020) 38:3121–9. doi: 10.1007/s00345-020-03132-z
Crossref Full Text | Google Scholar
34. Lin T, Li K, Liu H, Xue X, Xu N, Wei Y, et al. Enhanced recovery after surgery for radical cystectomy with ileal urinary diversion: a multi-institutional, randomized, controlled trial from the Chinese bladder cancer consortium. World J Urol. (2018) 36:41–50. doi: 10.1007/s00345-017-2108-3
留言 (0)