Hepatocellular carcinoma (HCC) is one of the most common cancers in China. Approximately 60-70% of patients have locally advanced or metastatic disease at diagnosis (1). It is a highly lethal and invasive cancer of the liver, and only a small percentage of patients are eligible for potentially curative therapies. Despite the range of localized treatments available to patients, systemic therapy often becomes the treatment of choice for patients who are inoperable and receive other localized treatments. Targeting immune checkpoint inhibitors (ICIs) play an important role in the treatment of HCC and are the main systemic therapeutic option for patients with advanced, metastatic or progressed disease. Recently, several new systemic treatment regimens have been identified from a number of clinical trials for the upfront treatment of advanced and metastatic HCC. However, until recently, there have been no well-recognized and consistent systemic therapies available for patients with HCC who have disease progression on or after first-line targeted therapy and ICIs. Multiple second-line studies are underway, but thus far, there are no definitive results. Some studies have explored new immune checkpoints that show promise as potential new avenues for second-line treatment in liver cancer, such as some emerging phagocytosis checkpoints (2, 3) and bispecific antibodies targeting immunomodulatory checkpoints for cancer therapy (4, 5).
Lenalidomide is an immunomodulatory and antineoplastic agent that was first used for the treatment of multiple myeloma. It is a thalidomide derivative that has similar but more potent activity as an antineoplastic agent. Lenalidomide has immunomodulatory, anti-inflammatory, antiangiogenic and antineoplastic effects. The mechanism of action of these agents in treatment is not well defined but may be related to inhibition of tumor necrosis factor (TNF) alpha, a potent proinflammatory cytokine, or to stimulation of T and NK cell activity (6, 7). Recently, one study revealed that lenalidomide targets the CRL4CRBN ubiquitin ligase to activate the Notch and interleukin-2 pathways in tumor-infiltrating CD8+ T cells. These T cells respond to PD-1-blocking antibodies even in the absence of the CD28 costimulatory receptor after treatment with lenalidomide. Lenalidomide can clinically enhance PD-1 therapy efficacy when treating solid tumors infiltrated with abundant CD28+CD8+ T cells. Molecular glue degraders, such as lenalidomide and pomalidomide, benefit individuals with cancer not only by killing cancer cells directly but also by promoting T-cell-based ICIs (8). We first propose that the efficacy of lenalidomide may be enhanced by combining or following ICIs. Our patient had HCC progression after first-line targeted therapy and ICIs and achieved a better tumor response and longer survival with lenalidomide, and lenalidomide regained efficacy after anti-PD-1 therapy and has not yet progressed.
Case presentationChief complaintsThe patient was a 54-year-old male whose chief complaint was anorexia, and he was subsequently diagnosed with HCC for 3 weeks.
History of present illnessThe patient was a 54-year-old man who was diagnosed with HCC on June 7, 2020, with a large mass on his left liver lobe (Figure 1A). The levels of alpha-fetoprotein (AFP) and antagonist-II (PIVKA-II) in this patient were significantly greater.
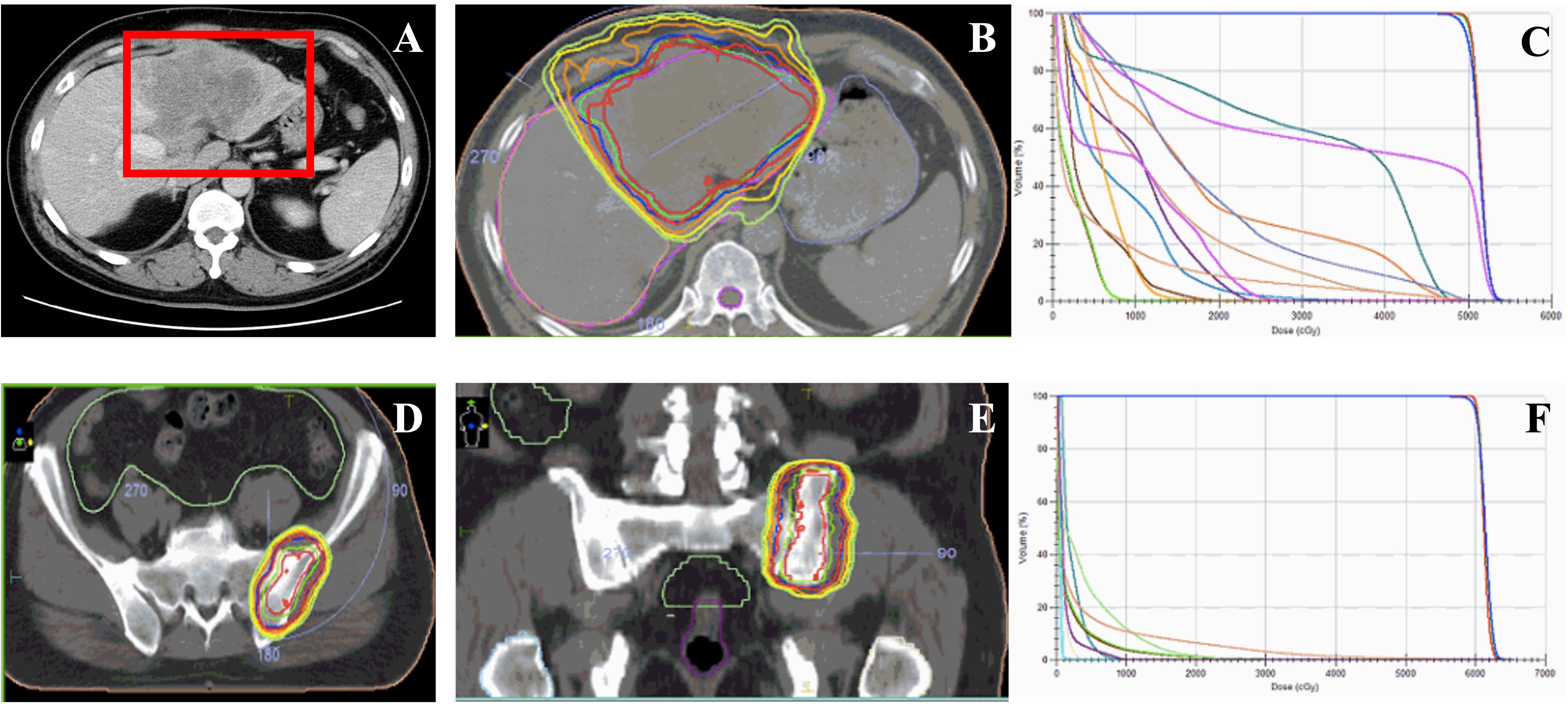
Figure 1. Image of the first patient during the initial treatment and the irradiation treatment plan of the bone metastasis of patient after progressing. (A) Liver images of the patient during the course of disease at diagnosis. (B) The target volume and the dosimetric distribution of the irradiation of the liver malignant. (C) The Dose-Volume Histogram (DVH) of the target volume and organ at risk (OAR) for evaluations of treatment plan. (D, E) The target volume of irradiation on the metastasis of the left ilium after three months of lenvatinib treatment. (F) The Dose-Volume Histogram (DVH) of the target volume and organ at risk (OAR) for evaluations of treatment plan.
Personal and family historyNo special notes.
Treatment and efficacyAll ancillary tests revealed that the patient had a portal vein cancerous embolus of the VP3 type at the time of initial diagnosis, and there was no indication for surgery. The patient initially received radiation therapy (RT) targeted to the liver tumor (50 Gy in 25 fractions) from June 25, 2020, to July 7, 2020 (Figures 1B,C). Moreover, the patient received lenvatinib (8 mg daily), and the treatment efficacy was evaluated for PR within 3 months until September 2020. Then, a bone scan revealed new metastasis in the left ilium. Radiotherapy was added to the metastasis of the left ilium at 30 Gy in 10 fractions (Figures 1D–F) with continuous targeted therapy consisting of lenvatinib and an added anti-PD-1 agent (camrelizumab, 200 mg). On March 15, 2021, hepatic arterial infusion chemotherapy (HAIC) was administered with the FOLFOX regimen because the intrahepatic lesions were still active. Systemic treatment was well tolerated, with a continuous tumor response in the liver for approximately 1 year until June 2021. Magnetic resonance imaging of the liver revealed progression of the liver lesion with new metastases in the right hepatic lobe and enlargement of the original tumor lesion (Figures 2A, B). Then, lenalidomide was added on June 25, 2021, accompanied by continuous lenvatinib treatment (8 mg daily) and anti-PD-1 therapy (camrelizumab 200 mg) until now. The patient achieved significant disease control after 3 weeks, 3 months and 6 months of lenvatinib and anti-PD-1 plus lenalidomide treatment (Figures 3A–C). Throughout the entire treatment process, the only drug-related adverse reaction observed was a second-degree decrease in the platelet count.
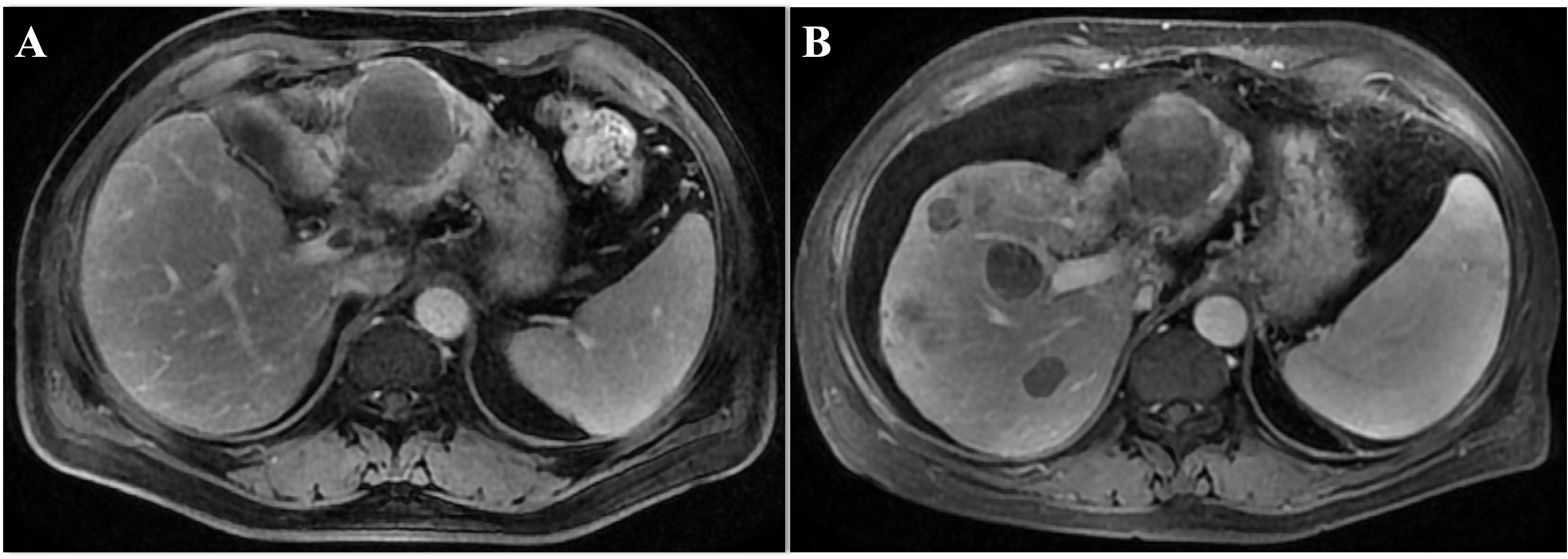
Figure 2. The image of the case during the initial treatment. (A) The original tumor size of liver shrinked significantly 1 week after HAIC and anti-PD-L1 treatment. (B) The enlarged primary tumor of the left lobe of liver and the new lesion appeared in the right lobe after Lenvatinib and anti-PD-L1 treatment. MRI, magnetic resonance imaging.
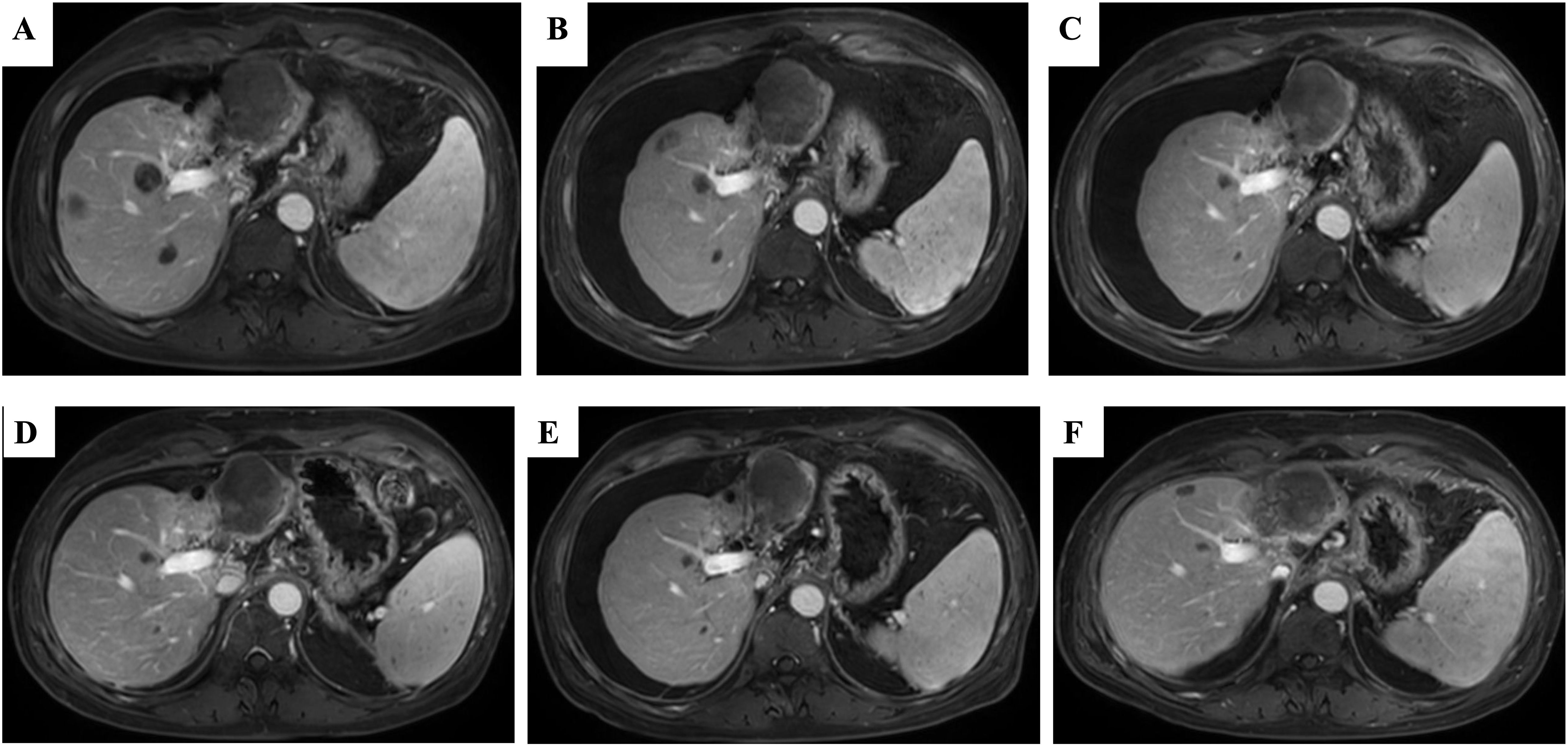
Figure 3. The liver MRI after lenalidomide interruption followed by the initiating of anti-PD-1 treatment and Lenvatinib again. (A) The remains of tumor regression after 3 weeks of Camrelizumab plus Lenvatinib. (B) The remains of tumor were continuous regressed after 3 months of Camrelizumab plus Lenvatinib. (C) The lesions of liver nearly disappeared after 6 months of the combination of lenalidomide and Lenvatinib. (D–F) The disease is still responding until now so far. MRI, magnetic resonance imaging.
Outcomes and follow-upAfter 9 months, 1 year and 2 years of treatment with lenalidomide, anti-PD-1 therapy and targeted therapy, the disease still responded (Figures 3D–F). The tumor markers AFP and PIVKA-II were continuously at low levels after combination with lenalidomide (Figures 4A, B).
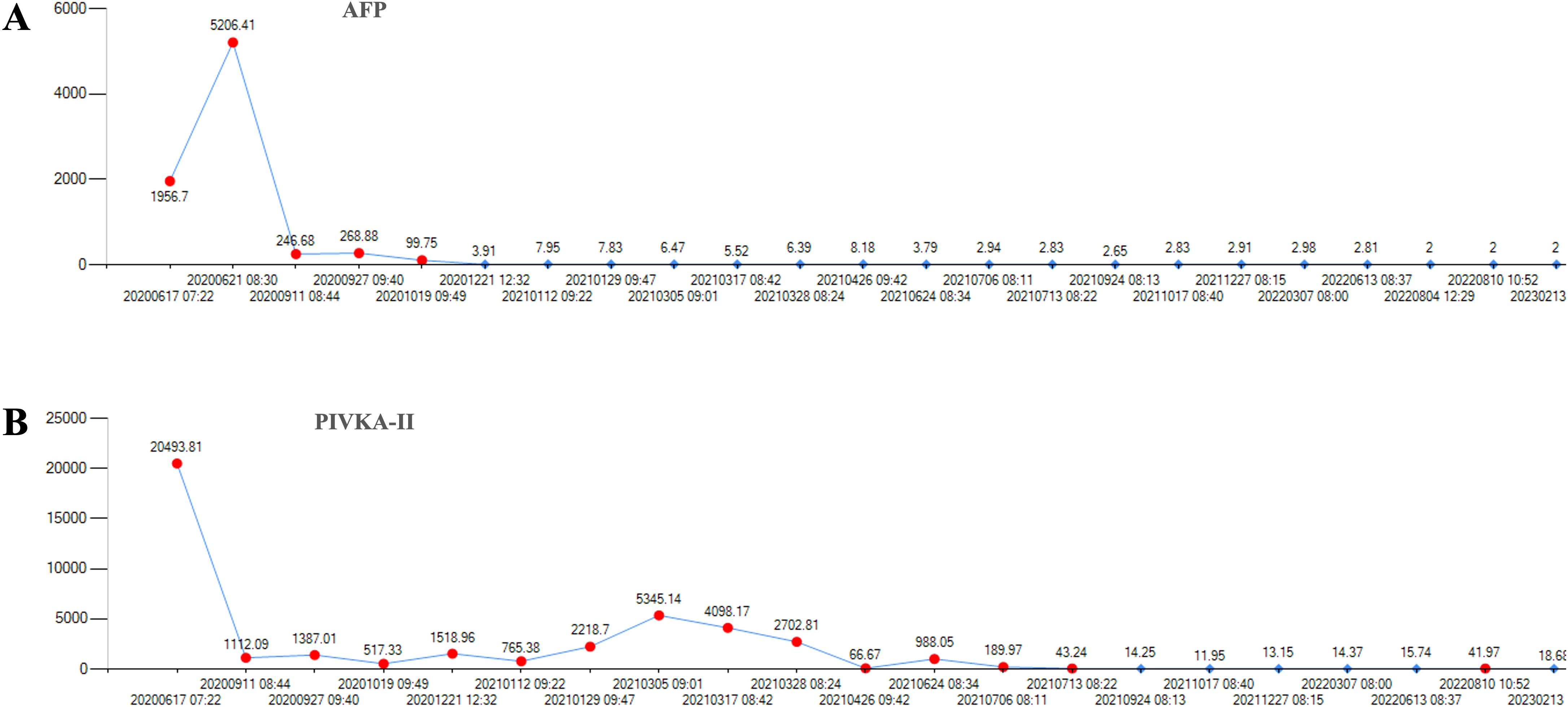
Figure 4. The level of the tumor markers of AFP and PIVKA-II of the first patient during the course of disease. (A) There was a continuous decline of AFP after lenalidomide and remained low level until the last follow-up date. (B) The level of PIVKA-II was declined after lenalidomide treatment. AFP, alpha-fetoprotein. PIVKA-II, vitamin K absence or antagonist-II.
In the case report, treatment consent was obtained from the patient, and she is satisfied with the therapeutic schedule and the results so far. We have de-identified the details such that the identity of the patient may not be ascertained in any way. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
DiscussionThere are no subsequent-line systemic therapy options for patients with HCC who experience disease progression after first-line treatment with ICIs. All the clinical trials for second-line treatment are based on sorafenib as the initial treatment. In our case, we presented the disease progression of an HCC patient after ICIs and lenvatinib. After the addition of lenalidomide to ICIs and lenvatinib treatment, the tumor shrunk again and has been controlled for more than 3 years, with no progression to date. At the initial treatment, the patient achieved an approximately 9-month DOR from September 2020 to June 2021 through camrelizumab plus lenvatinib. The progression-free survival (PFS) of the patient was much longer than that previously reported in the IMbrave 150 trial (6.9 months) (9) and the RESCUE trial (5.5 months) (10). A comparison of the combination of lenvatinib and pembrolizumab versus lenvatinib alone as a first-line treatment was completed in a randomized phase III trial, LEAP-002. Although the studies did not meet the primary endpoints (OS and PFS), the ORR was high, and the OS was long; therefore, in practice, this regimen is still used as the first-line treatment option for HCC in many medical centers in China because of its relatively high ORR and reduced risk of gastric bleeding (11). Unfortunately, patients inevitably progress from ICIs and targeted therapy.
Treatment with targeted ICIs is the mainstream first-line treatment for HCC, but there are few clinical data on the optimal treatment after first-line targeted therapy and ICIs. At present, the results of more second-line studies are mainly based on the failure of first-line administration of sorafenib. Therefore, there is an urgent need to find an effective second-line treatment for HCC after the failure of first-line targeted therapy and ICIs.
We considered the immune microenvironment of HCC and the mechanism of action of ICIs. The main inhibitory immune checkpoint receptors that naturally restrain T-cell activity and play vital roles in maintaining self-tolerance include PD-1, CTLA4, LAG3 and TIM3, whereas CD28, GITR and OX40 are examples of costimulatory immune checkpoint proteins that have been shown to enhance T-cell expansion (12). The interaction between PD-L1 and PD-1 leads to broad dephosphorylation of T-cell-activating kinases (13), resulting in T-cell inactivation; thus, PD-1–PD-L1 blockade restores effector CD8+ T-cell function (14). CTLA4 inhibition acts at the T-cell–antigen-presenting cell immune synapse by promoting unopposed interactions of B7 costimulatory ligands with CD28, leading to increased activation of naive CD4+ and CD8+ T cells and rebalancing of the effector and regulatory compartments within the tumor microenvironment (TME) (15, 16). In addition, programmed cell death protein 1 (PD-1) checkpoint blockade therapy requires the CD28 costimulatory receptor for CD8+ T-cell expansion and cytotoxicity. However, CD28 expression is frequently lost in exhausted T cells and during immune senescence, limiting the clinical benefits of PD-1 ICIs in individuals with cancer (17, 18).
Lenalidomide has immunomodulatory, anti-inflammatory, antiangiogenic and antineoplastic effects. Recently, one study using a mouse model of cereblon knockdown showed that lenalidomide reinstates the antitumor activity of CD28-deficient CD8+ T cells after PD-1 blockade. Lenalidomide redirects the CRL4Crbn ubiquitin ligase to degrade Ikzf1 and Ikzf3 in T cells and unleashes paracrine interleukin-2 (IL-2) and intracellular Notch signaling, which collectively bypasses the CD28 requirement for activation of intratumoral CD8+ T cells and inhibition of tumor growth by PD-1 blockade. This research suggested that PD-1 ICIs can benefit from a combination of lenalidomide and solid tumors infiltrated with abundant CD28- T cells (8). Based on these findings, we propose that lenalidomide may be an option when patients develop immune resistance.
In this case, the patient received lenalidomide combination therapy after disease progression following first-line targeted therapy and regained efficacy with ICIs, with tumor control for more than 3 years. The mechanism for this observed activity of ICIs in patients after progression remains unclear but might at least in part be due to the regulation of the TME. The use of lenalidomide alone or in combination with ICIs can allow patients to reobtain efficacy from ICIs. However, there are no data demonstrating the effect of lenalidomide alone or in combination on ICI resistance in HCC patients. This clinical practice provides new insight into the underlying mechanism and clinical trial.
ConclusionsOur case was the first international study demonstrating that the combination of lenalidomide and ICIs can be very therapeutic for HCC patients who have progressed after first-line therapy, with a low adverse effect profile and a good safety profile. Our case also shows that lenalidomide can increase the efficacy of ICIs, even if the patient is resistant to ICIs at the start of treatment. The novelty of our case is the prolonged use of ICIs in combination with an immunomodulatory drug, which improved survival and delayed resistance to ICIs. This is a case report, and further studies are needed to validate which patients may benefit from lenalidomide. In conclusion, this protocol represents a new therapeutic option for patients with HCC after progression who are receiving targeted immunologic first-line therapy.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by Beijing Tsinghua Changgeng Hospital, Tsinghua University, Beijing, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsTL: Writing – original draft. YZ: Writing – original draft. KL: Writing – original draft. GL: Writing – review & editing. GXL: Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe thank all the faculty members in our department and the patients, and their families involved in the article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther. (2023) 8:104. doi: 10.1038/s41392-023-01365-z
PubMed Abstract | Crossref Full Text | Google Scholar
3. Yang B, Chen K, Liu X, Liu W, Ma Y, Tian X, et al. Advance in tumor immunotherapy: establishing a new paradigm for oncological treatment. Trans Surg Oncol. (2023) 1:30–43.
4. Zhang T, Lin Y, Gao Q. Bispecific antibodies targeting immunomodulatory checkpoints for cancer therapy. Cancer Biol Med. (2023) 20:181–95. doi: 10.20892/j.issn.2095-3941.2023.0002
PubMed Abstract | Crossref Full Text | Google Scholar
5. Zhou R, Li S, Xiao X. Aryl hydrocarbon receptor nuclear translocator 2 as a prognostic biomarker and immunotherapeutic indicator for clear cell renal cell carcinoma. BIOCELL. (2023) 47:2397–408. doi: 10.32604/biocell.2023.030281
Crossref Full Text | Google Scholar
6. Kim EJ, Lee JG, Kim JY, Song SH, Joo DJ, Huh KH, et al. Enhanced immune-modulatory effects of thalidomide and dexamethasone co-treatment on T cell subsets. Immunology. (2017) 152:628–37. doi: 10.1111/imm.2017.152.issue-4
PubMed Abstract | Crossref Full Text | Google Scholar
7. Lenalidomide. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD (2012).
8. Geng CL, Chen JY, Song TY, Jung JH, Long M, Song MF, et al. Lenalidomide bypasses CD28 co-stimulation to reinstate PD-1 immunotherapy by activating Notch signaling. Cell Chem Biol. (2022) 29:1260–72 e8. doi: 10.1016/j.chembiol.2022.05.012
PubMed Abstract | Crossref Full Text | Google Scholar
9. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
PubMed Abstract | Crossref Full Text | Google Scholar
10. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer Res. (2021) 27:1003–11. doi: 10.1158/1078-0432.CCR-20-2571
PubMed Abstract | Crossref Full Text | Google Scholar
11. Finn RS, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, et al. LBA34 - Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. (2022) 33:S808–S69. doi: 10.1016/j.annonc.2022.08.031
Crossref Full Text | Google Scholar
13. Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. (2012) 209:1201–17. doi: 10.1084/jem.20112741
PubMed Abstract | Crossref Full Text | Google Scholar
14. Ahn E, Araki K, Hashimoto M, Li W, Riley JL, Cheung J, et al. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci U S A. (2018) 115:4749–54. doi: 10.1073/pnas.1718217115
PubMed Abstract | Crossref Full Text | Google Scholar
15. Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. (2005) 175:7746–54. doi: 10.4049/jimmunol.175.11.7746
PubMed Abstract | Crossref Full Text | Google Scholar
16. Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. (2009) 206:1717–25. doi: 10.1084/jem.20082492
PubMed Abstract | Crossref Full Text | Google Scholar
17. Di J, Liu M, Fan Y, Gao P, Wang Z, Jiang B, et al. Phenotype molding of T cells in colorectal cancer by single-cell analysis. Int J Cancer. (2020) 146:2281–95. doi: 10.1002/ijc.v146.8
PubMed Abstract | Crossref Full Text | Google Scholar
18. Kim KH, Kim HK, Kim HD, Kim CG, Lee H, Han JW, et al. PD-1 blockade-unresponsive human tumor-infiltrating CD8(+) T cells are marked by loss of CD28 expression and rescued by IL-15. Cell Mol Immunol. (2021) 18:385–97. doi: 10.1038/s41423-020-0427-6
留言 (0)