
Algorithm 1. mLZC Calculation Algorithm.
3.2.3 PermEnPermutation entropy serves as a specialized metric for quantifying time series random. This is achieved by generating distinct permutation modes through comparisons with neighboring data points. The computation entails the following steps:
Initially, the time delay τ and the embedding dimension m are established using the approach in Ibáñez-Molina et al. (2015), which to draw upon Recurrence Quantification Analysis (RQA).
The next step involves the reconstruction of the feature space. This entails arranging the component vector of the reconstruction matrix in ascending order of value. This step assists in identifying the pattern within the pattern group composed of m. The reconstruction matrix is following:
R =[x(1)x(1+τ)⋯x(1+(m-1)τ)⋮⋮⋮x(k)x(k+τ)⋯x(k+(m-1)τ)⋮⋮⋮x(K)x(K+τ)⋯x(K+(m-1)τ)] (9)where K = N−(m−1)τ; k = 1, 2, ⋯ , K and x(k) denotes the EEG data voltage value. This procedure generates a pattern sequence, which encapsulates the patterns identified within the dataset, as shown:
T=[t(1),t(2),⋯,t(k),⋯,t(K)] (10)where t(k)∈ and t(k) indicates the sequence number that matches the arrangement pattern. Subsequently, the Shannon entropy formula is applied as follows:
Hp=-∑l=1m!plln pl (11)where pl denotes the probability of each permutation pattern occurring and l ∈ . Finally, the permutation entropy is estimated using the aforementioned formula. This metric provides valuable insights into quantifying the inherent complexity within the time series data.
PermEn=Hpln(m!) (12) 3.2.4 mPermEnPermutation entropy can only reveal the randomness of brain discharge activities by detecting the randomness and dynamic displacement in time series, which has not been much researched on the deep-seated functional dynamic changes of the brain. In this study, we proposed a feature to characterize microstate randomness, and calculate a index, mPermEn, by using the probability distribution of the permutation pattern of each microstate subsequence. It effectively captures the randomness of microstate permutation patterns, representing the complexity of brain cognitive changes.
There is a phenomenon of sampling points belonging to the same microstate class for a period of time, which can seriously affect the probability distribution of several permutation patterns. Therefore, before calculating mPermEn, we removed several sampling points adjacent to the same microstate class in the microstate sequence (8). A new sequence of microstates is obtained:
MSv=[ms(1),ms(2),⋯,ms(u),⋯,ms(U)] (13)where ms(u) ≠ ms(u − 1), ms(u) ≠ ms(u + 1) and U ≤ N. We set the embedding dimension m to be the same as the number of microstate classes, and keep the time delay τ consistent with PermEn calculation. Therefore, we can attain a reconstruction matrix R as (9). And we also construct a mm × m dimensional permutation pattern matrix X and a zero vector of 1 × mm dimensional as index vector Y:
X=[a11a12⋯a1ma21a22⋯a2ma31a32⋯a3m⋮⋮⋮amm1amm2⋯ammm]mm×m (14) Y=[0,0,⋯,0]1×mm (15)where ajh=⌈j/mm-h⌉modm, j ∈ , h ∈ . Each row of the reconstructed matrix R is then compared with each row of the permutation pattern matrix X to determine which permutation pattern the reconstructed matrix R belongs to, and add 1 to the corresponding position of the index vector Y.
Finally, calculate the probability distribution for each permutation pattern and obtain mPermEn using the Equations 11, 12. The calculation process of mPermEn is outlined in Algorithm 2:

Algorithm 2. mPermEn Calculate Algorithm.
3.3 Spectral featuresIn this study, absolute power was computed for four distinct frequency bands: δ (1–4 Hz), θ (4–8 Hz), α (8–12 Hz), and β (12–30 Hz), along with the TBR, which is commonly used in neurological disease assessment (Luo et al., 2023; Group et al., 2023). The spectral analysis was carried out in two sequential steps. The initial step aimed to identify significant differences in frequency bands between the preictal and interictal states. Subsequently, the analysis sought to identify channels exhibiting significant differences within the specific frequency bands.
The power of each segment was computed using FFT, followed by the summation of power within each frequency band to yield the power of that band. Averaging across subjects and channels yielded a matrix of dimensions Ngroup * Nsubj * Nfre, where Ngroup represents interictal and preictal groups, Nsubj signifies the number of subjects, and Nfre corresponds to the count of frequency bands and TBR. By statistically analyzing the power matrix of these groups, differential frequency bands between the interictal and preictal periods were identified.
Once a distinct frequency band was ascertained, recalculating the absolute power within that band resulted in a matrix of dimensions Ngroup * Nsubj * Nchannel, where Nchannel refers to the count of EEG recording electrodes. Statistical analysis of this power matrix across groups highlighted channels exhibiting frequency band-specific differences.
3.4 Predictive classificationFor the seizure prediction task, we undertook both individual-level classifications over the normalized feature set.
In the individual-level classification task, we focused on classifying each subject's data into two states. To avoid evaluation bias caused by model overfitting, we first set aside 10% of the data as the test set, while the remaining 90% is used for training and validation. We applied 5-fold cross-validation to partition the training and validation sets, and employed grid search to optimize the hyperparameters of the support vector machine. This entire process was repeated 10 times, and the final performance evaluation was based on the average performance of the test set over the 10 iterations.
We primarily quantified classifier performance using metrics such as accuracy, sensitivity, false positive rate (FPR), and the area under the curve (AUC). In traditional classification tasks, accuracy is often considered a key performance metric. However, in this study, detecting the preictal class is critical due to its lower occurrence compared to the interictal class. As the preictal class is treated as the positive class, sensitivity becomes more important than specificity and accuracy in evaluating the proposed method. FPR represents the average number of false seizure predictions per hour of EEG recording, calculated as the number of predicted seizure events within 1 hour that do not overlap with actual reference seizures. AUC was used to assess the overall classifier performance, with higher values indicating better classification and discrimination.
In addition, to evaluate the classification effectiveness across each feature category, we separately inputted microstate parameters, nonlinear features, and spectral features into the SVM classifier. In the end, there are epochs of multiple lengths extracted for seizure prediction to explore the effect of data length on microstate parameters, nonlinear features and spectral features.
3.5 Statistical analysisIn this study, the R (v4.3.0) (Rahlf, 2017) was used for statistical analyses to identify and elucidate features with significant differences between the interictal and preictal states. Initially, the Kruskal-Wallis test was performed across clusters of microstate topographic maps to identify variations between the interictal and preictal conditions. Subsequently, the Shapiro-Wilk normal distribution test was performed on the each feature. Paired samples t-test was conducted for data that met the normal distribution, otherwise Wilcoxon signed rank sum test was used. Throughout the experiment, a trend emerged where the significance of outcomes increased with an increase in sample size. This phenomenon results in, with a substantial sample size, the significance indicated by the α level of 0.05 deviates from the real-world context. To address this, the G*Power software (Faul et al., 2009) was used to adjust the α significance level. The determination of α was guided by the desired effect size, power, and the extant sample size, ensuring that it accurately and scientifically mirrored the real-world significance. Cohen'sd effect size (Terpou et al., 2022) was also adopted to quantitatively express the distinction between the interictal and preictal states.
4 Results and discussions 4.1 Prediction performanceWe compiled the microstate parameters, nonlinear features, and spectral features into a consolidated feature matrix, which was then utilized as input for the SVM classifier. The results yielded promising individual-level classification metrics, including an average accuracy of 94.80%, sensitivity of 93.82%, FPR of 0.061/h, and AUC of 0.98 on FH-ZJU dataset; accuracy of 92.32%, sensitivity of 93.22%, FPR of 0.078/h, and AUC of 0.95 on Siena Scalp EEG dataset.
Table 2 portrays a comparison of the performance of existing methods of predicting seizures with scalp EEG dataset in recent years. At present, the most widely used dataset for seizure prediction is the CHB-MIT dataset (Lu et al., 2023a), whose EEG dataset of patients with medically uncontrollable seizures was collected by the Children's Hospital Boston in collaboration with the Massachusetts Institute of Technology and contains 22 subjects (5 males and 17 females). It can be seen from the table, majority of the researches achieved a sensitivity of 91% or more, implying that their methods are good at seizure prediction. One of the more notable performances is the method of Usman et al. (2021) and Lu et al. (2023b). Fusing the automatic features extracted by convolutional neural network (CNN) with manually extracted temporal features, Usman et al. (2021) trained using SVM, CNN and LSTM respectively, and achieved a sensitivity of 96.28% over CHB-MIT dataset. Lu et al. (2023b) used 3D CNN model to automatically extract information from EEG signals after STFT and later used Bi-LSTM for classification. Finally, a sensitivity of 98.40% and an accuracy of 97.95% were achieved on the CHB-MIT dataset. Both of them achieve a excellent performance by automatically extracting features using CNNs, however, the involved perspectives embracing time or spectral attributions are slightly single.
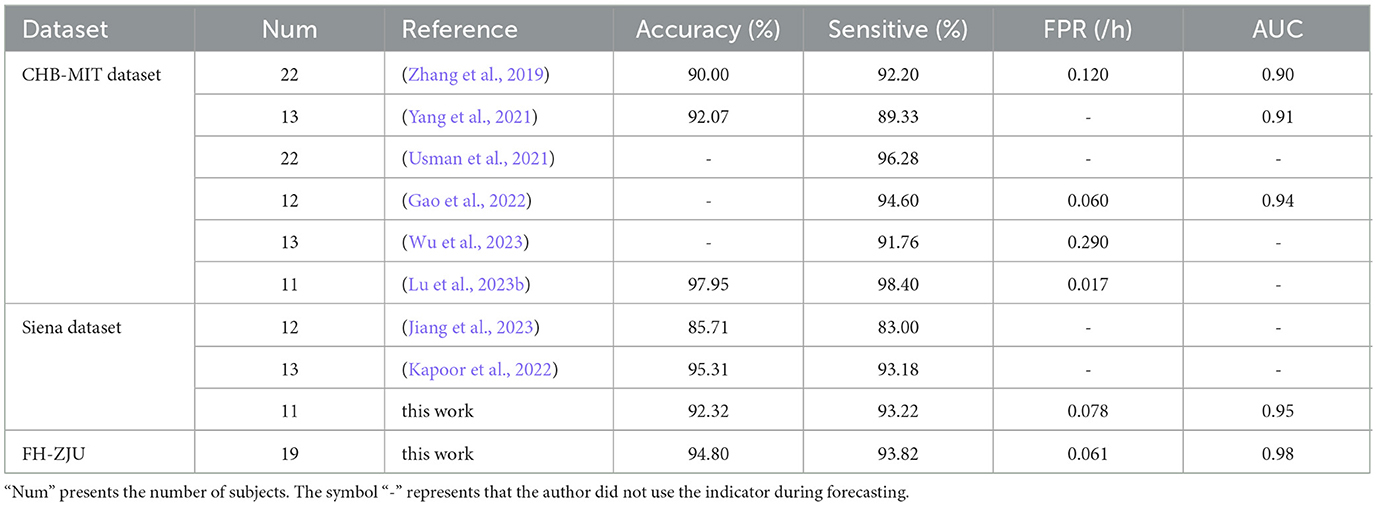
Table 2. Comparison of existing individual-level seizure prediction methods using scalp EEG signals.
Since the microstate analysis method used in this paper requires channel localization for single-lead EEG data. Therefore, we tested the proposed method on the Siena Scalp EEG dataset and compared it with previous works using the same dataset. Jiang et al. (2023) proposed a method based on frequency-domain analysis and phase-amplitude coupling (PAC) combined with a random forest classifier for seizure prediction and ultimately achieved an accuracy of 85.71% and a sensitivity of 83% on Siena EEG dataset. Kapoor et al. (2022) used a seizure prediction algorithm consisting of hybrid optimization control integrated classifier to classify wavelet and entropy based features in each frequency band and finally achieved accuracy of 95.31% and sensitivity of 93.18% on Siena dataset. Similar to Jiang et al. (2023) and Kapoor et al. (2022), this study does not employ a data-driven prediction approach. Instead, it bases predictions on the features that exhibit the most significant differences between the interictal and preictal periods. However, unlike these previous works, this study not only incorporates spectral information and nonlinear properties but also integrates the microstate temporal dynamics properties, achieving higher sensitivity.
To further assess the predictive efficacy of different feature types, we subjected microstate parameters, nonlinear features, spectral features, and their combined fusion features (referred to as “fusion features”) to classification at the individual level. The results as displayed in Table 3. Besides, to better compare the classification performance between different features visually, the results are shown in the radar plot of Figure 2. The figure shows that whether in the FH-ZJU dataset or the Siena dataset, it is with the same trend. Apart from fusion features, microstate features demonstrating the most pronounced classification performance, followed by nonlinear features and spectral features. The accuracy of fusion features and microstate parameters was more than 10% higher than that of nonlinear and spectral features across both the FH-ZJU and Siena datasets. Besides, the accuracy of fusion and microstate features was quite similar, with differences of only 2.18% on the FH-ZJU dataset and 3.79% on the Siena dataset. It highlights that the fusion feature matrix primarily derives its potency from microstate features. This means that the microstate temporal dynamic properties of EEG data are more suitable for seizure prediction tasks than nonlinear and spectral information. Besides, it can be seen from the performance of fusion features that the complementary information from multiple feature types can significantly improve the prediction performance.
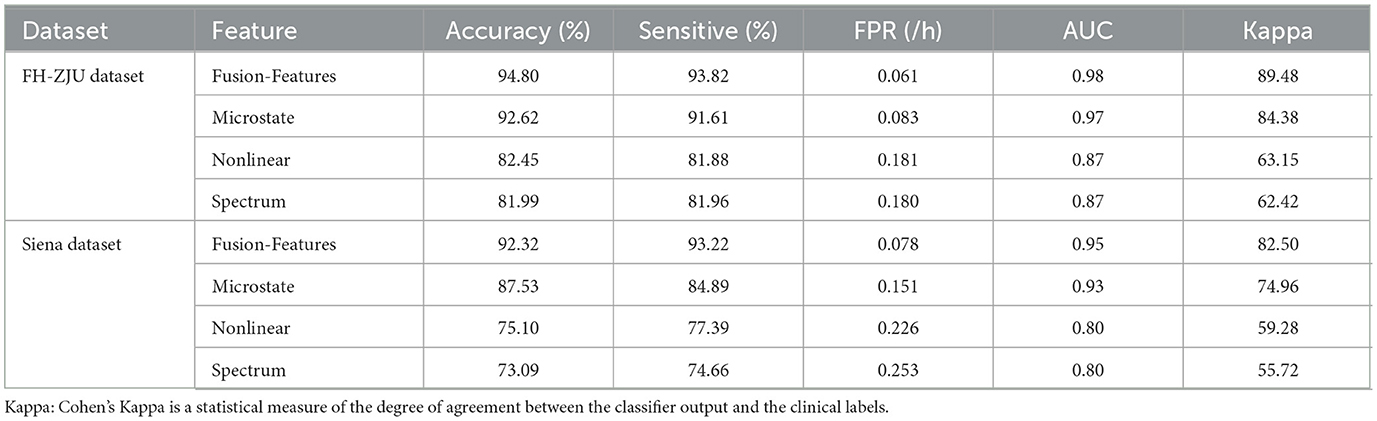
Table 3. Individual-level predictive performance results for multiple types of features.
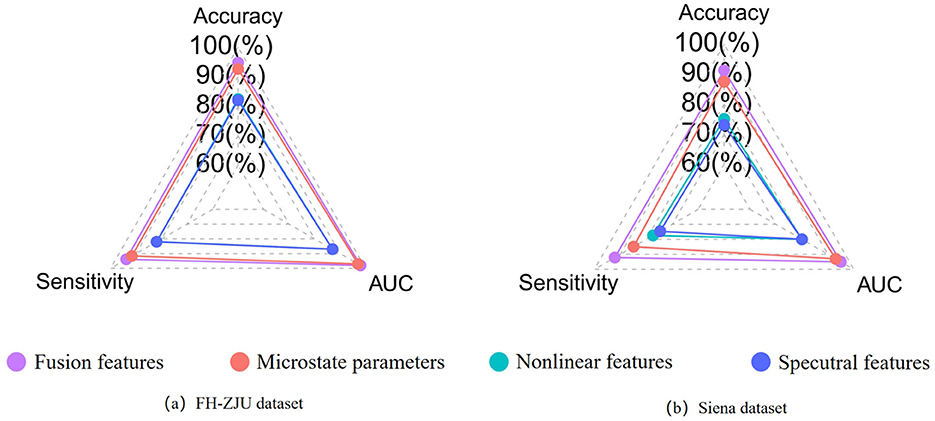
Figure 2. (a) Comparison of the individual-level performance of multiple types of features on the FHZJU dataset. (b) Comparison of the individual-level performance of multiple types of features on the Siena dataset.
Furthermore, we conducted subject-individual prediction experiments with different data lengths on the FH-ZJU dataset to verify the influence of data lengths on these features. From Figure 3a, it is seen that 3s data length have the optimum performance, 4s and 5s had a similar performance between that of 3 s and 2 s. To explore why the fundamental reason for the optimal performance of 3s data length, we separately conducted experiments on each features with different lengths at the individual-level. The results of microstate parameters, nonlinear features and spectral features are depicted respectively in Figures 3b–d. Comparing the four bar charts, we found that the distribution of microstate parameters in Figure 3b is nearly close to that of fusion features in Figure 3a, which reveals that the advantage of fusion features with 3s data length mainly comes from microstate features with 3s data length. These results gave a further verification on the significance of microstate temporal dynamic properties in seizure prediction. From Figures 3c, d, we can see that the results of different fragment lengths of nonlinear features and spectral features hold steady, which indicated that the nonlinear features and spectral features are less sensitive to the data length.
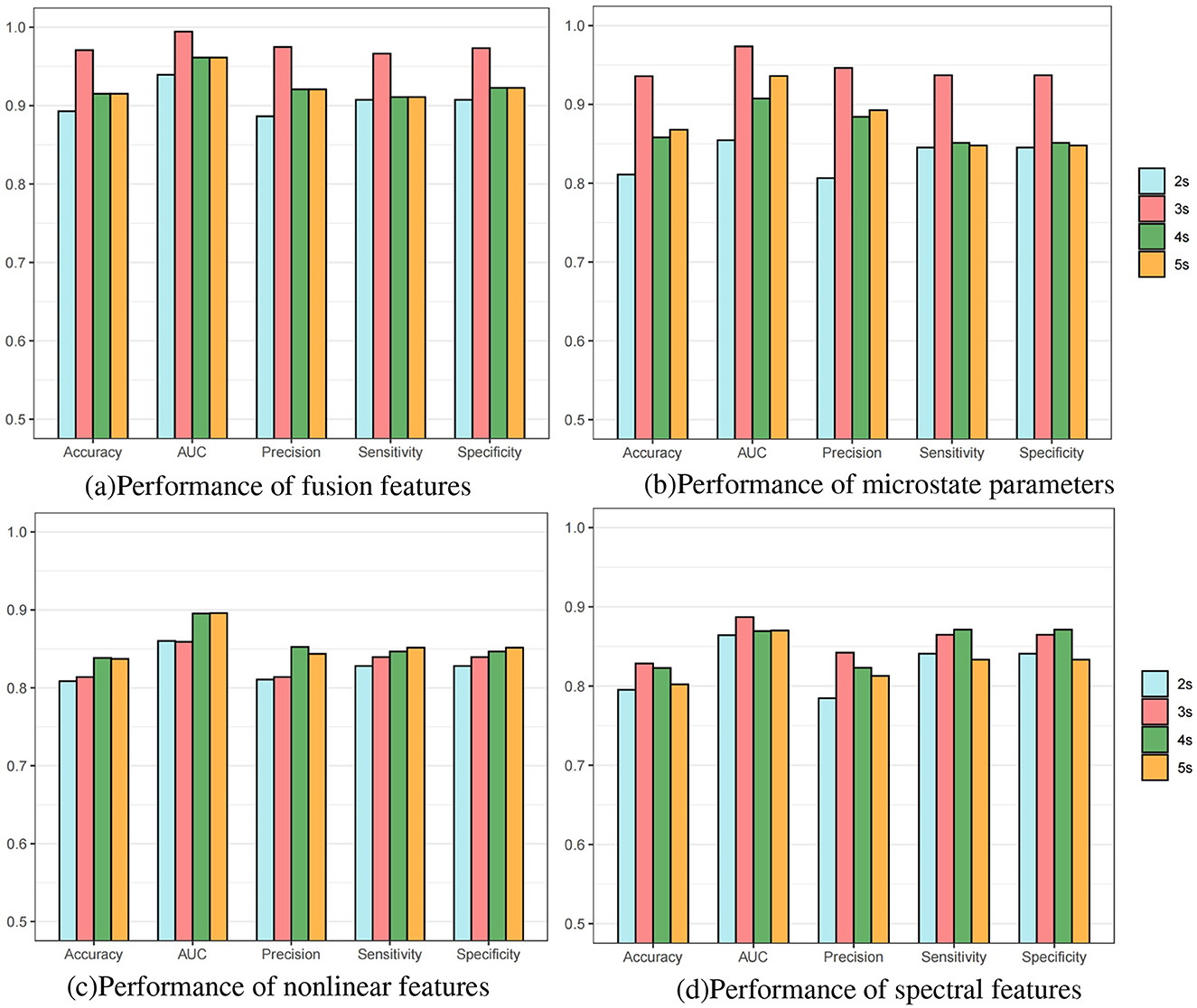
Figure 3. Bar charts of individual-level predictive performance of different length epochs on FH-ZJU dataset. (a) Performance of fusion features. (b) Performance of microstate parameters. (c) Performance of nonlinear features. (d) Performance of spectral features.
Notably, despite using a basic SVM model in this experiment, the prediction performance was remarkably high. This outcome can likely be attributed to two key factors. First, the dataset used in this study contained relatively few seizure events, and SVMs are well-suited for binary classification tasks with small sample sizes, allowing the model to maintain reliable prediction accuracy even with limited data. Second, the length of the preictal prediction window also significantly impacts the model's performance (Lu et al., 2021). Longer prediction windows may provide more advanced warnings but could introduce additional noise and uncertainty, while shorter windows may improve prediction accuracy by focusing on more immediate preictal changes. Therefore, optimizing the length of the prediction window is crucial for further enhancing the model's predictive capabilities.
4.2 Microstate properties between interictal and preictalMicrostate analysis of scalp electroencephalogram in the interictal and preictal groups yielded four canonical microstate topographic maps, as shown in Figure 4. These maps are consistent with those reported in previous studies on neurological disorders and are characterized as follows: class A (right-frontal and left-posterior topographies), class B (left-frontal and right-posterior topographies), class C (midline and frontal-occipital topographies), and class D (midline and frontal topographies). The four canonical microstate structures, often aligned with inherent brain functional networks (class A linked to the auditory network, class B to the visual imagination network, class C to the salience network, and class D to the attention network), exhibit general consistency between interictal and preictal states. The microstate maps showed overall consistency between the interictal and preictal states, accounting for 76.11% and 78.12% of the global explained variance (GEV) in the interictal and preictal groups, respectively. Consistent with Takarae et al. (2022), Kruskal-Wallis tests indicated no statistically significant differences between the interictal and preictal groups for any of the microstate maps [p(A) = 0.78, p(B) = 0.53, p(C) = 0.92, p(D) = 0.80].
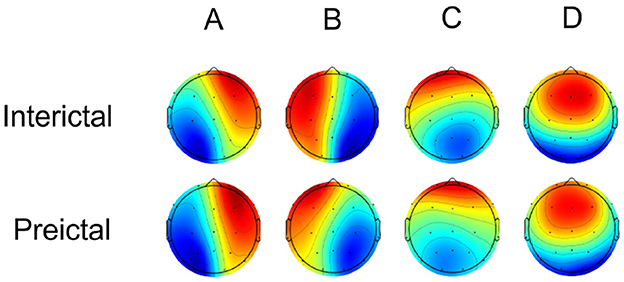
Figure 4. Four canonical microstate topographic maps of Interictal and Preictal.
Three parameters were obtained to capture the dynamic shifts in thought atom by applying microstate map backfitting to EEG data. We set the effect size, the power, and the sample size to 0.3, 0.95 and 600, and the adjustment of α was adjusted to 0.0005 using G*Power software. The test results and Cohen'sd value highlighted significant alterations in microstate dynamics between the interictal and preictal states, as depicted in Table 4. We thought that only when the P-value is less than 0.0005 and the absolute value of Cohen'sd exceeded 0.3, there are significant difference between preictal and interictal. The findings revealed that during the preictal phase, class A exhibited markedly elevated duration and coverage compared to its interictal counterpart. Conversely, class C displayed considerably reduced duration and coverage relative to interictal observations. Furthermore, the analysis of occurrence characteristics revealed that class A and class B microstates were lower in preictal states than interictal states, while occurrences of class D microstates were higher in preictal states than interictal states. Based on these phenomena, we hypothesize that seizures may result from changes that make microstate C more likely to shift to microstate A. We will continue to explore this point in our next work.
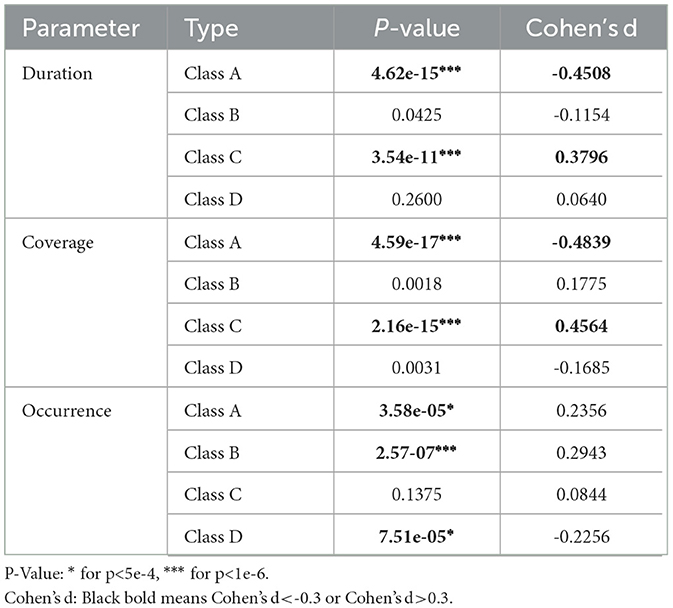
Table 4. Statistics of microstate parameters between interictal and preictal states.
4.3 Nonlinear attributes between interictal and preictalTo quantify the nonlinear dynamic changes between interictal and preictal states, six nonlinear features were extracted: mLZC, LZCmean, LZCmedian, LZCmid_p, mPermEn, and PermEn. As shown in Figures 5a, b, the test revealed that preictal complexity generally exhibited lower values compared to interictal complexity, along with greater numerical dispersion, which was consistent with Lehnertz (2008) as well as Babloyantz and Destexhe (1986), suggesting that epileptogenicity involves complexity reduction. Seizure activity is characterized by weakened nonlinearity, whereas interictal EEG demonstrates higher nonlinearity. Furthermore, we hypothesize that synchronized abnormal neuron firing seizures leads to this reduction in nonlinearity. In the 3 minutes preceding a seizure, nonlinearity displayed a decreasing trend, indicating a gradual loss of autonomous brain function. Additionally, individual EEG variations in the preictal state were greater than those in the interictal state, where complexity values tended to cluster within a normal range.
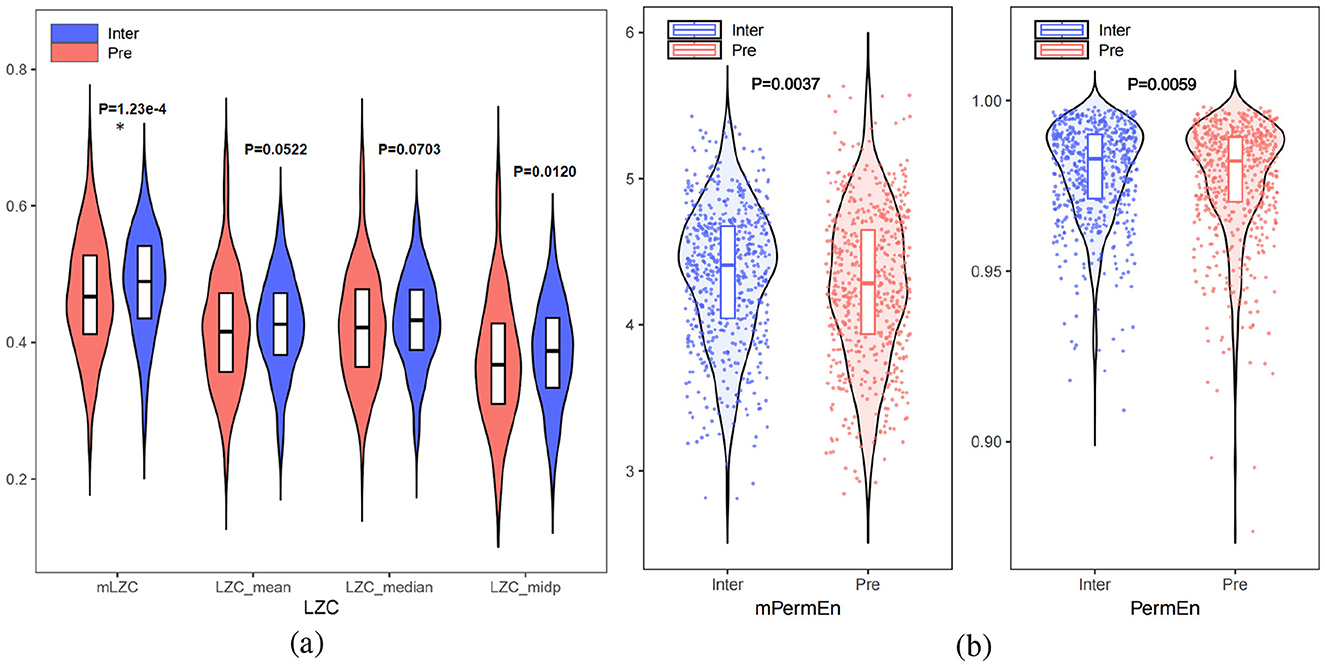
Figure 5. (a) Comparison of LZCmean, LZCmedian LZCmid_p with mLZC between interictal and preictal. (b) Comparison of PermEn and mPermEn between interictal and preictal (* for p <5e-4).
As shown in Figures 5a, b, the proposed microstate-based features, mPermEn (p = 0.0037) and mLZC (p = 1.23e-4), more effectively distinguished between interictal and preictal states compared to the EEG-based features PermEn (p = 0.0059), LZCmean (p = 0.0522), LZCmedian (p = 0.0703), and LZCmid_p (p = 0.0120). This highlights that mPermEn and mLZC aptly capture the complexity changes in brain cognitive activity, going beyond the traditional exploration of epilepsy pathology based solely on EEG signal complexity.
4.4 Spectral information between interictal and preictalFor statistical analysis of spectral characteristics, we calculated the power of each frequency band and adjusted the significance level α to 0.05. The paired t-test results indicated no significant difference in absolute power across frequency bands between interictal and preictal states (Figure 6a). However, upon closer examination, the δ bands showed decreased power in preictal states, while θ band power and TBR exhibited an increasing trend prior to seizures. Among the frequency bands, the δ band displayed the most substantial variation between the interictal and preictal states. We further analyzed differences in the δ band across different channels (Figure 6b), revealing significant disparities in the right frontal brain regions, specifically in the F4 (p = 0.0203), C4 (p = 0.0372), and F8 (p = 0.0113) channels.
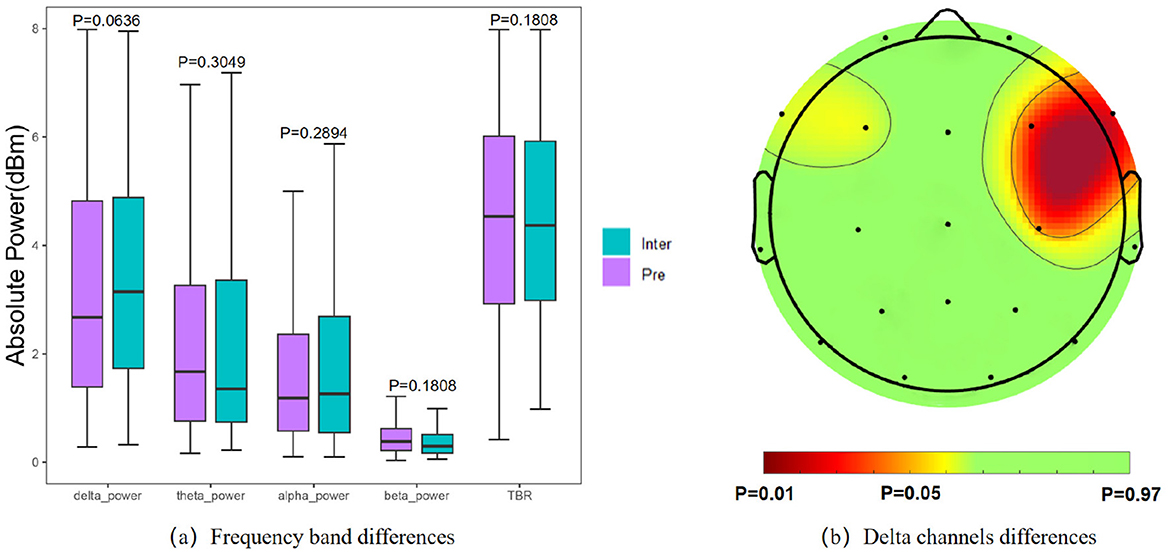
Figure 6. Results of statistical analysis of spectral features. (a) Comparison of power in each frequency band. (b) Significantly different of the δ band power on each channel.
A comparison of the statistical analysis results of the microstate parameters, nonlinear features and spectral features reveals that the microstate parameters exhibited the most significant differences between interictal and preictal phases, followed by the nonlinear features, which aligns with the predictive results. These findings underscore that epileptic seizures result in abnormal changes in brain microstate dynamics.
5 ConclusionIn this paper, we employed cognitive dynamic attributes to delineate the preictal state. By integrating nonlinear and spectral characteristics, a remarkable performance was achieved on both the FH-ZJU and Siena dataset. Besides, we proposed a novel microstate-based nonlinear feature, mPermEn, to effectively capture and articulate the randomness at the microstate level between interictal and preictal states. Comparative analysis revealed that changes in cognitive pattern dynamics using microstates better characterized the preictal period than nonlinear attributes and spectral information. Moreover, microstate properties within 3-second fragments proved more suitable for seizure prediction tasks. In all, microstate analysis not only elucidates temporal dynamic alterations in neurological diseases but also serves as a foundational approach for other EEG analysis methods, offering substantial potential for exploring the brain's cognitive mechanisms.
6 LimitationThe research method employed in this article has certain limitations. Specifically, relying solely on LZC, permutation entropy, and power as nonlinear and spectral features limits our ability to comprehensively capture such information. Consequently, our findings only preliminarily suggest that cognitive dynamic attributes may be superior to nonlinear and spectral metrics in characterizing the preictal phase. Moving forward, we will use additional, more representative features in these perspective characterization.
As the aim of this article is to introduce brain cognitive dynamic attributes into seizure prediction, assessing their potential as seizure biomarkers. To this end, we chose a basic SVM classifier rather than more intricate models. In future work, we plan to explore complex models to enhance seizure prediction accuracy.
Data availability statementThe datasets presented in this article are not readily available because part of the data involves patient privacy. Requests to access the datasets should be directed to Fangni Chen, chenfangni@zust.edu.cn.
Ethics statementThe studies involving humans were approved by the Ethics Committee of the Fourth Affiliated Hospital, Zhejiang University School of Medicine (registration number: K20190031). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributionsWS: Conceptualization, Formal analysis, Methodology, Project administration, Software, Visualization, Writing – original draft. YC: Data curation, Investigation, Project administration, Resources, Writing – review & editing. FC: Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. WT: Project administration, Validation, Writing – review & editing. LZ: Funding acquisition, Supervision, Writing – review & editing. JW: Funding acquisition, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Key Research and Development Project of Zhejiang Province (2022C03043), “Pioneer” and “Leading Goose” R&D Program of Zhejiang Province (2023C03195), the key projects of major health science and technology plan of Zhejiang Province (WKJ-ZJ-2129), and the National Natural Science Foundation of China (61601409).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesBagdasarov, A., Roberts, K., Bréchet, L., Brunet, D., Michel, C. M., and Gaffrey, M. S. (2022). Spatiotemporal dynamics of EEG microstates in four-to eight-year-old children: age-and sex-related effects. Dev. Cogn. Neurosci. 57:101134. doi: 10.1016/j.dcn.2022.101134
PubMed Abstract | Crossref Full Text | Google Scholar
Baud, M. O., Proix, T., Gregg, N. M., Brinkmann, B. H., Nurse, E. S., Cook, M. J., et al. (2023). Seizure forecasting: bifurcations in the long and winding road. Epilepsia 64, S78–S98. doi: 10.1111/epi.17311
PubMed Abstract | Crossref Full Text | Google Scholar
Bréchet, L., Brunet, D., Birot, G., Gruetter, R., Michel, C. M., and Jorge, J. (2019). Capturing the spatiotemporal dynamics of self-generated, task-initiated thoughts with eeg and fMRI. Neuroimage 194, 82–92. doi: 10.1016/j.neuroimage.2019.03.029
PubMed Abstract | Crossref Full Text | Google Scholar
Chu, C., Zhang, Z., Wang, J., Li, Z., Shen, X., Han, X., et al. (2023). Temporal and spatial variability of dynamic microstate brain network in early Parkinson's disease. NPJ Parkinson's Dis. 9:57. doi: 10.1038/s41531-023-00498-w
留言 (0)