Ovarian cancer (OC) ranks as the fifth most common cause of mortality due to cancer in women, with a survival rate of fewer than 50% over a period of five years (1). In the majority of instances, the main approach to treating ovarian cancer involves combining cytoreductive surgery with systemic chemotherapy (2, 3). Although the majority of patients experience a complete response to primary treatment, most will eventually experience a recurrence (4). The condition is associated with a pessimistic prognosis, as the average length of survival is less than 12 months (5).
Targeted therapy is now widely recognized as a successful treatment for various forms of cancers (6). Targeted therapy, such as anti-angiogenesis inhibitors (Ais) and poly ADP ribose polymerase inhibitors (PARPi), as an adjuvant therapy alongside chemotherapy and radiation therapy, can significantly improve treatment outcomes (7). Three PARPis, namely olaparib, niraparib, and rucaparib, have been granted approval by the US Food and Drug Administration (FDA) for use as maintenance therapy in OC patients (8). Olaparib, niraparib, and rucaparib have been approved for maintenance and third-line treatment, respectively, in Europe for platinum-sensitive, relapsed, BRCA1/2 mutated ovarian cancer after a complete or partial response (CR/PR) to platinum-based chemotherapy (9). Maintenance treatment with olaparib plus bevacizumab should be explored for patients with recently diagnosed advanced ovarian cancer, regardless of clinical risk, according to the PAPLA-1 and OVARIO trials (8). In the PAOLA-1 study, the median progression-free survival (PFS) reported by investigators was 22.1 months for olaparib plus bevacizumab and 16.6 months for placebo plus bevacizumab (10). The combination of niraparib with bevacizumab for maintenance therapy resulted in a primary endpoint of 18-month PFS rate of 62% in the intention-to-treat (ITT) population, with a median PFS of 19.6 months (11). Due to the notable clinical advantages of combining Ais with PARPi observed in ovarian cancer patients, there has been a rise in the number of clinical trials investigating the safety and effectiveness of this combination for the maintenance treatment of advanced ovarian cancer.
In the present study, we aimed to systematically assess the available evidence in the literature regarding the efficacy and safety of maintenance Ais combined with PARPi for patients with advanced ovarian cancer.
2 Material and methods2.1 Search strategyThe current meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 standards. This study has been registered at PROSPERO with a registration number of CRD42024543590. A systematic search was conducted in four databases, namely PubMed, Embase, Web of Science, and the Cochrane Library, to retrieve literature published until January 15, 2024. The search method used the following terms: “ovarian cancer,” “PARP inhibitor,” “angiogenesis inhibitors,” and “randomized controlled trial.” We also conducted a thorough manual examination of the bibliographies of the identified papers, as well as pertinent reviews and meta-analyses, in order to uncover any new research that fit the criteria for inclusion. Supplementary Material 1 provided a comprehensive overview of the search record.
2.2 Inclusion and exclusion criteriaInclusion criteria: (1) patients diagnosed with advanced ovarian cancer, fallopian tube cancer, or primary peritoneal cancer; (2) at least one cohort of patients was administered maintenance Ais combined with PARPi; (3) At least one of the following outcomes was reported: ORR, PFS, OS, Grade ≥ 3 AEs; (4) Study types: randomized controlled studies, single-arm trials.
Exclusion criteria: (1) other types of articles, such as case reports, publications, letters, reviews, meta-analyses, editorials, pharmacological intervention, animal studies and protocols; (2) other diseases; (3) irrelevant studies; (4) failed to extract data; (5) duplicate patient cohort.
2.3 Selection of studiesThe process of literature selection, which involved removing duplicate entries, was conducted using EndNote (Version 21; Clarivate Analytics). The initial search was undertaken by two independent reviewers. The duplicate records are eliminated, the titles and abstracts were assessed to ascertain their relevance, and each study was categorized as either included or excluded. We arrived at a resolution through the consensus. If the parties were unable to reach an agreement, a third reviewer acted as a mediator.
2.4 Data extractionTwo autonomous reviewers conducted a thorough examination of the title and abstract, followed by a comprehensive reading of the entire text. The discrepancies were resolved through consultation with a third investigator. The collected data consist of the first author’s name, year of publication, study area, trail ID, study design, sample size, intervention, age of participants, trial phase, study period, median follow-up duration, ORR, Grade ≥ 3 AEs, Kaplan-Meier curves for OS and Kaplan-Meier curves for PFS.
2.5 Risk of bias assessmentTwo unbiased reviewers evaluated the quality assessment of the included studies. We utilized the modified Jadad scale (12) as well as Cochrane Collaboration risk of bias assessment tool (ROB) to assess the quality of randomized controlled trials in this analysis. The single-arm trials were evaluated using the methodological index for non-randomized studies (MINORS) (13).
2.6 Statistical analysisThe selection of duplicates of studies included was conducted using EndNote (Version 21; Clarivate Analytics). All analyses were performed using Stata 16.0. The “meta” package and IPDformKM package were utilized in the analysis. GetData Graph Digitizer software was used to extract data from articles containing Kaplan-Meier curves, and individual data were reconstructed using the IPDformKM package. The established method by Guyot et al. was used to reconstruct individual patient-level data (14). Continuous variables were compared using the weighted mean difference (WMD) with a 95% confidence interval (CI). Relative ratio (RR) with 95% CI was used to compare binary variables. The medians and interquartile ranges of continuous data were converted to means and standard deviations. Statistical heterogeneity between included studies was calculated using the Cochrane ‘Sq test and the I2 index (I2 >50% indicating significant heterogeneity). When there was high heterogeneity among studies, the random effects model was adopted, otherwise the fixed effects model was adopted (15). P values < 0.05 was considered statistically significant. Finally, a sensitivity analysis was performed to determine the impact of individual studies on the aggregated results and to test the reliability of the results.
3 Results3.1 Search resultsFigure 1 illustrates the process of selecting and incorporating literature. We initially discovered a grand total of 1,224 studies. After eliminating unnecessary research, a total of 755 papers were kept. After assessing the titles and abstracts, a grand total of 24 publications were deemed inappropriate and thus excluded. Following a thorough examination of the entire text, a total of nine studies were included for inclusion in this meta-analysis.
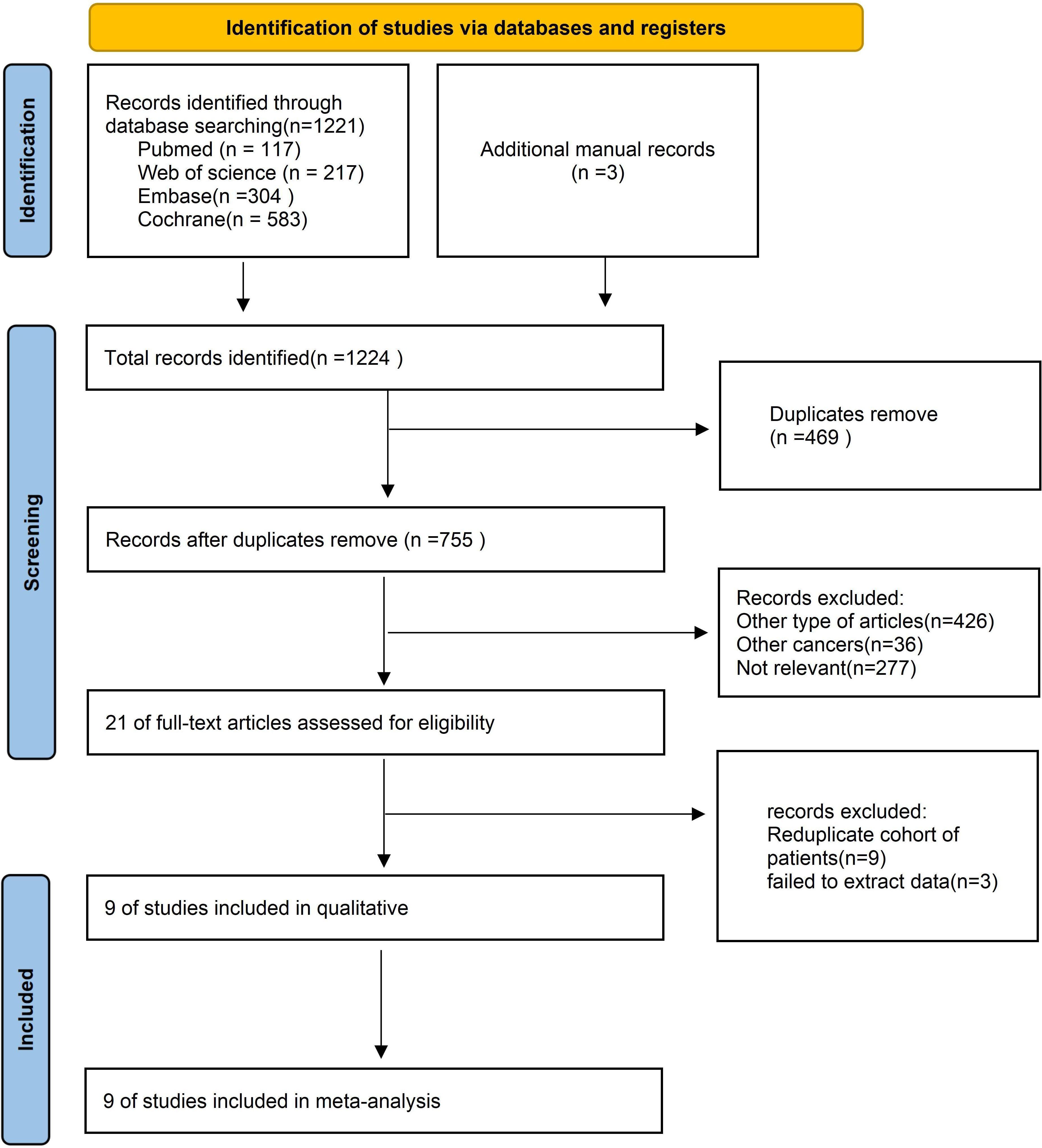
Figure 1. Flow chart of literature search strategies.
3.2 Patient characteristics and quality assessmentTable 1 presents detailed data on patient characteristics and quality assessment. A total of 9 articles were included in this article, including 6 RCTs (10, 16–20) and 3 single-arm trials (11, 21, 22), with a total of 1074 patients diagnosed with diagnosed with advanced ovarian cancer. The authors, registration ID, year, region, study design, study arm, patients, age, and median follow-up for each study are shown in Table 1. The meta-analysis focused exclusively on the data of patients who received Ais combined with PARPi. A subgroup analysis was conducted to address inconsistencies in the patient inclusion criteria across various trials, encompassing platinum-resistant recurrent ovarian cancer (17, 18, 21, 22), platinum-sensitive recurrent ovarian cancer (16, 19, 20), and newly diagnosed advanced ovarian cancer (10, 11). The specific regimens and doses in each trial were provided in Table 1. Six trials used Olaparib (10, 16–18, 20, 21) and three used Niraparib (11, 19, 22). We employed the modified Jadad scale to evaluate the quality of the RCT literature for quality assessment. The single-arm studies were evaluated using the MINORS methodology. All trials were considered to be of high quality (Table 1). Figure 2 presents a concise overview of the risk of bias assessment results for RCTs using ROB.
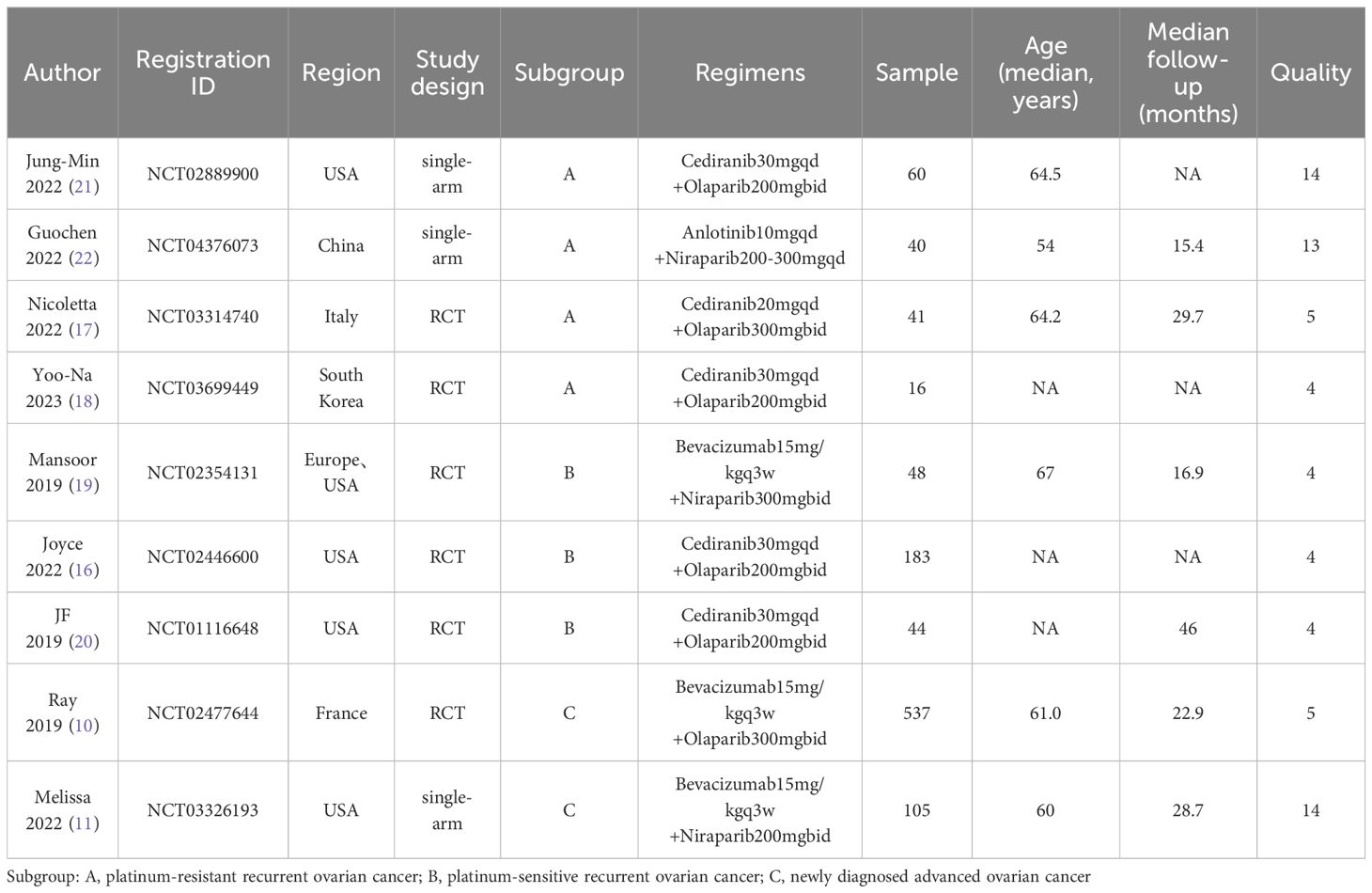
Table 1. Characteristics of included studies and patients.
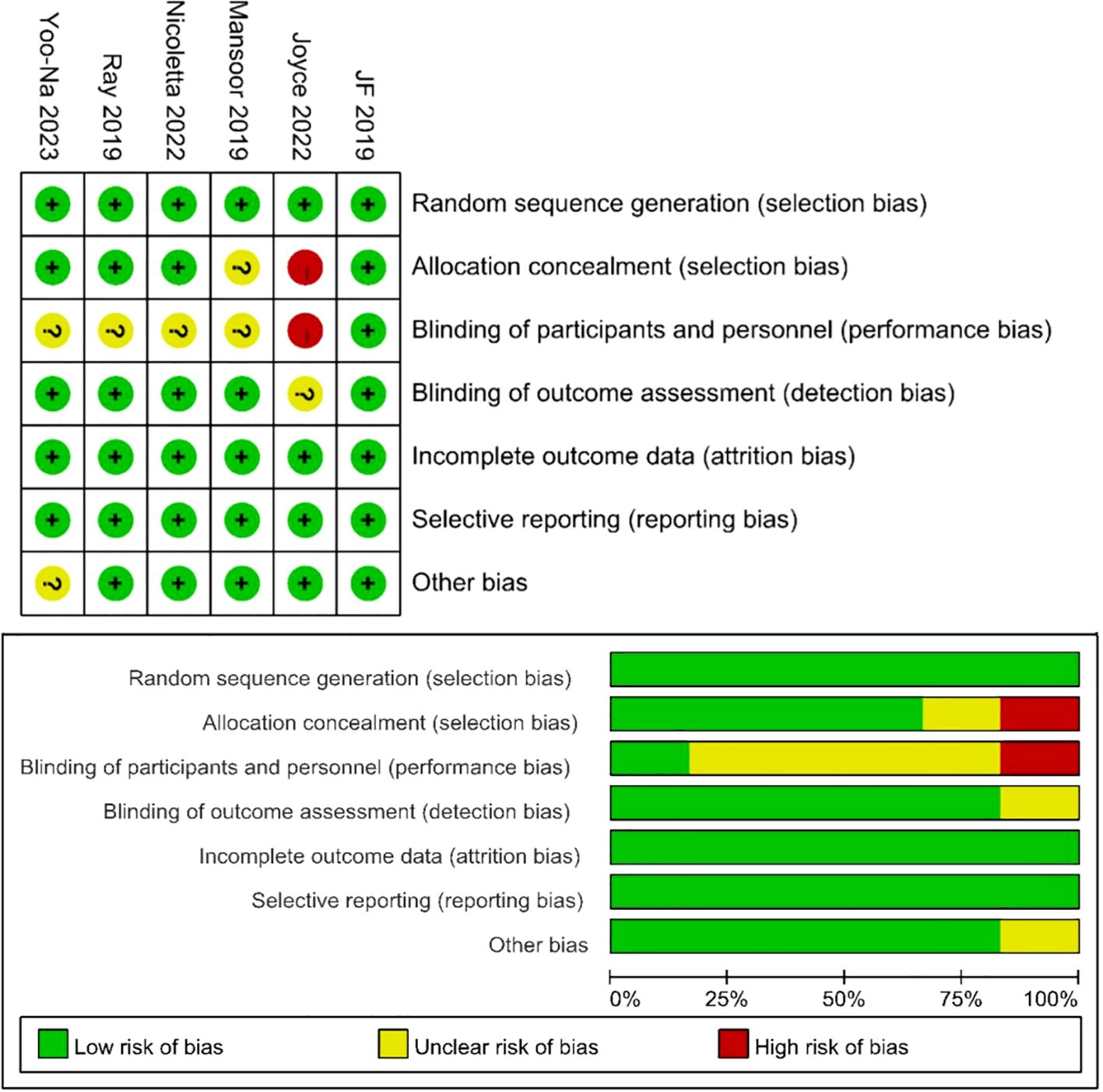
Figure 2. Risk of bias assessment for the RCT studies.
3.3 ORRFigure 3 presents the result of ORR. The ORR of patients with advanced ovarian cancer who received Ais combined with PARPi was 57% (95% CI, 35% to 77%). In subgroup analysis, the ORR of patients with platinum-resistant recurrent ovarian cancer, patients with platinum-sensitive recurrent ovarian cancer and patients with newly diagnosed advanced ovarian cancer was 30% (95% CI, 12% to 52%), 70% (95% CI, 61% to 78%) and 59% (95% CI, 55% to 63%), respectively.
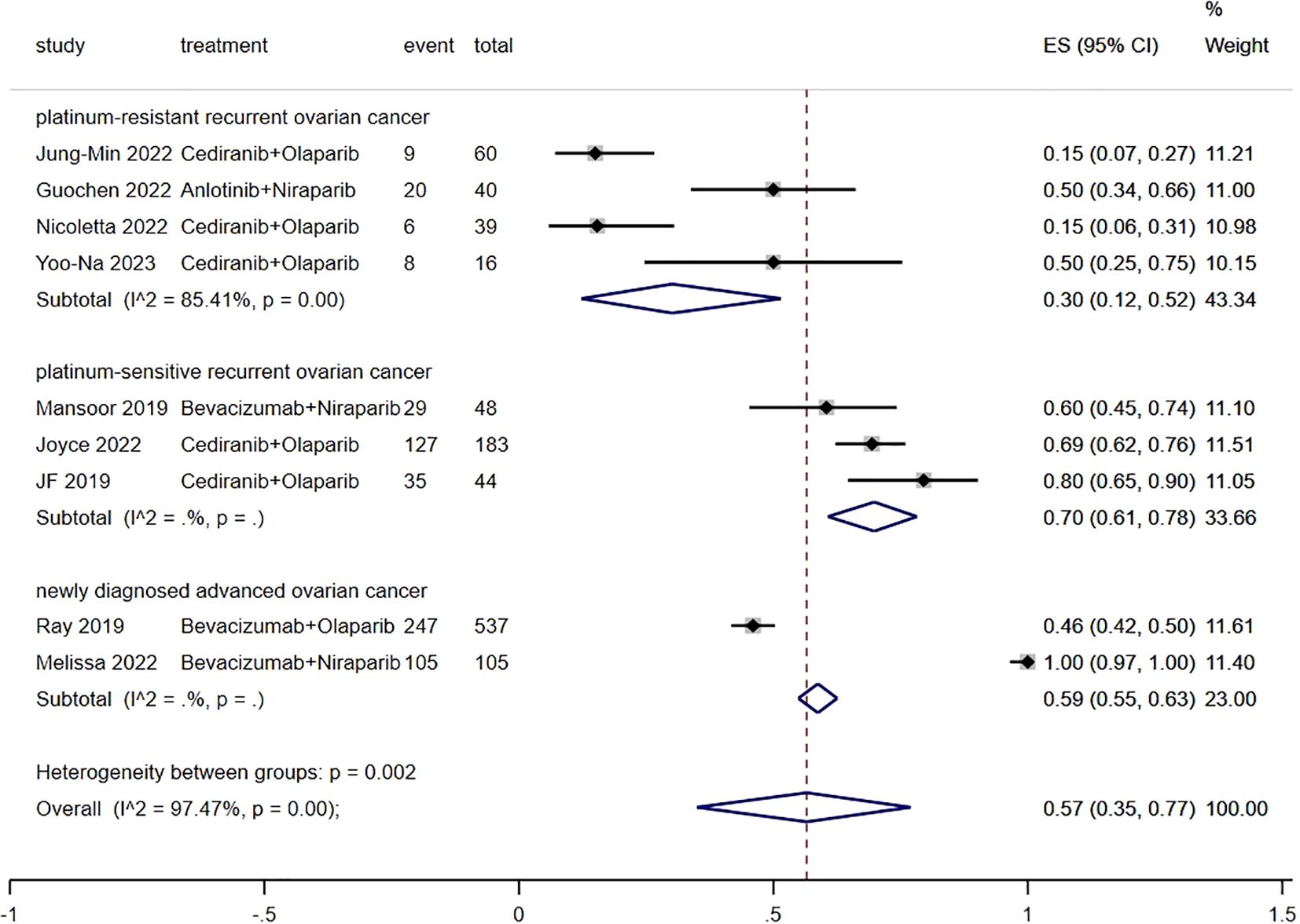
Figure 3. Forest plot of the meta-analysis for ORR.
3.4 PFSFollowing the reconstruction of the cohort, we conducted an additional evaluation of PFS using a Kaplan-Meier curve (Figure 4). The median PFS of patients with platinum-resistant recurrent ovarian cancer, patients with platinum-sensitive recurrent ovarian cancer and patients with newly diagnosed advanced ovarian cancer were 5.8 (95% CI, 5.3 to 7.1) months, 12.4 (95% CI, 10.6 to 13.2) months and 22.4 (95% CI, 21.5 to 24.2) months, respectively.
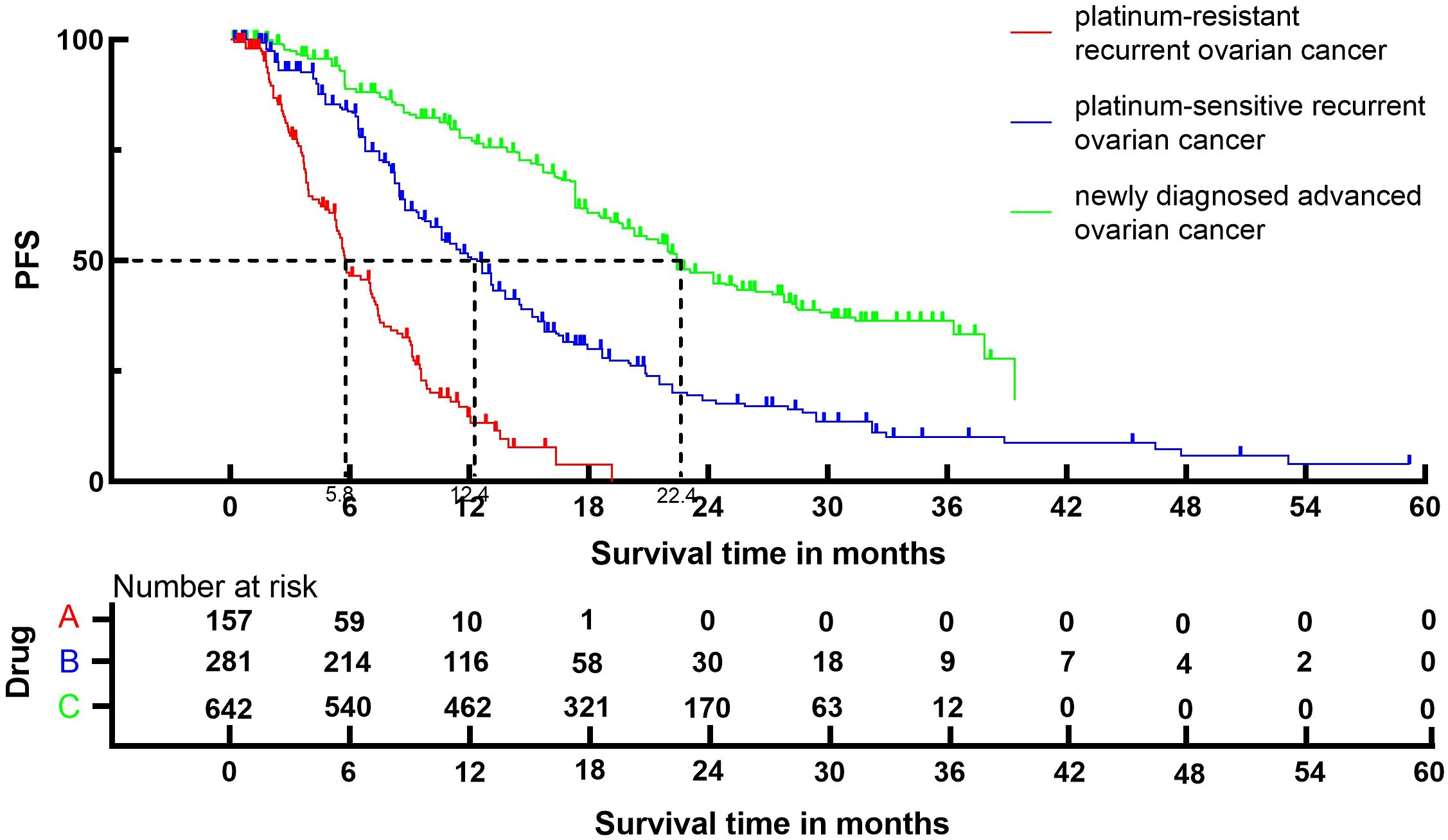
Figure 4. Kaplan-Meier curves for PFS.
3.4 OSFollowing the reconstruction of the cohort, we conducted an additional evaluation of OS using a Kaplan-Meier curve (Figure 5). The median OS of patients with platinum-resistant recurrent ovarian cancer, patients with platinum-sensitive recurrent ovarian cancer and patients with newly diagnosed advanced ovarian cancer were 15.5 (95% CI, 12.3 to 24.8) months, 40.8 (95% CI, 33.4 to 45.2) months and 56.3 (95% CI, 49.0 to 62.0) months, respectively.
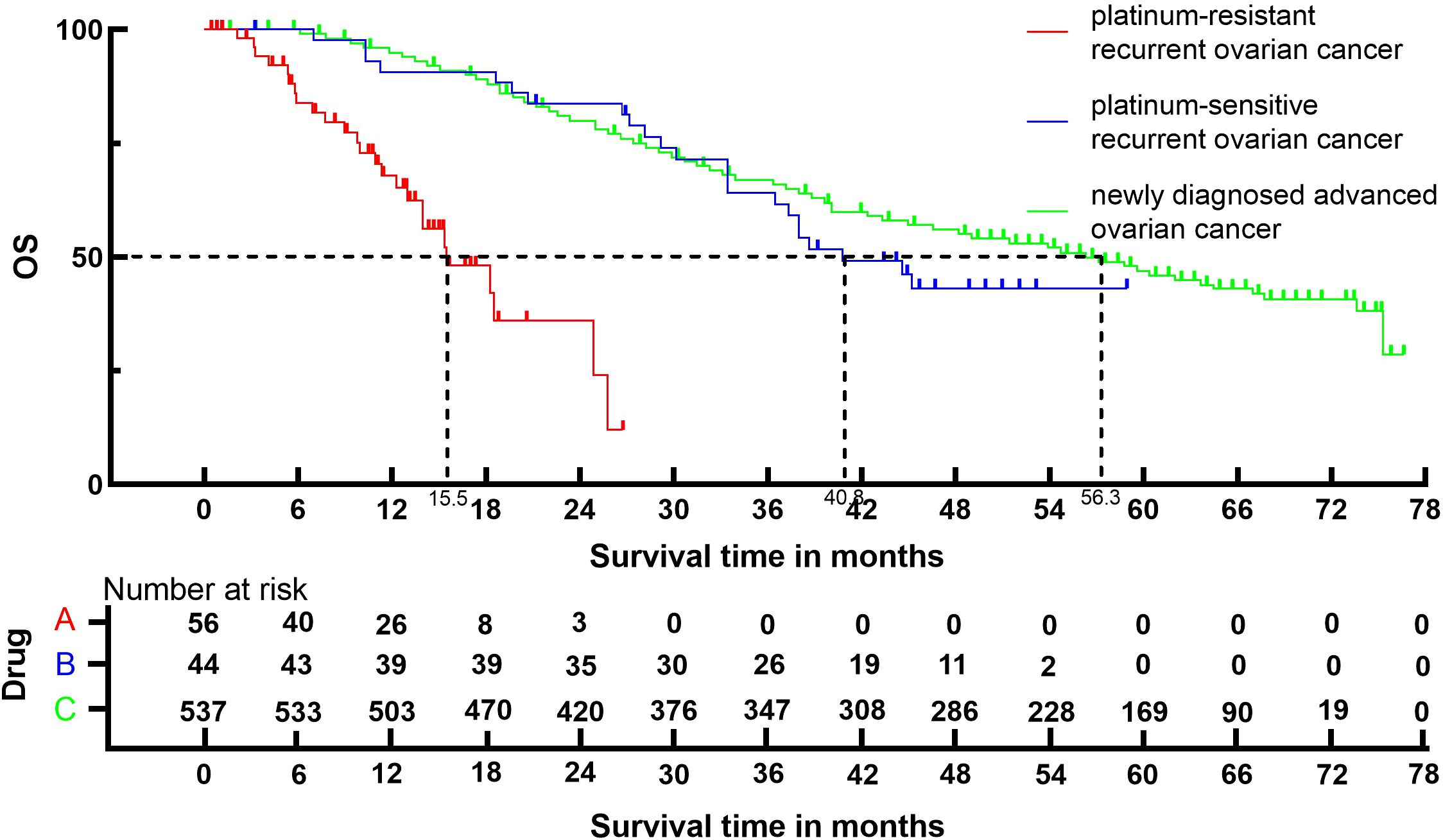
Figure 5. Kaplan-Meier curves for OS.
3.5 Grade≥ 3 TRAEs rateThe Grade≥ 3 TRAEs rate was found to be 0.22 (95% CI, 0.13 to 0.33) (Figure 6) among patients with advanced ovarian cancer who received Ais combined with PARPi. In subgroup analysis, the Grade≥ 3 TRAEs rate in patients with platinum-resistant recurrent ovarian cancer, patients with platinum-sensitive recurrent ovarian cancer and patients with newly diagnosed advanced ovarian cancer was 0.21 (95% CI, 0.18 to 0.24), 0.13 (95% CI, 0.10 to 0.17), and 0.42 (95% CI, 0.39 to 0.45) respectively.
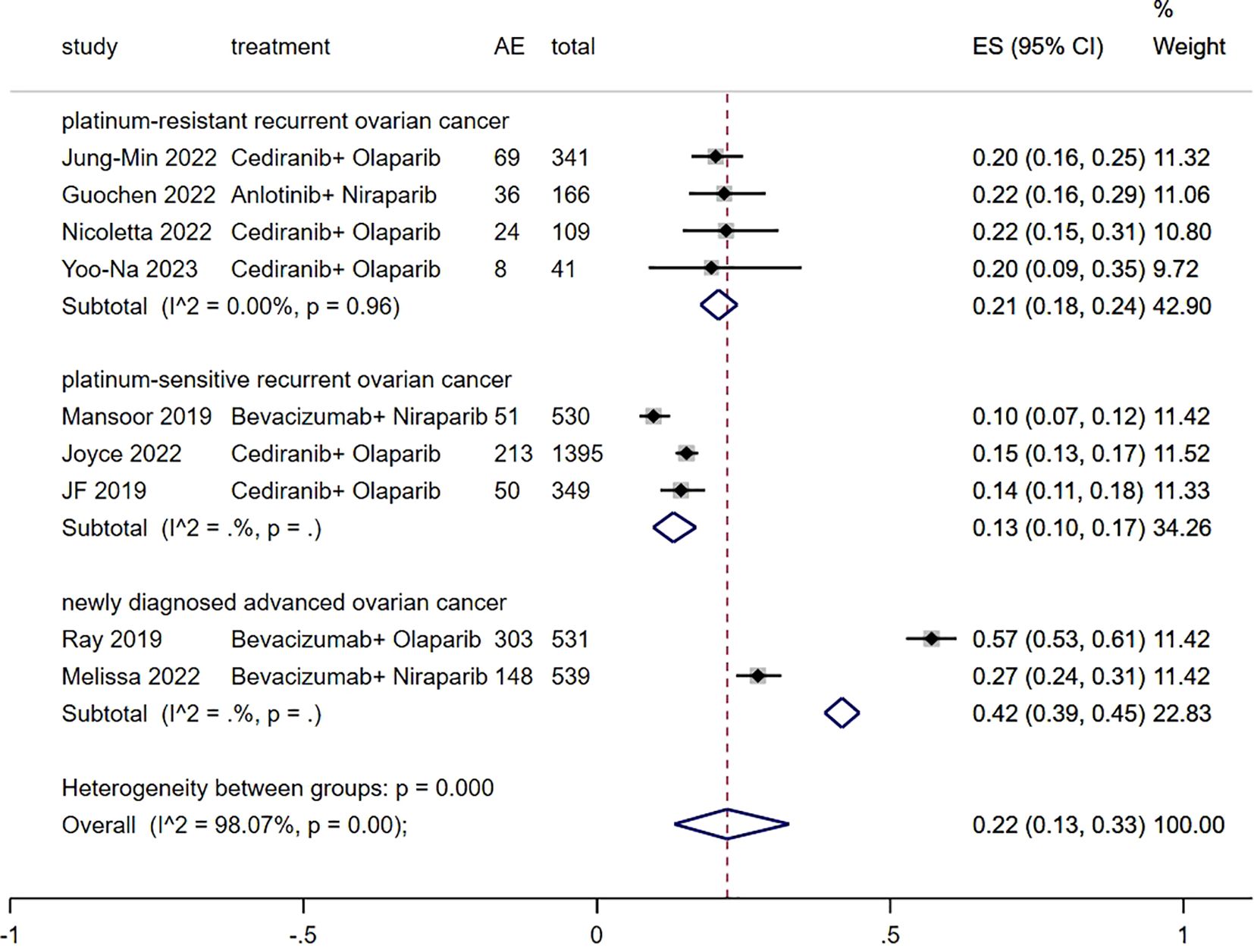
Figure 6. Forest plot of the meta-analysis for Grade≥ 3 AEs rate.
4 DiscussionThere has been an increase in the number of clinical trials studying the safety and effectiveness of maintenance combining Ais with PARPi for treating advanced ovarian cancer, due to the significant therapeutic benefits found in patients. In this study, we performed the first systematic review and meta-analysis to thoroughly evaluate the effectiveness and safety of maintenance combining Ais with PARPi for patients with advanced ovarian cancer.
The findings from our study revealed that patients with advanced ovarian cancer who were administered Ais plus PARPi had an ORR of 57%, indicating the favorable antitumor efficacy of the combined treatment. A subgroup analysis was performed to address discrepancies in the patient inclusion criteria among different trials, including patients with platinum-resistant recurrent ovarian cancer, platinum-sensitive recurrent ovarian cancer, and newly diagnosed advanced ovarian cancer. The ORR of patients with platinum-resistant recurrent ovarian cancer, patients with platinum-sensitive recurrent ovarian cancer and patients with newly diagnosed advanced ovarian cancer was 30%, 70% and 59%, respectively. This finding suggests that the resistance to Ais combined with PARPi may be linked to resistance to platinum. The outcomes of OS and PFS demonstrated that the combination of Ais and PARPi yielded more favorable oncological prognosis for patients with platinum-sensitive recurrent ovarian cancer, as opposed to patients with platinum-resistant recurrent ovarian cancer. Specifically, the notable advantage of Ais and PARPi for patients with advanced ovarian cancer was evident in terms of PFS and OS, confirming that the combination therapy should be recommended as the first-line therapy for patients with advanced ovarian cancer.
Ais play a crucial role in malignancy treatment (23) by disrupting the formation of new blood vessels, which tumor cells require for growth and metastasis (24). Specifically, Cediranib acts as a potent inhibitor of vascular endothelial growth factor receptors (VEGFRs), effectively blocking endothelial cell proliferation and survival (25). Bevacizumab, a monoclonal antibody, directly neutralizes VEGF, preventing its interaction with receptors on the endothelial cell surface (26). Anlotinib, a multi-targeted kinase inhibitor, not only inhibits VEGFR but also acts on other pathways like PDGFR and FGFR, reinforcing its anti-angiogenic effects in advanced ovarian cancer treatment (27). Ais or PARPi exhibit substantial efficacy as monotherapy for recurrent ovarian cancer, but, prolonged usage of a single agent can lead to the development of drug resistance (9). Previous research suggests that Ais plus PARP inhibition may boost anticancer efficacy (28, 29). Studies have demonstrated that antiangiogenic drugs have multiple effects on homologous recombination deficiency (HRD), including reducing angiogenesis, creating hypoxia in tumor microenvironment, and downregulating BRCA1/2 and RAD51, two of the most important components in HRD (29–31). Bevacizumab is linked to an elevated occurrence of hypoxia-induced HRD deficiency in tumor cells (32). Cells exhibiting heightened HRD may display more vulnerability to PARPi, whereas Ais have the ability to bind with PARPi, resulting in mutually reinforcing anticancer effects (28). Homologous recombination repair deficiency is a frequent feature of high-grade serous ovarian, fallopian tube and peritoneal carcinoma (HGSC) and is associated with sensitivity to PARP inhibitor (33). Additionally, PARPi may possibly have a role in the process of angiogenesis. Although vascular growth factor is present, the deletion of PARP1 in mice resulted in a decrease in angiogenesis, indicating a possible antiangiogenic action of PARPi (34). The upregulation of PARPi in human epithelial ovarian cancer tissues is correlated with parameters such as a high pathological grade and the spread of cancer cells to the lymph nodes, indicating that PARPi may play a role in the advancement of ovarian cancer (35). But PARP-1 is overexpressed and may also stimulate angiogenesis in epithelial ovarian cancer cells by increasing the expression of VEGFA (35). Preclinical studies have demonstrated that the combination of PARPi and Ais exhibits a synergistic effect, effectively inhibiting the invasion of ovarian cancer cells and the creation of microvascular endothelial tubes (29, 36). PARPi and Ais modify the genetic makeup of tumor cells, both directly and indirectly, in order to enhance the effectiveness of therapy (36). Furthermore, PARP inhibitors could be used in combination with other targeted therapies, including immunotherapies such as PD-1/PD-L1 inhibitors, to enhance the ability to attack tumor cells (37). However, the precise mechanisms underpinning these combinations remain poorly understood and may vary depending on the specific anti-angiogenic drugs involved. Additional research is required to clarify the precise mechanism through which this combination produces its anticancer effects.
With respect to safety, the rate of Grade≥ 3 TRAEs was determined to be 22%, indicating that the occurrence of negative effects from the combination therapy was deemed acceptable. In clinical trials examining the use of Ais alone, the most often seen Grade≥ 3 TRAEs were high blood pressure, blood clotting events, low levels of neutrophils, and bleeding outside of the central nervous system (35, 36). In clinical trials examining the use of PARPi alone, the most often seen Grade≥ 3 TRAEs were anemia, thrombocytopenia, neutropenia, fatigue, and nausea (38–40). Our results showed that the most often seen Grade≥ 3 TRAEs of the combined medication were stomach pain, hypertension, and anemia, which align with the adverse reactions typically associated with monotherapy.
The present study had numerous strengths. First, our study was an updated systematic review and meta-analysis to evaluate the effectiveness and safety of PARPi in conjunction with Ais for advanced ovarian cancer. Though Wei Y et al. conducted a meta-analysis about effectiveness and safety of PARPi plus Ais for advanced ovarian cancer, there were serious drawbacks in the previous meta-analysis (34). Four of the seven studies included in the previous meta-analysis contained duplicate patients from the PAOLA-1 trials, which led to a significant bias (34). Our study performed an expanded search strategy with additional search terms to ensure a comprehensive literature review, and nine trials studying PARPi plus Ais for advanced ovarian cancer were included, which resulted in more accurate results. Additionally, the IPDformKM package was utilized to reconstruct Kaplan-Meier curves for PFS and OS, providing a clear and comprehensible representation of oncological outcomes. Furthermore, a subgroup analysis was performed to resolve discrepancies in the patient inclusion criteria observed in different studies, which encompassed platinum-resistant recurrent ovarian cancer, platinum-sensitive recurrent ovarian cancer, and newly diagnosed advanced ovarian cancer. This study provides empirical support to inform the practical application of Ais in combination with PARPi for the management of patients with advanced ovarian cancer.
Undoubtedly, our study has specific limitations. First, the sample size was quite small. The analysis included only nine trials, involving a total of 1074 individuals diagnosed with advanced ovarian cancer who were treated with Ais plus PARPi. Second, the inclusion of single-arm clinical trials resulted in indirect comparisons between different treatment protocols. We failed to perform the meta-analysis of HR value estimates from Kaplan–Meier curves regarding PFS or OS. One reason was that several single-arm trials did not provide HR value since there was not a controlled group. Another reason was that the regimens of the control groups in different RCTs were not the same. As an alternative method, Kaplan-Meier curves for OS and PFS were reconstructed using the IPDformKM package, which presented an intuitive representation for oncological outcomes. This method has been reported previously by Guyot et al. (14). Third, the nine studies exhibited significant variation in terms of research technique, patient characteristics, and treatment regimens. Therefore, the explanation of our findings requires a certain level of caution.
In conclusion, the combination of PARPi and Ais is both viable and safe for treating advanced ovarian cancer. The findings of our study validate the recommendation of combination therapy as the primary treatment option for individuals diagnosed with advanced ovarian cancer. Our findings show improved survival and progression-free survival, as well as a reduction in treatment-related adverse events, in platinum-resistant recurrent ovarian cancer and in newly diagnosed advanced ovarian cancer. However, these results need to be further validated in a larger sample size and with longer follow-up.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributionsRH: Conceptualization, Data curation, Formal analysis, Writing – original draft. FJ: Conceptualization, Data curation, Formal analysis, Writing – original draft. LH: Investigation, Methodology, Project administration, Writing – original draft. YQ: Investigation, Methodology, Project administration, Writing – original draft. ZL: Software, Validation, Writing – original draft. MH: Software, Supervision, Validation, Writing – original draft. CL: Software, Supervision, Validation, Writing – original draft. JB: Funding acquisition, Resources, Visualization, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Laboratory Construction Project of Guangxi Health Commission (ZPZH2020007), the Scientific Research Foundation of Guangxi University of Science and Technology (20Z13), the Scientific Research Foundation of Guangxi Health Commission (Z-B20220927) and the Scientific Research Foundation of Guangxi Health Commission (Z-B20220930).
AcknowledgmentsEveryone who contributed significantly to this study has been listed.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1477105/full#supplementary-material
References2. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:191–226. doi: 10.6004/jnccn.2021.0007
PubMed Abstract | Crossref Full Text | Google Scholar
3. Kwolek DG, Gerstberger S, Tait S, Qiu JM. Ovarian, uterine, and vulvovaginal cancers: screening, treatment overview, and prognosis. Med Clin North Am. (2023) 107:329–55. doi: 10.1016/j.mcna.2022.10.016
PubMed Abstract | Crossref Full Text | Google Scholar
5. Indini A, Nigro O, Lengyel CG, Ghidini M, Petrillo A, Lopez S, et al. Immune-checkpoint inhibitors in platinum-resistant ovarian cancer. Cancers (Basel). (2021) 13:1–18. doi: 10.3390/cancers13071663
PubMed Abstract | Crossref Full Text | Google Scholar
9. Jiang X, Li X, Li W, Bai H, Zhang Z. Parp inhibitors in ovarian cancer: sensitivity prediction and resistance mechanisms. J Cell Mol Med. (2019) 23:2303–13. doi: 10.1111/jcmm.14133
PubMed Abstract | Crossref Full Text | Google Scholar
10. Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. (2019) 381:2416–28. doi: 10.1056/NEJMoa1911361
PubMed Abstract | Crossref Full Text | Google Scholar
11. Hardesty MM, Krivak TC, Wright GS, Hamilton E, Fleming EL, Belotte J, et al. Ovario phase ii trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecol Oncol. (2022) 166:219–29. doi: 10.1016/j.ygyno.2022.05.020
PubMed Abstract | Crossref Full Text | Google Scholar
12. Bañares R, Albillos A, Rincón D, Alonso S, González M, Ruiz-del-Arbol L, et al. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: A meta-analysis. Hepatology. (2002) 35:609–15. doi: 10.1053/jhep.2002.31354
PubMed Abstract | Crossref Full Text | Google Scholar
13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
PubMed Abstract | Crossref Full Text | Google Scholar
14. Liu N, Zhou Y, Lee JJ. Ipdfromkm: reconstruct individual patient data from published kaplan-meier survival curves. BMC Med Res Methodol. (2021) 21:111. doi: 10.1186/s12874-021-01308-8
PubMed Abstract | Crossref Full Text | Google Scholar
15. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
PubMed Abstract | Crossref Full Text | Google Scholar
16. Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, et al. Olaparib with or without cediranib versus platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer (Nrg-gy004): A randomized, open-label, phase iii trial. J Clin Oncol. (2022) 40:2138–47. doi: 10.1200/jco.21.02011
PubMed Abstract | Crossref Full Text | Google Scholar
17. Colombo N, Tomao F, Benedetti Panici P, Nicoletto MO, Tognon G, Bologna A, et al. Randomized phase ii trial of weekly paclitaxel vs. Cediranib-olaparib (Continuous or intermittent schedule) in platinum-resistant high-grade epithelial ovarian cancer. Gynecol Oncol. (2022) 164:505–13. doi: 10.1016/j.ygyno.2022.01.015
PubMed Abstract | Crossref Full Text | Google Scholar
18. Kim YN, Joung JG, Park E, Kim JW, Lee JB, Lim J, et al. Randomized, two-arm, noncomparative phase 2 study of olaparib plus cediranib or durvalumab in hrr-mutated, platinum-resistant ovarian cancer: A substudy of kgog 3045. Int J Cancer. (2023) 153:2032–44. doi: 10.1002/ijc.34696
PubMed Abstract | Crossref Full Text | Google Scholar
19. Mirza MR, Åvall Lundqvist E, Birrer MJ, dePont Christensen R, Nyvang GB, Malander S, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (Nsgo-avanova2/engot-ov24): A randomised, phase 2, superiority trial. Lancet Oncol. (2019) 20:1409–19. doi: 10.1016/s1470-2045(19)30515-7
PubMed Abstract | Crossref Full Text | Google Scholar
20. Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Overall survival and updated progression-free survival outcomes in a randomized phase ii study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann Oncol. (2019) 30:551–7. doi: 10.1093/annonc/mdz018
PubMed Abstract | Crossref Full Text | Google Scholar
21. Lee JM, Moore RG, Ghamande S, Park MS, Diaz JP, Chapman J, et al. Cediranib in combination with olaparib in patients without a germline brca1/2 mutation and with recurrent platinum-resistant ovarian cancer: phase iib concerto trial. Clin Cancer Res. (2022) 28:4186–93. doi: 10.1158/1078-0432.Ccr-21-1733
PubMed Abstract | Crossref Full Text | Google Scholar
22. Liu G, Feng Y, Li J, Deng T, Yin A, Yan L, et al. A novel combination of niraparib and anlotinib in platinum-resistant ovarian cancer: efficacy and safety results from the phase ii, multi-center annie study. EClinicalMedicine. (2022) 54:101767. doi: 10.1016/j.eclinm.2022.101767
PubMed Abstract | Crossref Full Text | Google Scholar
23. Ansari MJ, Bokov D, Markov A, Jalil AT, Shalaby MN, Suksatan W, et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun Signal. (2022) 20:1–23. doi: 10.1186/s12964-022-00838-y
PubMed Abstract | Crossref Full Text | Google Scholar
25. Heckman CA, Holopainen T, Wirzenius M, Keskitalo S, Jeltsch M, Ylä-Herttuala S, et al. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res. (2008) 68:4754–62. doi: 10.1158/0008-5472.CAN-07-5809
PubMed Abstract | Crossref Full Text | Google Scholar
26. Chase JL. Clinical use of anti-vascular endothelial growth factor monoclonal antibodies in metastatic colorectal cancer. Pharmacotherapy. (2008) 28:23–30. doi: 10.1592/phco.28.11-supp.23S
PubMed Abstract | Crossref Full Text | Google Scholar
27. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. (2018) 11:1–11. doi: 10.1186/s13045-018-0664-7
PubMed Abstract | Crossref Full Text | Google Scholar
29. Lim JJ, Yang K, Taylor-Harding B, Wiedemeyer WR, Buckanovich RJ. Vegfr3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of brca1 and brca2. Neoplasia. (2014) 16:343–53.e1-2. doi: 10.1016/j.neo.2014.04.003
PubMed Abstract | Crossref Full Text | Google Scholar
30. Bindra RS, Schaffer PJ, Meng A, Woo J, Måseide K, Roth ME, et al. Down-regulation of rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. (2004) 24:8504–18. doi: 10.1128/mcb.24.19.8504-8518.2004
PubMed Abstract | Crossref Full Text | Google Scholar
32. Chan N, Pires IM, Bencokova Z, Coackley C, Luoto KR, Bhogal N, et al. Contextual synthetic lethality of cancer cell kill based on the tumor microenvironment. Cancer Res. (2010) 70:8045–54. doi: 10.1158/0008-5472.Can-10-2352
PubMed Abstract | Crossref Full Text | Google Scholar
33. Miller RE, Leary A, Scott CL, Serra V, Lord CJ, Bowtell D, et al. Esmo recommendations on predictive biomarker testing for homologous recombination deficiency and parp inhibitor benefit in ovarian cancer. Ann Oncol. (2020) 31:1606–22. doi: 10.1016/j.annonc.2020.08.2102
PubMed Abstract | Crossref Full Text | Google Scholar
34. Tentori L, Lacal PM, Muzi A, Dorio AS, Leonetti C, Scarsella M, et al. Poly(Adp-ribose) polymerase (Parp) inhibition or parp-1 gene deletion reduces angiogenesis. Eur J Cancer. (2007) 43:2124–33. doi: 10.1016/j.ejca.2007.07.010
PubMed Abstract | Crossref Full Text | Google Scholar
36. Ivy SP, Liu JF, Lee JM, Matulonis UA, Kohn EC. Cediranib, a pan-vegfr inhibitor, and olaparib, a parp inhibitor, in combination therapy for high grade serous ovarian cancer. Expert Opin Investig Drugs. (2016) 25:597–611. doi: 10.1517/13543784.2016.1156857
PubMed Abstract | Crossref Full Text | Google Scholar
37. Della Corte LA-O, Foreste V, Di Filippo C, Giampaolino P, Bifulco G. Poly (Adp-ribose) polymerase (Parp) as target for the treatment of epithelial ovarian cancer: what to know. Expert Opin Investig Drugs. (2021) 30:543–54. doi: 10.1080/13543784.2021.1901882
PubMed Abstract | Crossref Full Text | Google Scholar
38. Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. (2018) 379:2495–505. doi: 10.1056/NEJMoa1810858
留言 (0)