Sialylation, the enzymatic addition of sialic acid to glycoproteins and glycolipids, is a key post-translational modification that plays a significant role in cancer biology. The sialic acid moiety, often found at the terminal position of glycan chains on cell surfaces, influences a wide range of biological processes, including but not limited to cell signaling (1–5), proliferation (6–9), immune responses (10), and cellular interactions (11–14). Aberrant sialylation has been recognized as a hallmark of cancer (15), contributing to tumor progression, immune evasion (16), and metastasis (17).
The molecular basis of sialylation involves a family of enzymes known as sialyltransferases, which catalyze the transfer of sialic acid to glycan structures. These enzymes are responsible for catalyzing different linkages, such as α2,3-, α2,6-, and α2,8-sialylation, each playing distinct roles in cellular behavior (18–26). In cancer, dysregulated sialylation often results in hypersalivation, which can mediate various oncogenic processes. For instance, the upregulation of sialyltransferases ST6GAL1 has been linked to increased cancer cell aggressiveness, enhanced survival, and resistance to apoptosis in cancers such as breast, colon, prostate, and brain cancers (18, 23, 24, 27, 28).
New evidence of where sialylation occurs outside the cell membrane has also emerged as an important factor in cancer. This form of sialylation contributes to cancer cell proliferation and metastasis by altering the glycocalyx, a dense layer of glycoproteins and glycolipids on the cell surface (29, 30). A sialylated glycocalyx not only provides a protective barrier against the immune system but also mediates cancer cell detachment and migration (31) by increasing mechanical tension at the cell membrane (32). The mechanical properties of the glycocalyx, such as its stiffness and bulk, influence membrane dynamics and signal transduction, promoting cancer cell invasion and metastatic potential (33–35).
Sialylation also plays a critical role in immune evasion, with sialic acid residues acting as ligands for Siglecs, a family of immune inhibitory receptors. By engaging Siglecs on immune cells, cancer cells can transmit inhibitory signals that prevent immune-mediated destruction (16, 36). For example, hypersialylated cancer cells expressing CD24 can bind to Siglec-10 on macrophages, effectively creating a “don’t eat me” signal that prevents phagocytosis (10, 16, 37–40). This immune evasion mechanism underscores the potential of targeting sialylation pathways as a therapeutic strategy in cancer treatment. Given its widespread impact on cancer biology, sialylation serves as a promising biomarker for cancer diagnosis and prognosis. Aberrant sialylation patterns have been associated with more aggressive tumor phenotypes and poor patient outcomes (27, 41–43), making it a valuable target for early detection and therapeutic intervention (44–46). Overall, this review aims to highlight the multifaceted roles of sialylation in cancer, emphasizing its contribution to tumor progression, immune evasion, and potential as a therapeutic target.
The molecular basis of sialylationSialylation is a well-regulated biological process that involves the addition of negatively charged sialic acid sugar residues to the glycoproteins and glycolipids at the terminal position of the glycan (N-, O-linked glycan or glycolipids) to mediate protein stability, cell–cell communication, and immune response (47). Sialic acids are a family of α-keto acids with unique structural features, containing nine-carbon backbone sugar molecules with an amino group at position 5 and a carboxyl group at position 1 (48). More than 50 sialic acid isoforms have been found in nature, with the most abundant being N-acetylneuraminic acid (Neu5Ac) (49, 50). N-acetylneuraminic acid (Neu5Ac) is derived from cytidine monophosphate sugar nucleotide CMP-Neu5Ac and can undergo acetylation, methylation, and other modifications. Sialylation is mediated by sialyltransferases that catalyze the transfer of sialic acid from the charged donor molecule, cytidine monophosphate–sialic acid (CMP–sialic acid), to the growing glycan chains in either an α2,3, α2,6, or α2,8 linkage depending on the activity of the sialyltransferases and the substrate glycan (48, 51).
Sialic acid biosynthesis progresses through a four-step process involving three different enzymes. GNE is the bifunctional enzyme that catalyzes the initial two steps in the biosynthesis pathway (Figure 1). This pathway begins in the cytosol with the formation of N-acetylmannosamine (ManNAc) from UDP-GlcNAc using the epimerase function of the GNE, followed by the subsequent phosphorylation of ManNAc to N-acetylmannosamine-6-phosphate (ManNAc-6-P) and conversion to N-acetylneuraminic acid-9-phosphate (Neu5Ac-9-P). Dephosphorylation of Neu5Ac-9-P produces free Neu5Ac, which is then transported to the nucleus for subsequent activation into CMP-Neu5Ac by CMP–sialic acid synthetase (CMAS) (51). After transport to the Golgi, the active CMP-Neu5Ac donors are used by sialyltransferases to catalyze the addition of sialic acid to the glycoconjugates. Subsequent secretion of these sialylated glycans to the cell surface significantly contributes to the biophysical properties of cells through the negative charge carried by the sialic acid (52).
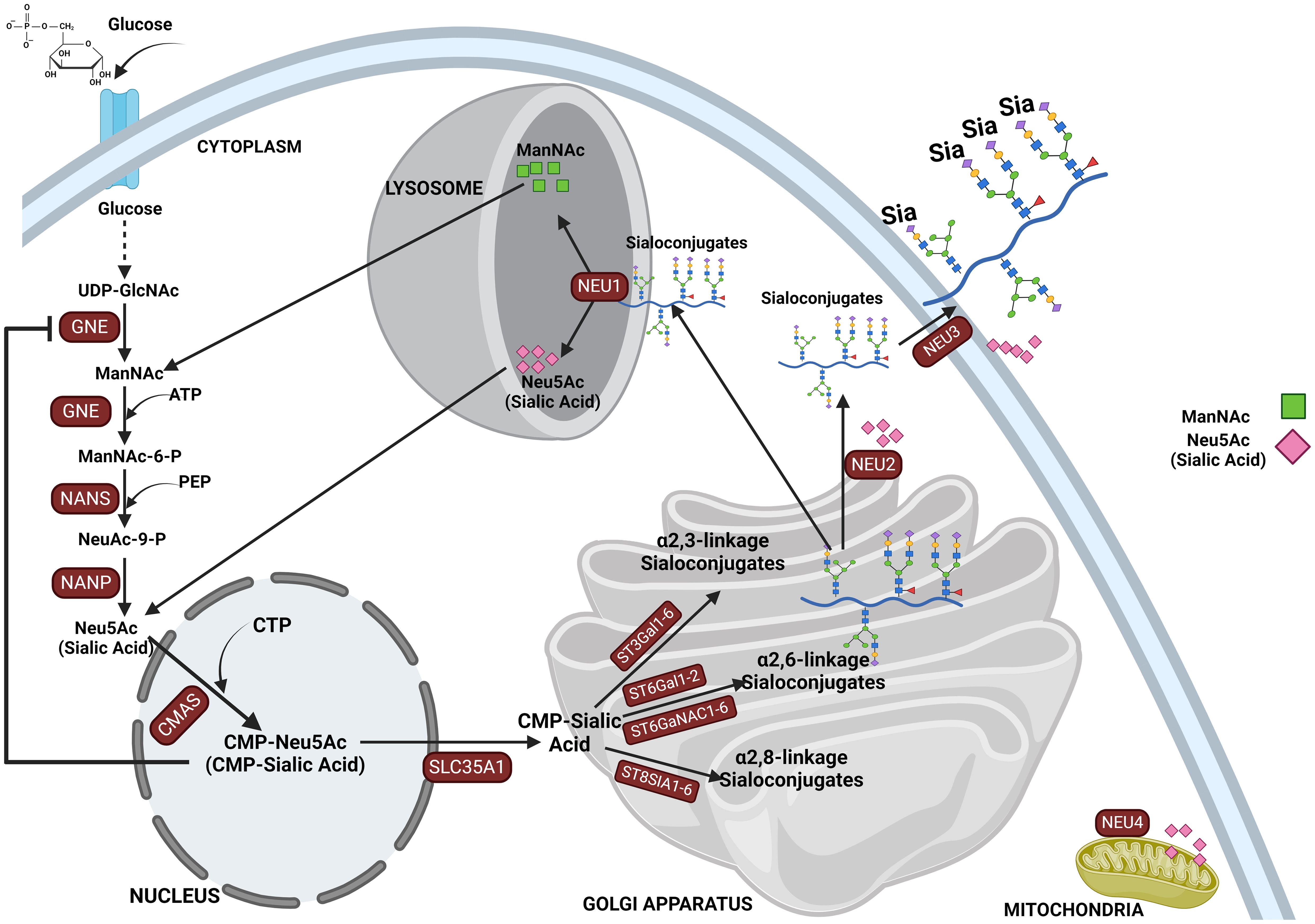
Figure 1. This figure depicts the sialylation pathway, illustrating the sequential biochemical steps involved in the synthesis and recycling of sialic acids. The pathway begins with the nucleotide sugar UDP-GlcNAc, produced via the hexosamine pathway, which is converted to ManNAc by UDP-GlcNAc 2-epimerase (GNE). ManNAc serves as the metabolic precursor for sialic acid synthesis, leading to the production of Neu5Ac in the cytosol. Neu5Ac then enters the nucleus, where it is converted into CMP-Neu5Ac. CMP-Neu5Ac is subsequently transported into the Golgi apparatus, where it is utilized by various sialyltransferases (ST3GAL1-6, ST6GAL1-2/ST6GALNAC1-6, and ST8SIA4) to synthesize α-2,3-, α-2,6-, and α-2,8-linked sialoglycoproteins or gangliosides. The final step in the pathway involves the recycling of sialosides by neuraminidases, which break them down into sialic acid monomers for reuse. This comprehensive depiction highlights the critical processes of sialic acid biosynthesis, modification, and recycling in cellular glycosylation.
Post-glycosylation modifications in α-linked sialoglycoconjugates are common in mammalian cells, with O-acetylation of N-acetylneuraminic acid (Neu5Ac) being the most frequent. O-acetylation is an important modification, occurring at positions C4, C7, C8, and C9 on N-acetylneuraminic acid (Neu5Ac) (53, 54). More frequently, they occur at positions C7, C8, and C9 across various species. However, it is also possible for O-acetylation to take place at the C4 position, which is directly connected to the pyranosyl ring, though this form is less common. These modifications influence the stability, recognition, and function of glycoproteins and glycolipids, impacting cellular interactions via immune modulation, pathogen binding, and overall cell signaling processes in various biological systems (55). In mammals, acetylation of sialic acid occurs at position C7 or C9 and is mediated by the enzyme CASD1, which utilizes acetyl-CoA as the acetyl donor. Following acetylation, sialyltransferases transfer O-acetylated sialic acids onto glycoproteins and glycolipids, which are then processed through the secretory pathway. Notably, some O-acetylated glycoproteins and glycolipids carrying sialic acids may remain within the Golgi for reasons not yet defined. However, deacetylation is carried out by the sialic acid esterase (SIAE), which is found both inside and outside of cells (54).
There are several reports of an increased O-acetylated sialic acid in cancers, which are implicated in tumor progression by inhibiting immune surveillance and enhancing oncogenic signaling. An O-acetylated sialyl-Tn has been reported to be involved in ovarian cancer-associated antigenicity. It was shown that modification of Sia with O-acetyl groups was critical for the recognition (56). 9-O-Ac-GD3, a modified ganglioside, is also found in several other cancers, including neuroblastoma (57), acute lymphoblastic leukemia (ALL) (58, 59), medulloblastoma (60), and breast cancer (61), but are typically rare in healthy adult tissues. Furthermore, sialic acids exist with different other modifications in which the hydroxyl groups may either be methylated or esterified with acetyl, lactyl, phosphate, or sulfate groups (62). It is critical to note that methylation of sialic acids plays a crucial role in cancer progression including signaling and cancer migration. Methylated sialic acid affects cancer cell adhesion and detachment mechanisms that are critical for metastasis. Specifically, they contribute to the mechanical stress and electrostatic forces that influence cancer cell migration. Recent findings highlight the association between the elevated production of sialic acids and increased methylation in cancer cells. This methylation alters the sialic acid’s role in cell-surface interactions, facilitating cancer cells’ ability to evade immune detection and promoting metastatic behavior (63, 64).
Several sugars make up both N-, O-linked, and glycolipid glycans, among which N-acetylneuraminic acid (Neu5Ac) is the most common sialic acid in humans (65–67) and holds great importance since they are termed “terminal” or capping sugars. Sialyltransferases catalyze the bond between sialic acid and glycan receptors, mediating the glycosidic bond formation between the sialic acid donor at Carbon-2 and the glycan receptor at Carbon-3, Carbon-6, or Carbon-8 hydroxyl positions, and are thus named ST3, ST6, or ST8, respectively, depending on the carbon number of the glycan onto which sialic acid is added (48, 65, 68) (see Table 1). The level of 2,6 sialylation is an important marker of cancer progression as α2,6-galactoside sialyltransferase 1 (ST6Gal1) (90) upregulation has been linked to aggressiveness of cancer cells (18, 27, 33, 44, 91–93) including colorectal (92, 94), gastric carcinoma (95), lungs (96), and brain (18). Additionally, another 2,6 siayltransferase, ST6GalNAc1, has been reported to regulate cancer cell adhesion and invasion in prostate cancer (46, 91).
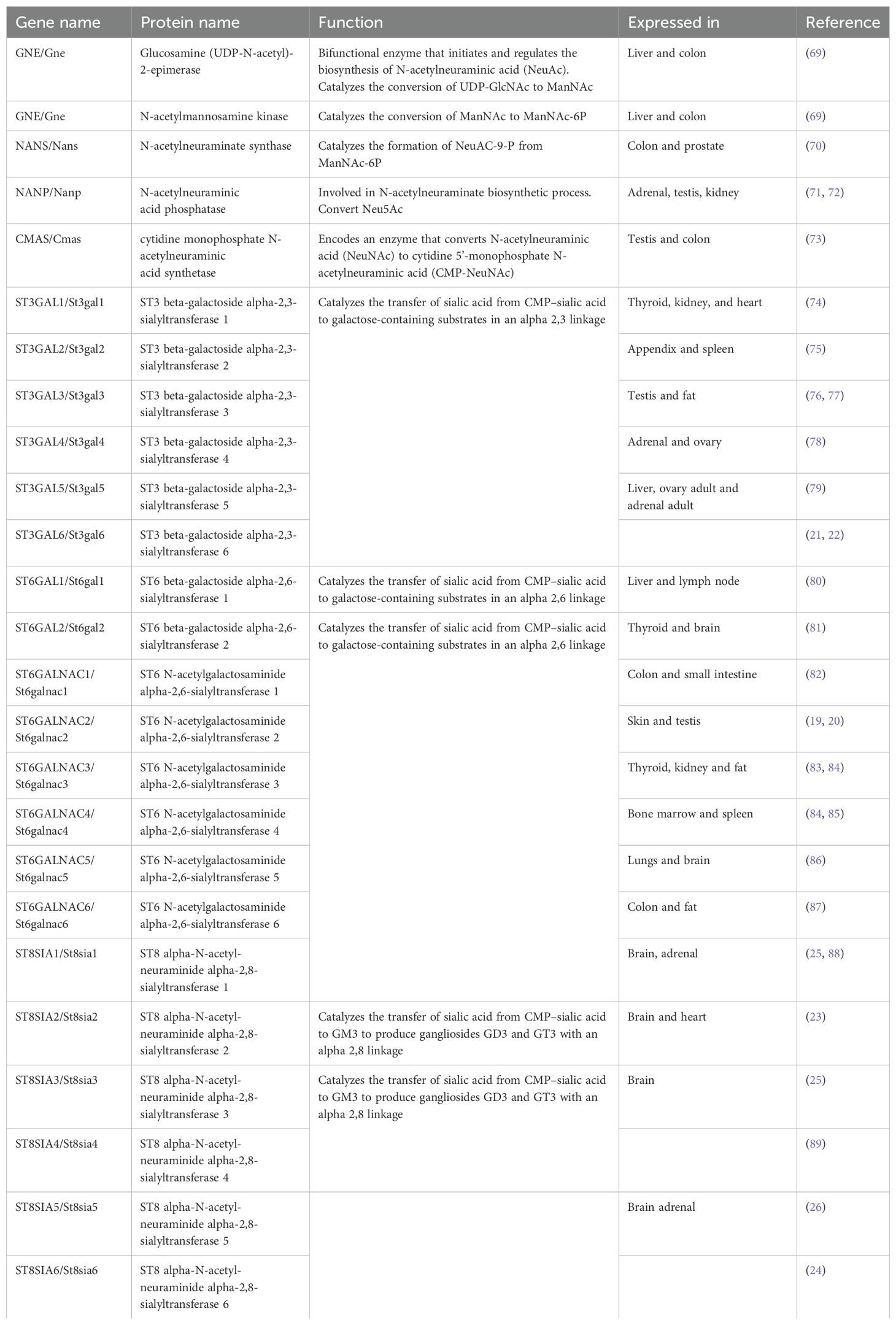
Table 1. Sialyltransferases and related genes.
Sialic acids are critical in various physiological and pathological processes, including cell–cell interaction (11, 12), protein stability, regulation of immune responses (16), pathogen recognition and infection, cell migration, and cancer progression (49, 65, 97). There are several reported cases where abnormal expression of sialyltransferases has led to aberrant expression of sialoglycans on glycoproteins, influencing various pathological conditions (98, 99), such as sialuria and hereditary inclusion body myopathy (HIBM). The latter is caused by mutations in the bifunctional GNE enzyme (UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase), which plays a crucial role in the biosynthesis of sialic acids (100). Following the sialylation of glycolipids and glycoproteins, they are released into the lysosome by sialidases and then to the cytosol for recycling or broken down by Neu5Ac lyase into ManNAc and pyruvate (101). Mutation in neuraminidase 1, NEU1 (sialidase 1), a lysosomal enzyme that plays a crucial role in the catabolism of sialo-glycoconjugates, leads to lysosomal storage disorder sialidosis, while abnormal NEU1 activity has been implicated in cancer progression, inflammation, and immune response (102, 103). Aside from lysosomal sialidase NEU1 (102), other human sialidases have been identified to include the cytosolic sialidase NEU2 (104), the plasma membrane-associated sialidase NEU3 (105), and mitochondrial membrane-associated sialidase NEU4 (106) (see Figure 1). Overall, this highlights the critical role of proper sialic acid biosynthesis and how dysregulation of this process at any step can lead to a wide variety of diseases and a more malignant phenotype in cancer (43, 107).
Extrinsic sialylationIn addition to the classic intracellular pathway of sialylation, there is new evidence to suggest that sialyltransferases can “extrinsically” catalyze the addition of sialic acid residues outside of the cell membrane (108), and this plays a role in a wide variety of cell processes including cancer progression (109). It has been long known that sialyltransferases (and other glycosyltransferases) exist outside the cell membrane (110), but extrinsic glycosylation was thought to be impossible due to a lack of activated sugar donors. It was not until the Lau Lab demonstrated that the activated sugar donors (29, 30), along with the glycosyltransferases (111) themselves, needed to complete the extrinsic glycosylation reaction could be found in abundance in platelets (29, 112). Subsequent work has implicated extrinsic sialylation in the maintenance of hematopoietic stem cells (113), the production of granulocytes through sialylation of the M-CSF receptor (93, 114), the proper development of B cells in the spleen (115–117), protection against radiation-induced gastrointestinal damage (118), and IgG sialylation (119, 120).
Neu5Gc and its incorporation into the glycocalyxIt is crucial to note that humans lack the ability to produce N-glycolylneuraminic acid (Neu5Gc) due to an inactivating mutation in the CMAH gene, which is irreversible. CMAH is the only enzyme responsible for the biosynthesis of Neu5Gc in deuterostome (121). Furthermore, note that no human genes have homology to CMAH. However, small amounts of Neu5Gc have been found in human tissues, including cancer cells (122–124). This is because humans can incorporate Neu5Gc from dietary sources, especially red meat and dairy products, into their cell surface glycoconjugates (125–127). This incorporation is most prominent in rapidly dividing tissues, such as epithelial cells and carcinomas (127). Once ingested, Neu5Gc is metabolically incorporated into the glycocalyx of cancer cells. The presence of Neu5Gc in cancer cells contributes to changes in their immunogenicity, making them susceptible to interactions with circulating anti-Neu5Gc antibodies (126). Despite the absence of endogenous Neu5Gc synthesis, most humans possess natural antibodies (IgA, IgM, and IgG) targeting Neu5Gc. These antibodies are formed in response to dietary Neu5Gc, making Neu5Gc a Xeno-autoantigen (126, 127). The presence of these antibodies is thought to be a response to dietary exposure or to bacteria scavenging Neu5Gc from the diet and incorporating it into their glycolipids. Anti-Neu5Gc antibodies can contribute to chronic inflammation when they recognize and bind to Neu5Gc present in the human glycocalyx, particularly in cancer cells in a process called xenosialitis, and may promote tumor progression or influence the inflammatory tumor microenvironment (128). Since humans cannot synthesize Neu5Gc, the presence of this non-human sialic acid on cancer cell surfaces renders these cells immunogenic, and become recognizable by the immune system as foreign, potentially leading to immune-mediated destruction of cancer cells. However, chronic inflammation is a well-known driver of cancer progression. The release of inflammatory cytokines such as IL-6, TNF-α, and IL-1β creates a tumor-promoting environment that favors cancer cell survival and growth (129, 130).
Aberrant sialylation as a hallmark of cancerFor many years, the onset of many cancer types has been recognized to stem from genetic mutations, but more recent emphasis has been placed upon post-translational hallmarks from alterations in biochemical pathways such as sialylation (20, 27, 35, 48, 131–133). Sialylation is a post-translational addition of sialic acid residue to glycoproteins as terminal monosaccharide, which modifies its structure, activity, and longevity and dictates many aspects of a cell’s interactions with the extracellular matrix (ECM) (46, 134). Sialic acids attach in either an α-2,3, α2,6, or α 2,8 linkage to galactose, usually terminating the glycans of glycoconjugates that cover the surface of cancer cells, creating a dense covering of sialylated glycans such as sialyl Lewis-A, -X, sialyl Tn antigen, or the GM2 ganglioside (SLeA, SLeX, STn, and GM2) (45).
Altered sialylation of several glycoproteins has been implicated in several health issues and diseases including cancer (45, 97, 135, 136). Hypersialylation plays a significant role in cancer development and progression by promoting cancer aggression and metastasis, immune evasion enhancing cancer cell survival, and resistance to therapy (41, 131, 137–139). The increase in α2,6 sialylation of N-glycans is driven by the sialyltransferases ST6GAL1, which is overexpressed in numerous cancer types and are fundamental for tumor growth, metastasis, immune evasion, and drug resistance (27). Overexpression of ST6GALNAC1 in the MDA-MB-231 breast cancer line has also been shown to promote the invasion and migration of breast cancer cells via the EMT pathway (140) while higher levels of ST8SIA4 promote tumorigenicity in the same cancer cell line (141). Blockade of ST6GAL1 has been shown to inhibit the metastatic spread of prostate cancer to bone (142). Overexpression of α(2-6)-sialic acids in pancreatic adenocarcinoma cell lines mediates increased adhesion to ECM while overexpressed α(2-3)-sialic acids contribute to increased migration (31). In human thyroid cancer, there is an increased expression of sialylated fibronectin (143) and SLeA antigen (144). Adrenal cancer also shows an overall increase in total cellular, cytoplasmic, and total plasma sialic acid content (145) (146). Aberrant upregulation of polysialylation on the neural cell adhesion molecule and serum sialic acids (147, 148) is critical in the progression of pituitary and brain cancer (149) (see Table 2). In the brain, there is still limited information on what sialic acid is relatively overexpressed and on which glycan is the sialylation phenotype prominent.
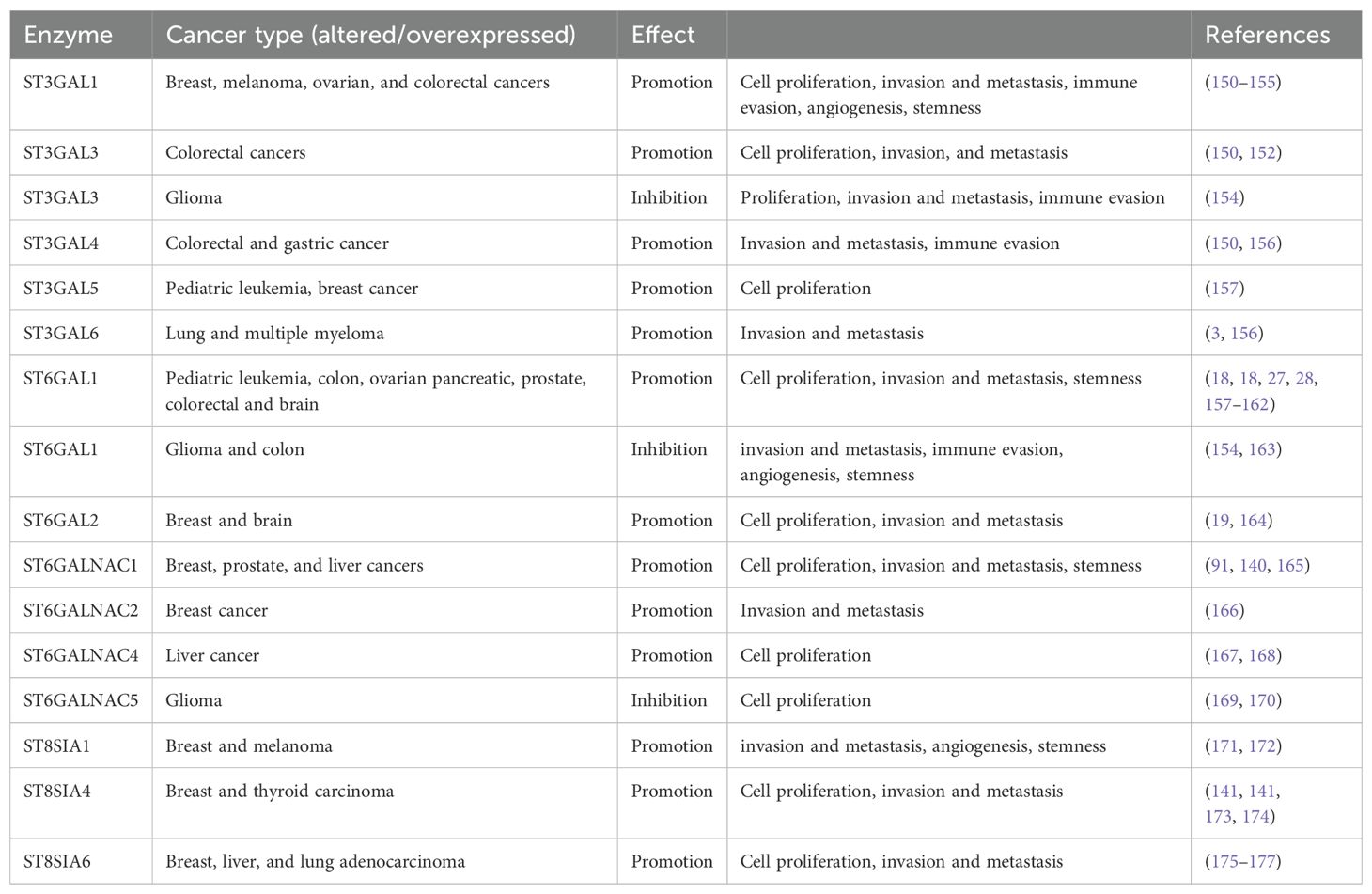
Table 2. Sialyltransferase expression in various cancer.
Sialic acid mediates immune evasion in tumors; masking selectin and Siglec bindingThe presence of sialic acids on cell surfaces influences various cellular behaviors that are crucial for cancer metastasis, including cell adhesion, signaling, and, most importantly, immune evasion. Heavy aberrant O-glycosylation on the surface of mucin residues correlates with metastatic invasion and immune evasion of tumor cells (99, 178–180). Hypersialylated cancer cell surfaces are prime ligands for sialic acid binding lectins (known as Siglecs) on immune cells. The hypersialylated cell surface protein CD24 binds with Siglec-10 on macrophages to prevent tumor cells from undergoing phagocytic death by acting as a “don’t eat me” signal (37). Increased sialyl Lewis antigens on tumor surfaces make the immune system recognize them as migrating leukocytes, not cancer, enabling them to evade the system and colonize other tissues and organs (45). In addition, aberrant expression of sialic acids on cancer cells prevents complement activation, extending longer on the cell surface and serving as a physical barrier to prevent NK cells from accessing their receptors on the cell surface. This, in turn, disables major killing mechanisms of cytotoxic T cells, modulates macrophage function, and dampens dendritic cell activation and function.
Siglec regulates immune surveillance of cancer, and one of the main results of aberrant sialylation is the loss of Siglec expression in cancer cells, preventing cancer cells from attack by the immune system (33). Siglecs play a critical regulatory role in innate and adaptive immune response via the recognition of mammalian species-specific sialylated glycans (181), as well as regulating cancer immune surveillance (182) (Figure 2). Hypersialylation on cancer cells increases sialic acid-binding receptors, aiding immune evasion, and helps to camouflage cancer cells by binding to Siglec receptors on immune cells, transmitting inhibitory signals, and promoting cancer cell survival and proliferation (15, 44). Some Siglecs can also deliver activation signals that enhance antitumor responses, and this interaction affects immune responses, including inflammation (183). Cancer cells have a prominent glycocalyx (35), and they need to evade the NK cells to proliferate, migrate, and metastasize. Siglec-7 and Siglec-9 are inhibitory receptors that bind sialic acid-containing ligands on tumors to dampen the activation of NK cells. Increased expression of Siglec-7 and Siglec-9 ligands on various cancer cells have been shown to decrease their susceptibility to NK cell-mediated killing (36, 184). Siglec-15 on macrophages suppresses the immune microenvironment in PD-L1-negative non-metastatic lung adenocarcinoma (38). It was also reported that inflammatory responses are attenuated or weakened when the activity of sialic acid binding to Siglecs receptors is increased (185). Sialic acid is attached to the outermost glycolipids and glycoproteins on the surface of tumor cells to bind receptors like Siglecs (186).
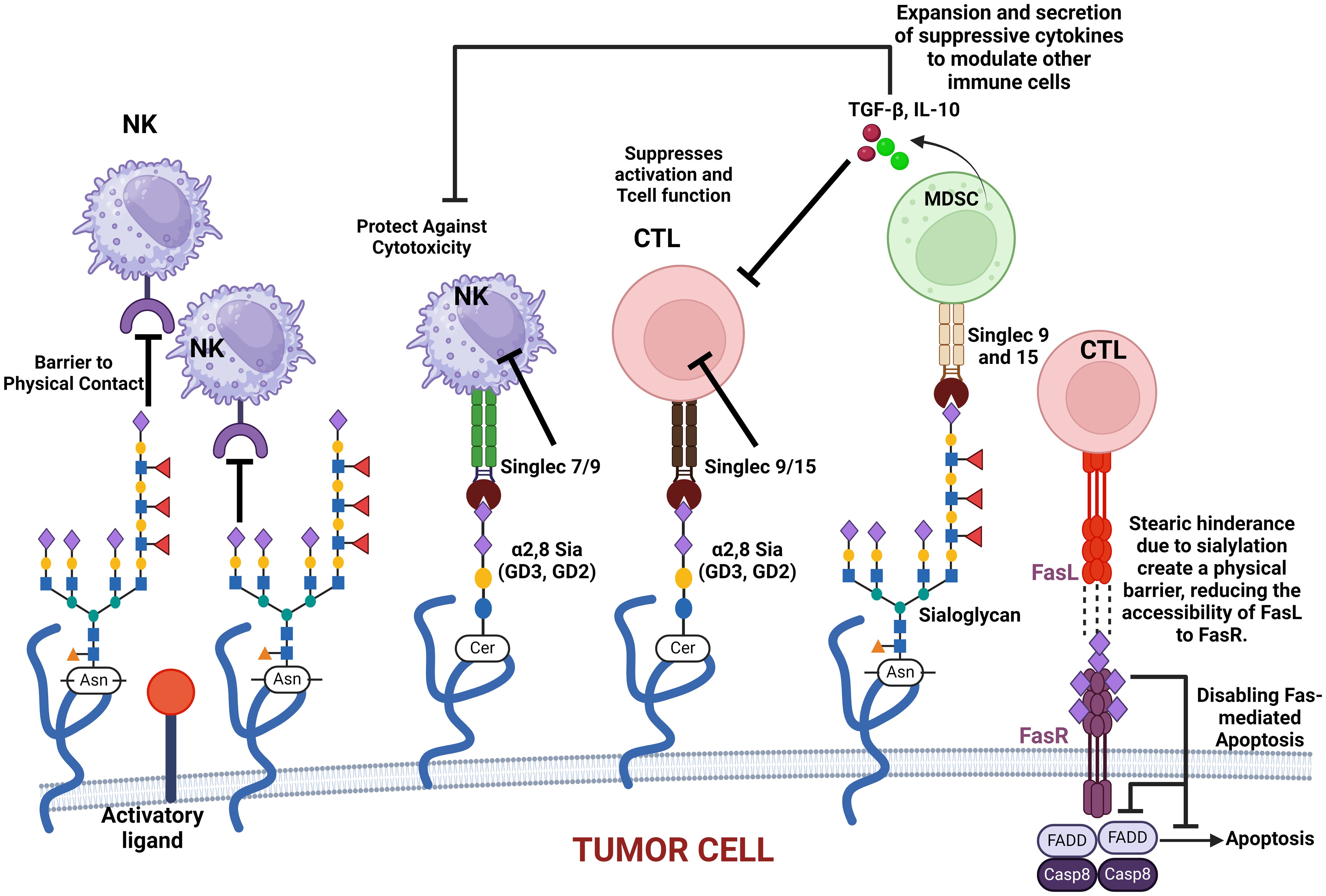
Figure 2. This figure illustrates how various sialylated glycoproteins on the cell membrane interact with immune cell surface components, such as Siglecs, to modulate immune responses. These interactions can protect cancer cells against cytotoxic activity, suppress T-cell activation, and promote the expansion and secretion of modulatory cytokines. Additionally, sialylated glycoproteins create a physical barrier that impedes immune cell contact. The figure also highlights the role of sialylated Fas receptor (FasR) in providing steric hindrance, preventing Fas ligand (FasL) access, and ultimately disabling apoptosis in cancer cells. These mechanisms collectively contribute to immune evasion and cancer progression.
It is critical to note that O-acetylation of sialic acids at position C7, C8, or C9 of sialic acids on the surface of glycoproteins alters their structural conformation and charge, effectively masking the binding of these glycoproteins to key recognition molecules, such as selectins and Siglecs (sialic acid-binding immunoglobulin-type lectins). When sialic acids are O-acetylated, particularly at C9, it sterically hinders or alters the conformation of the sialylated glycans, masking the recognition motifs required for selectin binding. This results in the inhibition of selectin–glycan interaction. When sialic acids are deacetylated, they modulate immune-mediated cytotoxicity via the sialic acid–Siglec pathway (187, 188). Selectins (such as E-, P-, and L-selectins) typically mediate cell adhesion in processes like leukocyte trafficking and cancer metastasis by recognizing sialylated structures like sialyl Lewis X (SLeX) (see Figure 3). However, O-acetylation at position C9 of sialic acids can block the recognition of SLeX by selectins, reducing cell adhesion and metastasis potential (189). This modification confers a selective advantage to tumor cells by enabling them to evade immune surveillance and promoting their survival and mobility within the body. Similarly, Siglecs, particularly those involved in immune suppression (like Siglec-7 and Siglec-9), depend on the recognition of sialylated glycans. O-acetylation disrupts their ability to bind to these glycans, helping cancer cells escape immune responses that would otherwise be triggered by Siglec–sialic acid interactions.
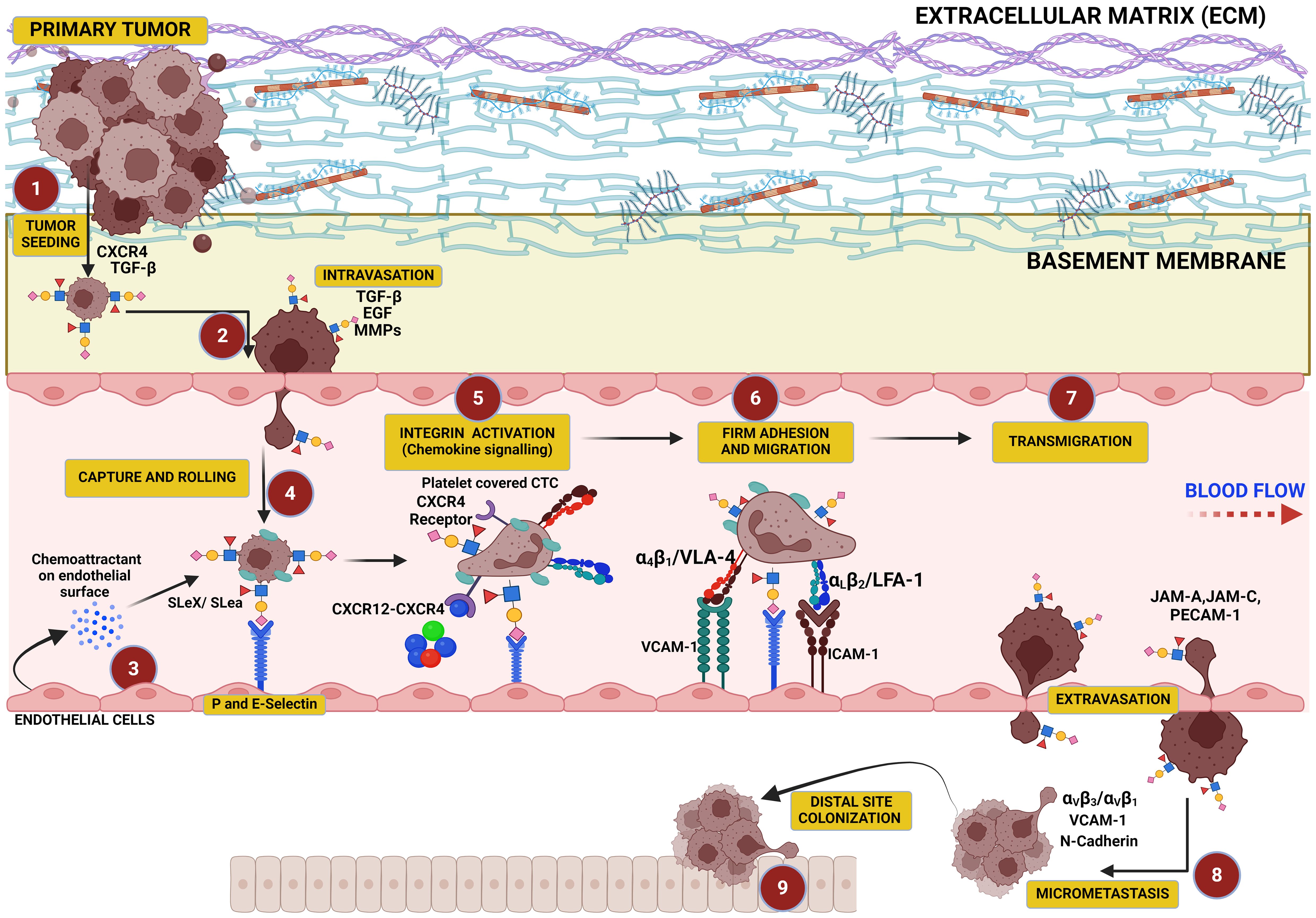
Figure 3. This figure illustrates the critical role of hypersialylation in driving the metastatic cascade. Overexpression of sialoglycans on cancer cells increases local mechanical tension within the primary tumor, leading to cell–cell and cell–matrix repulsion that facilitates tumor cell dissociation. These cells intravasate into the vasculature, where sialyl Lewis X (SLex) on their surface binds to selectins on endothelial cells, mediating their capture, tethering, and rolling along the vascular wall. This interaction precedes cellular migration and transmigration through the endothelium, culminating in colonization at distal sites.
Overall, O-acetylated sialic acids regulate the dynamics of glycoproteins on the cell surface to influence the formation of glycoprotein lattices and their association into signal-transducing microdomains. By modulating the interaction of glycoproteins with galectins and other lectins, O-acetylation influences how these glycoproteins cluster and move within the plasma membrane to allow for longer retention of sialic acids on the cell surface and, thus, stable glycoprotein networks that aids cellular proliferation, migration, and immune evasion. These microdomains are sometimes referred to as glycosynapses and are very crucial for organizing receptors and other signaling molecules into functional complexes that can transduce signals across the cell membrane.
Sialic acids’ role as galectin binding modifiersGalectins are a family of β-galactoside-binding proteins. They modulate various cellular processes, including cell–cell adhesion, immune responses, and tumor progression. It is critical to note that sialic acids play a crucial role in regulating galectin binding to glycoproteins by modifying the exposure of galactose residues. Galectins can be found both inside and outside the cell, with extracellular galectins exerting their functions via an interaction with cell surface oligosaccharides.
Overexpressed sialic acids can inhibit galectin binding by masking galactose residues on the glycan structures. This occurs because galectins preferentially bind to galactose and N-acetyllactosamine (LacNAc) sequences on glycans. When sialic acids cap these sequences, it prevents galectin binding. This sialic acid-mediated inhibition of galectin binding plays a role in cancer progression (190). For example, tumors with high levels of sialylation may avoid galectin-mediated cell–cell interactions, promoting tumor immune evasion and metastasis (191, 192). In contrast, reduction of sialylation can enhance galectin binding, potentially promoting galectin-dependent signaling and tumor cell apoptosis (193).
While there is considerable research interest in galectins, relatively few studies have focused on a critical enzyme that inhibits galectin signaling, namely, β-galactoside α2,6-sialyltransferase (ST6Gal-I). ST6Gal-I catalyzes α2,6-linked sialic acid to the terminal galactose of N-linked glycans, a modification that prevents galectin from binding to β-galactosides, a mechanism for tumor cell survival and immune evasion (190), and this enzyme is highly expressed in various cancer types including colon (194, 195), breast (196), cervical (197), leukemia (157), and brain tumors (198). High levels of ST6Gal-I are strongly associated with increased tumor metastasis and poor clinical prognosis (199, 200). Experimental evidence have shown that α2,6-sialylation of surface receptors by ST6Gal-I prevents Galectin-3 (Gal-3) from binding and initiating apoptotic pathways in epithelial tumor cells (201, 202). Notably, Gal-3, like ST6Gal-I, is also upregulated in various cancers (203–207). This presents a paradox, as upregulation of ST6Gal-I creates a sugar structure that inhibits Gal-3 binding, raising the question of why tumor cells would upregulate both Gal-3 and the sugar structures that block its interaction. To explore this paradox, recent studies have forced the overexpression of ST6Gal-I in SW48 cells (a colon epithelial cell line deficient in both α2,3- and α2,6-sialyltransferases) and examined the effects of recombinant Gal-3 exposure on apoptosis (208) and their results demonstrate that ST6Gal-I-mediated α2,6-sialylation provides a survival advantage to tumor cells by inhibiting Gal-3-induced apoptosis, underscoring the enzyme’s role in tumor progression and resistance to immune-mediated cell death.
Sialic acid as a cancer biomarkerSialylation levels and patterns are altered during cancer progression, indicating the potential of sialylated molecules as cancer biomarkers (33). One contributing factor to increased cancer cell signaling is the presence of sialic acid on the glycocalyx. Sialic acid on glycoproteins and glycolipids is known to mediate cell signaling (1). Multiple emerging studies have shed light on the relationship between the presence of sialic acid on these glycans and a more aggressive phenotype of specific cancers (18, 97, 99, 136, 140, 142, 209–211). Most evidently, sialylated glycans regulate cell transduction pathways through its nature to adhere to neighboring cells (through sialyl Lewis antigens and singles) for direct cell–cell signaling, which serves as an essential function for cancer progression (212). The sialic acid sugar also promotes overall tumor progression, not just at the cellular level, but at other levels that can prevent apoptosis, enhance metastasis, and develop resistance to therapy (209). Because of this, sialic acid and sialic acid binding proteins serve as potent biomarkers for all types of cancer for metastasis or invasive cancer spread. In cancers like glioblastoma, inflammation helps tumor cells invade secondary tissues by breaking down the blood–brain barrier. Understanding these mechanisms highlights the role of glycosylation in cancer and suggests targeting hypersialylated glycans as a potential strategy for more effective anti-cancer treatments (213).
Hypersialylation in cancer can be attributed to several mechanisms such as aberrant overexpression of sialyltransferases, varying sialidase/neuraminidase levels, and substrate availability, which all contribute to the rates of sialyation in cancer cells (214). Overexpression of these enzymes aids cancer cells in escaping apoptosis because of the sialylation of specific receptors that mediate apoptosis, such as the Fas receptor. Sialylation of the Fas receptor inhibits the internalization of Fas, blocking the formation of the complexes required for apoptosis (Figure 2) (65, 158). Neuraminidases can cleave sialic acid residues from glycans on the glycocalyx, and the overexpression of NEU 1 and NEU 2, found in lysosomes and the cytoplasm, respectively, has been found to inactivate apoptotic pathways of cancer cells (215). Higher sialic acid substrate availability on the glycans themselves leads to the increased activity of sialic acid production pathways. This alteration in metabolic sialic acid production can lead to increased amounts of sialic acid in cancer cells, which can encourage metastasis (216).
Sialylation of the cancer glycocalyx mediates aggressionIn most cancer cells, glycocalyx signatures are usually characterized by upregulated glycosylation (96, 132, 133, 217–221), leading to increased proliferation, migration, and immune evasion as well as invasive capacities (2, 6, 99, 220–222). The size and structure of this glycocalyx are critical not only in defining the tumor cell’s ability to proliferate, migrate, and metastasize but also to evade immune surveillance (8, 99, 223, 224). Three enzymes are crucial in the initiation and/or extension of the three main classes of glycan on the glycoprotein or glycolipids in the cellular glycocalyx. In mammalian cells, Core 1 β 1,3 Galactosyltransferase-specific molecular chaperone (COSMC) and Alpha-1,3-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase gene (MGAT1) are critical in catalyzing the chain extension of N- and O-linked sugars, respectively, while UDP-glucose ceramide glucosyltransferase (UGCG) catalyzes the initiation of glycosphingolipid (GSL) sugars, all playing a crucial role in cell signaling and metastasis (138, 225–227). Although the biosynthesis of core glycans is different, chain extensions are similar and often capped by terminal additions of sialic acid and/or fucose (228). Overexpression of sialylated O-glycans is a feature of cancer cell aggression (99), and its knockdown or knockout inhibits tumor growth, invasion, metastasis, and immune evasion (4, 99, 229, 230). Expression of ST8SIA4 in the MDA-MB-231 breast cancer line is associated with breast cancer metastasis (141). N-glycans are also heavily glycosylated in cancer to promote cell motility and loss of contact inhibition (231, 232) and protected against immune responses not only in pancreatic tumors but also in tumors of the lung, ovary, and bladder (233). In the brain, GSLs are implicated in tumor progression (234) as well as in immune evasion (10, 235, 236). Sialic acids are attached to either O-, N-linked glycolipid glycans at their galactose (Gal) or N-acetylgalactosamine (GalNAc) units via α-2,3 or α-2,6 bonds, or to other sialic acid moieties via α-2,8 or α-2,9 bonds (Figure 4) by specific sialyltransferases depending on the bond (47, 49).
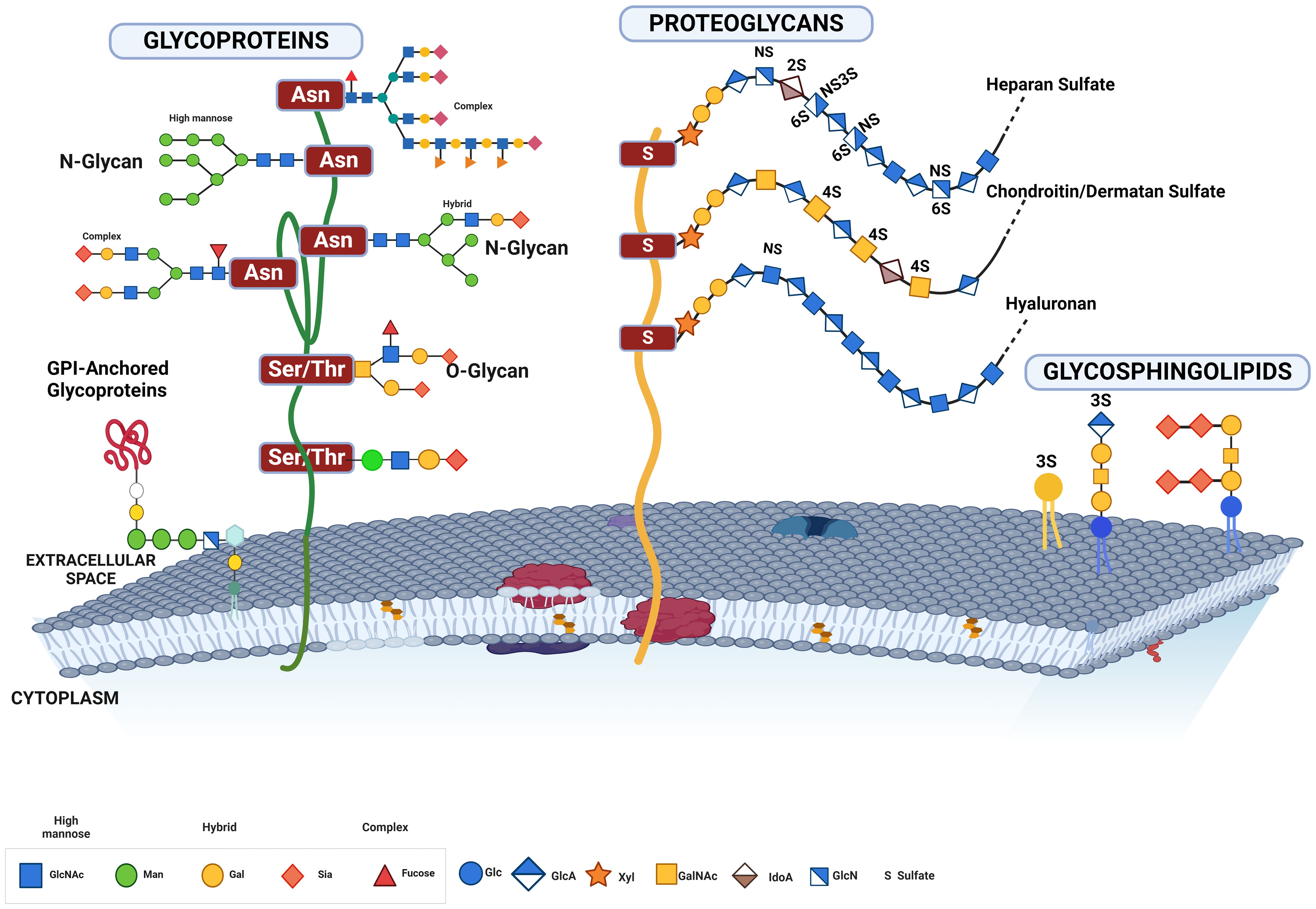
Figure 4. This figure illustrates the various forms of glycosylated proteins present on the cell membrane, highlighting their structural diversity and attachment modes. Depicted are glycoproteins with N-glycans and O-glycans, distinguished by their attachment to specific amino acid residues. N-glycans are attached to asparagine residues, while O-glycans are linked to serine or threonine residues. The figure also includes proteoglycans, which have long, unbranched glycosaminoglycan chains, GPI-anchored glycoproteins, which are tethered to the membrane via glycosylphosphatidylinositol anchors, and glycolipids, which consist of carbohydrate moieties attached directly to ceramide lipids within the membrane. This comprehensive depiction underscores the complex and varied nature of glycosylation on the cell surface, essential for numerous cellular functions and interactions.
Hypersialylated glycans mediate prolonged survival in cancerCancer cells often have high levels of sialylation (237), which are often associated with malignancy and poor prognosis in patients (94, 95). Increased sialylation can increase local negative charges (as sialic acid is the only monosaccharide to carry a charge) on the cancer cell membrane to physically disrupt cell–cell adhesion and promote detachment from the tumor mass through electrostatic repulsion, which ultimately leads to membrane bending (13) (Figure 5). In N-glycans, the mannosyl (alpha-1,3-)-glycoprotein beta-1,2-N-acetylglucosaminyltransferase (MGAT) family of enzymes including MGAT1, MGAT4A, and MGAT5A are upregulated in many cancers, to fuel the loss of contact inhibition, increased cell motility, invasion, and metastasis (42, 233, 238–243). MGAT1, MGAT2, MGAT4, and MGAT5 sequentially add N-acetylglucosamine (GlcNAc) residues to the core mannose residues of N-glycans, resulting in highly branched complex and hybrid N-glycans. Increased activity of MGAT enzymes leads to more complex and branched N-glycan structures on glycoproteins to provide more sites for sialyltransferases to add sialic acid residues. Increased branching structures facilitate the attachment of multiple sialic acids, leading to hypersialylation.
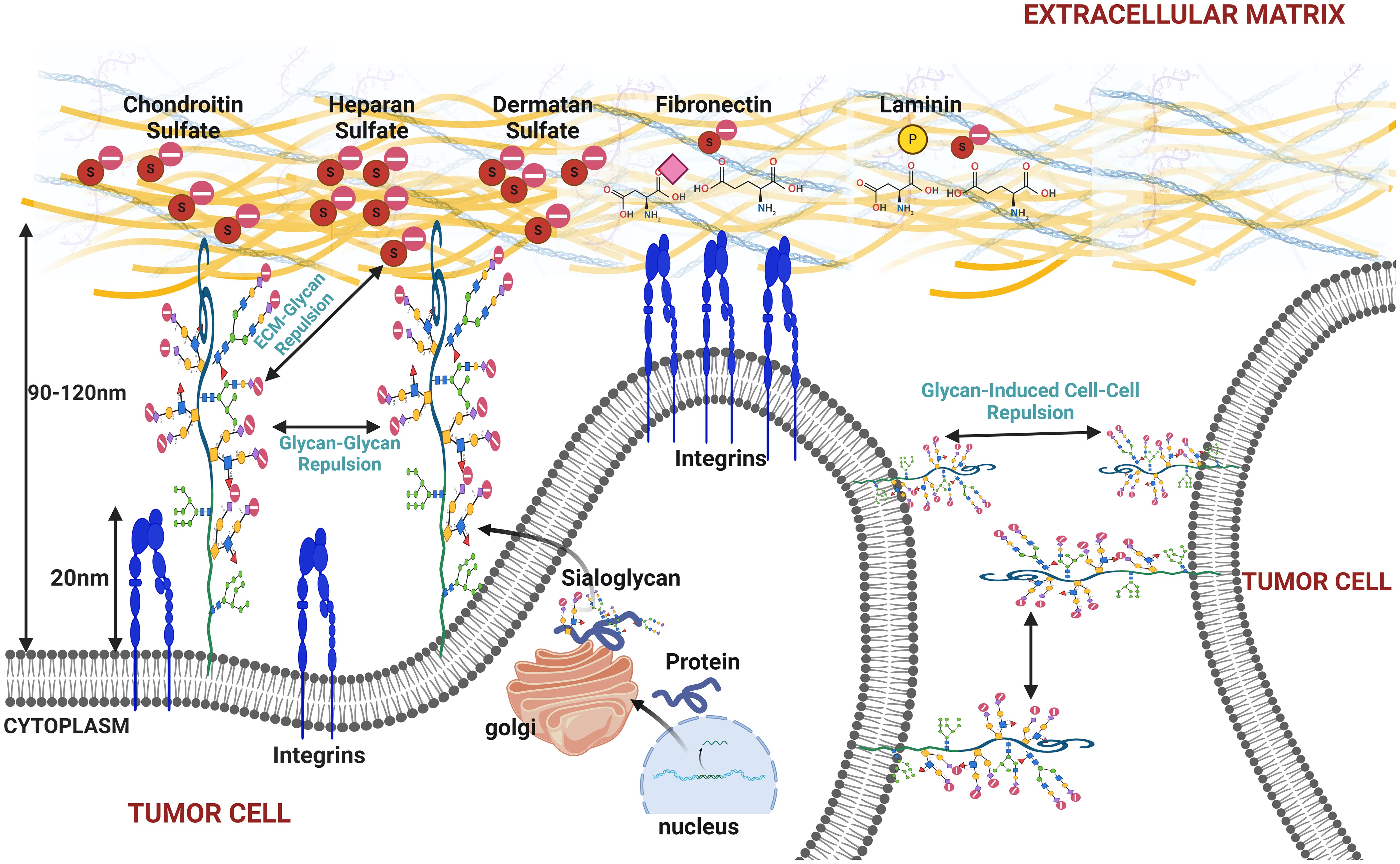
Figure 5. This figure illustrates the effects of increased sialylation on the cell membrane under cancerous conditions, highlighting how heightened sialylation can promote membrane bulging, cell detachment from the extracellular matrix (ECM), and tumor cell disaggregation. The figure depicts the repulsive interactions between sialylated glycoproteins on the cell membrane, causing glycan–glycan repulsion and resulting in membrane bulging. Additionally, it shows how sialylated glycans interact with the ECM components and neighboring tumor cells, leading to glycan–ECM and glycan-induced cell–cell repulsion. These mechanisms contribute to cancer cell detachment and increase metastatic potential, emphasizing the role of sialylation in cancer progression and metastasis.
In O-glycans, COSMC is a specific molecular chaperone required for the proper folding and function of the enzyme C1GALT1. In cancers, dysregulation of COSMC has been reported to cause the accumulation of Tn antigen to form the sialyl-Tn antigen. Dysregulated COSMC causes T-synthase (C1GALT1) to be misfolded and degraded, leading to aberrant glycosylation (244, 245). Mucin overexpression in epithelial cells increases the number of glycosylation sites to increase sialylation and fuel resistance against NK cells (99). Overexpression of COSMC in human colon cancer cells significantly enhances cell migration, invasion, and cancer survival (246–248).
In cervical and breast cancer, overexpression of UDP-glucose ceramide glucosyltransferase (UGCG) led to increased synthesis of glucosylceramide and subsequently more complex GSLs to fuel cell proliferation and glycolysis via the PI3K/AKT pathway (7, 9, 249). The increased availability of precursor molecules facilitates the synthesis of gangliosides, which are often hypersialylated in cancer cells. GSLs are expressed in the brain with a bulk of sialic acids to form gangliosides (250–252), and their aberrant expression drives tumor growth and survival (252–254).
The composition of the cellular glycocalyx modulates membrane dynamics in cancerThe most consistent finding in glycobiology-based cancer research is the priming of cell surface with a robust sugar glycocalyx to mechanically foster cell growth and survival. Upregulation of glycoproteins in cancer is a recurrent event (138, 225–227) to enhance integrin-dependent cell growth and survival (228, 255) and promotes a mesenchymal-like phenotype (217). In many cancer types (breast, brain, lungs, and prostate), glycosylation events are increased to enable addition of more (bulkier) sugars on the glycan including the terminal sialic acid being the most important. More “bulky” glycan structures on the glycocalyx components help to mechanically apply tension the cell membrane and provide local repulsive forces within the membrane vicinity and between adjacent cells, leading to membrane bulging and cell dissociation from matrix and from other tumor cells. These more bulky glycans in many types of cancer extend further (>50 nm) from the cell surface than the integrins (~30 nm) (8), and because of the desirability of most cancer cells to attach to the ECM through their integrins, there is a forced ECM–integrin interaction. This serves to mechanically induce a feedback pull on both the ECM and the nucleus of the tumor cell and subsequently promote the activation genes involved in cancer cell proliferation and metastasis (14, 159, 217, 256–261). Engineering of O-glycans on the cancer glycocalyx demonstrated that a thick and dense glycocalyx could trigger complete cellular detachment from the ECM to facilitate prolonged cell survival (236). Several research lines have applied gene editing to affect the enzymatic function of specific precursor sugars of the entire glycan tree to make cancer cells susceptible to immune attack (262–265).
The cancer cell glycocalyx is heavily decorated by glycan structures (266), and this glycan bulk induces an upregulation of both mesenchymal and bulky glycocalyx-related genes to drive aggression (217). A bulky glycocalyx have also been shown to drive metastasis by increasing cell cycle progression (8). Other reports showed that an increase in the size of the glycocalyx with the MUC1 ecto-domain was sufficient to drive metastatic potential in an in vivo model of breast cancer (8) as well as promoting immune evasion in epithelial cells that had increased MUC1 expression (99). Recent reports further revealed that increased MUC1 expression is a precursor to hypersialylation and immune evasion. Overexpression of mucins in the MCF10A epithelial cell line increases the glycocalyx bulk to evade immune detection while its knockout abrogated the glycocalyx bulk in the ZR-75-1 breast cancer cell line (99).
As our knowledge in this area continues to expand, it has become increasingly evident that the glycocalyx of tumor cells is closely associated with its ability to migrate (267–270). A tumor cell’s glycocalyx serves to tension their membrane and enhance integrin clustering and activate downstream pathways involved in tumor proliferation and invasion (8, 223, 271, 272), as well as extravasation (17, 273, 274). Other studies indicated that mucin degradation in ZR-75-1 breast cancer cell line led to an increased NK-mediated cytotoxicity (99) while genetic disruption of the glycocalyx in melanoma cells tends to be anti-metastatic (275). Recently, synthetic mucin glycopolymers of low and high densities were expressed on epithelial cell membrane to increase the relative size and density of mucin and modulate plasma membrane dynamics (236). Glycocalyx-mediated tensioning has been identified in many cancer types including glioma cells, which cause glioblastoma multiforme (GBM) to adopt a more mesenchymal and lethal phenotype with high migration velocity (217). This finding is consistent with other studies where increase in mucin glycosylation (O-glycan) correlates with metastatic invasion and immune evasion (99, 178–180).
Sialylation-induced mechanical tension modulates signaling in cancerPhysical stress induced on the cell membrane by glycan capped by sialic acid can impact cellular behavior to reorganize and activate surface receptors’ integrins. This tension-activated integrin pulls on the ECM ligands, causing a tension-mediated glycocalyx–integrin feedback loop to promote mesenchymal-like phenotype in most cancer cells that overexpresses mucin glycoprotein. This, in turn, has a pulling mechanical effect on the nucleus to promote downstream signaling with growth factor receptors, a positive factor to G1 cell cycle progression (217, 223, 276). Overexpression of mucin glycoproteins in cancer cells potentiate more glycosylation hotspots for O- and N-glycosylation, which are often capped with an excess of sialic acid and/or fucose at the terminal end of the sugar glycan. Increased expression of mucin in MCF-10A breast epithelial cell line correlates with sialic acid expression (99) while increased sialic acids on glycoproteins have been reported to stimulate not just integrin-FAK mechanosignaling (8, 217) (Figure 6). Sialylation events such as overexpression of ST6GalNAcII mediates the invasive properties of breast carcinoma through the PI3K/Akt/NF‐κB signaling pathway (166). ST6GALNAC1 expression has been shown to promote the invasion and migration of breast cancer cells via the EMT pathway (140).
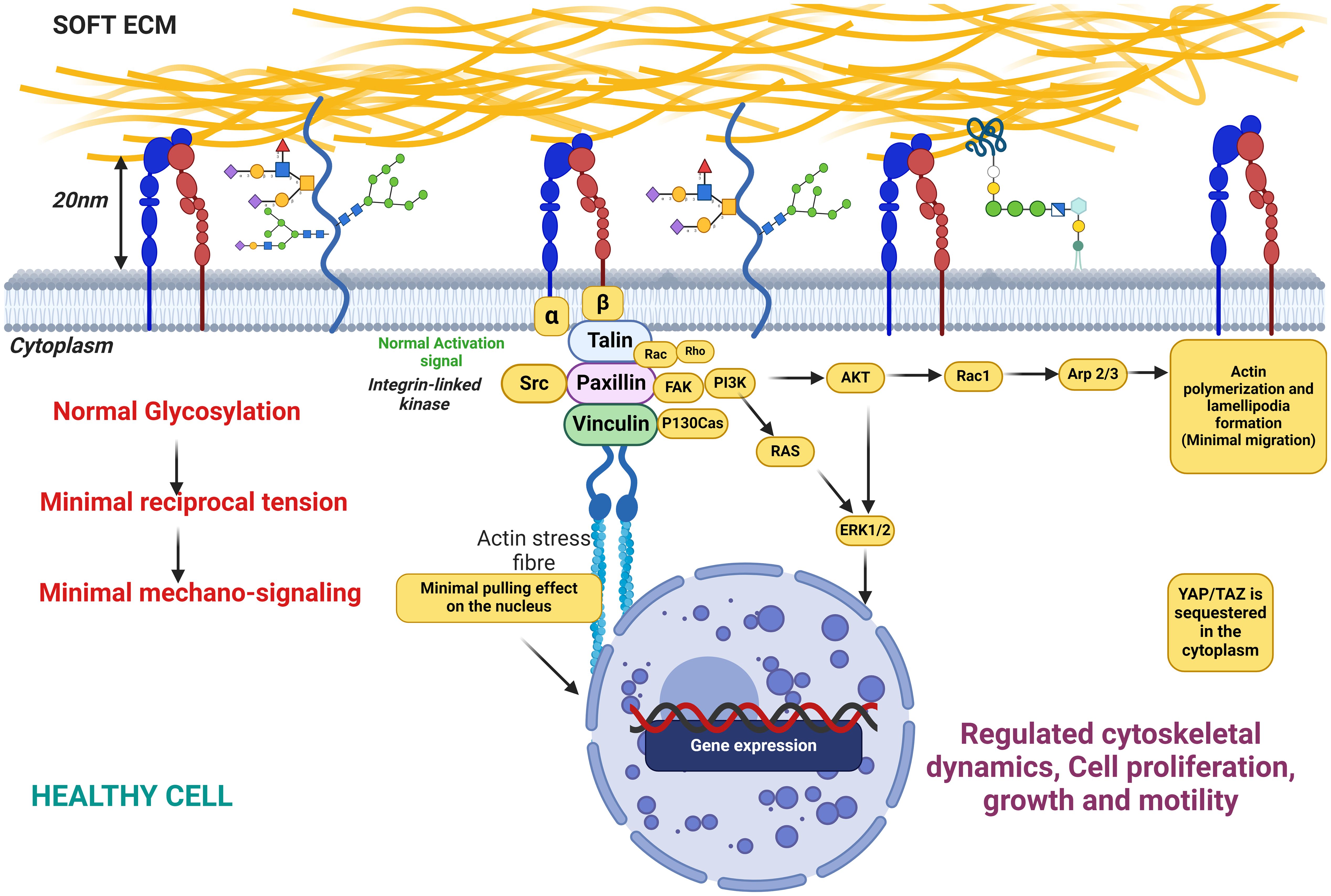
Figure 6. This figure illustrates the state of cellular glycosylation on the membrane under healthy conditions, characterized by minimal reciprocal membrane tension and low mechanosignaling activity. The figure also depicts the distribution and structure of glycosylated proteins on the cell membrane, highlighting the role of glycosylation in maintaining cellular functions in a healthy physiological state. Key components include glycoproteins, the actin cytoskeleton, and signaling molecules, demonstrating their interplay in preserving normal cellular behavior with minimal mechanical stress to the membrane.
Other studies have shown that MUC1 is heavily sialylated and mice deficient in MUC1 resist tumor formation (277) while primary tumor xenografts that overexpress MUC1 grow and metastasize more aggressively (268). The implication of this finding is that tumors with bulky glycans on their mucins may foster tumor progression and aggression. Heavily sialylated mucins have been reported to bend the cancer cell membrane and stimulate integrin-FAK mechanosignaling. This occurs due to the negatively charged sialic acids populating the membrane, creating a repulsive effect in the local vicinity of the cancer cell membrane and pulling all the integrins and other surface receptors apart. As a result, focal adhesions are formed and the receptor integrins are forced to bind and pull on the ECM as well as the intracellular cytoskeleton to mediate a stiffer ECM. The subsequent autophosphorylation of tyrosine pY397-FAK assembly, which is an activation signal to other downstream mechanosignaling events involved in cell migration, proliferation, and aggression (8, 217, 223, 261), then occurs. FAK assembly disseminates adhesion and tensional information from focal adhesions to the rest of the cell via autophosphorylation at tyrosine 397, as well as increased phosphorylation at tyrosine 925 (278). The cell senses increased substrate stiffness through integrins to induce assembly of focal adhesions and further cause activation of focal adhesion kinase (FAK) (34, 279). This leads to enhanced MAPK activation, such as Akt phosphorylation, which then promotes G1 cell cycle progression through expression of proteins like the cyclins (8). The AKT pathway and MAPK pathway can influence each other’s activity through crosstalk and regulatory interactions. AKT can directly or indirectly modulate the activation and function of various MAPKs, including ERK1/2, JNK, and p38 MAPK. Staining for pY397-FAK, cyclin D1, and pAKT substrate demonstrated that overexpression of the MUC1 ectodomain increases mechanosignaling, cell cycle progression, and MAPK activity (280).
Taken together, increased sialylation drives integrin clustering through kinetic funneling, leading to increased signaling from focal adhesion-associated proteins such as FAK in combination with growth factor signaling.
Altered sialylation in brain cancerOwing to the brain’s importance in controlling the central nervous system and overseeing most of the body’s functions, tumor growth in the brain has unique cell types, immune context, anatomy, and metabolic limitations as the tumor microenvironment of the brain is significantly different compared to other parts of the body. This unique microenvironment, protected by the blood–brain barrier, favor
留言 (0)