Lung cancer is the most commonly occurring cancer worldwide, with the highest mortality rate according to the data released by the World Health Organization (WHO) cancer agency in February 2024, and approximately 80%–90% of newly diagnosed lung cancers are non-small cell lung cancer (NSCLC) (1). With the rapid development of precision medicine, targeted therapies based on oncogenic driver genes have changed the treatment mode of NSCLC for more than a decade, especially for non-squamous cell carcinomas of NSCLC. Rearranged during transfection (RET) is an oncogenic driver gene located on the long arm of autosome 10 (10q11.2) and encodes a transmembrane glycoprotein receptor tyrosine kinase, which accounts for approximately 1%–2% in NSCLC (2). After interacting with ligands from the glial cell line-derived neurotrophic factor (GDNF) family, it forms a dimer and autophosphorylates on several tyrosine residues in a specific RET cytoplasmic domain (3, 4), ultimately resulting in the expansion of intracellular signaling and transcriptional pathways such as RAS/MAPK, JAK/STAT, and PI3K/AKT, which promote survival, proliferation, and migration (3, 5). Since its discovery, RET and its receptors have been implicated in a variety of cancers, including thyroid cancer, lung adenocarcinoma, colorectal cancer, and chronic monocytic leukemia (6, 7). Among them, the majority of RET fusion-positive NSCLC patients tend to be relatively young (<60 years of age), never smoked or lightly smoked, with the histological type of adenocarcinoma, with brain metastases at the initial diagnosis (5).
RET fusion-positive NSCLC has not benefited greatly from chemotherapy (8) or immune checkpoint inhibitors (ICIs) (9) although they have been widely used in patients with driver gene-negative NSCLC and achieved breakthrough progress. In the early phase of targeted therapy for RET fusion-positive NSCLC, the first-generation multikinase inhibitors (MKIs) with auxiliary RET activity such as sorafenib, cabozantinib, and vandetanib were commonly used. However, these MKIs were eliminated due to a lack of specificity, resulting in numerous off-target toxicities and a low objective response rate (ORR) (10). Thanks to its well tolerability, robust intracranial activity, and high specificity, the new RET-TKIs pralsetinib and selpercatinib have ushered in a new era of RET precision therapy. The U.S. Food and Drug Administration (FDA) approved pralsetinib and selpercatinib 3 years ago for the treatment of RET fusion-positive NSCLC. Adverse drug reactions (ADRs) are a significant factor influencing the therapeutic impact and course of patients receiving targeted therapy, and RET-TKIs are no exception. According to the updated data from the ARROW and LIBRETTO-001 trials, the most frequent ADRs of pralsetinib with a grade of 3 or higher were anemia (12%), hypertension (12%), and neutropenia (20%) (11), while the most common grade 3 or worse ADRs of selpercatinib were hypertension (13.2%), increased alanine aminotransferase level (9%), and increased aspartate aminotransferase level (6.3%) (12). However, as novel targeted drugs, the clinical efficacy and safety of RET-TKIs are still being explored in real-world practice. This study has been approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University ( Ethics number: XJTU1AF2024LSYY-111).
Case presentationA 56-year-old man was admitted to our hospital in March 2019, with an incidental finding of a mass in the upper lobe of the right lung and elevation of tumor markers during a physical examination without any symptoms. He had a history of hypertension and colon polyp, without diabetes, coronary heart disease, and endemic mycosis exposure history. He did not smoke cigarettes or drink alcohol and had no family history of cancer or genetic disease. After exclusion of contraindications, the patient underwent thoracoscopic radical resection of the mass in the right upper lung lobe mass plus wedge resection of the right middle lung lobe on 21 March 2019 (intraoperative detection revealed that the mass was located in the anterior segment of the right upper lobe and invaded the right middle lobe). Postoperative pathological examination revealed adenocarcinoma (5 cm × 3 cm × 2 cm), with invasion of the visceral pleura, middle lobe of the right lung, and lymph node metastasis. According to the 8th American Joint Committee on Cancer Staging System (13), the patient’s postoperative staging was pT4N1M0, stage IIIA. Carboplatin and pemetrexed adjuvant chemotherapy every 21 days for four cycles and thoracic radiation therapy (TRT) were given 4 weeks after surgery.
After being discharged from the hospital, the patient received regular follow-up examinations and remained disease-free for 36 months. Unfortunately, in July 2022, disease progression was revealed by chest computed tomography (CT) scan, ultrasound bronchoscopy (EUBS) needle aspiration biopsy, and right pleural effusion cell smear (see Figures 1, 2 for details). The biopsy tissue was sent for next-generation sequencing (NGS) with a 14-gene lung cancer panel, and KIF5B-RET fusion was detected. Additionally, co-alterations were not identified for EGFR, ALK, ROS1, MET, HER2, BRAF, or KRAS. The Eastern Cooperative Oncology Group (ECOG) performance status score was 1. The patient was treated with pralsetinib, which was administered orally at a dose of 400 mg once daily since July 2022.
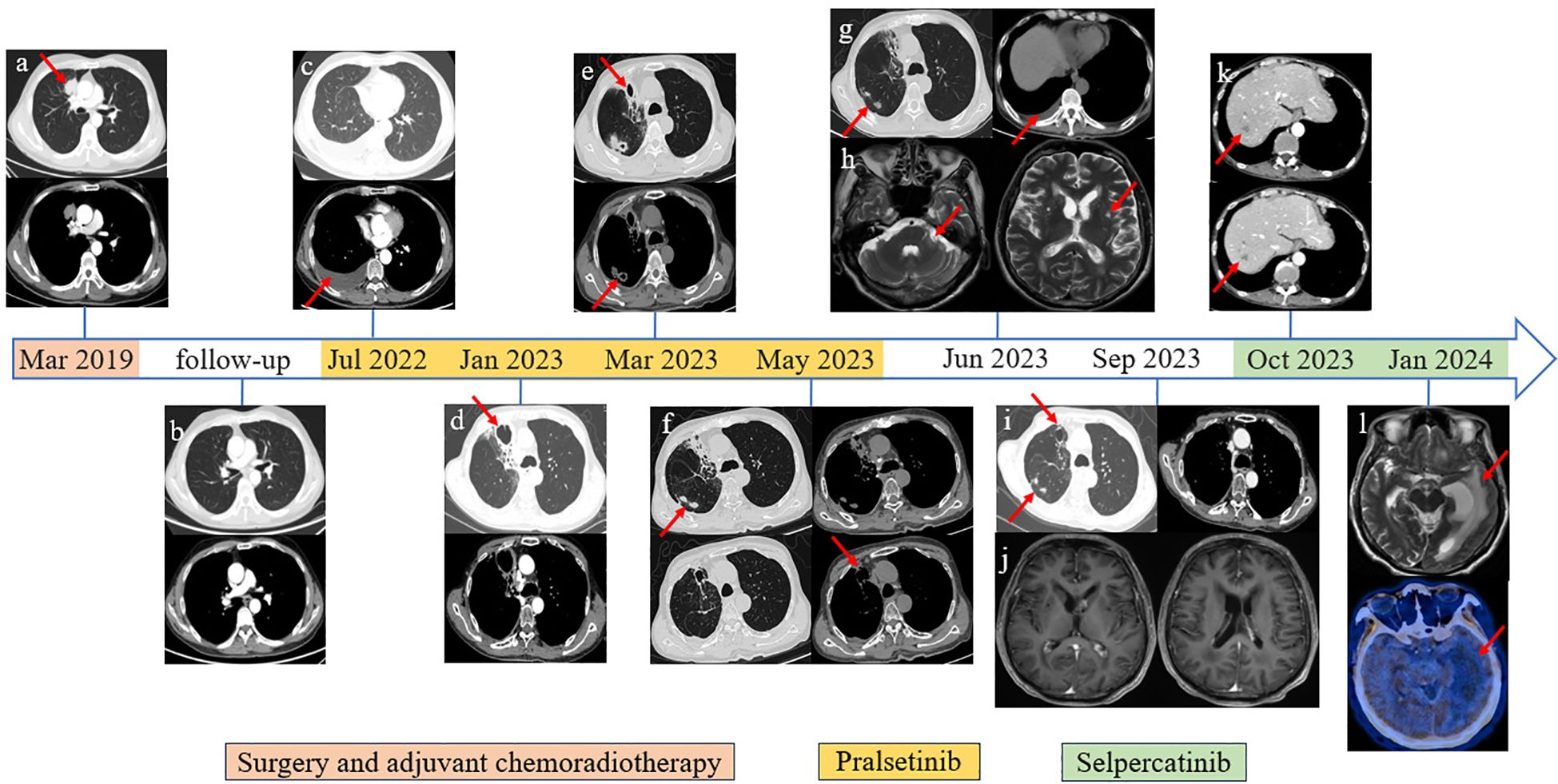
Figure 1. Imaging information throughout the disease course. (A) Primary lung tumor in the upper lobe of the right lung. (B) Follow-up chest CT scan image after surgery and adjuvant chemoradiotherapy. (C) Disease progression revealed by right pleural effusion cell smear. (D) Bronchial stenosis in the middle medial segment of the right lung, surrounding obstructive pneumonia and irregular cavity shadow, approximately 45 * 42 mm in size. (E) Cavity in the middle medial segment of the right lung was smaller than before, approximately 21 * 22 mm in size; newly added multiple irregular lesions with cavities. (F) The lesions on the middle medial segment of the right lung were unchanged. (G) The lesions were the same as above; recently presented right pleural effusion. (H) Abnormal enhancement in bilateral lateral ventricles and left cerebellar angle area. (I) The lesions on the middle medial segment of the right lung had shrunk. (J) The circularly enhancing lesion in bilateral lateral ventricles and left cerebellar angle area had shrunk. (K) Distant metastasis was discovered in the liver through the abdominal CT scan. (L) Cranial MRI revealed a new large edema on the left lateral ventricle compared with the previous examination, and further PET/CT (18F-FET) scan showed that there was no abnormal nuclide concentration in the edema area.
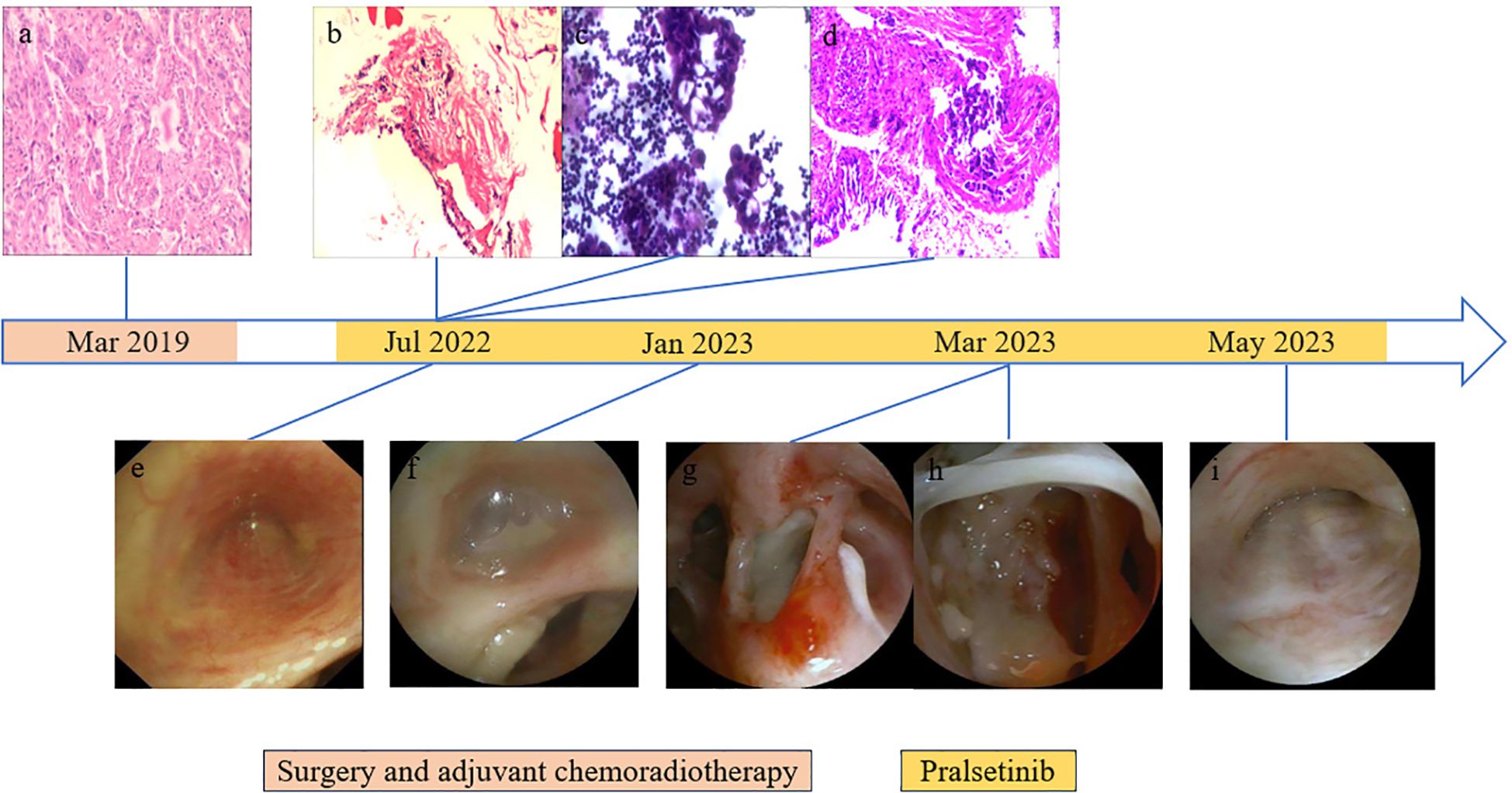
Figure 2. Results of endoscopic and histopathological examinations. (A) HE staining of surgical specimens showed lung adenocarcinoma. (B) Right pleural puncture pathology showed chronic inflammation of fibrous adipose tissue and a little skeletal muscle tissue. (C) On the right side, pleural effusion smear and sediment paraffin section found gland cancer cells; HE staining shows CK7(+), CEA(+), TTF-1(+), NapsinA (+), CDX2(−), CR(−), and D2-40(−). (D) Biopsy of lesions in the middle lobe of the right lung showed a small amount of poorly differentiated adenocarcinoma infiltration in the bronchial mucosa and a very small number of heterologous glandular epithelial cells in the cellulose exudation. HE staining indicated HER2(0), ALK(−), and PD-L1 (TPS: +1%). (E) Bronchoscopy showed mucosal eminence and lumen occlusion of the right middle lobe bronchus. (F) Right middle lobe bronchial tube lumen occluded with viscous discharge. (G) Excessive purulent discharge obstructing the lumen was seen in the right intermediate bronchus. (H) White camouflaged secretions can be seen in the dorsal segment of the lower lobe of the right lung covering the blocked lumen. (I) Bronchoscopy showed lumen occlusion of the right middle lobe bronchus.
From January 2023 to May 2023, the patient went to the hospital several times due to a fever accompanied by cough and sputum. The test results of respiratory virus antibodies detection, atypical pathogen antibodies detection, COVID-19 nucleic acid test, sputum cytology, and sputum culture were all negative. The value of serum 1,3-β-d-glucan testing (G) and serum galactomannan testing (GM) increased. Chest CT scan showed multiple irregular cavities. Pseudomonas aeruginosa, Aspergillus fumigatus, Candida albicans, and Pneumocystis jirovecii were found in the bronchoalveolar lavage fluid, which was consistent with prior reports (14). During hospitalization, the patient received combined antimicrobial therapy with discontinuation of pralsetinib, his temperature returned to normal, and his symptoms such as cough and sputum were improved. After discharge from the hospital, he received voriconazole combined with a reduced pralsetinib dose of 200 mg once daily to control the primary disease, and intermittent fever occurred repeatedly (see Supplementary Table 1 for details).
On June 2023, the patient visited the hospital once again due to dizziness with nausea, vomiting, and difficulty walking. Confusion and slurred speech, choking when drinking water and difficulty in swallowing, partial limb numbness, and meningeal irritation signs were not presented during the physical examination. The ECOG performance status score was 4. The chest CT scan revealed an additional pericardial and pleural cavity effusion. Cranial magnetic resonance imaging (MRI) showed abnormal enhancement in bilateral lateral ventricles and left cerebellar angle area, considering the possibility of central nervous system (CNS) infection. Lumbar puncture and NGS detection of cerebrospinal fluid proved CNS infection (see Supplementary Tables 2, 3 for details). He received antifungal therapy in conjunction with dehydrated cranial pressure, and pralsetinib was discontinued in June 2023. After being discharged from the hospital, the patient continued taking voriconazole for 12 weeks, and the follow-up chest CT scan and brain MRI showed lesions to be decreasing. Due to the fact that the patient suffered from recurrent infections, which significantly impacted his physical wellbeing, and the discontinuation of pralsetinib led to the elevation of tumor markers, the decision was made to transition to selpercatinib at a dose of 160 mg twice daily from October 2023.
Follow-up CT scan and tumor markers showed that the tumor did not progress, but dizziness accompanied by limb weakness appeared again in January 2024. The patient’s cranial MRI revealed a new large edema on the left lateral ventricle compared with the previous examination, and further PET/CT (18F-FET) scan showed that there was no abnormal nuclide concentration in the edema area, which was not consistent with neoplastic changes, as shown in Figures 1, 3. Combined with the previous history of intracranial Aspergillus fumigatus infection, consideration of the intracranial lesions as recurrent CNS infections was not excluded. The patient and his family requested discharge, and since then, the patient’s general condition deteriorated and he died in March 2024.
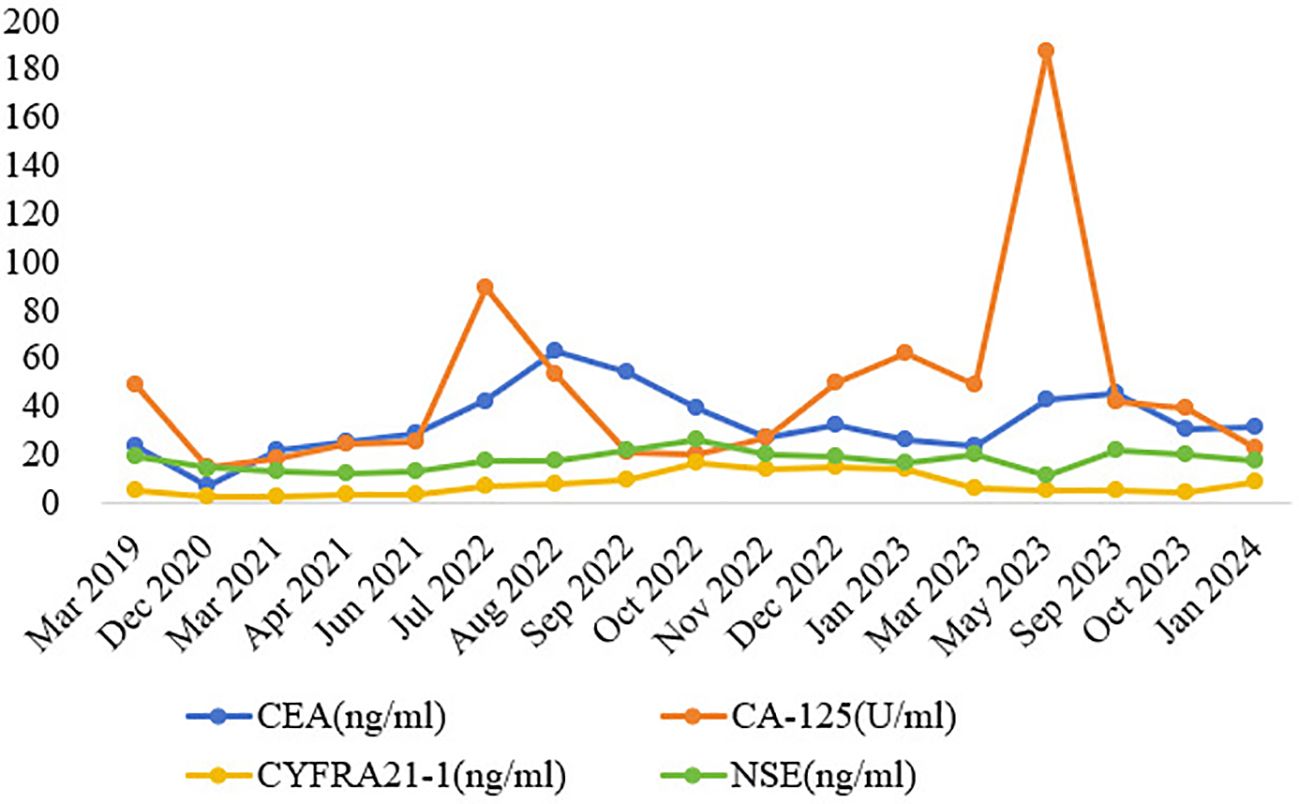
Figure 3. Dynamic monitoring of tumor markers during treatment. Note: CEA, carcinoembryonic antigen; CA-125, carbohydrate antigen 125; CYFRA21-1, cytokeratin 19 fragment; NSE, neuron-specific enolase.
DiscussionPralsetinib and selpercatinib are potent and highly selective RET-TKIs that target RET alterations, including fusion and mutation, irrespective of the tissue of origin. Recently, the results from clinical trials demonstrated significant clinical benefits and tolerable toxicity of pralsetinib and selpercatinib, respectively. According to the updated data from the ARROW and LIBRETTO-001 trials, pralsetinib had an ORR of 72% for treatment-naive patients and 59% for patients with prior platinum-based chemotherapy, and the median progression-free survival (mPFS) rates were 13.0 and 16.5 months, respectively (11), while selpercatinib had an ORR of 84% for treatment-naive patients and 61% for patients with prior platinum-based chemotherapy, and the mPFS rates were 22.0 and 24.9 months, respectively (12).
This patient is a middle-aged man with RET fusion-positive NSCLC, without a history of chronic respiratory disease or smoking. A regular review of the chest CT scans showed no pulmonary infectious lesions. RET-TKIs provided significant benefits to patients, and a decline in tumor markers was observed, which is consistent with previous studies. At the same time, we observed an effect not previously documented in clinical trials. After 5 months of antitumor treatment with pralsetinib, the patient developed invasive pulmonary aspergillosis, and symptoms occurred repeatedly even after the reduction or discontinuation of pralsetinib, ultimately progressing to CNS infection. In light of the ADR of pralsetinib, the antitumor therapy was discontinued, and a powerful anti-infective therapy was initiated, which then controlled the infection. However, after 2 months of antitumor treatment with selpercatinib, large edema of the left lateral ventricle was detected without abnormal nuclide concentration in PET/CT (18F-FET), suggesting a correlation between the adverse reactions and the targeted therapy drug. Moreover, the patient in this case had been regularly taking nifedipine controlled-release tablets to control blood pressure for many years, and the patient experienced no discomfort in the past. Furthermore, there was no record of adverse reactions to infection in its instructions. Therefore, the possibility of nifedipine controlled-release tablets causing invasive aspergillosis was not considered. In addition, the patient had no history of diabetes or long-term use of broad-spectrum antibiotics, glucocorticoids, or immunosuppressants during the treatment. Therefore, we concluded that the occurrence of invasive aspergillosis may be related to RET-TKIs.
We reviewed the literature on infections that occurred after treatment with RET-TKIs and identified six studies published locally and abroad, as shown in Table 1. Most of the reported opportunistic infections were associated with pralsetinib, whereas no association was reported with selpercatinib. Of these, Mycobacterium tuberculosis (MTB) infections were the most common, including pulmonary and extrapulmonary tuberculosis, and the average time to infection was approximately 3 months after starting RET-TKI therapy. In view of the above data, it is suggested that opportunistic infectious events may be a significant safety issue for patients who are receiving RET-TKIs. As reported in the updated data from the ARROW and LIBRETTO-001 trials, 26.5% and 4% of the patients treated with pralsetinib and selpercatinib developed pneumonia, respectively, involving multiple pathogens, including a variety of bacteria, cytomegalovirus, and influenza virus, but no fungal infection events had been recorded after treatment with pralsetinib or selpercatinib (11, 12). It is important to note that clinical trials typically enroll carefully selected patients with fewer comorbid conditions and immunosuppressive conditions than average patients, and there may be a higher incidence of opportunistic infection in the real world.
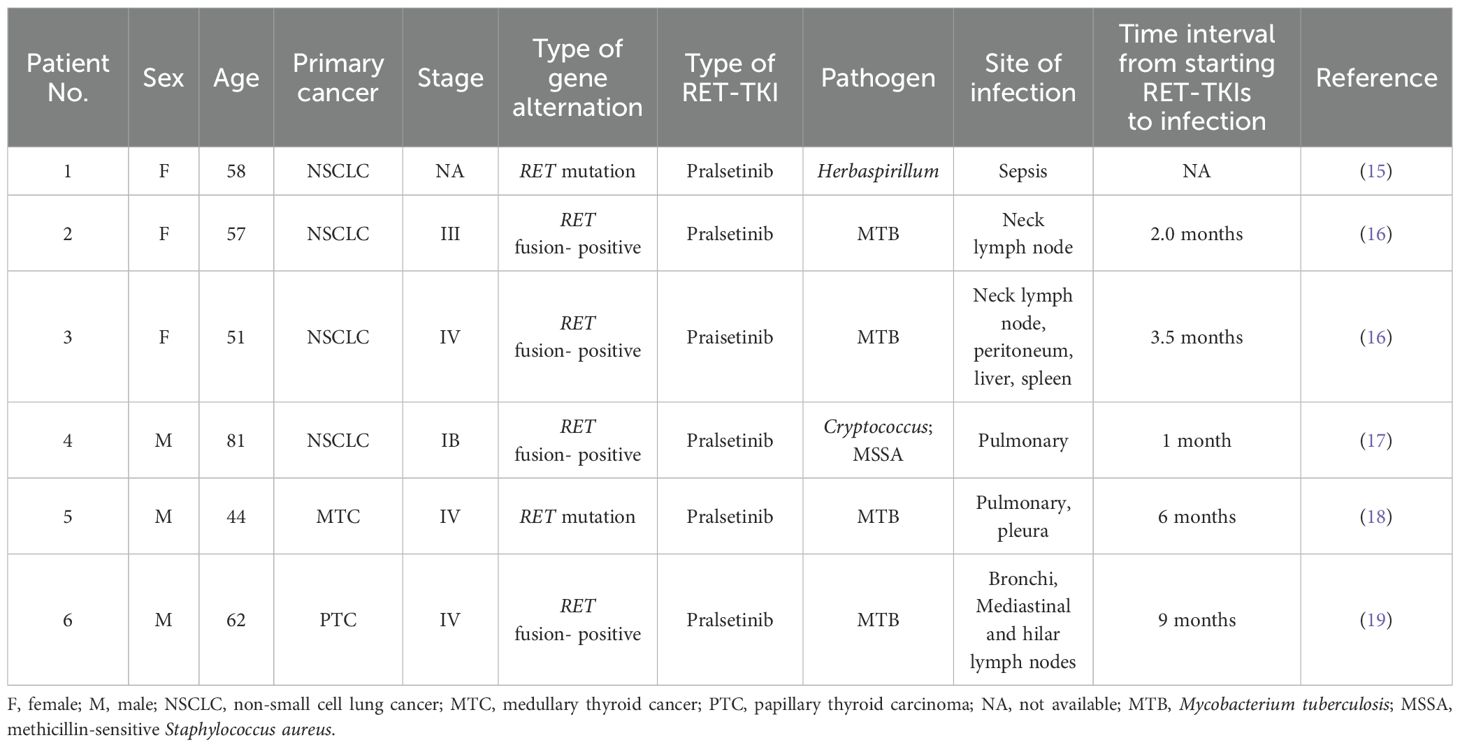
Table 1. Summary table of published literature on infections occurred after treatment with RET-TKIs.
The mechanism of RET-TKIs in causing invasive aspergillosis is not well understood, and there are no clinical cases reported to date, as the drugs have only recently entered the market. Although pralsetinib and selpercatinib are highly selective against RET kinases, they have also been shown to inhibit some non-RET kinases, such as JAK 1/2 and VEGFR (20). The JAK-mediated intracellular signaling pathway plays a key role in immune regulation and host defense (21). Treating autoimmune diseases or cancer with JAK inhibitors has been well documented to be associated with an increased frequency of infections, including fungal and mycobacterial infections and reactivation of shingles and viral hepatitis B (22–24). Therefore, RET-TKIs may cause invasive aspergillosis due to their off-target effect on JAK 1/2. In addition, the antifungal drugs voriconazole and fluconazole used during infections act as CYP3A inhibitors, resulting in increased exposure to RET-TKIs, which may be attributed to recurrent infection events.
However, there are several factors that may influence our conclusion. A previous study found that lower body mass index (BMI), underlying interstitial lung disease, and a history of lung cancer surgery or radiation pneumonia were identified as risk factors for pulmonary infections (25). Nevertheless, Meng’s research systematically reviewed existing publications on NSCLC treated with radiotherapy and TKIs, and it suggested an acceptable risk of severe treatment-related pneumonitis and rare mortality in patients with NSCLC (26). In brief, further prospective studies with a larger number of patients and a longer-term follow-up are warranted to support our conclusion that RET-TKIs may cause invasive aspergillosis.
Infection during antitumor therapy will not only prolong a patient’s hospitalization time but also increase hospitalization expenses, and even in severe cases, the infection quickly spreads to other organs and often leads to death. Additionally, it will lead to a dose reduction of antitumor drugs, delay of time, and even suspension of treatment, which will affect the antitumor efficacy and the patient’s survival time. Therefore, it is crucial to manage infection events during antitumor therapy. In this case, the patient’s symptoms improved after pausing pralsetinib and receiving a powerful anti-infective therapy. To avoid affecting the timing of antitumor treatment and in consideration of drug safety, the antitumor treatment regimens were replaced by another RET-TKI—selpercatinib—after the infection was absolutely controlled. However, selpercatinib caused a new large edema of the left lateral ventricle, and the patient’s general condition deteriorated since then, and he died in a few months. In conclusion, this case describes real-world experiences of using RET-TKIs in patients with RET fusion-positive NSCLC, underscoring the importance of adequate baseline assessment and ongoing monitoring of immune function, infection biomarkers, and chest CT scans to inform future clinical practice. If there is a fever or respiratory symptoms that cannot be explained by the primary disease, it is important to consider ADRs.
As mentioned above, RET fusion-positive NSCLC patients are prone to brain metastases. A single-center RET retrospective study from South Korea analyzed 59 treated RET fusion-positive NSCLC patients, and the results showed that a total of 17 patients (28.8%) had a brain lesion at the initial diagnosis, while 11 additional patients (18.6%) developed brain metastases during follow-up (27). Therefore, when RET fusion-positive NSCLC patients have single or multiple lesions on brain MRI, it is necessary to distinguish them from the primary tumor with brain metastases, which depends on medical history, clinical manifestations, and appropriate imaging examinations. CT is limited in the differential diagnosis of brain abscess and brain metastases, while MRI-enhanced scan is more sensitive than CT or MRI plain scan, and it is easier to detect more and smaller lesions at the early stage. In addition, diffusion-weighted imaging (DWI) and PET/CT provide valuable evidence for the differential diagnosis of benign and malignant diseases of the CNS, which can help identify lesion properties and avoid patients undergoing unnecessary invasive procedures such as puncture or craniotomy. Clinicians should improve their understanding of CNS infection when treating tumor patients and avoid misdiagnosing CNS infection lesions as the progression of tumor brain metastases.
ConclusionPralsetinib and selpercatinib have been on the market for a short time in China. Although they have achieved remarkable efficacy in the treatment of RET fusion-positive NSCLC, it is still necessary to pay attention to the ADRs. This article presents a rare case of invasive aspergillosis induced by RET-TKIs in the treatment of RET fusion-positive NSCLC, suggesting that clinicians should pay attention to identifying ADRs, opportunistic infections, and tumor progression throughout the antitumor targeted therapy so as not to delay antitumor treatment.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (Ethics number: XJTU1AF2024LSYY-111). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsKS: Visualization, Writing – original draft, Writing – review & editing. YL: Data curation, Investigation, Validation, Writing – review & editing. YM: Data curation, Investigation, Validation, Writing – review & editing. ZH: Investigation, Methodology, Visualization, Writing – review & editing. YJZ: Investigation, Methodology, Visualization, Writing – review & editing. YXZ: Investigation, Methodology, Visualization, Writing – review & editing. XL: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing. TT: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing. ZR: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing. YY: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. XF: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by The Science and Technology Plan Project of Shaanxi Province (No. 2020JM-384).
AcknowledgmentsWe thank the Biobank of the First Affiliated Hospital of Xi’an Jiaotong University for providing clinical data.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1431908/full#supplementary-material
References1. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:iv192–237. doi: 10.1093/annonc/mdy275
PubMed Abstract | Crossref Full Text | Google Scholar
2. Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, et al. RET fusions define a unique molecular and clinicopathologic subtype of non–small-cell lung cancer. J Clin Oncol. (2012) 30:4352–9. doi: 10.1200/JCO.2012.44.1477
PubMed Abstract | Crossref Full Text | Google Scholar
3. Addeo A, Miranda-Morales E, den Hollander P, Friedlaender A, Sintim HO, Wu J, et al. RET aberrant cancers and RET inhibitor therapies: Current state-of-the-art and future perspectives. Pharmacol Ther. (2023) 242:108344. doi: 10.1016/j.pharmthera.2023.108344
PubMed Abstract | Crossref Full Text | Google Scholar
4. Coulpier M, Anders J, Ibáñez CF. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase. J Biol Chem. (2002) 277:1991–9. doi: 10.1074/jbc.M107992200
PubMed Abstract | Crossref Full Text | Google Scholar
5. Servetto A, Esposito D, Ferrara R, Signorelli D, Belli S, Napolitano F, et al. RET rearrangements in non-small cell lung cancer: Evolving treatment landscape and future challenges. Biochim Biophys Acta (BBA) - Rev Cancer. (2022) 1877:188810. doi: 10.1016/j.bbcan.2022.188810
PubMed Abstract | Crossref Full Text | Google Scholar
6. Subbiah V, Yang D, Velcheti V, Drilon A, Meric-Bernstam F. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol. (2020) 38:1209–21. doi: 10.1200/JCO.19.02551
PubMed Abstract | Crossref Full Text | Google Scholar
7. Ballerini P, Struski S, Cresson C, Prade N, Toujani S, Deswarte C. RET fusion genes are associated with chronic myelomonocytic leukemia and enhance monocytic differentiation. Leukemia. (2012) 26:2384–9. doi: 10.1038/leu.2012.109
PubMed Abstract | Crossref Full Text | Google Scholar
8. Shen T, Pu X, Wang L, Yu Z, Li J, Zhang Y, et al. Association between RET fusions and efficacy of pemetrexed-based chemotherapy for patients with advanced NSCLC in China: A Multicenter Retrospective Study. Clin Lung Cancer. (2020) 21:e349–54. doi: 10.1016/j.cllc.2020.02.006
PubMed Abstract | Crossref Full Text | Google Scholar
9. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the immunotarget registry. Ann Oncol. (2019) 30:1321–8. doi: 10.1093/annonc/mdz167
PubMed Abstract | Crossref Full Text | Google Scholar
10. Drilon A, Hu ZI, Lai GY, Tan DSW. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. (2017) 15:151–67. doi: 10.1038/nrclinonc.2017.175
PubMed Abstract | Crossref Full Text | Google Scholar
11. Griesinger F, Curigliano G, Thomas M, Subbiah V, Baik CS, Tan DSW, et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. (2022) 33:1168–78. doi: 10.1016/j.annonc.2022.08.002
PubMed Abstract | Crossref Full Text | Google Scholar
12. Drilon A, Subbiah V, Gautschi O, Tomasini P, de Braud F, Solomon BJ. Selpercatinib in patients with RET fusion–positive non–small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol. (2023) 41:385–94. doi: 10.1200/JCO.22.00393
PubMed Abstract | Crossref Full Text | Google Scholar
14. Gao M, Zhang X, Yan H, Sun D, Yang X, Yuan F, et al. Pralsetinib-associated pneumonia in RET fusion-positive non-small cell lung cancer. Supportive Care Cancer. (2023) 31:671. doi: 10.1007/s00520-023-08125-3
PubMed Abstract | Crossref Full Text | Google Scholar
15. Dhital R, Paudel A, Bohra N, Shin AK. Herbaspirillum infection in humans: A case report and review of literature. Case Rep Infect Dis. (2020) 2020:1–6. doi: 10.1155/2020/9545243
PubMed Abstract | Crossref Full Text | Google Scholar
16. Lee Y-P, Jeong BH, Eun Y, Kang CI, Park S, Jung HA, et al. Extrapulmonary tuberculosis in patients with RET fusion-positive non-small cell lung cancer treated with pralsetinib: A Korean single-centre compassionate use experience. Eur J Cancer. (2021) 159:167–73. doi: 10.1016/j.ejca.2021.09.037
PubMed Abstract | Crossref Full Text | Google Scholar
17. An L, Pengzhi C, Wang J, Xuebing Q, Tingting L, Yanhong G, et al. Case report: Recurrent lung infections following treatment with pralsetinib for an elderly patient with RET-fusion positive NSCLC. Front Oncol. (2022) 12:1024365. doi: 10.3389/fonc.2022.1024365
PubMed Abstract | Crossref Full Text | Google Scholar
18. Fan B, Wang X, Ning Z. A case of tuberculosis infection after pralsetinib treatment for medullary thyroid carcinoma. Natl Med J China. (2023) 103:2624–6. doi: 10.3760/cma.j.cn112137-20230119-00111
Crossref Full Text | Google Scholar
19. Bolourchi S, Julka K. A case of endobronchial tuberculosis in a patient on pralsetinib therapy. Chest. (2023) 164:A4542. doi: 10.1016/j.chest.2023.07.2945
Crossref Full Text | Google Scholar
20. Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discovery. (2018) 8:836–49. doi: 10.1158/2159-8290.CD-18-0338
PubMed Abstract | Crossref Full Text | Google Scholar
21. Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou X, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. (2020) 80:106210. doi: 10.1016/j.intimp.2020.106210
PubMed Abstract | Crossref Full Text | Google Scholar
22. Adas MA, Alveyna E, Cook E, Dey M, Galloway JB, Bechman K. The infection risks of JAK inhibition. Expert Rev Clin Immunol. (2021) 18:253–61. doi: 10.1080/1744666X.2022.2014323
PubMed Abstract | Crossref Full Text | Google Scholar
23. Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis. (2018) 66:140–8. doi: 10.1093/cid/cix687
PubMed Abstract | Crossref Full Text | Google Scholar
25. Choi Y, Noh JM, Shin SH, Lee K, Um SW, Kim H, et al. The incidence and risk factors of chronic pulmonary infection after radiotherapy in patients with lung cancer. Cancer Res Treat. (2023) 55:804–13. doi: 10.4143/crt.2022.1305
PubMed Abstract | Crossref Full Text | Google Scholar
26. Meng Y, Sun H, Wang S, Yang H, Kong FM. Treatment-related pneumonitis of EGFR tyrosine kinase inhibitors plus thoracic radiation therapy in patients with non-small cell lung cancer: A systematic review and meta-analysis. Int J Radiat oncology biology Phys. (2024) 118:415–26. doi: 10.1016/j.ijrobp.2023.09.009
PubMed Abstract | Crossref Full Text | Google Scholar
27. Lee J, Ku BM, Shim JH, Choi YL, Sun J-H, Lee S-H, et al. Characteristics and outcomes of RET-rearranged Korean non-small cell lung cancer patients in real-world practice. Japanese J Clin Oncol. (2020) 50:594–601. doi: 10.1093/jjco/hyaa019
留言 (0)