Since the beginning of December 2019, the COVID-19 pandemic, caused by a novel virus known as SARS-CoV-2 that originated in Wuhan, China, has presented a major challenge to global health and healthcare systems. Although COVID-19 is predominantly associated with lung-related symptoms and distinct functional and morphological changes, it has become evident that the infection can also lead to a multi-systemic disease affecting various organs including heart (1–3).
There is growing evidence of COVID-19's harmful effects on the heart, including acute events like heart attacks and long-term consequences even after recovery. The precise mechanisms underlying the cardiac damage caused by COVID-19 remain incompletely understood. COVID-19 has been associated with various patterns of cardiovascular dysfunction, including myocarditis, ischemic heart disease (e.g., myocardial infarction), hypovolemia, RV dysfunction resulting from pulmonary embolism, and, in some cases, cardiovascular dysfunction due to superimposed bacterial or fungal sepsis (4). The pathological findings suggest that SARS-CoV-2 can trigger hyper myocardial inflammation by infecting cardiomyocytes, leading to myocyte necrosis. This, in turn, may contribute to an increased risk of acute myocardial infarction, heart failure, arrhythmia, cardiac arrest, and acute coronary syndrome. Furthermore, apart from the potential harm caused by the illness itself, certain medications used in the treatment of COVID-19 and drug interactions may also have specific side effects on the heart (5).
Despite the advancements in treatments for COVID-19, it is anticipated that long-term consequences of the disease, particularly those affecting the heart, will persist in survivors. Therefore, the investigation of myocardial dysfunction following recovery from COVID-19 plays a vital role in the development of post-discharge monitoring programs and the formulation of public health, economic, and social policies (6). Cardiac imaging studies can serve as valuable predictive tools and aid in the comprehension of the underlying mechanisms of cardiac involvement. Echocardiography has been recognized as an available, non-invasive, and informative diagnostic tool, to identify cardiac manifestation (7). Echocardiographic findings in individuals with COVID-19 may exhibit variability, ranging from specific regional wall motion abnormalities of the LV or RV to varying degrees of global cardiac dysfunction associated with myocarditis or a systemic dysregulated inflammatory response to viral infection. Echocardiography is therefore essential in distinguishing these patterns, guiding treatment strategies, and monitoring the clinical progression over time (8).
Existing research has extensively focused on the impact of acute COVID-19 on cardiac function and complications, with numerous reviews and studies providing valuable insights into this area (9). However, there is a lack of information in the research on the lasting impacts of COVID-19, known as long covid, on heart function. Previous reviews have tried to examine this connection, but they have been restricted in their coverage and have not carried out thorough meta-analyses. Furthermore, these reviews have not taken into consideration possible influencing factors like the severity of the initial COVID-19 infection, the time elapsed since the infection, and the presence of other medical conditions. Hence, it is essential to conduct more in-depth and thorough research that specifically looks into the lasting effects of COVID-19 on the heart, considering important factors. To tackle this issue, we carried out a systematic review and meta-analysis that focused on echocardiographic imaging to study the long-term impact of SARS-CoV-2 infection on heart function and the risk of future cardiac complications.
2 Materials and methodsThis systematic review and meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (10). The PRISMA checklist is provided as supplement (Supplementary S1 document). The protocol for this work was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (identifier: CRD42024481337). This review also followed the published protocol for evaluating risk factors and prognostic implications of imaging left ventricular diastolic dysfunction in individuals diagnosed with COVID-19, adhering to a systematic approach (8).
2.1 Eligibility criteriaTo be considered for inclusion, published studies had to meet the following criteria: (1) studies employed valid research designs with clearly defined methodology, (2) studies assessed cardiac function using Echocardiography in COVID-19 patient after recovery, (3) studies identified COVID-19 infection according to the World Health Organization interim guidance, (4) studies reported at least one echocardiographic parameter measuring myocardial function and/or structure, (5) studies excluded cases with pre-existing cardiac disease including ischemic heart disease, valvular disease, arrhythmias-conduction disorders, heart failure, cardiomyopathies, pericarditis, pericardial effusion, pulmonary hypertension, pulmonary embolism, sarcoidosis, amyloidosis, active cancer, recent pregnancy, postpartum. The overall exclusion criteria were as follows: (1) studies involved cases during acute stage of COVID-19, (2) studies evaluated cardiac function using any other imaging technique other than echocardiography, (3) studies reported as abstracts, case reports, case series, reviews, or practice guidelines.
2.2 Information sourcesA thorough search was conducted in the PubMed, Scopus, Web of Science, and Cochrane databases to locate relevant studies published until March 2024. Additionally, a manual search of the reference lists of the identified articles was carried out.
2.3 Search strategyThe search strategy of Scopus was conducted as follows: (TITLE-ABS-KEY (((“left ventric*” OR “right ventric*” OR “left cardiac*” OR “right heart” OR “right cardiac” OR “left heart” OR atri* OR myocardi* OR diastol* OR systol*) PRE/1 (dysfunction OR function OR remodeling OR impair* OR hypertroph* OR active* OR volume OR mass* OR dimension* OR diameter OR thickness OR index* OR “ejection time” OR “ejection fraction”)) OR echo OR echocardiograph*)) AND [TITLE-ABS-KEY (“covid-19” OR “sars cov 2”)]. The search strategy employed for PubMed, Web of Science, and the Cochrane Library was similar to that used for Scopus and its table is provided as supplement (Supplementary S2 document). Furthermore, three reviewers independently reviewed the reference lists of systematic reviews and selected studies to ensure that all pertinent articles were included in the analysis.
2.4 Study selectionThree reviewers independently assessed each title and abstract, and if the articles fulfilled the inclusion criteria, the full text was reviewed. The eligibility of the selected articles was then assessed by the same three reviewers through an evaluation of their full texts. Any discrepancies were resolved through discussion with a fourth reviewer. The study selection process was summarized using the PRISMA flow diagram.
2.5 Data extraction and data itemsFollowing the extraction of data, the information was gathered through Microsoft Excel spreadsheets. The subsequent dataset comprises: studies' basic characteristics (study design, year of publication, country, and first author), participant characteristics (age, body mass index, number of cases and control groups), echocardiographic indices and major findings of each study. Potential confounding factors were carefully considered to ensure the robustness of the study findings. These factors included severity of COVID-19 infection, persistent post-COVID symptoms, duration from COVID acute phase to echocardiography examination in recovery phase, presence of comorbid disease. Data related to these factors were extracted from the studies to address their potential influence on the findings.
2.6 Risk of bias assessmentROBINS-I was employed to evaluate the methodological quality and risk of bias for non-randomized control trials. This tool encompasses the assessment of seven potential sources of bias, including confounding bias, bias in participant selection, bias in intervention classification, bias due to deviations from intended interventions, bias resulting from missing data, bias in outcome measurement, and bias in the selection of reported results (11). Importantly, no studies were excluded based on the assessment of bias risk.
2.7 Outcome quality assessmentThe certainty of overall evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method (12). The assessment of evidence certainty for individual outcomes relied on five distinct criteria: (1) limitations of the study design; (2) consistency of results; (3) directness; (4) precision and (5) potential for publication bias. A decrement of one level in certainty was implemented for each unfulfilled criterion.
2.8 Synthesis methodsThe mean differences (MD) pooled the data, with 95% confidence intervals (CIs). The I2 statistic was used to analyze the interstudy statistical heterogeneity. To calculate the pooled effect, either fixed-effects or random-effects model was used according to the heterogeneity, study design and sample size. I2 values of 25%, 50%, and 75% were considered to represent low, moderate, and high levels of heterogeneity, respectively. Subgroup meta-analyses were conducted to uncover the underlying heterogeneity. A univariate meta-regression model was used to explore the impact of age and BMI as potential moderators. A sensitivity analysis was carried out in cases where the decision-making values had arbitrary or unclear ranges. Publication bias was assessed by visually inspecting funnel plots of MD vs. standard error. When at least 10 studies were available for analysis, Begg's tests and Egger's tests were employed to evaluate the potential publication bias. If there was an obvious publication bias, a trim-and-fill analysis was used to determine the underlying origin of the publication bias. All analyses were conducted using Comprehensive Meta-Analysis Version 3. P-value < 0.05 was considered significant in all tests.
3 Results 3.1 Study selectionThe study flowchart is shown in Figure 1; our search strategy revealed 2,602 studies in PubMed, 6,502 in Scopus, 2,994 in WOS and 42 in Cochrane. After removing duplications, 5,942 studies underwent title assessment. Of these, 2,321 studies were eligible for abstract review. After surveying abstracts, 107 studies were perused for full text. Finally, 66 studies were qualified to be included in this systematic review and meta-analysis, and the rest did not meet the inclusion criteria; the reasons for their exclusions are provided in the supporting information section (Supplementary S3 document).
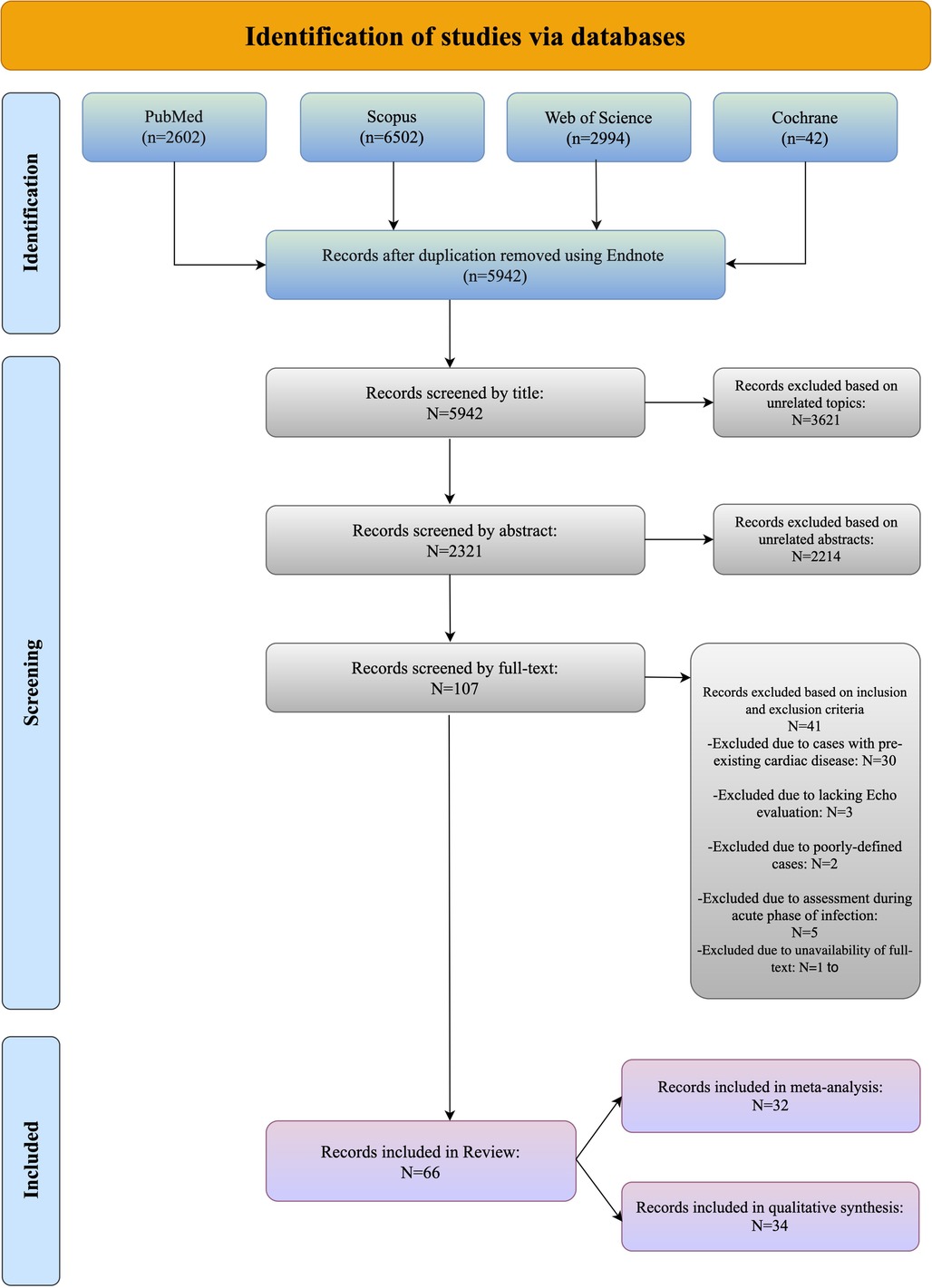
Figure 1. Identification of studies via databases.
3.2 Characteristics of studiesTable 1 presents the key features of the sixty-six studies (13–76) included in this research. The search process resulted in the identification of 66 studies, out of which 41 were designed as cohort studies, 16 were cross-sectional studies, 8 were case-control studies, and one study (20) was a combined cross-sectional and longitudinal cohort. The majority of these studies utilized real-time PCR (rt-PCR) as the diagnostic method for COVID-19, while a few employed the IgG antibody titer for diagnosis (32, 37). Most of the studies focused on adult patients who had recovered from COVID-19, whereas 6 studies specifically examined athletes who had overcome the disease (13, 17, 19, 29, 52, 75). A total of 32 studies were conducted comparing post-COVID patients to a control group of individuals who tested negative for COVID-19. All 32 studies were included in a meta-analysis, with the exception of one study (39) where patients and controls were not matched, and two studies (42, 43) where matching status was unknown. Four studies (16, 27, 28, 40) categorized COVID-19 cases into groups based on the severity of the infection. To maintain consistency with the other included studies, we treated these studies as separate entities, each having a common healthy control group. Furthermore, two studies (30, 43) assessed COVID-19 cases based on the presence of dyspnea symptoms in patients. These studies were also divided into two distinct studies. Three studies (16, 24, 37) divided COVID-19 cases based on the time duration between diagnosis and echocardiography. Similarly, one study (17) divided COVID-19 cases into male and female athletes. Each of these studies was separated into two distinct studies as well.

Table 1. Characteristics of included studies.
The severity of COVID-19 infection was not addressed in 12 studies (19, 29, 30, 32, 37, 45, 46, 48, 60, 66, 72, 77). Regarding the COVID-19 vaccination, only six studies (24, 29, 30, 46, 52, 64) provided information on the vaccination status of patients. It is noteworthy that data collection in most of the studies was conducted before the availability of any vaccines. Thirteen studies lacked information on post-COVID symptoms at the time of study enrollment (16, 20, 39, 40, 50, 51, 55, 62, 65, 66, 74, 77, 78). In most studies, the time interval between the acute phase of COVID-19 and echocardiography during the recovery phase was over 1 month, except for 3 studies (13, 16, 29) that were conducted within at least 10 days. Thirty studies reported the exclusion of patients with comorbid disease. On the other hand, three studies did not provide any information regarding the comorbid diseases (49, 66, 78). The primary focus of the studies pertained to the evaluation of LV function, with a secondary emphasis on RV function. A subset of studies also conducted concurrent assessments of both LV and RV function. Majority of the studies found significant changes in echocardiographic parameters, indicating subclinical alterations in the function of the LV and/or RV in post-COVID patients. However, 17 studies (17, 18, 27, 29, 30, 37, 41, 44, 46, 47, 49, 64, 68, 69, 73, 74, 78) did not report any significant findings.
3.3 Studies' risk of biasFigure 2 depicts a summary of the RoB-1 assessment. The overall risk of bias was found to be low to moderate. A low percentage (<15%) of serious risk of bias was identified in various domains, including confounding, selection of participants, classification of interventions, deviations from intended interventions, and missing data bias. Moderate risk of bias (25%–50%) was noted in confounding and deviations from intended interventions. There was no significant bias detected in the selection of reported results. Among 32 studies enrolled in the meta-analysis, five were found to have a serious risk of confounding bias (19, 30, 32, 37, 45). These studies did not provide information on the severity of COVID-19 infection in the patients. Additionally, 12 studies were rated as having a moderate risk of bias due to the presence of comorbid diseases that could impact heart function (15, 16, 20–22, 24, 27, 28, 35, 38, 40, 41). Three studies found a serious risk of bias in participant selection due to an unmatched case-control group (39, 42, 43), while five studies indicated a moderate risk due to the inclusion of specific populations such as athletes, women, and young adults that may not accurately represent the general population (13, 17, 19, 31, 36). Concerning bias due to classification of interventions, one study (21) found a serious risk of bias in comparing echo findings between two groups with reduced and normal-LVGLS, while two (24, 43) were deemed to have moderate risk due to incorrectly classifying post-COVID patients and comparing echo measures between them instead of with controls. Three studies demonstrated a moderate risk of bias due to deviations from intended interventions (30, 34, 39). Their main focus was on evaluating cardiopulmonary function rather than cardiac alone. Seven studies were found to have a moderate risk of bias due to missing data, and they reported small amounts of echocardiographic indices (15, 25, 30, 34, 37, 39, 43). Regarding bias in outcome measurement, 10 studies (14, 19, 22, 24, 26, 27, 33, 36, 42, 45) found a moderate risk of bias in reporting certain echo indices that deviated from the ranges reported in other studies (detailed in Table 2). Thirty-four studies only had post-COVID cases. Regarding the confounding factors, one study had a serious risk of bias as it did not provide information about the comorbid diseases of the patients (66). Twenty studies had moderate risks due to the presence of comorbid diseases (29, 51, 57, 59, 61–63, 67–69, 71, 72, 74, 76, 77) and lack of information on the severity of COVID-19 infection (29, 46, 48, 49, 72, 77). Considering the risk of bias in participant selection, 6 studies had moderate risks for reasons of inclusion of specific populations such as athletes (29, 52, 75) and having no classification and comparison among patients (49, 55, 68). Regarding bias due to deviations from intended interventions, one study had a serious risk of bias as its main focus was on hepatic abnormalities rather than cardiac alone (60). Twenty-one studies revealed moderate risk due to laboratory and biomarker evaluations, electrocardiogram evaluations, various surveys and lifestyle changes, return to play evaluation of athletes, chest computer tomography, post-COVID-19 functional status scale, cardio-ankle vascular index, ankle-brachial index, myocardial work analysis, walk test, pulmonary function tests, and cardiopulmonary exercise tests (48, 49, 51–59, 65, 67–71, 74–77). Bias due to missing data was serious in 3 studies as they reported small amounts of echocardiographic indices (48, 62, 74). Figure 3 represents the traffic light plot of risk of bias assessment for each included study.
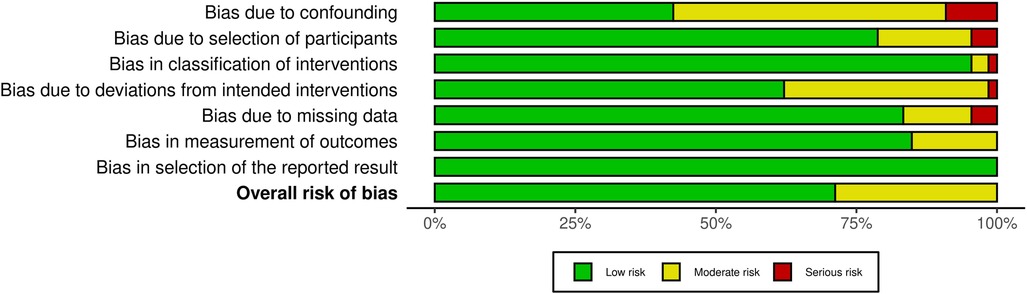
Figure 2. Overall risk of bias.
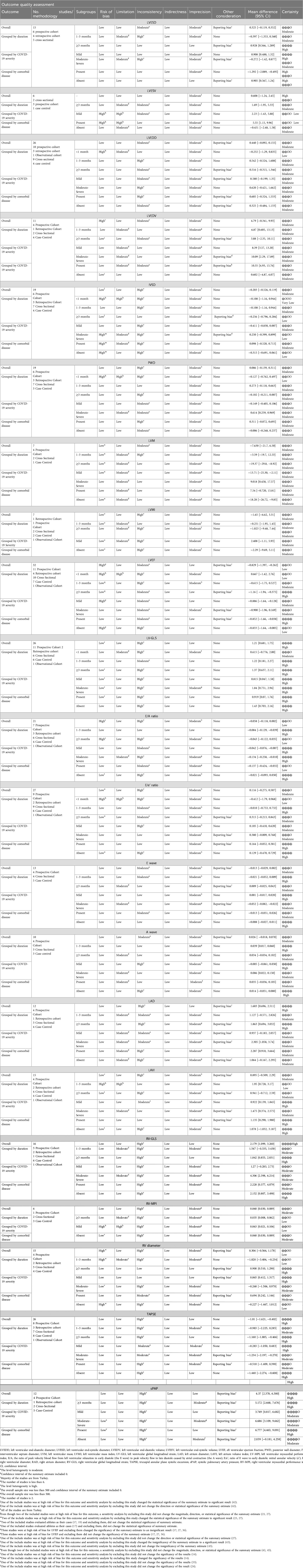
Table 2. GRADE approach.
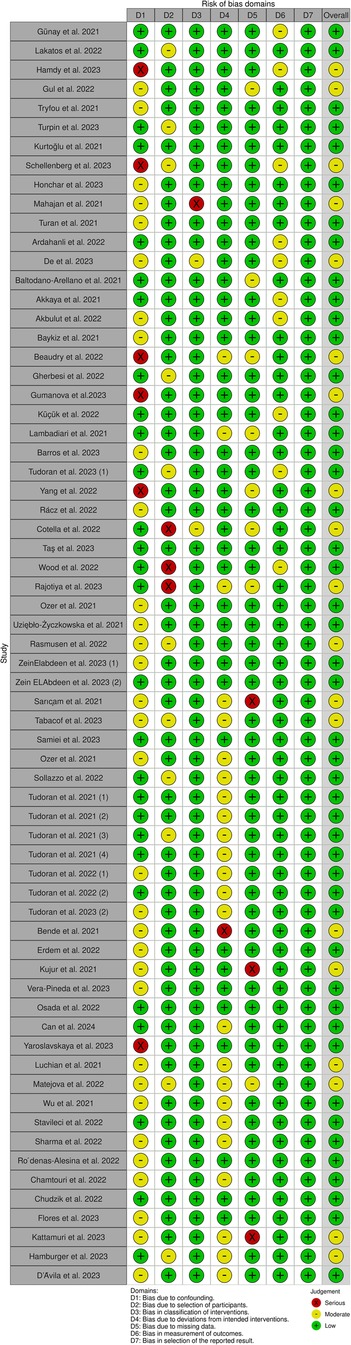
Figure 3. Risk-of-bias assessment (traffic light plot).
3.4 Outcome quality assessmentThe certainty of evidence for outcomes, as assessed by GRADE framework, is delineated in Table 2. The meta-analysis indicates a moderate level of certainty in the majority of outcomes, primarily attributable to the inherent susceptibility to bias in observational studies. Outcomes with low certainty are typically caused by a small number of studies, significant heterogeneity, and the existence of potential biases.
3.5 Result of synthesis 3.5.1 Overall outcomesAmong the echocardiographic measures of LV systolic function, LV-GLS and LVEF were found to be significantly different between the two groups being compared. The analysis of 26 studies showed a notable decrease in LV-GLS (less negative) in post-COVID patients (n = 1,810) compared to controls (n = 1,254), with a mean difference of 1.21 [95%CI (0.681, 1.75), p = 0.000, I2 = 91%]. Post-COVID patients (n = 2,173) exhibited a lower LVEF compared to controls (n = 1,770), with a MD of −0.829 [95%CI (−1.397, −0.262), p = 0.004, I2 = 73%]. Additionally, the meta-analysis of 12 studies revealed that LAD was significantly increased in post-COVID patients (n = 833) comparing to controls (n = 892) with a MD of 1.603 [95%CI (0.696, 2.511), p = 0.001, I2 = 80.7%]. However, LAVI was not significantly different comparing two groups with a MD of 0.895 [95% CI (−0.509, 2.29), p = 0.211, I2 = 82.7%]. In terms of RV evaluation, post-COVID patients showed significantly lower RV-GLS (less negative) and higher RV-MPI values compared to controls, with mean differences of 2.179 [95%CI (1.099, 3.260), p = 0.000, I2 = 85.4%] and 0.060 [95% CI (0.030, 0.089), p = 0.009, I2 = 99%], respectively. No significant differences were found in the diastolic and geometric indices of the left ventricle between the two groups being compared (Table 3). Forest plots are provided in supporting information (Supplementary S4 document).
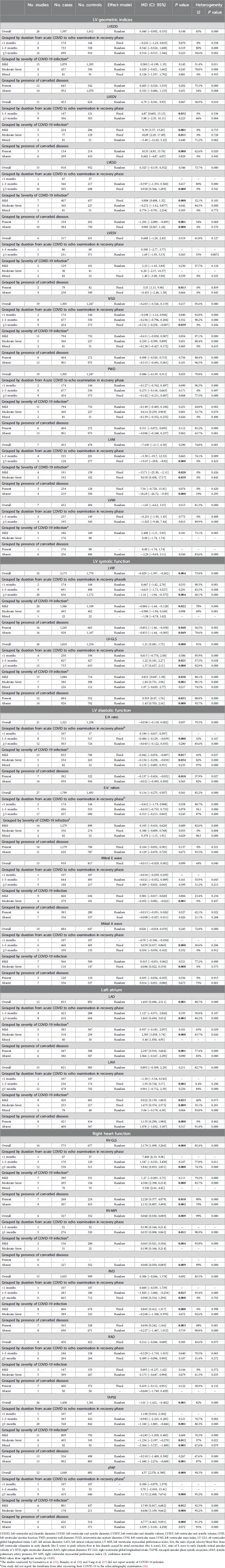
Table 3. Result of synthesis.
3.5.2 Subgroup analysis 3.5.2.1 Grouped by duration from acute COVID to echo examination in recovery phaseThe subgroup meta-analysis found that among LV geometric indices, LVESD was notably higher in post-COVID patients (n = 505) compared to controls (n = 698) for a duration of ≥3 months, showing a MD of 0.928 [95% CI (0.566, 1.289), p = 0.000, I2 = 0%]. Moreover, post-COVID patients exhibited a significant decrease in IVSD and LVM compared to controls for a duration of ≥3 months, with a MD of −0.132 [95% CI (−0.258, −0.007), p = 0.039, I2 = 0%] and −19.37 [95%CI (−29.8, −8.92), p = 0.000, I2 = 0%], respectively.
In terms of systolic function lasting ≥3 months, LVEF was found to be significantly lower in post-COVID patients (n = 836) compared to controls (n = 1,172), with a MD of −1.16 [95% CI (−1.94, −0.375), p = 0.004, I2 = 60.1%]. Furthermore, post-COVID patients exhibited a significantly decreased LV-GLS (less negative) compared to controls for durations of both 1–3 months and ≥3 months, with MDs of 1.22 [95% CI (0.181, 2.27), p = 0.021, I2 = 57.5%] and 1.37 [95% CI (0.637, 2.11), p = 0.000, I2 = 92.9%], respectively.
There were significant differences in the E/A ratio and mitral A wave among diastolic function indices. Within a period of ≥3 months, post-COVID patients exhibited a significant reduction in the E/A ratio and an increase in the mitral A wave compared to the control group. The MDs were −0.084 [95%CI (−0.129, −0.039), p = 0.000, I2 = 32%] for the E/A ratio and 0.039 [95% CI (0.017, 0.060), p = 0.000, I2 = 30.6%] for the mitral A wave. Additionally, post-COVID patients (n = 410) exhibited a significant elevation in LAD in comparison to the control subjects (n = 604), over a duration of ≥3 months, with a MD of 1.863 [95% CI (0.694, 3.032), p = 0.002, I2 = 86.2%]. However, a meta-analysis of 2 studies showed a significant increase in LAVI in post-COVID patients (n = 236) compared to controls (n = 174) within a timeframe of 1–3 months, with a MD of 1.95 [95% CI (0.728, 3.17), p = 0.002, I2 = 8.4%].
In subgroup meta-analysis of RV function, RV-MPI, RVD and sPAP were significantly higher in post-COVID patients compared to control group for a duration of ≥3 months, with MDs of 0.035 [95% CI (0.008, 0.062), p = 0.012, I2 = 98.9], 0.900 [95% CI (0.510, 1.290), p = 0.000, I2 = 0%] and 5.172 [95%CI (2.668, 7.676), p = 0.000, I2 = 95.2%], respectively. Moreover, a significant decrease in TAPSE and RV-GLS (less negative) were observed in post-COVID patients compared to controls with a MD of −1.160 [95% CI (−1.885, −0.466), p = 0.001, I2 = 80.3%] and 1.842 [95%CI (0.853, 2.831), p = 0.000, I2 = 74.3%], respectively. Detailed information is provided in Table 3.
3.5.2.2 Grouped by severity of COVID-19 infection 3.5.2.2.1 Mild COVID-19 infectionIn terms of mild COVID-19 infection and LV geometric indices, significant increase was observed in LVEDV and LVESD in post-COVID patients compared to controls with MDs of 8.39 [95% CI (3.57, 13.20), p = 0.001, I2 = 0%], and 0.908 [95% CI (0.488, 1.32), p = 0.000, I2 = 32.1%], respectively. LVM was significantly lower in post-COVID patients (n = 191) compared to controls (n = 139), with a MD of −13.71 [95 CI% (−25.30, −2.11), p = 0.020, I2 = 0%]. Moreover, significant changes in systolic function were observed in mild infection cases. Post-COVID patients reveled to have a decrease in LVEF and LV-GLS (less negative) compared to control groups, with MDs of −0.886 [95% CI (−1.64, −0.128), p = 0.022, I2 = 78%] and 0.815 [95% CI (0.047, 1.58), 0.038, I2 = 88.1%], respectively. Among LV diastolic indices, E/A ratio was significantly lower and LAVI was significantly increased in mild infection compared to controls, with MDs of −0.042 [95% CI (−0.076, −0.007), p = 0.017, I2 = 45%] and 0.922 [95% CI (0.139, 1.845), p = 0.023, I2 = 46%], respectively. RVD and sPAP were significantly higher in post-COVID patients compared to controls with MDs of 0.865 [95% CI (0.412, 1.317), p = 0.000, I2 = 0%] and 3.749 [95% CI (0.817, 6.682), p = 0.012, I2 = 0.012, I2 = 92.3%], respectively. Detailed information is provided in Table 3.
3.5.2.2.2 Moderate and/or severe COVID-19 infectionPost-COVID patients exhibited higher values of LVEDV, PWD, and LVM compared to the control group. The MDs for LVEDV, PWD, and LVM were 10.09 [95% CI (2.29, 17.89), p = 0.011, I2 = 0%], 0.614 [95% CI (0.259, 0.969), p = 0.001, I2 = 52.7%], and 9.018 [95% CI (0.458, 17.57), p = 0.039, I2 = 0%], respectively. Concerning systolic function, there was no significant difference in LVEF between the two groups, as indicated by a MD of −0.900 [95% CI (−1.96, 0.169), p = 0.098, I2 = 69%]. Conversely, LV-GLS exhibited significantly lower (less negative) values in post-COVID patients in comparison to the control group, with a MD of 1.84 [95% CI (0.751, 2.94), I2 = 90.1%]. Among LV diastolic indices, E/A ratio and mitral E wave values were significantly decreased and mitral A wave was significantly increased in post-COVID patients compared to controls. The MDs for E/A, E wave and A wave were −0.134 [95% CI (−0.258, −0.010), p = 0.034, I2 = 82%], −0.052 [95% CI (−0.082, −0.022), p = 0.001, I2 = 0%] and 0.086 [95%CI (0.022, 0.150), p = 0.008, I2 = 0%], respectively.
Additionally, significantly higher values were found in both LAD and LAVI in post-COVID patients compared to controls. The MD for LAD was 2.305 [95% CI (1.058, 3.74), p = 0.000, I2 = 63.7%], and for LAVI it was 1.475 [95% CI (0.374, 2.575), p = 0.009, I2 = 32.1%].
Regarding RV indices, post-COVID patients showed significantly increased value in sPAP with MDs of 4.306 [95% CI (2.398, 6.214), p = 0.000, I2 = 83.7%]. Moreover, TAPSE and RV-GLS values were significantly lower in post-COVID patients compared to controls with MDs of −1.234 [95% CI (−2.197, −0.270), p = 0.012, I2 = 57%] and 6.686 [95% CI (3.109, 9.662), p = 0.000, I2 = 95.2%], respectively. Detailed information is provided in Table 3.
3.5.2.3 Grouped by presence of comorbid diseasesPost-COVID patients with comorbidities showed higher values of LVEDV and LVESV compared to comorbid-matched control group with MDs of 10.35 [95% CI (4.93, 15.76), p = 0.000, I2 = 62.6%] and 5.55 [95% CI (1.15, 9.96), p = 0.013, I2 = 0%], respectively. There was a significant decrease in LVESD in post-COVID patients with comorbidities and an increase in cases without comorbidities compared to their comorbid-matched controls with MDs of −1.292 95% CI [−2.089, −0.495], p = 0.001, I2 = 43.2%) and 0.905 [95% CI (0.567, 1.24), p = 0.000, I2 = 0%], respectively. LVEF exhibited a significant decrease in post-COVID patients with comorbidities and those without comorbidities when compared to their comorbid-matched controls. The MDs were −0.852 [95% CI (−1.66, −0.038), p = 0.040, I2 = 56.5%] and −0.833 [95%CI (−1.64, −0.005), p = 0.049, I2 = 79.6%], respectively. Furthermore, LV-GLS was significantly decreased (less negative) in both groups of post-COVID patients, with MDs of 0.919 [95% CI (0.07, 1.76), p = 0.033, I2 = 88.8%] and 1.43 [95% CI (0.703, 2.16), p = 0.000, I2 = 93.7%] compared to their respective controls. E/A ratio was significantly lower in post-COVID patients with comorbidities compare to its comorbid-matched controls, with a MD of −0.137 [95% CI (−0.424, −0.032), p = 0.010, I2 = 57.9%]. Significantly higher values of both LAD and LAVI were observed in post-COVID patients with comorbidities compared to their matched controls. The MDs were 2.287 [95% CI (0.910, 3.664), p = 0.001, I2 = 77.4%] and 1.135 [95% CI (0.290, 1.980), p = 0.008, I2 = 0%], respectively.
Regarding RV function, RV-GLS was notably decreased (less negative) in both post-COVID patients with and without comorbidities compared to their controls, with MDs of 2.228 [95% CI (0.377, 4.079), p = 0.018, I2 = 90%] and 2.152 [95% CI (0.807, 3.498), p = 0.002, I2 = 79%]. Additionally, post-COVID patients without comorbidities presented higher values of RV-MPI with a MD of 0.060 [95% CI (0.030, 0.089), p = 0.009, I2 = 99%], compared to matched-controls. In post-COVID patients without comorbidities, TAPSE values were significantly lower, whereas no significant difference was found in cases with comorbidities when compared to their matched controls. The MDs were −1.440 [95%CI (−2.296, −0.585), p = 0.001, I2 = 87.4%] and −0.337 [95% CI (−1.213, 0.540), p = 0.452, I2 = 76%], respectively. Moreover, sPAP presented higher values in post-COVID patients with comorbidities and no significant result in cases without comorbidities compared to their matched controls with MDs of 6.777 [95% CI (4.463, 9.091), p = 0.000, I2 = 91.2%] and 2.039 [95%CI (−0.181, 4.258), p = 0.072, I2 = 91%], respectively. Detailed information is provided in Table 3. Forest plots are provided in supporting information (Supplementary S4 document).
Table 4 represent the summary of quantitative synthesis.
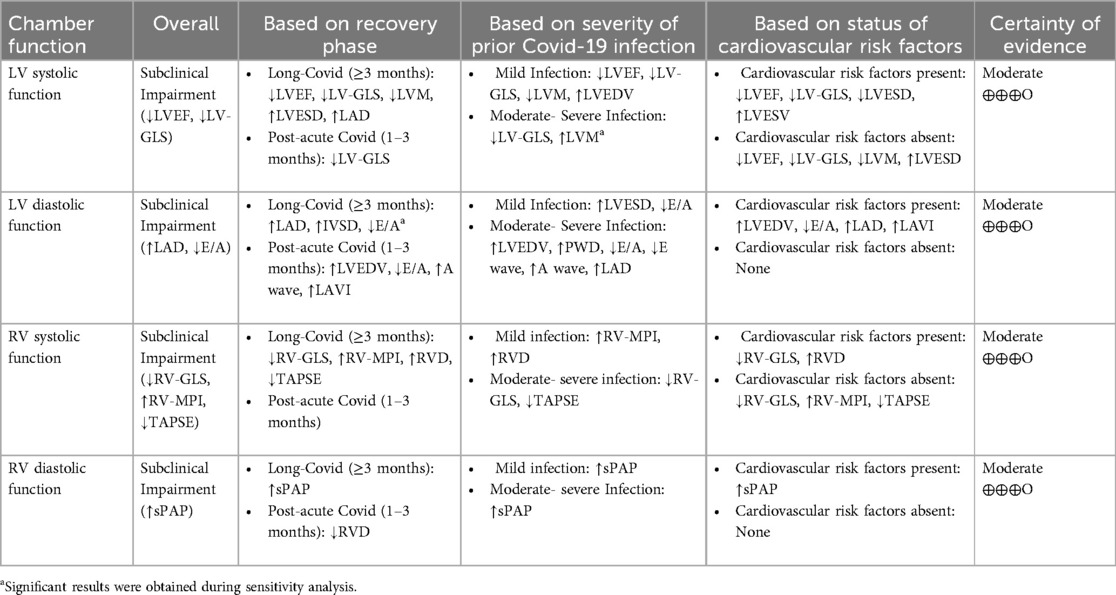
Table 4. Summary of quantitative synthesis.
3.6 Sensitivity analysis 3.6.1 LVEDVThe study by Wood et al. (42), showed a high risk of bias for LVEDV in overall result of synthesis. Excluding this study revealed a significant difference between two groups of comparison with a MD of 4.732 [95% CI (1.367, 8.096), p = 0.006, I2 = 46.3%]. However, no significant difference was observed between two groups when grouped by duration ≥3 months and absence of comorbidities with MDs of 5.727 [95% CI (−0.209, 11.66), p = 0.059, I2 = 59.7%] and 1.964 [95% CI (−3.076, 7.00), 0.445, I2 = 57%], respectively.
3.6.2 IVSDThe studies by Ardahanli et al. (23) and Akbulut et al. (27) were found to have a high risk of bias for IVSD for in overall result of synthesis and duration of ≥3 months. Excluding these studies did not change the direction, or statistical significance of the summary estimate with MDs 0.011 [95% CI (−0.147, 0.170), p = 0.891, I2 = 77%] and 0.135 [95%CI (−0.124, 0.394), p = 0.307, I2 = 88.5%], respectively. However, excluding these studies revealed significant difference between two groups of comparison in moderate-severe COVID-19 infection, presence and absence of comorbid disease with MDs of 0.539 [95%CI (0.281, 0.798), p = 0.000, I2 = 77%] and 0.320 [95%CI (0.019, 0.620), p = 0.037, I2 = 88.5%], −0.083 [95%CI (−0.143, −0.023), p = 0.007, I2 = 36%], respectively.
3.6.3 LVMTwo studies (17, 19) were at high risk of bias for LVM due to involving athletes as their cases. A sensitivity analysis by excluding them did not change the direction, or statistical significance of the summary estimate with effect size of −5.78 [95%CI (−27.2, 15.3), p = 0.597, I2 = 83%].
3.6.4 LVMIThe study by Turpin et al. (17) was deemed to have a high risk of bias for LVMI due to the inclusion of athletes as study participants. However, excluding this study did not change the direction, or statistical significance of the summary estimate with an effect size of −0.722 [95%CI (−6.575, 5.123), p = 0.809, I2 = 86.9%]. Furthermore, subgroup analyses focusing on mild COVID-19 infection and the absence of comorbid diseases also showed no change in the significance of the results when excluding this study. The effect sizes for mild COVID-19 infection and absence of comorbid diseases were 2.07 [95%CI (−7.21, 11.36), p = 0.622, I2 = 80.2%] and −1.06 [95%CI (−10.82, 8.70), p = 0.831, I 2 = 89%], respectively.
3.6.5 LVEFThree studies, conducted by Turpin et al. (17), Tudoran et al. (36) and Akbulut et al. (27), were deemed to have a high risk of bias in relation to LVEF. In a sensitivity analysis focusing on overall, mild COVID-19 and cases without comorbidities, the exclusion of these studies resulted in a change in the significance of the summary estimate. The effect size was found to be −0.499 [95% CI (−0.935, 0.037), p = 0.070, I2 = 63%] for overall cases, −0.229 [95% CI (−0.842, 0.383), p = 0.463, I2 = 62%] for mild cases, and −0.036 [95% CI (−0.686, 0.613), p = 0.913, I2 = 56.5%] for cases with absent comorbidities. However, excluding these studies did not change the direction, or statistical significance of the summary estimate for meta-analysis of duration ≥3 months with a MD of −0.693 [95% CI (−1.298, −0.087), p = 0.025, I2 = 47%].
3.6.6 LV-GLSAkkabulut et al. (27) was found to have a high risk of bias in the meta-analysis of LV-GLS for both overall results and durations of ≥3 months. Conducting a sensitivity analysis by excluding this study did not affect the significance of the results, with effect sizes of 1.43 [95%CI (0.900, 1.961), p = 0.000, I2 = 91%] and 1.78 [95%CI (1.049, 2.516), p = 0.000, I2 = 92%], respectively. Furthermore, excluding this study did not alter the significant findings in the subgroup analysis of severity of COVID-19 infection. The effect sizes remained significant at 1.021 [95%CI (0.265, 1.776), p = 0.008, I2 = 87%] for mild infection and 2.289 [95%CI (1.201, 2.314), p = 0.000, I2 = 89%] for moderate-severe infection.
3.6.7 E/A ratioHamdy et al. (45) showed a high risk of bias in relation to this specific outcome. A sensitivity analysis was conducted by removing this study, changed the significancy of summary estimate for overall outcome and a duration of ≥3 months. The effect sizes were −0.079 [95%CI (−0.127, −0.032), p = 0.001, I2 = 64.6%] and −0.079 [95%CI (−0.141, −0.018), p = 0.011, I2 = 71.8%], respectively. However, excluding this study did not change the direction, or statistical significance of the summary estimate for the absence of comorbid diseases, with an effect size of −0.053 [95%CI (−0.109, 0.002), p = 0.061, I2 = 63.2%].
3.6.8 E/e' ratioHamdy et al. (45) and Wood et al. (42) were found to have a high risk of bias regarding this outcome. Excluding these studies did not change the direction, or statistical significance of the summary estimate for the overall outcome and duration of ≥3 months. The effect sizes remained at 0.092 [95%CI (−0.229, 0.412), p = 0.575, I2 = 76.3%] and 0.333 [95%CI (−0.094, 0.759), p = 0.126, I2 = 72.4%] for each respective outcome.
3.6.9 LAVIHamdy et al. (45) was found to have a high risk of bias for the outcome. Excluding this study did not change the direction, or statistical significance of the summary estimate for the overall outcome and duration of ≥3 months, with effect sizes of 0.578 [95%CI (−0.361, 1.517), p = 0.227, I2 = 51.4%] and 0.674 [95%CI (−0.077, 1.424), p = 0.079, I2 = 42.2%], respectively. The exclusion of this study also did not alter the lack of significance for the absence of comorbid disease, with an effect size of 0.214 [95%CI (−1.261, 1.688), p = 0.776, I2 = 73.4%].
3.6.10 RV-MPIThe study by Günay et al. (14) had a high risk of bias for this particular outcome. A sensitivity analysis was conducted by removing this study did not change the significancy of summary estimate for the overall outcome, showing an effect size of 0.035 [95% CI (0.008, 0.062), p = 0.012, I2 = 98.9%].
3.6.11 RVDThe study by Günay et al. (14) was found to have a high risk of bias for this particular outcome. Excluding this study changed the statistical significancy of summery estimates for the overall outcome, duration of 1–3 months, and the absence of comorbid disease, with effect sizes of 0.654 [95%CI (0.321, 0.987), p = 0.000, I2 = 17%], −0.277 [95%CI (−1.046, 0.493), p = 0.481, I2 = 21.3%] and 0.607 [95%CI (0.115, 1.099), p = 0.016, I2 = 0%], respectively. However, the sensitivity analysis for moderate-severe COVID-19 infection did not alter the direction or statistical significance of the summary estimate of the results. The effect sizes for these outcomes and 0.444 [95%CI (−0.099, 0.987), p = 0.109, I2 = 61.8%], respectively.
3.6.12 sPAPKüçük et al. (33) had a high risk of bias for moderate to severe COVID-19 infection. A sensitivity analysis that excluded this study showed that the result remained significant with an effect size of 8.016 [95%CI (6.800, 9.232), p = 0.000, I2 = 26.5%]. De et al. (24) was also at high risk of bias for the presence of comorbid disease. However, excluding this study in a sensitivity analysis did not change the direction or statistical significance of the summary estimate, with an effect size of 8.097 [95%CI (7.08, 9.113), p = 0.000, I2 = 0%].
Forest plots of sensitivity analysis are provided in supporting information (Supplementary S5 document).
3.7 Meta-regressionThe results of the univariate meta-regression showed a significant positive correlation between MDs of RV-GLS and age. The effect size was 0.150 [95% CI (0.027, 0.272), p = 0.016, R2 = 0.32]. Moreover, MDs of TAPSE was negatively correlated with post-COVID patients' age with an effect size of −0.077 [95%CI (−0.152, −0.003), p = 0.04, R2 = 0.09]. There were no other significant correlations observed between echocardiographic variables and age or BMI. Detailed information is presented in Table 5. Scatter plots are provided in supporting information (Supplementary S6 document).
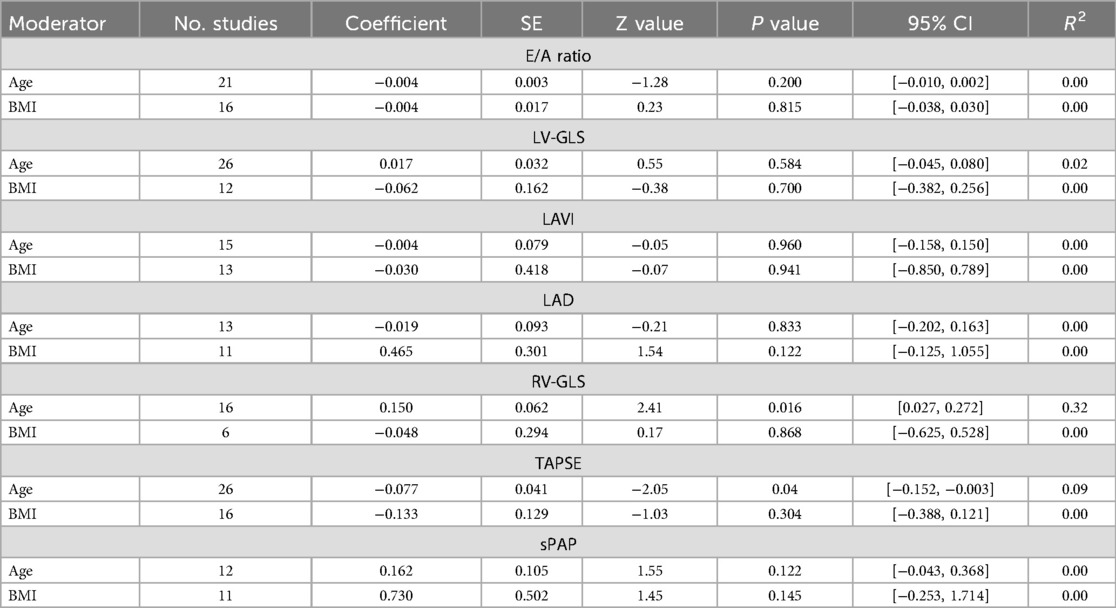
Table 5. Meta-regression results between echocardiographic indices and baseline characteristics of patients.
3.8 Publication biasA clear publication bias was observed when examining LVEF, LAVI, LAD and sPAP. After applying Duval and Tweedie's trim and fill method, it was determined that 9 studies needed to be added on the right side of the scatter plot for LVEF analysis. Following this adjustment, the effect size was calculated to be −0.120, with a 95%CI of (−0.711, 0.471). In the case of LAVI analysis, 5 studies needed to be imputed on the right side of the scatter plot, resulting in a summary effect size of 1.92, with a 95%CI of (0.689, 3.168). For LAD, 4 studies needed to be added on the left side of the scatter plot. The adjusted effect size was calculated 0.800 with a 95%CI of (−0.115, 1.716). Lastly, for sPAP correction analysis, 5 studies required imputation on the left side of the scatter plot, leading to a summary effect size of 1.29, with a 95%CI of (0.882, 1.717). Funnel plots and findings of Egger's and Begg's tests for all indices are provided in supporting information (Supplementary S7 document).
4 DiscussionIn the present systematic review and meta-analysis, we performed a pooled analysis of 66 studies to evaluate the effect of SARS-CoV-2 infection on cardiac function in post-COVID-19 survivors without a prior history of cardiac issues or abnormalities. Following strict inclusion and exclusion criteria, we identified 32 studies that met the eligibility criteria for meta-analysis. This meta-analysis revealed significant myocardial alterations in individuals who have recovered from COVID-19 when compared to control groups. Furthermore, differences were observed in the function of the right and left ventricles in post-COVID patients compared to controls, especially in subgroup analyses based on the time since the onset of acute COVID-19 and echocardiogram evaluation during recovery, the severity of the initial infection, and the presence of comorbidities.
4.1 Definition“Long COVID” or “post-COVID syndrome” is the term used to describe the ongoing presence of symptoms after a SARS-CoV-2 infection, lasting for weeks or months, regardless of whether the virus is still present in the body. These symptoms can persist or come back intermittently and may consist of either lingering symptoms from the initial COVID infection or new symptoms (79). The National Institute for Health and Care Excellence (NICE), the Scottish Intercollegiate Guidelines Network, and the Royal College of General Practitioners have collaborated to develop guidelines for individuals who have recuperated from COVID-19 but are still facing symptoms. They have coined the terms “post-acute COVID-19” for symptoms persisting 4–12 weeks after the initial infection and “long-COVID” for symptoms lasting beyond 12 weeks (80).
4.2 Echocardiographic evaluation of long COVIDThe current systematic review and meta-analysis found that chronic COVID-19 patients exhibit impaired cardiac function in both the right and left sides of the heart. Unlike in previous reviews and meta-analyses, these patients did not have any history of cardiac disease and/or comorbidities that could affect their cardiac function.
4.2.1 Left ventricular functionLV systolic dysfunction has been observed as a consequence of acute COVID-19 infection. Multiple studies have shown significant reductions in LVEF after 3 months of recovery from COVID-19, across a spectrum of symptoms and severity levels (24, 36, 39, 40). Additionally, there have been reports of reduced LVEF in chronic COVID-19 survivors, although these studies lacked a control group (70, 71, 75, 81).
LV-GLS provides valuable insight into LV function and is considered a more precise measure compared to LVEF (82). Long COVID patients, with and without a control group, were found to have reduced (less negative) LV-GLS (24, 25, 28, 31, 33, 34, 38, 66, 72, 77). However, there were reports of studies with no significant findings of LVEF and LV-GLS in long-COVID cases (26, 27, 37, 41, 74, 76, 78). The present meta-analysis revealed that individuals with long-COVID had significantly lower LV-GLS and LVEF compared to the control group. Unlike LVEF, decreased LV-GLS was also observed in COVID-19 patients with both mild and moderate-severe infections. Furthermore, reduced LVEF and LV-GLS were observed in COVID-19 patients with and without comorbidities compared to their matched groups.
Several studies have reported LV diastolic dysfunction in addition to LV systolic dysfunction. Long-COVID patients were found to have lower E/A and E/e' ratios compared to the control group (24, 28, 32, 36, 38, 40). In a study conducted by Sharma and colleagues (71), it was found that individuals with moderate to severe cases of COVID-19 had a greater likelihood of experiencing left ventricular diastolic dysfunction compared to those with mild cases when assessed through echocardiography six months post-infection (71). However, the present meta-analysis did not find any significant differences in E/e', E/A, mitral A wave, and mitral E wave between long-COVID patients and the control group.
Diastolic dysfunction is characterized by an irregular filling pattern in the left ventricle, often resulting in significant elevations in end-diastolic pressure during the filling of the ventricle (83). Left atrium enlargement is a key indicator of the structural remodeling process that occurs in reaction to chronically elevated LV end-diastolic pressure, typically resulting from diastolic dysfunction (83). In the current meta-analysis, it was found that LAD was significantly higher in long-COVID patients compared to the control group. However, there were no significant differences observed in LAVI between the two groups. In subgroup analysis, LAD and LAVI were increased in patients with history of moderate-severe COVID-19 infection and comorbid disease compared to their matched controls.
Additionally, abnormal LV shape can be a sign of both systolic and diastolic dysfunction. Several studies have shown that patients with long-lasting COVID-19 symptoms have significant alterations in LV geometric measurements (27,
留言 (0)