Sarcoidosis is a multi-system granulomatous disease of uncertain etiology, variable population prevalence, and protean clinical manifestations (1). Cardiac sarcoidosis (CS) can present as sudden cardiac death (SCD), heart failure (HF), arrhythmias, and/or conduction disorders (1, 2). The prevalence of symptomatic cardiac involvement has been historically reported as 5%, though asymptomatic cardiac involvement may affect 20%–25% of patients with pulmonary or systemic sarcoidosis (1, 3). With use of contemporary multi-modality cardiac imaging and updated diagnostic guidelines, however, rates of hospitalization for sarcoidosis have increased (4–8) Immunosuppressive and novel non-steroidal treatment approaches can potentially delay the progression of cardiac sarcoidosis, underscoring the need for early diagnosis and treatment (1, 9).
CS is diagnosed when cardiac symptoms develop in a patient with a diagnosis of systemic sarcoidosis or when HF, SCD, or arrhythmias of unclear etiology are ultimately attributed to granulomatous inflammation (7, 8). The manifestations of CS are non-specific and overlap with other disorders (10–12). For this reason, a high index of suspicion for CS is needed to diagnose CS. To date, there is limited data assessing differences in the primary clinical presentation of CS from non-CS cardiac disorders that could aid clinicians in the diagnostic process (13). We hypothesized that differences in clinical presentation, demographics, testing, and outcomes can be identified between hospitalizations with vs. without a secondary diagnosis of CS. Therefore, we compared hospitalizations for primary diagnoses suggestive of CS between cases with and without a CS secondary diagnosis in a national readmissions database.
Methods Population and data sourceWe retrospectively analyzed hospitalizations in the Healthcare Cost and Utilization Projection (HCUP) Nationwide Readmissions Database (NRD) from 2016 through 2019 (14). This database is a United States (US) all-payer administrative dataset constructed from the individual state inpatient hospitalization databases, developed and made available by the US Agency for Healthcare Research and Quality (AHRQ). The NRD is a de-identified dataset; hospitalizations (inpatient admissions) are the units of measurement. For each admission, the NRD includes hospital characteristics, demographics, comorbidities, primary and secondary diagnosis codes, procedures performed, in-hospital outcomes [length of stay (LOS), mortality], and total charges. Clinical information prior to admission, medication data, and clinical information not coded by ICD codes during the in-hospital stay are not included in the NRD. If a patient readmitted within the calendar year, a unique identifier is added to the NRD record to allow for tracking between the index admission and subsequent readmissions within the calendar year. The study interval was chosen to align with the introduction of ICD-10 codes in October 2015. At the time of data analysis, 2019 was the most recent year of the NRD dataset that was available from HCUP. We chose to include data through the end of 2019 to avoid potential confounding effects of the COVID-19 pandemic in 2020 and later.
The study cohort, comorbidities, and procedures were identified using International Classification of Diseases, 10th Revision, Clinical Modification codes (ICD-10-CM) and Procedure codes (ICD-10-PCS) (Supplementary Table S2). At least two sources were cross-referenced for each code category; sources included published medical literature, HCUP clinical classification software refined, and internal programmatic quality and operations codes list.
The study cohort consisted of hospitalizations with a primary discharge diagnosis of HF/cardiomyopathy, cardiac arrest, general arrhythmia, ventricular arrhythmia (VA), supraventricular arrhythmia, or heart block in the first visit within the calendar year (Figure 1). Hospitalizations with a primary diagnosis of CS were excluded from the analysis cohort. For the 30-day readmission outcome, we excluded cases from December to avoid lack of 30-day follow-up, since cases cannot be tracked across years in the NRD. This study was approved by the Providence St. Joseph Health institutional review board, with waiver of informed consent.
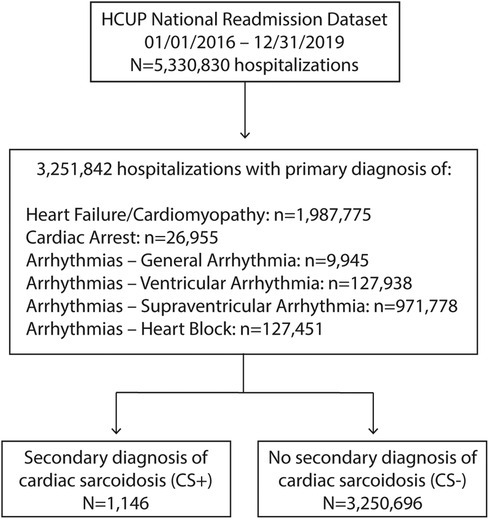
Figure 1. Study selection flow chart. CS, cardiac sarcoidosis; HCUP, health care utilization project.
Study aimsThe primary aim was to compare demographics and clinical characteristics of patients hospitalized with a primary diagnosis suggestive of CS who also had a secondary diagnosis of CS (CS+) vs. those who do not (CS−). As outlined above, the study dataset included hospitalizations with one of the six primary diagnoses suggestive of CS during the study time frame. Those hospitalizations were then sub-grouped into the presence (CS+) or absence (CS−) of cardiac sarcoidosis (ICD-10: D86.85) as a secondary diagnosis. The secondary aims were to (1) compare in-hospital procedures, outcomes, and 30-day readmissions between subgroups, and (2) to examine temporal trends in admissions, in-hospital mortality, and 30-day readmissions between the subgroups.
Statistical analysisDemographics, in-hospital procedures, 30-day readmissions, and outcomes within the calendar year were summarized for each group. Length of stay was reported as median and interquartile range (IQR) (25th, 75th percentile) based on non-normal distribution of the variable. All other continuous variables were summarized using descriptive statistics (n, mean ± SD). Categorical variables were summarized using frequencies and percentages. Prevalence estimates were weighted using survey analysis methods with “DISCWT” as the weight variable, “HOSP_NRD” as the clustering variable, and accounting for the different strata in the NRD design using the “NRD_STRATUM”, following AHRQ recommendations. This approach aimed to ensure accurate national representative estimates for the US hospitalizations (14).
To balance potential confounding factors between CS+ and CS− cases, a propensity score matching (PSM) method was implemented. Multivariable logistic regression was used to calculate propensity scores and estimate the probability of cardiac sarcoidosis diagnosis based on age, sex, year of admission, comorbidities listed in Table 1, urban/rural hospital type, and teaching/non-teaching hospitals. A CS+ case was matched with a CS− case using the greedy nearest-neighbor matching algorithm without replacement. The caliper was set at 0.25. Covariate balance between cardiac sarcoidosis and non-cardiac sarcoidosis was assessed using standardized mean differences (SMD).
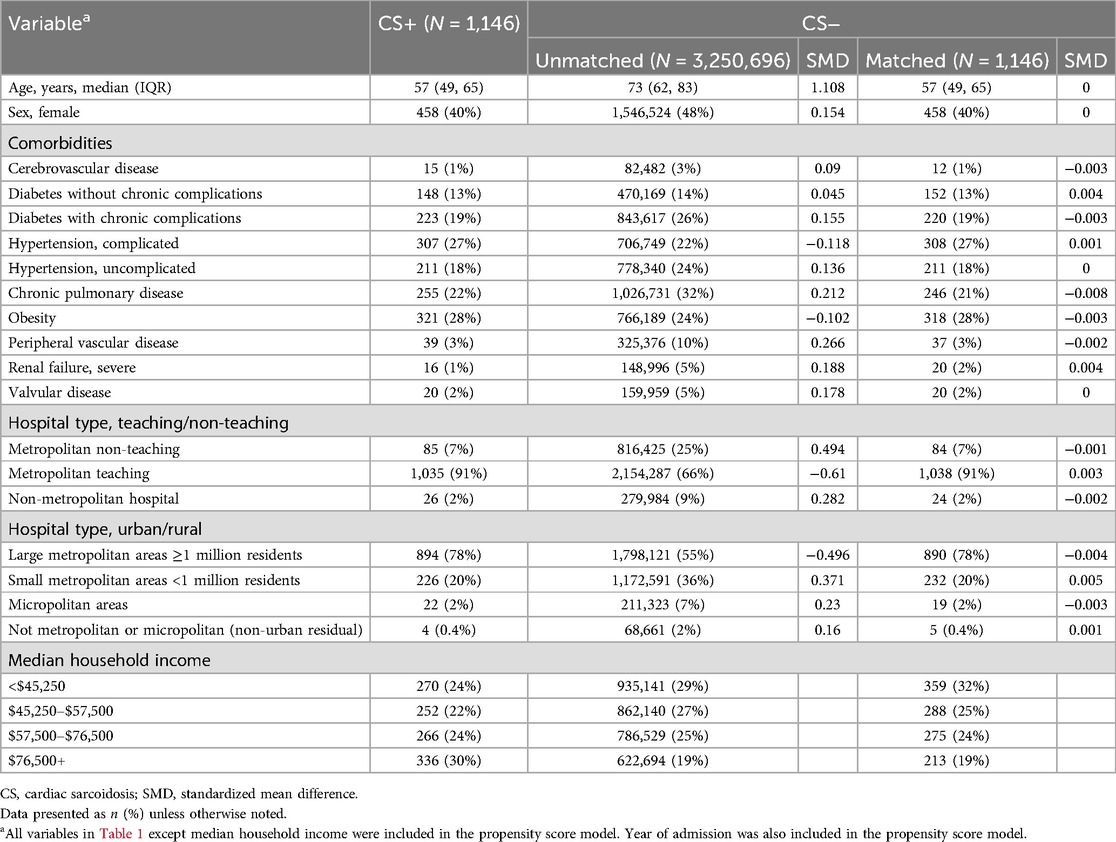
Table 1. Demographics.
Comparisons of the outcomes were performed after PSM. Categorical variables were compared with McNemar tests. Continuous variables were described using paired t-tests. Medians were compared using Wilcoxon's rank sum test. Missing data rates were <1% for all variables except median household income (1.4%). Significance was considered at p-values <0.05. All analyses were conducted using SAS version 9.4 (SAS Institute Inc). Figures were designed and edited in SAS, GraphPad Prism 9, and Adobe Illustrator 2022.
ResultsBetween 2016 and 2019, there were 1,146 hospitalizations with a primary diagnosis suggestive of CS plus secondary diagnosis of CS (CS+) and 3,250,696 hospitalizations in patients with a primary diagnosis suggestive of CS without a secondary diagnosis of CS (CS−) (Figure 1). The CS+ population increased modestly each year over the study period (Figure 2). Over these same four years, CS as any diagnosis increased over time, from 37.7 per million in 2016 to 68.0 per million in 2019. The six primary diagnoses suggestive of CS also increased over the study period, from 39,155 per million in 2016 to 44,155 per million in 2019.
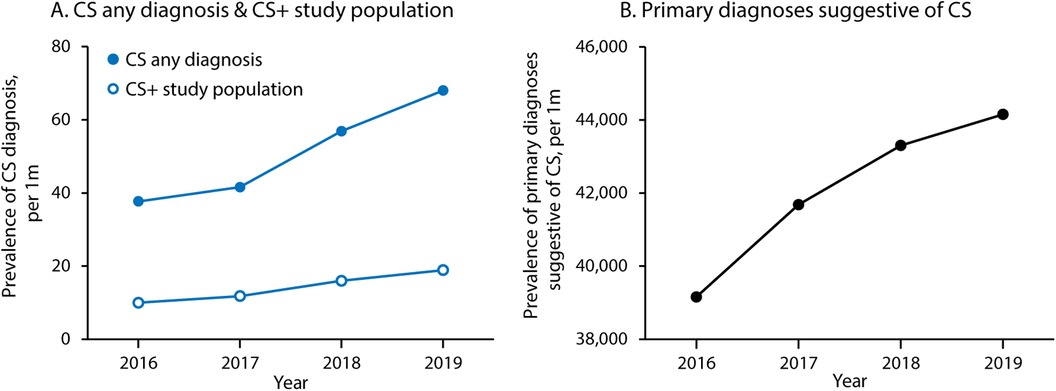
Figure 2. Trends in cardiac sarcoidosis and related diagnoses over time. (A) Shows prevalence of CS as any diagnosis (primary or secondary) and within the study population (cases with one of the six primary diagnoses suggestive of CS and a secondary diagnosis of CS). (B) Shows prevalence of the six primary diagnoses suggestive of CS (heart failure/cardiomyopathy, cardiac arrest, general arrhythmia, ventricular arrhythmia, supraventricular arrhythmia, and heart block). CS, cardiac sarcoidosis.
The CS+ cohort included patients who were younger (57 vs. 73 years old) and more likely male (60% vs. 52%) (Table 1). Cases without CS had higher rates of nearly all comorbid conditions, including hypertension, diabetes mellitus, obesity, valvular disease, peripheral vascular disease (PVD), and renal dysfunction (Table 1). Based on All Patients Refined Diagnosis Related Groups (APR-DRG) risk of mortality, CS+ cases had a higher likelihood of dying (major and extreme likelihood 55% vs. 50%) (Supplementary Table S1). CS+ hospitalizations occurred more often at a metropolitan teaching hospital (91% for the CS+ cohort vs. 66% for the CS− cohort).
PSM resulted in a well-matched CS population, with absolute standardized mean differences of less than 0.1 for all matching variables (Table 1; Supplementary Figure S1). After matching, demographics, comorbidities, and hospital characteristics were nearly equal between the groups (Table 1). Median household income was not included as a matching variable and remained higher in the CS+ group after matching ($76,500+, 30% vs. 19%, CS+ vs. CS−).
After matching, hospitalizations in the matched CS+ group more often presented with a primary diagnosis of VA (36% vs. 8%) or heart block (12% vs. 3%) (Central Illustration). Those in the matched CS- group more often presented with a primary diagnosis of HF/cardiomyopathy (52% vs. 42%) or supraventricular arrhythmia (36% vs. 10%).
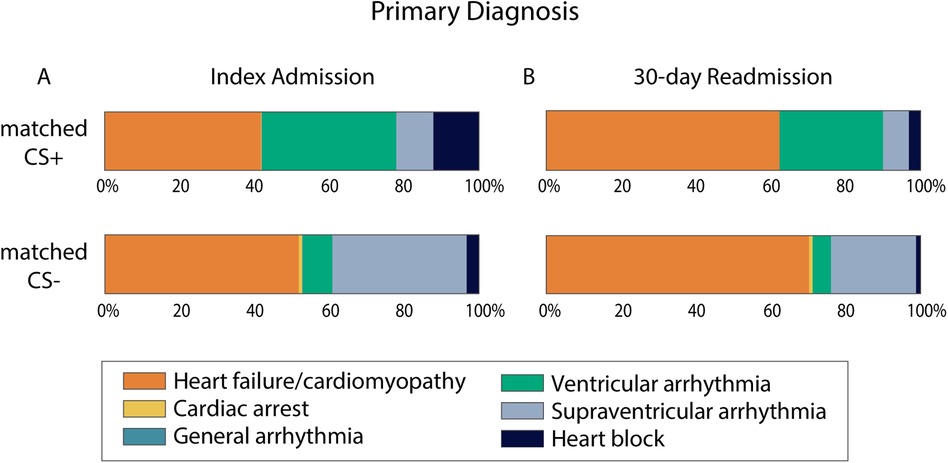
Central Illustration. Primary diagnosis for admissions with and without secondary diagnosis of cardiac sarcoidosis. Percent of cases in the matched CS+ and matched CS− groups that presented with each of the six primary diagnoses that are suggestive of CS. Index admission cases are summarized in panel A. 30-day readmission cases are summarized in panel B. For 30-day readmissions, N = 162 for the matched CS+ group and N = 160 for the matched CS− group. CS, cardiac sarcoidosis.
In-hospital procedures were performed more frequently in the matched CS+ group compared to the matched CS- group, including right heart catheterization (RHC) (17% vs. 8%, p < .0001), endomyocardial biopsy (4% vs. 0.6%, p < .0001), ventricular assist device (VAD) implantation (2% vs. 0.5%, p = 0.004), and permanent pacemaker insertion (31% vs. 6%, p < .0001) (Table 2). Furthermore, the matched CS+ cohort had significantly longer length of stay (4 (2–8) days vs. 3 (2–6) days, p < .0001) (Table 3). Nevertheless, in-hospital mortality during the index admission remained similar between the matched CS+ and matched CS- cohorts (2% vs. 3%, p = 0.08). All-cause 30-day readmission did not differ between groups (14% vs. 14%, p = 0.9), nor did the number of readmissions over the calendar year (p = 0.5269). Over the study period, in-hospital mortality during index admission and 30-day readmission rates were variable and did not statistically differ between cohorts (Supplementary Figure S2).
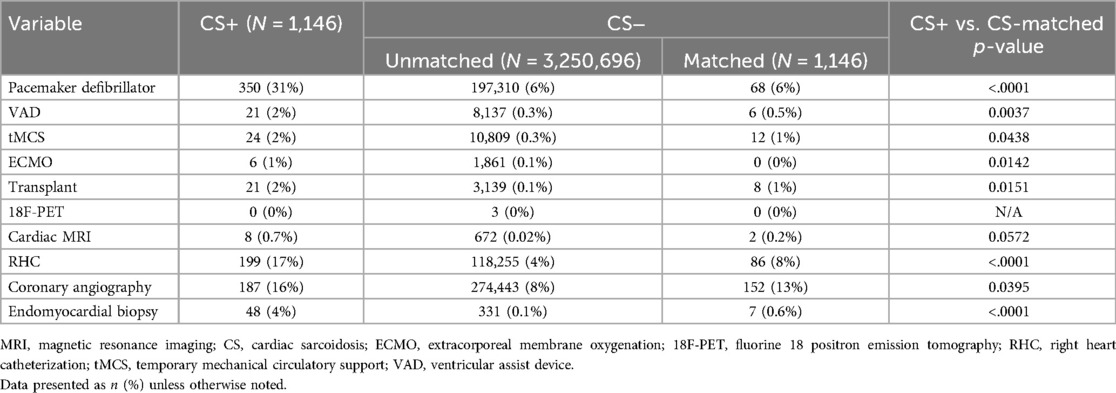
Table 2. In-hospital procedures.
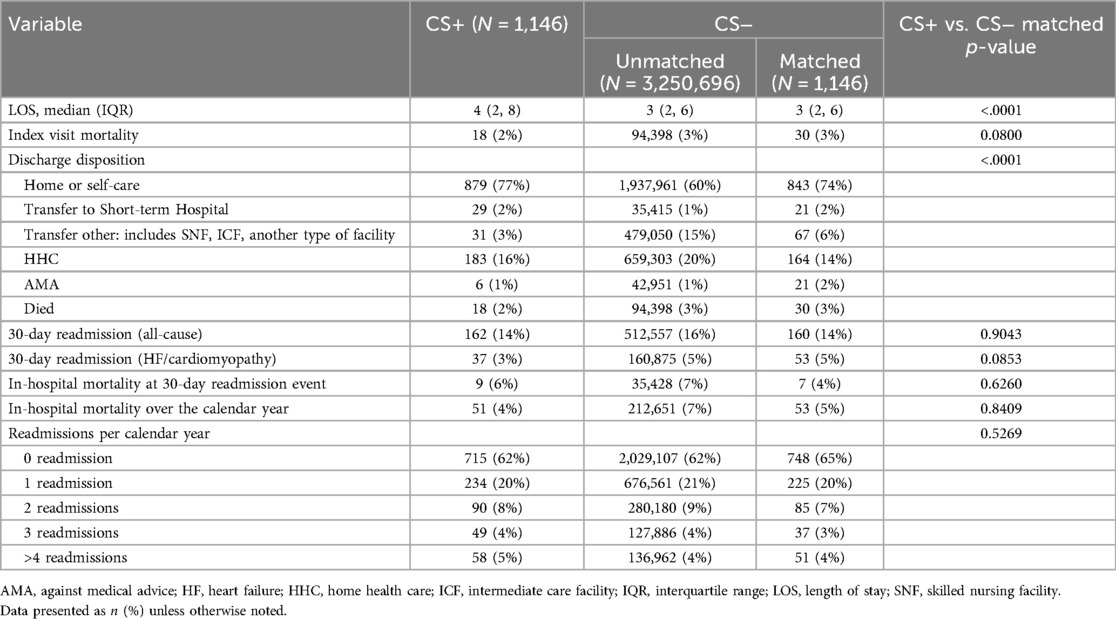
Table 3. Outcomes.
DiscussionIn this analysis of the Nationwide Readmissions Database, we identified primary diagnoses suggestive of CS (cardiac arrest, HF, arrhythmia, conduction disease), then defined cohorts of hospitalizations based on presence (CS+) or absence (CS-) of CS as a secondary diagnosis and compared propensity matched CS+ and CS− cohorts. This analysis yields several important observations. First, the CS+ cohort was comprised of patients who were significantly younger, more often male, and had fewer cardiac and non-cardiac comorbidities (valvular disease, hypertension, chronic obstructive pulmonary disease, PVD, or severe renal failure). Second, hospitalizations for matched CS+ cases more frequently related to a primary diagnosis of ventricular arrhythmia or heart block, whereas hospitalizations in the matched CS- cohort had higher rates of HF. Third, the matched CS+ cohort had higher rates of invasive diagnostic procedures (endomyocardial biopsy, RHC, coronary angiography) and invasive therapies [implantation of pacemaker/defibrillator, left ventricular assist device (LVAD), or heart transplant]. Fourth, despite having a higher rate of major likelihood of dying, cases in the matched CS+ cohort had the same in-hospital mortality and all-cause and HF-specific readmission rates at 30 days.
In contrast to prior publications that analyzed administrative datasets beginning with a primary diagnosis of sarcoidosis, we defined the study population by selecting primary diagnoses that could represent manifestations of CS. The rationale for this analysis was to identify demographic and clinical features that may assist clinicians to identify CS patients amongst a population presenting with HF/cardiomyopathy, cardiac arrest, arrhythmias, or heart block (e.g., primary diagnosis suggestive of CS).
In taking this analytic approach, our study yields insights that could potentially refine clinical suspicion for CS when confronting patients with unexplained HF, cardiac arrest, or arrhythmias. In particular, we found a younger and predominantly male patient population with higher rate of presentations with VT or conduction block in the cohort with a secondary CS diagnosis. The results of this analysis have potential implications for diagnosing and treating CS. Specifically, our results indicate that CS should be strongly suspected in younger, male patients presenting with ventricular arrhythmias or conduction system disease and should prompt additional diagnostic testing, including fluorine-18 fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG PET/CT) scans or cardiac magnetic resonance imaging (MRI). Patients suspected of having CS are more likely to require RHC and endomyocardial biopsy. Additionally, permanent pacemaker and durable LVAD are more frequently indicated in CS+ patients.
Our findings contrast with an earlier study of sarcoidosis hospitalizations by Patel et al. using the National Inpatient Sample from 2005 to 2014. Because of the earlier time frame, the authors identified sarcoidosis and cardiovascular manifestations using ICD-9 diagnosis codes, which are less extensive than ICD-10 codes (4). They reported a female predominance and heart failure and arrhythmias (not further specified) as the major cardiac causes of admission in sarcoidosis. The differences in findings between the current study and that of Patel et al. may be explained by the fact that Patel et al. defined their study population as any diagnosis of sarcoidosis, excluded ischemic heart disease hospitalizations, and used a different and a less contemporary administrative database. Additionally, in February 2017, the Japanese Circulation Society published new guidelines for the diagnosis and treatment of CS that elevated 18F-FDG PET/CT tracer uptake in the myocardium or late gadolinium enhancement by MRI from minor to major diagnostic criteria (7). In August 2017, the Society of Nuclear Medicine and Molecular Imaging and the American Society of Nuclear Cardiology published a consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring (15). These guideline statements may have increased the use of MRI or PET, which could increase the incidence of CS as has been observed in Finland, but we cannot draw any causal inferences from our analysis (16).
Due to the challenges of diagnosis and evolving diagnostic criteria, the true prevalence of isolated cardiac sarcoidosis is difficult to ascertain. In a retrospective study of 286 patients with suspected CS by Takaya et al, 7.3% of patients were diagnosed with isolated CS and 22% diagnosed with systemic CS utilizing updated Japanese Circulation Society guidelines. There were no demographic differences between patients with and without CS. In a secondary analysis of the ILLUMINATE-CS Japanese registry study, clinical characteristics and prognosis were compared between patients with isolated cardiac sarcoidosis and cardiac sarcoidosis with concomitant systemic disease. The primary outcome was a combined endpoint of all-cause death, hospitalization for heart failure, or fatal ventricular arrhythmia events. Among 475 patients with CS (mean age, 62.0 ± 10.9 years; female sex, 59%), 25.1% were diagnosed with isolated CS. Isolated CS patients had a higher prevalence of hospitalization for heart failure and lower left ventricular ejection fraction than those with systemic cardiac sarcoidosis. Isolated CS was a significant risk factor for the primary outcome in an unadjusted model, but this association was not maintained in a multivariable model.
The in-hospital mortality, 30-day readmission rates, in-hospital mortality over the calendar year, and readmissions per calendar year (Table 3) were similar amongst the unmatched study cohorts despite higher APR-DRG major mortality risk in the CS+ cohort. This finding warrants further investigation, though it may be related to the younger age and fewer non-cardiac comorbidities of the CS+ cohort, as well as the higher rate of admission to urban teaching hospitals. The lack of difference in mortality is particularly noteworthy given the fact that the CS+ cohort underwent invasive diagnostic and therapeutic procedures at a higher rate, including temporary mechanical circulatory support, ECMO, and heart replacement therapies (LVAD or transplant), suggesting a more severe clinical trajectory amongst a subset of CS+ patients. The observation that LOS was longer for CS+ patients compared to CS− patients is notable given the fact that CS+ patients were found to have a lower HF/cardiomyopathy incidence relative to CS− patients in this analysis. Longer LOS may also have been related to the higher rates of invasive procedures in the CS+ patients, which in turn could be related to the subsequent diagnosis of CS. Given the limitations of the current dataset, it is unclear what variables may have contributed to differences in LOS. This finding warrants further investigation and verification with additional datasets. It is possible that tertiary or quaternary diagnoses not captured in NRD may explain the LOS difference given the fact that sarcoidosis represents a systemic disease which places patients at risk for significant comorbidities. Additionally, delays in diagnosis or more severe disease presentation amongst CS+ patients may contribute to these observed differences.
We performed propensity score matching using covariates of age, sex, year, and clinically relevant comorbidities to minimize the effects of confounding. The very low SMD for all covariates indicates a highly balanced match that limits the effects of potential confounders. The major observations of the study remained significant after matching, strengthening confidence in our findings. The heightened risk of adverse outcomes for patients with CS has been previously studied in several national European registries. A Danish registry analysis demonstrated an increased lifetime hazard for HF, atrial/ventricular arrhythmias, heart block/need for pacemaker, and all-cause mortality in patients with systemic sarcoidosis (17). In a nationwide 18-year registry inclusive of 351 patients with CS in Finland, Ekstrom and colleagues identified atrioventricular block and VA as common presenting manifestations. Sudden death accounted for 80% of all deaths (18). Similarly, Takaya et al. showed that the rate of cardiac death of hospitalization for HF was higher in patients with isolated CS than systemic CS. Taken together with our finding that VA is more frequent in the CS+ cohort, these observations highlight the urgency of appropriate diagnostic testing and therapeutic interventions.
Finally, we report a rising prevalence of CS during the study period (37.7 per million in 2016 to 68.0 per million in 2019). This observation likely underestimates the actual disease prevalence as this analysis is limited to inpatients. A rising prevalence of CS in this North American registry corroborates recent observations from Europe (19). Though the reasons underlying this observation cannot be determined from the present analysis, it is reasonable to surmise that heightened awareness of CS amongst clinicians, coupled with increased use of advanced cardiac imaging (cardiac MRI and FDG-PET/CT scans), has contributed to higher rates of diagnosis. Clinically manifested CS represents a fraction of the total disease burden, underscoring the need for clinicians to seek out the diagnosis of CS in order to prevent significant morbidity and mortality (19).
LimitationsThere are limitations to this observational study. First, as with all administrative databases, the NRD is inherently subject to potential coding errors, in particular for procedures that are typically coded using current procedural termination (CPT) codes which are not available in the NRD, such as endomyocardial biopsy, cardiac MRI, and PET scans. In addition, the NRD does not include medication information or information prior to the index hospital admission, such as patients’ clinical treatment, procedures, or previous hospitalization data. Follow up studies using clinically adjudicated datasets that follow the clinical trajectory of CS patients would be useful. Second, the NRD excludes interstate hospitalizations and does not link admissions across calendar years, which could underestimate readmission rates. Third, we excluded cases with a primary diagnosis of CS to enrich for patients diagnosed during the index hospitalization. However, we are unable to determine with certainty the timing of an initial CS diagnosis nor were we able to ascertain how the diagnosis of CS was made [based on histological analysis, advanced cardiac imaging (cardiac MRI and/or FDG PET/CT imaging), etc.]. Finally, because the NRD is a US national database and lacks information about regional differences and patient race/ethnicity, we are unable to estimate these potential sources of differences.
ConclusionsIn a nationally representative cohort of US hospitalizations for heart failure, ventricular arrythmias, heart block, or cardiac arrest, cases with secondary diagnosis of CS (CS+) were more likely to be younger males and to present with ventricular tachycardia (VT) or heart block compared to patients without a secondary CS diagnosis (CS−). CS+ cases were more likely to undergo invasive diagnosis and therapeutic procedures, including temporary mechanical circulatory support and heart replacement therapies. Despite having higher predicted mortality risk, in-hospital mortality and 30-day readmission rates were similar to CS− cases. These findings could inform diagnostic and therapeutic approaches for clinicians confronting patients with unexplained HF or arrhythmias.
Data availability statementThe dataset presented in this article is available for purchase from the Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project (HCUP). Requests to access the dataset should be directed to https://hcup-us.ahrq.gov/tech_assist/centdist.jsp.
Ethics statementEthical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributionsJA: Conceptualization, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. KS: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. H-FL: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. TP: Writing – original draft, Writing – review & editing. MW: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. FS: Conceptualization, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1475181/full#supplementary-material
Supplementary Figure S1 | Standardized Mean Difference Plot. The CS− unmatched population is shown in blue and the CS− population after propensity score matching is shown in red. The two dashed lines represent the recommended limits of −0.25 and 0.25 for the standardized mean differences.
Supplementary Figure S2 | Index Event Mortality (A) and 30-day Readmission (B) Over Time. CS, cardiac sarcoidosis.
Supplementary Table S1 | Additional Comorbidities. CS, cardiac sarcoidosis; APR-DRG, all patients refined diagnosis related groups. Data presented as n (%) unless otherwise noted.
AbbreviationsAHRQ, agency for healthcare research and quality; APR-DRG, all patients refined diagnosis related groups; CPT, current procedural terminology; CS, cardiac sarcoidosis; CS+, patients hospitalized with a primary diagnosis suggestive of cardiac sarcoidosis who also had a secondary diagnosis of cardiac sarcoidosis; CS−, patients hospitalized with a primary diagnosis suggestive of cardiac sarcoidosis without a secondary diagnosis of cardiac sarcoidosis18F-FDG PET/CT = fluorine-18 fluorodeoxyglucose-positron emission tomography/computed tomography; HCUP, healthcare cost and utilization projection; HF, heart failure; ICD, international classification of diseases; IQR, interquartile range; LOS, length of stay; LVAD, left ventricular assist device; MRI, magnetic resonance imaging; NRD, nationwide readmissions database; PSM, propensity score matching; PVD, peripheral vascular disease; RHC, right heart catheterization; SCD, sudden cardiac death; SD, standard deviation; SMD, standardized mean difference; VA, ventricular arrhythmia; VAD, ventricular assist device; VT, ventricular tachycardia.
References1. Gilotra NA, Griffin JM, Pavlovic N, Houston BA, Chasler J, Goetz C, et al. Sarcoidosis-related cardiomyopathy: current knowledge, challenges, and future perspectives state-of-the-art review. J Card Fail. (2022) 28(1):113–32. doi: 10.1016/j.cardfail.2021.06.016
PubMed Abstract | Crossref Full Text | Google Scholar
2. Garg M, Gupta M, Patel NN, Bansal K, Lam PH, Sheikh FH. Predictors and outcomes of sudden cardiac arrest in heart failure with preserved ejection fraction: a nationwide inpatient sample analysis. Am J Cardiol. (2023) 206:277–84. doi: 10.1016/j.amjcard.2023.08.145
PubMed Abstract | Crossref Full Text | Google Scholar
4. Patel N, Kalra R, Doshi R, Arora H, Bajaj NS, Arora G, et al. Hospitalization rates, prevalence of cardiovascular manifestations, and outcomes associated with sarcoidosis in the United States. JAHA. (2018) 7(2):e007844. doi: 10.1161/JAHA.117.007844
PubMed Abstract | Crossref Full Text | Google Scholar
5. Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. (2014) 31(1):19–27.24751450
PubMed Abstract | Google Scholar
6. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. (2014) 11(7):1304–23. doi: 10.1016/j.hrthm.2014.03.043
PubMed Abstract | Crossref Full Text | Google Scholar
7. Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, et al. JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis – digest version. Circ J. (2019) 83(11):2329–88. doi: 10.1253/circj.CJ-19-0508
PubMed Abstract | Crossref Full Text | Google Scholar
8. Kawai H, Sarai M, Kato Y, Naruse H, Watanabe A, Matsuyama T, et al. Diagnosis of isolated cardiac sarcoidosis based on new guidelines. ESC Heart Fail. (2020) 7(5):2662–71. doi: 10.1002/ehf2.12853
PubMed Abstract | Crossref Full Text | Google Scholar
9. Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. (2001) 88(9):1006–10. doi: 10.1016/S0002-9149(01)01978-6
PubMed Abstract | Crossref Full Text | Google Scholar
10. Philips B, Madhavan S, James CA, te Riele AS, Murray B, Tichnell C, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy and cardiac sarcoidosis. Circ Arrhythm Electrophysiol. (2014) 7(2):230–6. doi: 10.1161/CIRCEP.113.000932
PubMed Abstract | Crossref Full Text | Google Scholar
12. Asimaki A, Tandri H, Duffy ER, Winterfield JR, Mackey-Bojack S, Picken MM, et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. (2011) 4(5):743–52. doi: 10.1161/CIRCEP.111.964890
PubMed Abstract | Crossref Full Text | Google Scholar
15. Writing group, Document reading group, EACVI Reviewers: This document was reviewed by members of the EACVI Scientific Documents Committee for 2014–2016 and 2016–2018. A joint procedural position statement on imaging in cardiac sarcoidosis: from the cardiovascular and inflammation & infection committees of the European association of nuclear medicine, the European association of cardiovascular imaging, and the American society of nuclear cardiology. Eur Heart J Cardiovasc Imaging. (2017) 18(10):1073–89. doi: 10.1093/ehjci/jex146
PubMed Abstract | Crossref Full Text | Google Scholar
16. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. (2015) 131(7):624–32. doi: 10.1161/CIRCULATIONAHA.114.011522
PubMed Abstract | Crossref Full Text | Google Scholar
17. Yafasova A, Fosbøl EL, Schou M, Gustafsson F, Rossing K, Bundgaard H, et al. Long-term adverse cardiac outcomes in patients with sarcoidosis. J Am Coll Cardiol. (2020) 76(7):767–77. doi: 10.1016/j.jacc.2020.06.038
PubMed Abstract | Crossref Full Text | Google Scholar
18. Ekström K, Lehtonen J, Nordenswan HK, Mäyränpää MI, Räisänen-Sokolowski A, Kandolin R, et al. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J. (2019) 40(37):3121–8. doi: 10.1093/eurheartj/ehz428
PubMed Abstract | Crossref Full Text | Google Scholar
19. Lehtonen J, Uusitalo V, Pöyhönen P, Mäyränpää MI, Kupari M. Cardiac sarcoidosis: phenotypes, diagnosis, treatment, and prognosis. Eur Heart J. (2023) 44(17):1495–510. doi: 10.1093/eurheartj/ehad067
留言 (0)