The internal carotid artery ascends to the base of the skull, traverses the petrous portion of the temporal bone, and gains entry into the cranial cavity through the foramina. And carotid artery disease typically refers to a range of conditions characterized by structural changes in the lumen of the carotid artery. The optimal management for coronary artery disease patients undergoing off-pump coronary artery bypass grafting (OPCAB), combined with carotid artery disease has been regarded as the “bottleneck” problem, requiring urgent resolution in the field of cardiac surgery (1–5). A conclusive advantage from carotid endarterectomy in mitigating stroke risks has been demonstrated for severe symptomatic carotid artery disease (6–8). However, a controversy persists regarding the management of severe asymptomatic carotid artery stenosis (ACAS) (9) and no definitive conclusions have been reached in the current guidelines for the optimal surgical management of patients with severe ACAS and coronary artery disease (CAD) (2, 10–12) (Table 1). In addition, the level of the evidence in the above guidelines is low. The root cause of the disputed conclusions for the optimal management of severe ACAS and CAD patients undergoing OPCAB, we thought that the relationship between severe ACAS and adverse 30-day postoperative outcomes, particularly the 30-day postoperative mortality in surgical patients, has not been determined by previous clinical data.
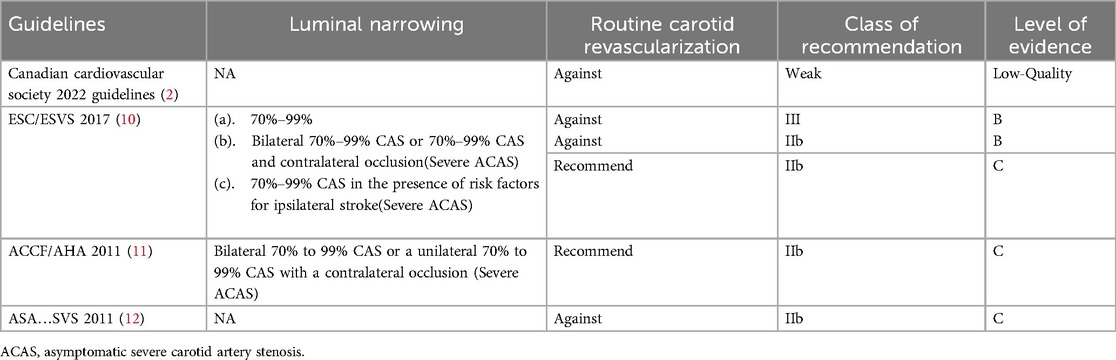
Table 1. Review of current guidelines for the surgical management of patients with ACAS and coronary artery disease.
In addition, the Sequential Organ Failure Assessment (SOFA) maximum was reported to be the gold standard for the prognostic evaluation of OPCAB (13–15), and the severe multi-organ dysfunction, measured by SOFA maximum ≥11, has been confirmed to predict the 30-day postoperative mortality in patients undergoing isolated OPCAB (13). Since the independent correlation between severe ACAS and 30-day postoperative mortality in patients undergoing OPCAB cannot be explored directly, it is limited by sample size. Therefore, it is imperative to advance the study endpoint by prioritizing severe multi-organ dysfunction over the 30-day postoperative mortality as the primary endpoint of our current research.
To catch the above knowledge gap, the current research aimed to demonstrate the independent association between preoperative severe ACAS and severe multi-organ dysfunction after OPCAB, which may further indicate the relationship between severe ACAS and adverse 30-day postoperative outcomes of patients undergoing OPCAB.
MethodsThe Ethics Committees of Beijing Anzhen Hospital, Capital Medical University (Beijing, China), approved the current observational research. Ethics number: 2024205x.
Patients’ selectionThis study included 562 patients without a history of stroke or Transient Ischemic Attacks (TIA) (asymptomatic), who underwent for an isolated OPCAB in the ward 2, center for operative treatment of coronary artery disease of Beijing Anzhen Hospital from January 2020 to December 2021. Patients with prior stroke or TIA, with previous or recent carotid intervention, undergoing other cardiac surgeries, such as aortic or valve operations, undergoing OPCAB combined with above surgeries were all excluded. All enrolled patients underwent carotid artery ultrasound prior to OPCAB. The specific enrollment of patients was shown in Figure 1. The information was extracted independently by two authors of the study from the medical records. Informed consent was obtained from the patient or their relatives on the day of admission.
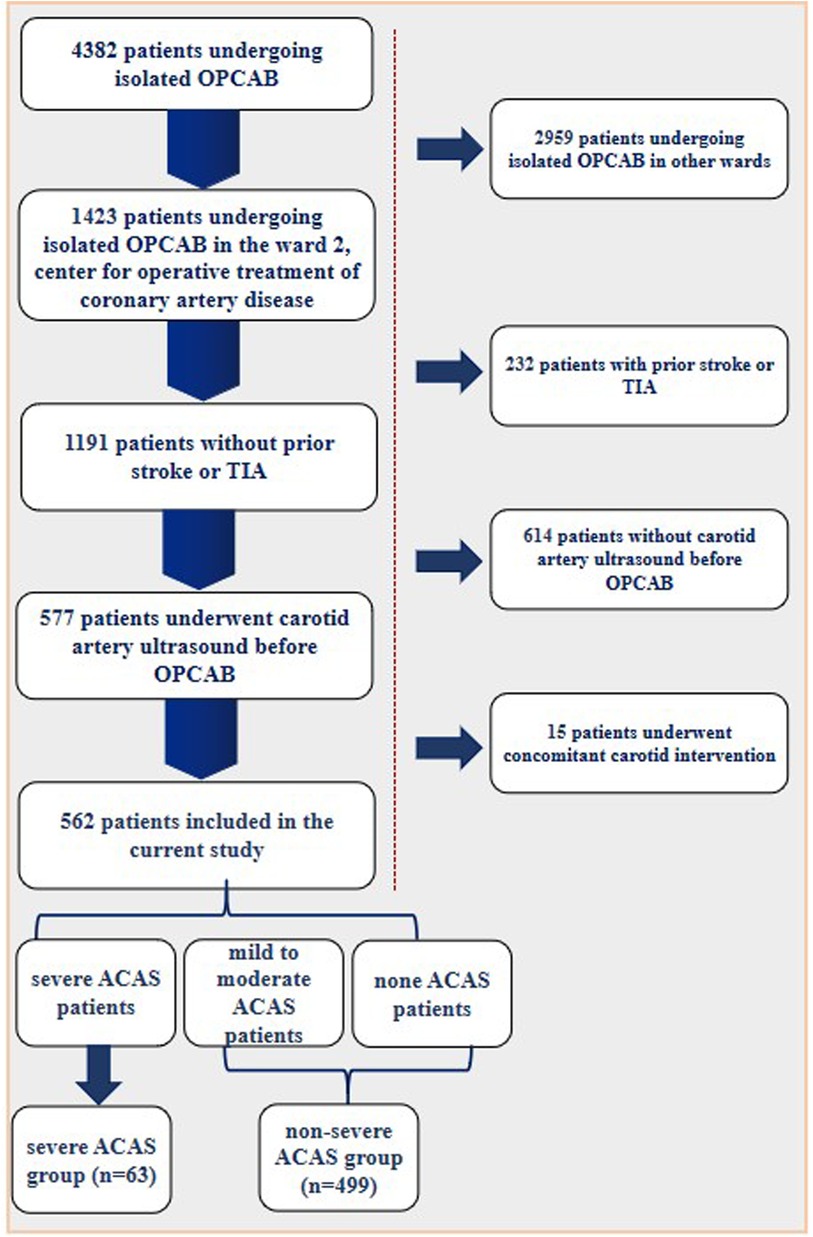
Figure 1. Patient enrollment flow diagram.
Definitions, patients’ grouping, and study endpointsBased on the latest guidelines for the carotid arterial disease (2, 10–12). The severity of CAS was defined according to the percentage of carotid arterial luminal stenosis, with the arterial lumen distal to the stenosis as the reference diameter. Severe CAS was defined as the presence of one or more of the following: (1) bilateral 70%–99% CAS; (2) unilateral 70%–99% CAS and contralateral occlusion; and (3) unilateral 70%–99% CAS in the presence of risk factors for ipsilateral stroke or TIA. The included asymptomatic individuals were divided into two groups according to the presence or absence of severe CAS: the severe ACAS group and the non-severe ACAS group (mild to moderate ACAS and no ACAS).
The primary endpoint was postoperative severe multi-organ dysfunction, measured by SOFA score ≥11 based on the latest literature (13, 14, 16). The SOFA score (Table 2) was counted daily from the day of the surgery to the day of discharge. The SOFA maximum was reported to be the gold standard for prognostic evaluation of OPCAB. Furthermore, the secondary endpoint was the 30-day postoperative stroke, which was defined as the 30-day postoperative neurologic deficit that did not resolve within 24 h and associated with a brain lesion (Brain lesions are defined as areas of brain tissue that have been compromised due to injury or disease. These lesions are typically identified through diagnostic imaging scans, such as MRI or CT scans. They can manifest in various forms and sizes, and their presence may indicate underlying neurological conditions.).
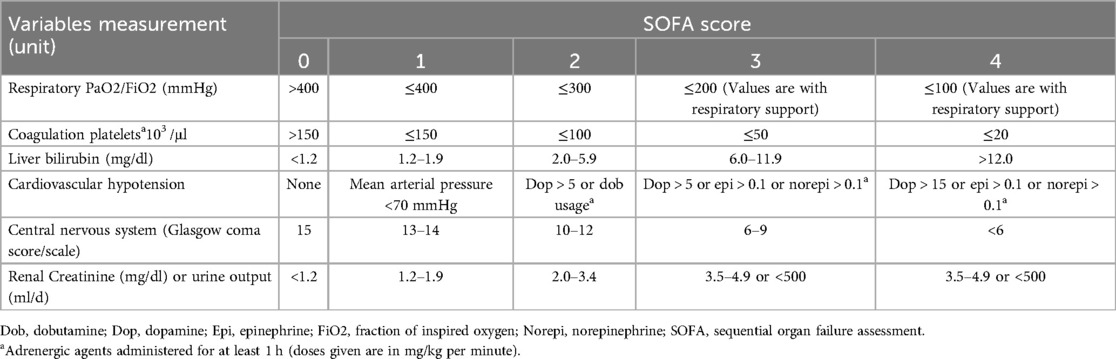
Table 2. The definition of SOFA score.
Other short-term postoperative complications include: (1) 30-day postoperative major adverse cardiovascular and cerebral events (MACCEs), which is defined as the presence of one or more of the following events: death, stroke, myocardial infarction (MI), or repeat revascularization; (2) new-onset of atrial fibrillation (AF) and ventricular arrhythmias (VA); (3) postoperative delirium; (4) pulmonary embolism; (5) pneumonia; (6) acute kidney injury (AKI); and (7) re-operation. The above events were recorded by our authors based on the definitions of the 30-day postoperative outcomes patients from the society of thoracic surgeons (STS) national cardiac database (17) and the 2021 ACC/AHA/SCAI guideline for coronary artery revascularization (18).
Statistical analysisStatistical analyses were performed using SPSS version 25.0 (IBM Corporation, Armonk, NY). Continuous and categorical variables were shown as mean ± SD and percentages, respectively. Student t test was used to compare continuous variables, and the chi-square test or Fisher exact test was used to compare categorical variables. The variables potentially associated with our endpoints were evaluated using univariate analysis. Variables that had a p-value of less than 0.05 in the univariate analysis were further assessed in the multivariate analysis. The power of the association between variables and outcomes was expressed as odds ratio (OR). All statistical tests were two-sided, and differences with p < 0.05 were considered to be statistically significant.
ResultsThe baseline characteristics of the study population are shown in Table 3. A total of 562 patients met the inclusion criteria for the current study. 63 (11.2%) suffered from severe ACAS. Compared to the non-severe ACAS group, patients with severe ACAS experienced a longer operation time (5.0 ± 1.6 vs. 4.2 ± 1.0, p < 0.0001), a higher EuroSCORE (11.0 ± 6.2 vs. 8.3 ± 3.7, p < 0.0001), a lower left ventricular ejection fraction (LVEF) (45.6 ± 19.5 vs. 53.7 ± 14.2, p < 0.0001), and had a higher prevalence of peripheral arterial disease (50.8% vs. 35.3%, p = 0.032) and heart failure (38.0% vs. 23.6%, p = 0.041). In addition, severe ACAS was found to be more common in current smokers (41.3% vs. 24.2%, p = 0.016).
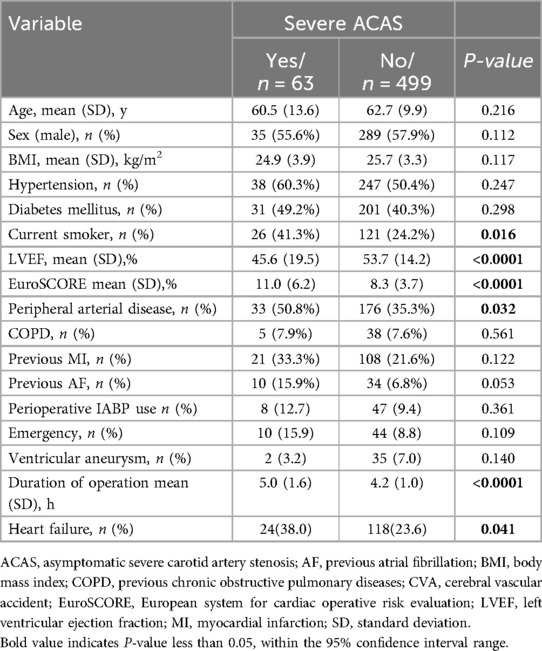
Table 3. Patients’ baseline characteristics.
Short-term postoperative events are summarized in Table 4. The SOFA maximum in the severe ACAS group was significantly higher than that in the non-severe ACAS group (9.76 ± 3.03 vs. 7.75 ± 2.96, p < 0.0001, (Figure 2), and a higher proportion of patients in the severe ACAS group exhibited severe multi-organ dysfunction (44.4% vs. 14.0%, p < 0.0001). In addition, severe ACAS was related to an increased rate of 30-day postoperative MACCEs (15.9% vs. 6.6%, p = 0.027), including a 30-day postoperative stroke (9.5% vs. 3.0%, p = 0.033). Severe ACAS was associated with an elevated risk of delirium (6.3% vs. 0.8%, p = 0.010), and AKI (12.7% vs. 1.6%, p < 0.0001). For the other short-term events, there were no significant differences between the two groups.
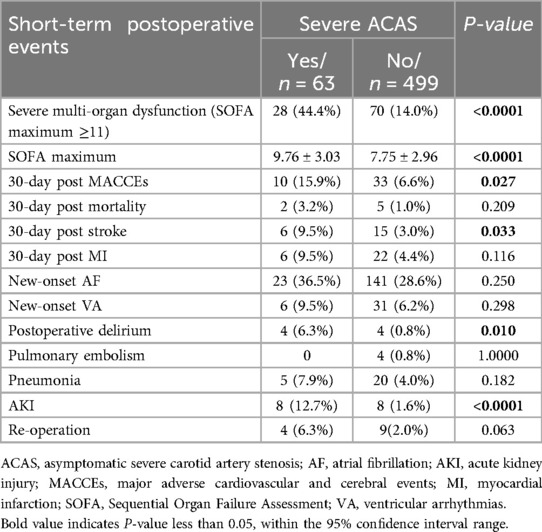
Table 4. Short-term postoperative events.
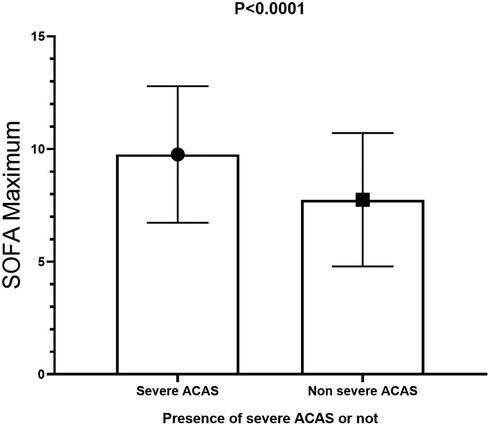
Figure 2. SOFA maximum in the severe ACAS group was significantly higher than that in the non-severe ACAS group. SOFA, sequential organ failure assessment; ACAS, asymptomatic severe carotid artery stenosis.
The results of the multivariate analysis (Table 5) demonstrated that severe ACAS may be independently associated with severe multi-organ dysfunction (OR, 7.37, 95% CI 4.80–14.30, p < 0.0001) after OPCAB. In addition, the higher body mass index (BMI) (OR, 1.35, 95%CI 1.25–1.46, p < 0.0001), higher EuroSCORE (OR, 1.07, 95% CI 1.02–1.12, p-0.010), peripheral arterial disease (OR, 2.10, 95% CI 1.27–3.48, p = 0.004), previous MI (OR, 2.26, 95% CI 1.39–3.69, p = 0.001), perioperative IABP use (OR, 3.42, 95% CI 1.74–6.69, p < 0.0001), emergency (OR 7.60, 95% CI 3.94–14.36, p < 0.0001), ventricular aneurysm (OR, 3.92, 95% CI 1.49–7.70, p = 0.004), and longer operating time (OR, 1.32, 95% CI 1.06–1.64, p = 0.013) were independent risk factors for severe multi-organ dysfunction. On the contrary, higher preoperative LVEF may be independently related to a decreased incidence of severe multi-organ dysfunction.
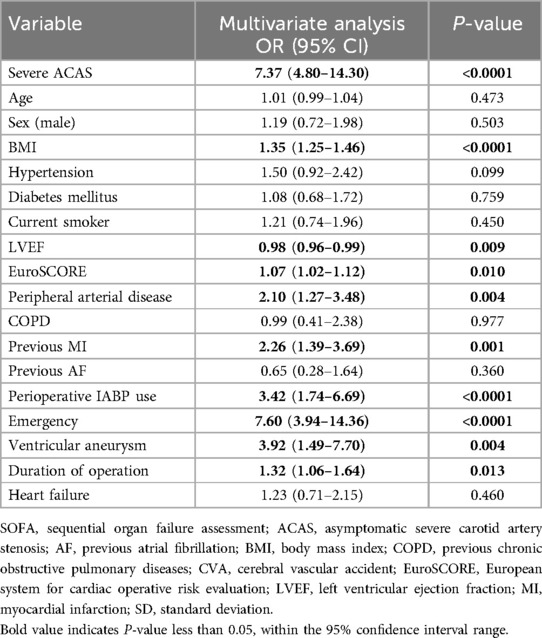
Table 5. Independent predictors of severe multi-organ dysfunction (SOFA maximum ≥11).
Based on the results of multivariate analysis (Table 6), the current research also demonstrated severe ACAS may be independently associated with 30-day postoperative stroke (OR, 2.83, 95% CI 1.03–7.75, p = 0,043). The male sex (OR 2.68, 95% CI 1.08–6.62, p = 0.033), emergency (OR, 10.67, 95% CI 3.62–31.40, p < 0.0001), and longer operating time (OR, 1.86, 95% CI 1.30–2.65, p = 0.001) were independent risk factors for 30-day postoperative stroke.
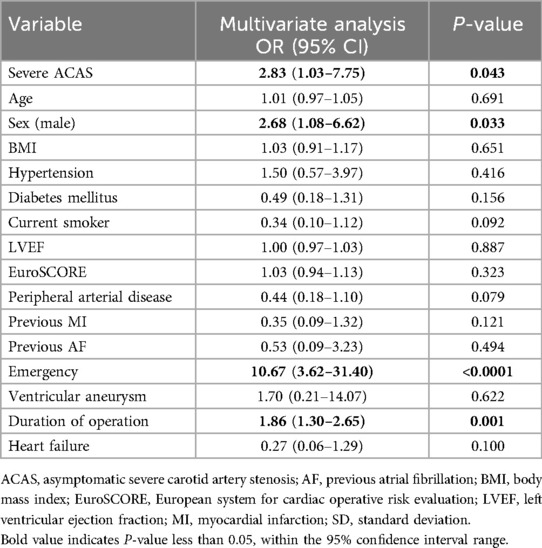
Table 6. Independent predictors of 30-day postoperative stroke.
DiscussionTo the best of our knowledge, this is the first single-center, retrospective observational study that demonstrate the correlation between severe ACAS and the incidence of multi-organ dysfunction after OPCAB. The key results of this study are that SOFA maximum in the severe ACAS group was significantly higher than that in the non-severe ACAS group, and the severe ACAS was independently associated with severe multi-organ dysfunction after OPCAB, which may be associated further with an increased rate of 30-day postoperative mortality and complications. In addition, we demonstrated that severe ACAS may be independently associated with 30-day stroke after OPCAB.
Although severe ACAS is associated with an increased risk of stroke and the European Society of Cardiology/American Heart Association guidelines classify individuals with severe ACAS as high-risk for MACCEs prevention, its potential impact on the 30-day adverse events after OPCAB remains incomprehensible. For the 30-day postoperative cerebral events, Santarpino et al. found that ACAS ≥ 90% was independently associated with 30-day stroke after OPCAB (4). However, Preop screening of CAS is routine in CABG and OPCAB, thus ACAS will be seen (4). On the contrary, Mahmoudi et al. found that severe ACAS is not an independent predictor for the 30-day postoperative stroke in patients undergoing OPCAB, and pre-operative screening for ACAS prior to OPCAB should be personalized and based on clinical discretion (19). A meta-analysis focusing on ACAS individuals revealed that patients with bilateral ACAS ≥ 50% had a stroke risk of 6.5% following cardiac surgery (20). No significant differences in the incidence of 30-day stroke after OPCAB between patients with and without severe ACAS had also been reported in other studies (21–23). In addition, at present there is a paucity of clinical studies examining the association between severe ACAS and 30-day postoperative mortality, as well as other organ-related events. In summary, firstly we thought the inconsistent criteria utilized for evaluating severe ACAS may contribute to the discrepant findings reported in the previous literature. There is a lack of literature regarding the definition of severe ACAS using the criteria recommended by current guidelines. Secondly, the limited sample size precludes directed the exploration of the independent correlation between severe ACAS and 30-day postoperative mortality in patients undergoing OPCAB. Based on the limited data available, it is imperative to employ a novel primary endpoint that makes it possible to investigate the association between severe ACAS and 30-day mortality, as well as complications after OPCAB.
As a robust predictor of 30-day outcomes following cardiac surgery, severe multi-organ dysfunction demonstrates superior accuracy, specificity, and independence from treatment when forecasting patient outcomes (14, 24). Four scoring systems were used to define multi-organ dysfunction: the Glasgow Coma Scale, poison severity score, Multiple Organ Dysfunction Score (MODS), and SOFA score. The SOFA score was adopted based on the following advantages: (1) A maximum SOFA score ≥11, namely severe multi-organ dysfunction, is the gold standard for evaluating 30-day mortality after OPCAB with a sensitivity of 95% (13); (2) the SOFA score is more feasible with cardiovascular and cerebrovascular function evaluation (24). The current research regarded the severe multi-organ dysfunction instead of the 30-day postoperative mortality as the first endpoint.
The potential adverse effects of severe ACAS on the 30-day postoperative mortality and organ-related complications may be attributed to the following factors. First, in severe ACAS patients undergoing OPCAB, the occurrence of 30-day cerebral events may be ascribed to the impairment in cerebral hemodynamics. Preexisting hemodynamic impairments serve as a key predictive factor for the occurrence of stroke/TIA (25). Severe ACAS, especially asymptomatic carotid artery occlusion, can lead to not only a reduction in the perfusion pressure on the ipsilateral side but a recruitment of secondary collaterals, both of which may be associated with hemodynamic impairment (3, 26). And the presence of hemodynamic impairment in both hemispheres has been reported in asymptomatic individuals with carotid artery occlusion. Secondly, the rupture of the severe ACAS that was associated with atherosclerotic plaque can result in distal embolism and subsequent 30-day postoperative mortality and stroke/TIA (3). Thirdly, atherosclerosis of severe ACAS is another significant cause of 30-day adverse outcomes after OPCAB (27). Kanemitsu et al. confirmed that the prevalence of complications was significantly higher in individuals with severe atherosclerosis who underwent OPCAB, compared to those who without severe atherosclerosis (28).
Whether severe ACAS patients will lead to a clinically meaningful benefit from a OPCAB combined carotid endarterectomy remains to be investigated further. Mahmoudi et al. identified that severe ACAS may lead to the development of 30-day postoperative adverse outcomes, but its role is unlikely to be a critical one in severe ACAS patients undergoing OPCAB. Therefore, they opposed the customary employment of OPCAB with carotid endarterectomy (19). In the Coronary Artery Bypass Graft Surgery in Patients with Asymptomatic Carotid Stenosis Study (CABACS), the authors compared with OPCAB alone, severe ACAS patients undergoing OPCAB with carotid endarterectomy had a similar five-year risk of mortality and stroke (29). They recommended OPCAB alone for severe ACAS individuals (29). Our findings supported personalized surgical management based on refined clinical evaluation for severe ACAS patients undergoing OPCAB. Since our results confirmed that severe ACAS was independently associated with severe multi-organ dysfunction after OPCAB, a prompt intervention for severe ACAS may improve the prognosis of patients undergoing OPCAB theoretically (RCTs verification required). However, it has been reported that the combination of such an intervention with OPCAB is associated with a higher incidence of adverse postoperative outcomes (1, 30). Therefore, the potential benefits and risks of OPCAB with carotid endarterectomy should be assessed and personalized. In addition, the role of multi-organ parameters, especially the cardio-cerebral factors, should be emphasized during the process of severe ACAS management. For the etiology of 30-day mortality or stroke, the North American Symptomatic Carotid Endarterectomy Trial (NASCET) (27) demonstrated that strokes of cardioembolic origins among the severe ACAS patients was probably underestimated and could not be prevented by carotid endarterectomy. And as discussed above, severe cardio-cerebral atherosclerosis is another significant cause of 30-day adverse outcomes after OPCAB.
It is worth mentioning that the current study demonstrated a longer operation time was associated with severe multi-organ dysfunction and an increased rate of 30-day MACCEs. Our thinking on this issue is that patients with ACAS have increased risk factors before surgery, such as more severe atherosclerosis and ischemic damage to brain blood flow. In addition, we also observed that the degree and number of coronary artery stenosis may be more complex in patients with ACAS. Therefore, the anesthesia time and surgical plan may be adjusted, and the final time may be longer. Previous study confirmed that a longer operation time is an independent predictor of longer ICU stay, regardless of factors such as the number of blood products transfused, CPB/myocardial ischemic times, and ejection fraction, which are indirect markers to assess the complexity level of OPCAB procedures (31). However, prospective studies are still needed to directly demonstrate whether the association between surgical duration and adverse outcomes is affected by the complexity of cardiovascular disease and OPCAB procedures.
Our study has some limitations. First, the results lack long-term follow-up. Therefore the findings are limited to the 30-day timeframe and cannot be extrapolated beyond it. In addition, it is a small, single-center, retrospective observational study. High quality, large-scale, and multicenter randomized controlled trials are required to further confirm the optimal management of severe ACAS patients undergoing OPCAB. In addition, the explanation of adverse outcomes in the presence of ACAS is multifactorial. For instance, a valuable study demonstrated the risk of 30-day MACCEs was significantly greater in patients with diabetes, without clinical cardiovascular disease, who have both a longer diabetes duration and significant ACAS, compared with those who with a shorter duration and/or nonsignificant ACAS (32). And the confounding factor, namely diabetes, has been eliminated through multivariate analysis in the current study. However, since this is a retrospective observational study, the possibility of some unmeasured confounding variables still cannot be ruled out.
ConclusionsSevere ACAS was independently associated with severe multi-organ dysfunction, measured by SOFA maximum ≥11 after OPCAB, which may be associated further with an increased rate of 30-day postoperative mortality and complications. Our data contributes to the growing evidence that highlights the importance of personalized assessment for potential advantages and disadvantages in prognosis of severe ACAS patients undergoing OPCAB with carotid endarterectomy. Nevertheless, the role of multi-organ parameters, especially cardio-cerebral factors, should be emphasized during the process of severe ACAS management.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by the Ethics Committees of Beijing Anzhen Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsTW: Data curation, Methodology, Writing – original draft, Writing – review & editing. CZ: Data curation, Methodology, Writing – original draft. JC: Data curation, Methodology, Project administration, Resources, Writing – original draft. KZ: Formal Analysis, Methodology, Project administration, Writing – original draft. RW: Methodology, Project administration, Writing – original draft. YX: Conceptualization, Data curation, Formal Analysis, Writing – original draft. RD: Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. JW: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Beijing Nova Program (No. Z201100006820088, No. 20220484174), Beijing Natural Science Foundation (L222098, 7232041). Beijing Association for Science and Technology's “Golden-Bridge Seed Funding Program” (ZZ22055).
AcknowledgmentsWe thank Elsevier Language Editing Services for English language editing. We also thank the methodology teacher Zhang Congcong for doing quality control for us.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsACAS, asymptomatic severe carotid artery stenosis; CAD, coronary artery disease; SOFA, sequential organ failure assessment; AF, previous atrial fibrillation; BMI, body mass index; COPD, previous chronic obstructive pulmonary diseases; CVA, cerebral vascular accident; EuroSCORE, European system for cardiac operative risk evaluation; LVEF, left ventricular ejection fraction; MI, myocardial infarction; SD, standard deviation; OPCAB, off-pump coronary artery bypass grafting; AF, atrial fibrillation; AKI, acute kidney injury; MACCEs, major adverse cardiovascular and cerebral events; VA, ventricular arrhythmias; TIA, transient ischemic attacks; CAS, carotid artery stenosis; STS, society of thoracic surgeons; OR, odds ratio; CI, confidence interval; MODS, multiple organ dysfunction score; NASCET, North American symptomatic carotid endarterectomy trial; CPB, cardiopulmonary bypass; CABACS, coronary artery bypass graft surgery in patients with asymptomatic carotid stenosis study.
References1. Hill MD, Shrive FM, Kennedy J, Feasby TE, Ghali WA. Simultaneous carotid endarterectomy and coronary artery bypass surgery in Canada. Neurology. (2005) 64:1435–7. doi: 10.1212/01.WNL.0000158477.55659.FE
PubMed Abstract | Crossref Full Text | Google Scholar
2. Primary Panel: Abramson BL, Al-Omran M, Anand SS, Albalawi Z, Coutinho T, de Mestral C, et al. Canadian cardiovascular society 2022 guidelines for peripheral arterial disease. Can J Cardiol. (2022) 38:560–87. doi: 10.1016/j.cjca.2022.02.029
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ruka E, Lesur O, Gingras M, Buruian M, Voisine E, Marzouk M, et al. Relationship between the degree of carotid stenosis and the risk of stroke in patients undergoing cardiac surgery. Can J Cardiol. (2022) 38:347–54. doi: 10.1016/j.cjca.2021.11.007
PubMed Abstract | Crossref Full Text | Google Scholar
4. Santarpino G, Nicolini F, De Feo M, Dalén M, Fischlein T, Perrotti A, et al. Prognostic impact of asymptomatic carotid artery stenosis in patients undergoing coronary artery bypass grafting. Eur J Vasc Endovasc Surg. (2018) 56:741–8. doi: 10.1016/j.ejvs.2018.07.042
PubMed Abstract | Crossref Full Text | Google Scholar
5. Naylor AR, Mehta Z, Rothwell PM, Bell PRF. Carotid artery disease and stroke during coronary artery bypass: a critical review of the literature. Eur J Vasc Endovasc Surg. (2002) 23:283–94. doi: 10.1053/ejvs.2002.1609
PubMed Abstract | Crossref Full Text | Google Scholar
6. North American Symptomatic Carotid Endarterectomy Trial Collaborators; Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. (1991) 325:445–53. doi: 10.1056/NEJM199108153250701
PubMed Abstract | Crossref Full Text | Google Scholar
7. Greenhall RM, Potter JF, Sharples DJ, Scholefield PF, Sterne J, Brown MM, et al. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European carotid surgery trial (ECST). Lancet. (1998) 351:1379–87. doi: 10.1016/S0140-6736(97)09292-1
PubMed Abstract | Crossref Full Text | Google Scholar
8. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. (1998) 339:1415–25. doi: 10.1056/NEJM199811123392002
PubMed Abstract | Crossref Full Text | Google Scholar
9. Drakopoulou M, Oikonomou G, Soulaidopoulos S, Toutouzas K, Tousoulis D. Management of patients with concomitant coronary and carotid artery disease. Expert Rev Cardiovasc Ther. (2019) 17:575–83. doi: 10.1080/14779072.2019.1642106
PubMed Abstract | Crossref Full Text | Google Scholar
10. Aboyans V, Ricco J-P, Bartelink M-LEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS). Eur Heart J. (2018) 39:763–816. doi: 10.1093/eurheartj/ehx095
PubMed Abstract | Crossref Full Text | Google Scholar
11. Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary. J Thorac Cardiovasc Surg. (2012) 143:4–34. doi: 10.1016/j.jtcvs.2011.10.015
PubMed Abstract | Crossref Full Text | Google Scholar
12. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/ SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Vasc Med. (2011) 16:35–77. doi: 10.1177/1358863X11399328
PubMed Abstract | Crossref Full Text | Google Scholar
13. Chang CH, Chen SW, Fan PC, Lee CC, Yang HY, Chang SW, et al. Sequential organ failure assessment score predicts mortality after coronary artery bypass grafting. BMC Surg. (2017) 17(1):22. doi: 10.1186/s12893-017-0219-9
PubMed Abstract | Crossref Full Text | Google Scholar
14. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. (2001) 286:1754–8. doi: 10.1001/jama.286.14.1754
PubMed Abstract | Crossref Full Text | Google Scholar
15. Antonelli M, Moreno R, Vincent JL, Sprung CL, Mendoça A, Passariello M, et al. Application of SOFA score to trauma patients. Intensive Care Med. (1999) 25:389–94. doi: 10.1007/s001340050863
PubMed Abstract | Crossref Full Text | Google Scholar
17. Mehta RH, Grab JD, O'Brien SM, Glower DD, Haan CK, Gammie JS, et al. Clinical characteristics and in-hospital outcomes of patients with cardiogenic shock undergoing coronary artery bypass surgery. Circulation. (2008) 117:876–85. doi: 10.1161/CIRCULATIONAHA.107.728147
PubMed Abstract | Crossref Full Text | Google Scholar
18. Writing Committee Members; Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization. J Am Coll Cardiol. (2022) 79:e21–e129. doi: 10.1016/j.jacc.2021.09.006
PubMed Abstract | Crossref Full Text | Google Scholar
19. Mahmoudi M, Hill PC, Xue Z, Torguson R, Ali G, Boyce SW, et al. Patients with severe asymptomatic carotid artery stenosis do not have a higher risk of stroke and mortality after coronary artery bypass surgery. Stroke. (2011) 42:2801–5. doi: 10.1161/STROKEAHA.111.618082
PubMed Abstract | Crossref Full Text | Google Scholar
20. Naylor AR, Bown MJ. Stroke after cardiac surgery and its association with asymptomatic carotid disease: an updated systematic review and meta-analysis. Eur J Vasc Endovasc Surg. (2011) 41:607–24. doi: 10.1016/j.ejvs.2011.02.016
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ghosh J, Murray D, Khwaja N, Murphy MO, Walker MG. The influence of asymptomatic significant carotid disease on mortality and morbidity in patients undergoing coronary artery bypass surgery. Eur J Vasc Endovasc Surg. (2005) 29:88–90. doi: 10.1016/j.ejvs.2004.07.011
PubMed Abstract | Crossref Full Text | Google Scholar
22. Baiou D, Karageorge A, Spyt T, Naylor AR. Patients undergoing cardiac surgery with asymptomatic unilateral carotid stenoses have a low risk of peri-operative stroke. Eur J Vasc Endovasc Surg. (2009) 38:556–9. doi: 10.1016/j.ejvs.2009.08.001
PubMed Abstract | Crossref Full Text | Google Scholar
23. Li Y, Walicki D, Mathiesen C, Jenny D, Li Q, Isayev Y, et al. Strokes after cardiac surgery and relationship to carotid stenosis. Arch Neurol. (2009) 66:1091–6. doi: 10.1001/archneurol.2009.114
PubMed Abstract | Crossref Full Text | Google Scholar
24. Sharif AF, Fayed MM. Evaluation of multiple organ dysfunction score (MODS) and the sequential organ failure assessment (SOFA) score as in-hospital outcome predictors among cases of hydrogen cyanamide exposure: a cross-sectional study. Environ Sci Pollut Res Int. (2021) 28:42161–76. doi: 10.1007/s11356-021-13655-6
PubMed Abstract | Crossref Full Text | Google Scholar
26. Hartkamp NS, Petersen ET, Chappell MA, Okell TW, Uyttenboogaart M, Zeebregts CJ, et al. Relationship between haemodynamic impairment and collateral blood flow in carotid artery disease. J Cereb Blood Flow Metab. (2018) 38:2021–32. doi: 10.1177/0271678X17724027
PubMed Abstract | Crossref Full Text | Google Scholar
27. Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. N Engl J Med. (2000) 342:1693–700. doi: 10.1056/NEJM200006083422302
PubMed Abstract | Crossref Full Text | Google Scholar
28. Kanemitsu S, Tanabe S, Ohue K, Miyagawa H, Miyake Y, Okabe M. Improve morbidity and mortality in coronary artery bypass graft surgery for severe atherosclerosis. Ann Vasc Dis. (2011) 4:93–8. doi: 10.3400/avd.oa.10.01044
PubMed Abstract | Crossref Full Text | Google Scholar
29. Knipp SC, Holst T, Bilbilis K, von Velsen O, Ose C, Diener H-C, et al. Five-year results of coronary artery bypass grafting with or without carotid endarterectomy in patients with asymptomatic carotid artery stenosis: CABACS RCT. Stroke. (2022) 53:3270–7. doi: 10.1161/STROKEAHA.121.037493
PubMed Abstract | Crossref Full Text | Google Scholar
30. Ricotta JJ, Wall LP, Blackstone E. The influence of concurrent carotid endarterectomy on coronary bypass: a case-controlled study. J Vasc Surg. (2005) 41:397–401. doi: 10.1016/j.jvs.2004.11.035
留言 (0)