Dear Editor,
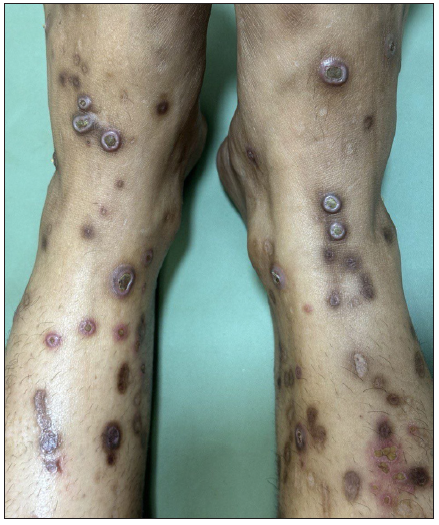
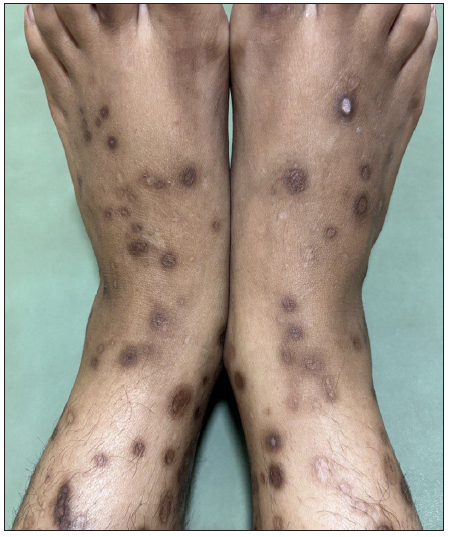
A 29-year-old woman, with multiple chronic conditions including type 1 diabetes mellitus, hypertension, pancreatitis, gastric ulcer, hypothyroidism, and depression, visited the dermatology department with generalised hyperkeratotic papules associated with intense itching for the past four years. Examination revealed multiple discrete papules and nodules with central keratotic plugs accompanied by excoriations and post-inflammatory hyperpigmentation. Her upper and lower extremities had most of the lesions, with a few on her lower back and face body surface area (BSA>20%); the Koebner phenomenon was also observed [Figure 1a].
Export to PPT
Export to PPT
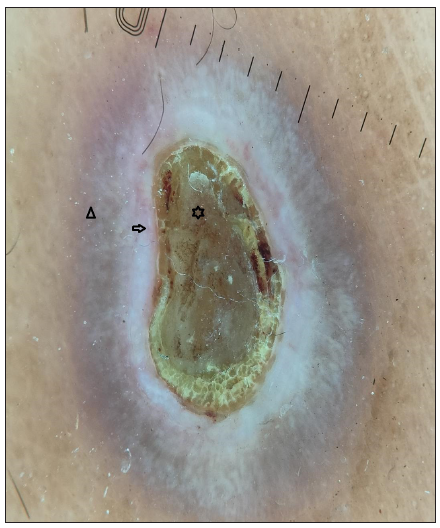
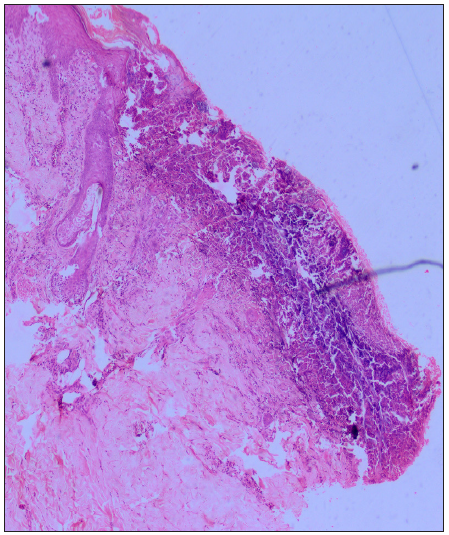
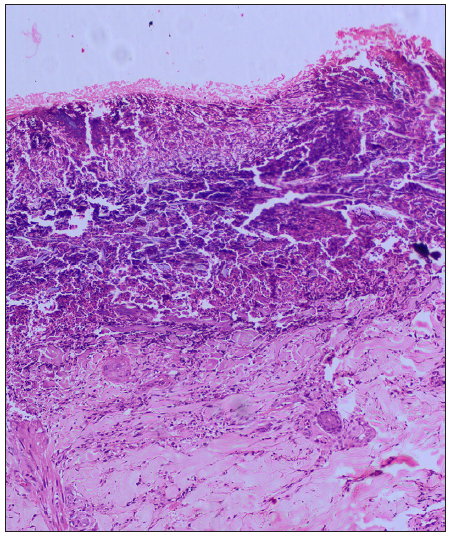
Dermoscopy (Dermlite DL5 in polarised mode) revealed four zones: a central keratotic plug with a structureless white area, a structureless pink area with dotted vessels, and a structureless brown area in concentric rings [Figure 1b]. Clinical differentials of perforating dermatosis (PD) and excoriated prurigo nodularis were considered. The haematological investigations revealed a significant elevation in blood glucose levels, with HbA1c reading of 11.5%. Complete haemogram, liver, and kidney function tests were normal, and anti-thyroid peroxidase or anti-islet cell antibodies were not detected. Other findings were atrophic pancreas with areas of calcification on computed tomography (CT), pangastritis, and duodenitis on upper gastrointestinal (GI) endoscopy. A punch biopsy from a keratotic papule was sent for histopathology, which depicted a cornified plug with basophilic debris embedded in an epidermal invagination with perifollicular inflammatory infiltrate [Figure 2a and 2b]. Special stains for collagen and elastin were negative. A final diagnosis of Kyrle’s disease (KD) with multisystem pathologies was made.
Export to PPT
Export to PPT
Based on the perforating dermatosis (PD) severity score suggested by Kawakami et al., the patient had an Eczema Area and Severity Index (EASI) of 12.2/36 and a pruritus score of 8/10, attaining a total score of 13 which comes under moderate (7–15) involvement.1
Considering the severity and its detrimental effect on the patient’s quality of life, systemic treatment was deemed appropriate. Systemic steroids and oral retinoids were not considered due to underlying comorbidities. Apremilast, a phosphodiesterase inhibitor was considered for treating this patient along with topical keratolytics. After three months of starting apremilast 30mg twice a day, the patient’s itch severity score improved significantly from 9 to 3. There has been a notable decrease in both new and existing lesions [Figure 3]. PD severity score also decreased from 13 to 4. Our patient has been maintaining the clinical response for the past eight months, without experiencing any adverse events.
Export to PPT
KD is a type of acquired perforating dermatosis (APD), commonly observed in adults (30–50 years), predominantly among females. The condition manifests as discrete hyperkeratotic papules and nodules, predominantly affecting the extensor surface of the limbs, accompanied by pruritus and koebnerisation. Excoriated prurigo nodularis, insect bite hypersensitivity, chronic folliculitis, and other APDs are among the diagnostic considerations.
The exact pathogenesis of KD remains unclear. Due to its frequent associations, underlying systemic disorders are considered as the main etiology. Some factors that contribute to this are uremia (renal diseases), hyperphosphatemia, elevated fibronectin levels, advanced glycated end products (diabetes), abnormal low-density lipoprotein (LDL) levels (diabetes and lipid disorders), and occasionally infections. The proposed final pathway involves a disruption in the epidermal keratinisation process, leading to the accumulation of abnormal keratin. This triggers inflammation, resulting in the expulsion of material via the epidermis followed by re-epithelisation.2,3 Histologically, the epidermis is hyperplastic with cup-shaped invagination and a degenerative basophilic material with plug formation and no collagen or elastic fibres.4
The treatment of KD poses challenges and often yields unsatisfactory outcomes. Treatment options include topical corticosteroids, keratolytics, retinoids, oral corticosteroids, immunosuppressants, and phototherapy, but with limited effectiveness. Oral upadacitinib (oral selective Janus kinase [JAK] 1 inhibitor) has emerged as a novel treatment approach because of its action on JAK/signal transducers and activators of the transcription (STAT) pathway.3
Apremilast (phosphodiesterase-4 inhibitor) prevents the degradation of cyclic adenosine monophosphate (cAMP), which results in an antagonistic effect on the production of proinflammatory cytokines (TNF-α, IL-23, interferon (IFN)-γ and an increase in anti-inflammatory mediators (e.g. IL-10).5 This broad-spectrum anti-inflammatory action leads to a reduction in inflammatory cell infiltration in the epidermis, the inflammatory response against keratin, normalisation of epidermal keratinisation preventing hyperkeratosis, and reduction in itch severity.6
We encountered only a single case report describing a patient with Down syndrome who had psoriasis, hidradenitis suppurativa (HS), and PD. The patient’s PD showed a remarkable response to apremilast, which was initially prescribed for the treatment of psoriasis and HS.7
Herein, we report the satisfactory response with apremilast in a patient of KD associated with various comorbidities. Given its wide range of immunomodulatory effects and favourable safety profile, it could be a therapeutic option for chronic disorders as in our case.
留言 (0)