The primary goals of fixed appliance orthodontic therapy are smile correction and tooth alignment. The brackets and archwires make up the majority of the fixed orthodontic appliances. Plaque retention is influenced by these appliances’ surface properties and design. In addition, these devices make it challenging for patients to maintain good dental hygiene and brushing, which raises the prevalence of caries.[1,2]
The duration and effectiveness of therapy are adversely affected by high frictional forces that come from the contact of the bracket and guiding archwire. Surface roughness is a crucial characteristic and affects the bacterial aggregation, friction, biocompatibility, color stability, cleanliness, and esthetics of the appliance.[3]
Because orthodontic tools have so many undercuts, plaque accumulates, changing the oral microbiota and increasing the risk of dental caries, gingival inflammation, periodontal disease, white spot lesions, and enamel demineralization.
Several metals have been used as bacteriostatic or bactericidal agents. One of them is silver. Silver and its salts have been used as a bactericidal agent for a long time now. Due to their unique chemical and physical characteristics, silver nanoparticles (Ag NPs) have been shown to offer an alternative for the creation of new antibacterial medications.[1]
Given that silver ions affect plaque accumulation and frictional forces in fixed appliance therapy and surface roughness affects orthodontic tooth movement, it is critical to evaluate and compare the surface topography and frictional resistance of the silver ion-coated and uncoated wire.[4]
In vitro tests have been performed in the past to compare silver-coated and untreated wire.[5] However, the mechanical properties of the archwires can be impacted in the oral cavity by various factors such as masticatory pressures, food and drink, and debris.
Hence, this study has been taken up to compare the changes occurring in the frictional resistance, surface roughness, and anti-microbial properties of silver-coated and uncoated stainless steel archwires in the oral cavity.
AimThe aim of the study is to evaluate and compare the frictional resistance, surface roughness, and microbial colonization of silver-coated and uncoated stainless steel archwire.
Objectives(i) To evaluate the frictional resistance, surface roughness, and anti-microbial properties of the silver-coated part of the stainless steel archwire.
(ii) To evaluate the frictional resistance, surface roughness, and anti-microbial properties of the uncoated part of the stainless steel archwire.
(iii) To compare the frictional resistance, surface roughness, and anti-microbial properties of the silver-coated and uncoated stainless steel archwire.
MATERIAL AND METHODS Inclusion criteriaPatients requiring therapeutic extraction of bilateral upper first premolars as a part of the treatment
Good oral hygiene and healthy periodontium (plaque index score of 0–1 according to Silness and Loe).
Exclusion criteriaPatients who do not give consent for the study
Poor oral hygiene
Patients with compromised periodontium
Nonextraction cases
Systemic illnesses such as diabetes mellites, patients on medication like phenytoin or immunosuppressants
Missing teeth in the anterior region.
Source of dataThe present study took place at the Department of Orthodontics, Manubhai Patel Dental College and Hospital. Patients who were registered in the Department of Orthodontics, Manubhai Patel Dental College and Hospital, Vadodara, and required fixed orthodontic treatment and fulfilled inclusion criteria were considered for this study.
Sample sizeAssuming a 0.5 difference in mean frictional resistance between 2 groups with a standard deviation of 0.7, 60 sites (30/group) were taken to achieve 95% confidence and 80% power.
This was a split-mouth in vivo study. Thirty patients undergoing orthodontic treatment were selected for the study. The maxillary teeth were well aligned, requiring extraction of 1st premolar as a part of treatment. Each participant was treated with a fixed pre-adjusted appliance. The system used was a metal 0.022 × 0.028” slot MBT bracket system of Ormco, USA.
The surface modification [Figure 1] of wires coated with silver ions was carried out by the thermal vacuum evaporation method with silver (10 nm size) using vacuum coating unit model no.- 15 F6 HINDHIVAC (Hind High Vacuum Co., Bangalore). 0.019 × 0.025” stainless steel archwires of G&H Orthodontics, Franklin, USA, were used. Pure silver (99.9%) was used to obtain a 10 nm coating on orthodontic wires.
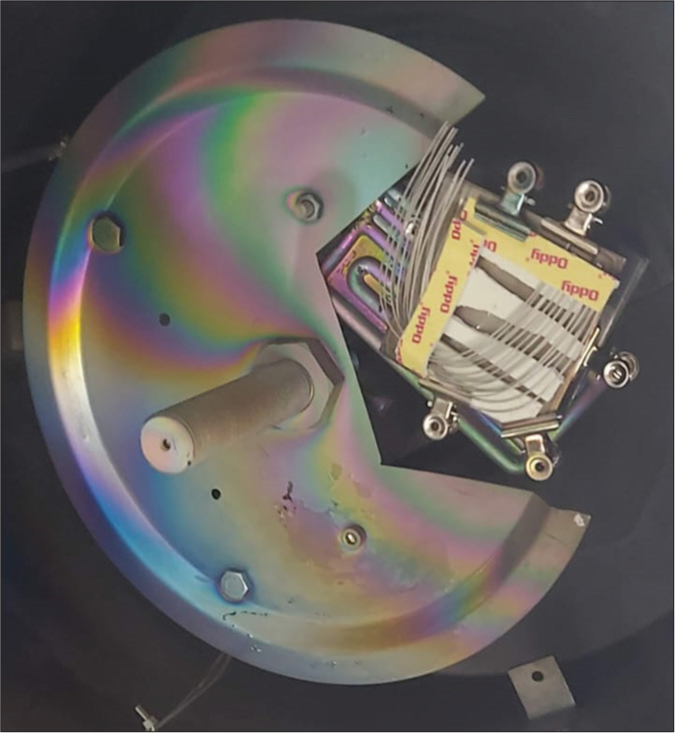
Export to PPT
Allocation methodParticipants were allocated into 2 sides; one side of the patient was the silver-coated side of the archwire, and the other was the uncoated side of the archwire [Figure 2]. Randomization was done between the right and left sides, and the wires were coded accordingly to ensure double blinding (patients and the investigator for the different parameters were blinded).
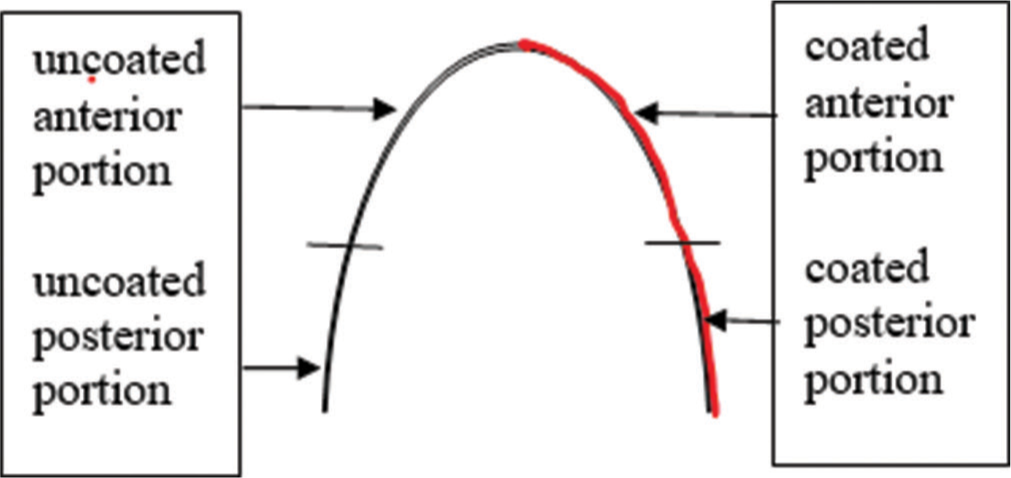
Export to PPT
The patients had undergone treatment with fixed appliances. Before commencing the study, the patients were treated with a wire sequence of 0.012” NiTi, 0.016” NiTi, 0.018” NiTi, 0.017” × 0.025” NiTi, as required in the case. Patients were then given 0.019” × 0.025” stainless steel (half-coated) wire for 6 weeks for torque expression.
Midlines were marked before retrieving the archwires. Castroviejo caliper (for precise measurements) was used to measure (a) the Distal aspect of the canine bracket on the one side to the distal of the canine bracket on the opposite side (anterior segment) and (b) the mesial of the second premolar (including the extraction site) to distal of the molar tube (posterior segment).
After completion of these 6 weeks, the wire was retrieved. This wire was then cut into anterior and posterior segments. The wire was stored in sterile vacuum-sealed dark pouches, which were coded according to the base code (code used for blinding).
The frictional resistance of wires was checked using a universal testing machine (UTM). The straight part of the posterior segment of the wire was cut up to 20 mm. This straight segment of each wire was then ligated to the bracket with stainless steel ligature instead of elastomeric ligature to decrease the friction. One end of the wire was fixed to the upper arm of UTM, and the other end of the wire was placed in the slot and secured with stainless steel ligature. The load cell registered the force levels required to move the wire along the bracket at a speed of 0.5 mm/min, as seen in a previous study done by Shah et al.[5] The unit for calculating the load values of frictional resistance was Newton (N).
The surface roughness of the wire was checked using a scanning electron microscope (SEM) at 3000× magnification at 15 kV. The wires were checked for irregularities on the surface.
To check the anti-microbial effect of silver coating, two brand of the cotton swab used for taking the sample swabs were then taken immediately after retrieval of the wire from the anterior segment: (1) from the side that is coated with silver and (2) on the uncoated side, demarcated by the marked midline. These swabs were taken to the microbiology laboratory to assess bacteria such as Lactobacillus acidophilus and Streptococcus mutans. Columbia Sheep Agar was used as the culture medium. They were incubated in a Sesw 28L incubator at 35–37°C for 24–48 h. The bacterial identification and colony-forming units were calculated using the Automated Vitek2 System (Biomerieux) [Figure 3].
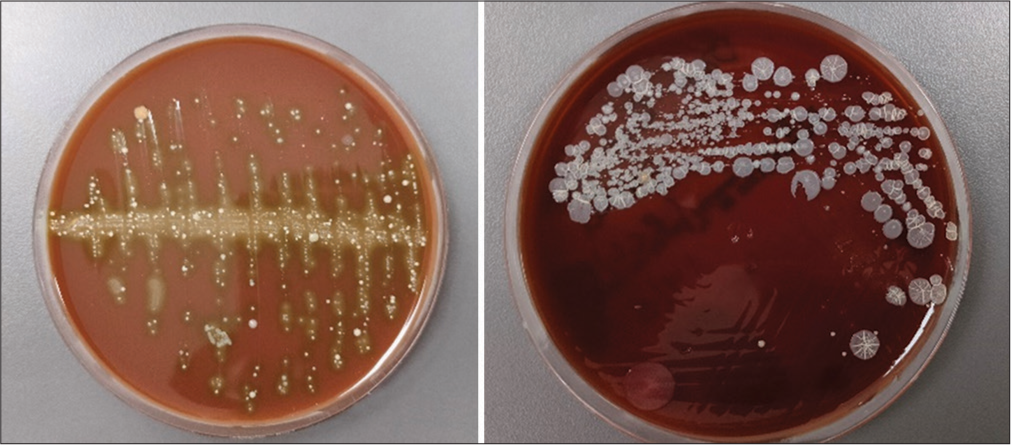
Export to PPT
When sent for these investigations, the sample was coded, such that double blinding was assured.
RESULTSForty-five patients were enrolled in the study, 15 were lost to follow-up, and 30 patients were included in the study. The archwires were retrieved after being placed passively in the oral cavity for 6 weeks. These were then sent for testing of frictional resistance, surface roughness, and microbial colonization. The results obtained from these interventions are given, respectively.
Owing to the split-mouth design, pairwise comparisons were considered for both categorical and continuous variables.
All the analysis was done using the Statistical Package for the Social Sciences version 26. A P < 0.05 was considered statistically significant. Normality was tested by the Shapiro– Wilk test. Inter-group comparisons of continuous variables were compared using the Wilcoxon signed-rank test or paired t-test based on the distribution of data. Inter-group comparisons of categorical variables were done using the McNemar–Bowker test.
For frictional resistance of the wireFrictional resistance was measured at two intervals in one cycle of the UTM machine for each sample: one at the point when the load is applied, i.e., load at the limit, and the second when the applied load is maximum, and applying any more load will cause permanent deformation.
Inter-group comparison of various frictional parameters for the silver-coated and uncoated stainless steel archwire is shown in [Table 1].
Table 1: Inter-group comparison of various frictional parameters.
Frictional resistance Coated Uncoated P-value Mean SD Mean SD Load at limit 1.17 0.52 1.28 0.68 0.721; NS Deflection at limit 5.00 0 4.99 0 - Maximum load 1.19 0.63 2.42 1.23 0.056; NS Deflection at maximum load 2.99 1.42 2.13 1.69 0.386; NSFor frictional resistance at the limit, the mean for the uncoated section was 1.28 ± 0.68 N, and for a coated section of the stainless-steel archwire was 1.17 ± 0.522 N at load at the limit. This showed that the load at the limit is more in the uncoated section, signifying more friction in the uncoated section, which was statistically non-significant (P = 0.721).
For the deflection of wire at this load, the mean for the uncoated section was 5.00 ± 0 mm, and for a coated section of the stainless-steel archwire was 4.99 ± 0 mm. This shows that the deflection at limit was similar in both sections.
For frictional resistance at maximum load, the mean for the uncoated section was 2.42 ± 1.69 N, and for a coated section of the stainless-steel archwire was 1.19 ± 0.63 N. This shows that the friction at maximum load was more in the uncoated section, but this increased friction was statistically not significant that more friction in the uncoated section was statistically non-significant (P = 0.056).
For the deflection of wire at maximum load, the mean for the uncoated section was 2.13 ± 1.69 mm, and for a coated section of the stainless-steel archwire was 2.99 ± 1.42 mm. This shows that the deflection at maximum load is less in the uncoated section, signifying more friction in the uncoated section, which was statistically non-significant (P = 0.386).
For microbial colonizationFrom [Table 2], we can conclude that most of the samples had heavy and moderate Streptococcus accumulation in uncoated wires, while most of the coated wires had moderate to scanty coating, which was significant (P = 0.024).
Table 2: Inter-group comparisons of bacterial growth in coated and uncoated wires.
Coated Uncoated P-value n % n % Streptococcus mutans Heavy 4 13.3 13 43.3 0.024; Sig Moderate 13 43.3 13 43.3 No growth 2 6.7 1 3.3 Scanty 11 36.7 3 10.0 Lactobacillus acidophilus No growth 29 96.7 27 90.0 0.5; NS Scanty 1 3.3 3 10.0As for Lactobacillus, the coated section showed no growth, and in the uncoated section, some samples did show a scanty growth of the same, resulting in a statistically non-significant difference between the groups (P = 0.5).
For surface roughnessA coated portion of wire under SEM is represented in [Figure 4a]. It shows rubbing marks on the surface of the wire.
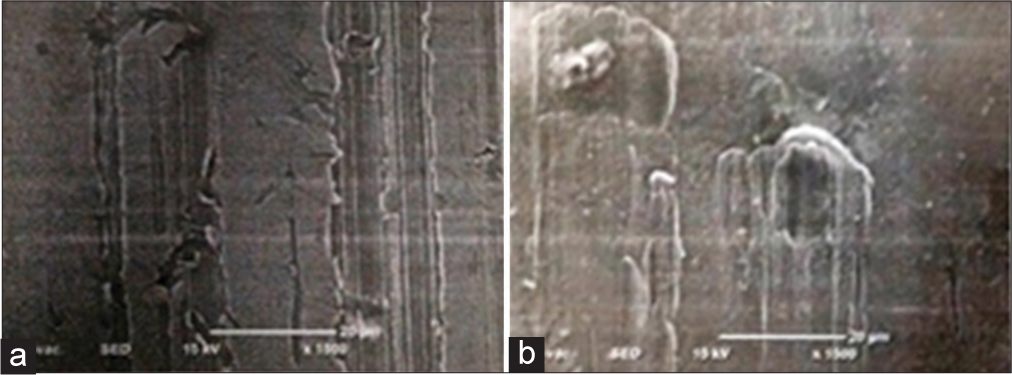
Export to PPT
The SEM image of an uncoated wire is shown in [Figure 4b]. Even this shows rubbing marks, but with dents, whorls, and unevenness were seen to be more on the surface.
DISCUSSIONThe use of fixed orthodontic appliances can accelerate the rate of plaque accumulation, which in turn results in enamel decalcification and gingival inflammation when not maintained properly. The relationship between oral bacteria and orthodontic treatment has been under study for a very long time. An increase in the incidence of dental caries and S. mutans bacterial counts was seen with fixed orthodontic appliances. Similarly, the placement of orthodontic appliances caused the colonization of periodontal pathogenic bacteria such as Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens, and Fusobacterium nucleatum.[6]
It had been demonstrated that altering the surface by doping it with appropriate elements such as gold, zinc, copper, platinum, silver, or a mixture of them would significantly boost the antibacterial characteristics. Among them, silver has been known to be the most potent antibacterial agent since the dawn of medicine. It has been successfully applied to biomedical engineering in a variety of forms (nanoparticles, nanocomposite, colloids, foams, polymers, fibers, etc.) to prevent the formation of biofilms and, subsequently, the frequency of infections. Promising antibacterial and tribological qualities such as wear resistance, friction reduction, and surface lubrication are possessed by silver. In addition to its antibacterial qualities, silver also works well against a variety of fungi and viruses and does not have the same resistance issues as certain antibiotics.[7]
Metals have additional difficulties as orthodontic biomaterials, such as tooth friction. Since a portion of the applied force is dispersed to overcome friction and a portion is sent to the supporting structures to induce tooth movement, friction control poses a significant challenge.
Force loss due to friction between the bracket and archwire might reach 50%.[8] The intended tooth movement is consequently restricted or slowed. The wire’s size, elasticity, and surface structure, including any surface treatments, are all significant factors in addition to the alloy composition of the archwire. White spot lesions are caused by plaque accumulation, which is influenced by surface roughness.
Many studies on coating the surface of archwire with various metals, such as silver, gold, zinc, and molybdenum, have been carried out in an effort to address these drawbacks and make these materials more appropriate for orthodontic applications. According to published research, archwire surface treatment could reduce friction by up to 46%.[9]
The mechanism of action of silver involves the continuous release of nanoparticles of silver ions for antimicrobial action. Owing to electrostatic attraction and affinity to sulfur proteins, silver ions can adhere to the cell wall and cytoplasmic membrane. The adhered ions can enhance the permeability of the cytoplasmic membrane and lead to disruption of the bacterial envelope. After the uptake of free silver ions into cells, respiratory enzymes can be deactivated, generating reactive oxygen species but interrupting adenosine triphosphate production. Reactive oxygen species can be a principal agent in the provocation of cell membrane disruption and deoxyribonucleic acid (DNA) modification. As sulfur and phosphorus are important components of DNA, the interaction of silver ions with the sulfur and phosphorus of DNA can cause problems in DNA replication and cell reproduction or even result in the termination of the microorganisms. Moreover, silver ions can inhibit the synthesis of proteins by denaturing ribosomes in the cytoplasm.
Moreover, Ag NPs have anti-inflammatory properties. For this, Ag NPs (i) decrease levels of vascular endothelial growth factor; (ii) reduce expression of the hypoxiainducible factor 1α, which acts on bacterial destruction and regulates expression of pro-inflammatory genes; (iii) prevent the hypersecretion of mucins (mucus glycoproteins); and (iv) suppress the production of pro-inflammatory cytokines such as interleukin-12 and tumor necrosis factor α and also provoke a decrease in the expression of the cyclooxygenase-2 gene at higher concentrations. Therefore, further studies regarding the use of silver hindering orthodontic tooth movement are required.
In one of his investigations, Ghasemi et al. electroplated the brackets with silver films ranging in thickness from 8 μm to 10 μm. However, they were unable to detect any appreciable increases in frictional resistance. They postulated that the friction decrease was prevented by the thick coating of deposited silver.[3] However, pure silver can now be reduced to nanometer-sized particles because of advances in nanoscience[8], which make it possible to coat fixed orthodontic appliances with an incredibly thin layer of silver. In our investigation, we applied a layer of 10 nm-thick Ag NPs to the stainless steel archwire. As seen in [Table 2], this layer showed antibacterial effects.
Antibacterial activity may also be impacted by Ag NP concentration. At a concentration of 0.004% w/w, Ag NPs smaller than 15 nm have demonstrated the most efficacy in inhibiting the growth of oral bacteria that cause decay of teeth and halitosis.[10] In our study, the Ag NP layer was 10 nm, and the anti-microbial investigation showed positive results.
Ag NPs can be coated on the stainless steel archwire[11] using a variety of techniques, including spin coating, chemical vapor deposition, atomic layer deposition, electroplating, and spray pyrolysis. In our investigation, argon gas was utilized as the sputtering gas to partially coat the stainless steel archwire using the sputtering technique of physical vapor deposition (PVD) in a vacuum coating unit. The PVD process has the advantage of being comparatively safe and applicable to orthodontic appliances.
The likelihood of silver toxicity was observed with ionic and nanocrystalline silver in humans at cumulative doses in the range of 70–1500 mg silver/kg body weight.[12] The amount of silver used for coating the stainless steel archwires in our study was in micrograms. Thus, it can be considered non-toxic.
In our study, the frictional resistance of a silver-coated stainless-steel wire was evaluated and compared with an uncoated counterpart. It was a split-mouth study.
It was seen that the load at the limit for frictional resistance was 1.171015 N in the coated wire and 1.282344 N in the uncoated section. In the study by Shah et al.[5] and by Shah et al.[13], similar measurements were of higher value when compared to our study.
It was seen that at maximum load for frictional resistance was 1.186799 N in the coated wire and 2.42451 N in the uncoated portion. In the study by Shah et al.[5] and by Shah et al.,[13] the values for the coated portion showed similar results.
It was seen that the deflection at maximum load for frictional resistance was 2.99305 mm in the coated wire and 2.13164 mm in the uncoated portion. In the study by Shah et al.,[13] the results were similar to our study.
Reduction in friction is beneficial for en masse retraction of the anterior teeth using the sliding mechanics. Thus, patients undergoing upper 1st premolar extraction were included in the study.
As for surface roughness, the uncoated wire was seen to be more rough when compared to the roughness of the silver-coated wire. This was also supported by the in vitro study by Shah et al.[13]
The anti-microbial activity was also performed. S. mutans and L. acidophilus were the organisms under study. As for S. mutans, samples taken from the coated section of the wire showed scanty to moderate growth of the bacteria, whereas the uncoated section showed moderate to heavy growth of the bacteria. The L. acidophilus showed no growth in the coated section but showed scanty growth in the uncoated section of the archwire.
In a study done by Bindu et al.,[14] the anti-microbial activity of Ag NPs was checked by coating on the stainless-steel molar bands. The coated bands showed a potent antimicrobial effect in the in vitro simulation of the oral environment.
In a study by Metin-Gürsoy et al.,[1] nanosilver coating was done on routine orthodontic brackets and bonded in albino rats. Here, the results showed inhibition of the S. mutans and reduction in caries, thus proving the antimicrobial effect of the Ag NPs.
In a study done by Ryu et al.,[15] they coated a specimen of stainless steel with silver. In this in vitro study, the high potency of silver ion as an antimicrobial agent was concluded against S. mutans and Aggregatibacter actinomycetemcomitans.
In a systemic review, Siva et al.[16] reviewed the antimicrobial effects of various nano-particle coatings on orthodontic brackets. They concluded that there was a significant decrease in the amount of plaque accumulation and a reduction in bacterial colony count.
Tawakal et al.[17] compared the antimicrobial effect of silver and silver chitosan nanoparticles on ceramic brackets. According to this study, these nano particles were seen to be effective against S. mutans.
All these in vitro studies[1,14-17] show results that are in accordance with our in vivo study. Thus, coating the orthodontic appliances with Ag NPs, in this case, stainless steel archwire, has a potent antimicrobial effect on oral microflora such as the S. mutans and L. acidophilus. This would reduce plaque accumulation and reduce the occurrence of white spot lesions and caries.
LimitationIn our study, the archwire was placed passively in the oral cavity, and no retraction forces were applied. Hence, the difference in the rate of retraction was not measured; measuring which may help us in knowing whether this statistically non-significant decrease in the frictional resistance is clinically relevant. Thus, further studies are required to gain a better knowledge about the benefits of AG-NPs.
CONCLUSIONWith this study, it was concluded that:
Silver coating the stainless steel archwire does not alter the frictional properties of the wire.
The surface of a silver-coated wire was smoother when compared to the uncoated stainless steel archwire.
Silver-coated wire showed better anti-microbial activities than the uncoated stainless steel archwire.
留言 (0)