S-palmitoylation is a highly conserved post-translational lipid modification widely present in eukaryotes. It plays crucial roles in various physiological processes by influencing protein structure, localization, transport, and function (Tabaczar et al., 2017). Based on the different acyl acceptors, protein palmitoylation can be classified into S-palmitoylation, N-palmitoylation, and O-palmitoylation (Zhang Y. et al., 2021). Among these modifications, S-palmitoylation involves the attachment of palmitic acid (PA) to target proteins via thioester bonds (Sobocińska et al., 2018). Due to the specificity of thioester bonds, this modification is a valuable reversible modification (Qu et al., 2021). S-palmitoylated proteins are predominantly membrane proteins, especially transmembrane and peripheral membrane proteins (Charollais and Van Der Goot, 2009). Upon modification, target proteins undergo changes in hydrophobicity and membrane-binding ability, thereby altering protein structure, localization, transport, and function. The combination of S-palmitoylation modification and its reverse reaction—depalmitoylation—leads to periodic changes in the structure and function of modified proteins, aligning with specific biological functions of individuals (Li et al., 2022).
As the most malignant primary tumor of the brain, Glioblastoma has long been a challenge for neurologists, oncologists, clinicians and so on Shergalis et al., 2018. Understanding its pathogenesis has been a focal and challenging aspect of scientific research. The significant role of zDHHC-mediated palmitoylation in GBM is increasingly recognized, particularly in key proteins associated with its occurrence and progression (Chen et al., 2020a). Increasing evidence suggests that palmitoylation or palmitoyltransferases could become novel targets for cancer therapy (Chen et al., 2018). This review comprehensively analyzes how zDHHC-mediated S-palmitoylation modification regulates the occurrence and development of GBM (Figure 1). Additionally, it explores the potential of developing protein S-palmitoylation-targeted drugs for more precise personalized therapy.
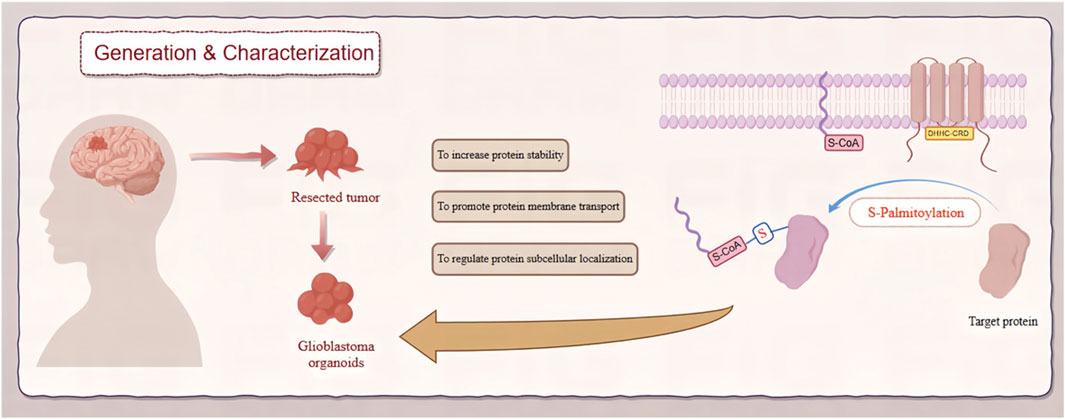
Figure 1. Role of zDHHC-mediated S-palmitoylation in GBM. This diagram depicts the involvement of zDHHC enzymes in S-palmitoylation, a post-translational modification, and its effects on the pathogenesis of GBM. zDHHC enzymes facilitate the addition of palmitoyl groups to specific substrate proteins, altering their localization, stability, and function. These modifications influence key signaling pathways that drive GBM progression and malignancy.
2 S-palmitoylationS-palmitoylation is a reversible protein lipid modification that widely exists in eukaryotic cells and participates in regulating downstream gene transcription, expression, and signal transduction, thereby influencing cellular activities (Resh, 2013). Discovered in the 1980s, palmitoylation is catalyzed by the protein acyltransferase (PAT) family, which attaches palmitic acid (PA), a 16-carbon saturated fatty acid, to cysteine residues on target proteins (Schmidt et al., 1979; Chen et al., 1985; Mitchell et al., 2006). PA, which is one of the important substrates involved in palmitoylation modification, is the most common saturated fatty acid in the human body and constitutes 20%–30% of total fatty acids and can be obtained from dietary sources like meat and dairy (Carta et al., 2017; Carta et al., 2015) or synthesized endogenously through de novo lipogenesis (DNL) from other metabolites (Song et al., 2018; Kersten, 2001). Of note, PA plays essential physiological roles, serving as a component of cell membranes in the form of phospholipids and sphingolipids, providing energy through β-oxidation, or being esterified into triglycerides for lipid storage (Carta et al., 2017). Changes in PA concentration directly affect the efficiency and function of palmitoylation (Pei et al., 2016; Wang et al., 2023). Upon modification, the hydrophobicity and affinity of target proteins for the cell membrane surface increase.
Unlike irreversible lipid modifications such as myristoylation or prenylation, which involve amide or thioether bonds, respectively, S-palmitoylation forms thioester bonds (Robert and Vagner, 2018; Yuan et al., 2020a). These bonds are less stable due to the larger atomic radius and weaker bonding energy of sulfur compared to oxygen (Ashenhurst, 2015; van Bergen et al., 2016). Additionally, sulfur atoms are prone to oxidation and reduction reactions, leading to the instability of thioester bonds and enabling proteins to cycle between palmitoylated and depalmitoylated forms, thus spatio-temporal control of protein function can be conferred (Ko and Dixon, 2018; Wang et al., 2020). Depalmitoylation, the reverse reaction of palmitoylation, is catalyzed by palmitoyl-protein thioesterases (PPTs), including acyl-protein thioesterases (APT1/2), palmitoyl-protein thioesterases (PPT1/2), or proteins containing α/β hydrolase domains (ABHD17A/B/C) (Hornemann, 2015). Overall, depalmitoylation serves as an essential complement to palmitoylation, necessary for protein function and cellular processes. The dynamic balance between palmitoylation and depalmitoylation allows cells to regulate protein activity, stability, and localization to adapt to different cellular environments and physiological needs. Many key proteins in cellular signaling pathways undergo palmitoylation, and depalmitoylation can alter their activity and stability, thereby affecting signal transduction. Through depalmitoylation, cells can timely adjust the activity of signaling pathways to adapt to different cellular stimuli.
3 ZDHHCThe enzymes catalyzing S-palmitoylation modification, known as PATs, are referred to as DHHC-PATs (Mitchell et al., 2006). In most cases, these enzymes contain a cysteine-rich domain (CRD) comprising 51 amino acids. Within the CRD, there’s a highly conserved catalytic domain called the aspartate-histidine-histidine-cysteine (DHHC) motif, which is crucial for maintaining the palmitoylation activity of the PAT molecule (Tabaczar et al., 2017; Gottlieb et al., 2015). The DHHC domain serves as the catalytic center of the enzyme, and mutations in this domain can weaken the palmitoyl transfer capability (Ko and Dixon, 2018; Resh, 2006a). Additionally, the CRD contains two zinc-binding sites that coordinate with two Zn2+ ions which do not directly participate in protein catalysis play a vital role in maintaining the integrity and functionality of the domain (Zmuda and Chamberlain, 2020; Stix et al., 2020). Due to the presence of the zinc finger DHHC domain, this class of PATs is also referred to as zinc finger DHHC domain-containing palmitoyl transferases (zDHHC).
Up to present, There are 23 zDHHC genes identified in the human genome, named zDHHC1-24 (excluding zDHHC10) (Ko and Dixon, 2018). These zDHHC proteins constitute the zDHHC protein family, primarily responsible for the catalytic activity of palmitoyl transferases (Linder and Deschenes, 2007). These enzymes are mainly localized in the endoplasmic reticulum (ER), Golgi apparatus, with a smaller fraction found on the plasma membrane (PM), mitochondria and perinuclear regions (Chen et al., 2021; Greaves and Chamberlain, 2011; Jansen and Beaumelle, 2022). The localization of DHHC-PATs partly depends on structure and motif. For example, lysine-based sorting signals on the sequences of DHHC4 and DHHC6 enable them to form KXX and KKXX motifs, thus guiding them to the ER membrane (Gorleku et al., 2011).
Structurally, the molecular structure of zDHHC proteins which as a type of polytopic integral membrane protein consists of an N-terminal domain facing the cytoplasm, 4-7 transmembrane domains (TMDs), and a C-terminal domain also facing the cytoplasm (Ohno et al., 2006). N-terminal and C-terminal sequences exhibit significant diversity, mediating protein-protein interactions and facilitating acyl transfer processes (Jiang et al., 2018; Malgapo and Linder, 2021). The TMDs collectively form a cavity resembling a tent, facilitating the binding of fatty acyl chains (Rana et al., 2018). The DHHC domain, crucial for maintaining catalytic activity, is located between the second and third TMDs (Jiang et al., 2018; Rana et al., 2019). Besides the DHHC motif, other conserved motifs such as Asp-Pro-Gly (DPG), Thr-Thr-x-Glu (TTxE), and PaCCT have been reported (Mitchell et al., 2006). The second serine in the TTxE motif can directly interact with the aspartate of the DHHC motif to form hydrogen bonds, while Asn266 in the PaCCT motif utilizes its hydrogen bonding capability to assist inside chain amide formation (Rana et al., 2018). This suggests that these conserved residues may also participate in substrate protein recognition and catalysis processes by making key contacts with the DHHC domain (Rana et al., 2018).
Some zDHHC proteins require interaction with auxiliary factors to form complexes, such as the DHHC9/GCP16 complex (Swarthout et al., 2005) and the DHHC6/Selk complex (Fredericks et al., 2014). Additionally, certain zDHHC enzymes require palmitoylation by other family members to initiate S-palmitoylation cascades, thereby regulating substrate palmitoylation (Abrami et al., 2017; Plain et al., 2020). Specific zDHHC molecules may exhibit unique substrate binding preferences, as evidenced by zDHHC13 and zDHHC17’s strong selectivity for synaptic-related proteins (Rodenburg et al., 2017; Zhang and Hang, 2017). Notably, zDHHC13 and zDHHC17 contain an ankyrin repeat domain (AR) within their N-terminal domains (Lemonidis et al., 2017), facilitating membrane localization and enhancing the palmitoylation activity of other zDHHC enzymes (Lemonidis et al., 2014; Lemonidis et al., 2015). These findings underscore the complex and efficient structure-function relationship within the zDHHC family, providing insights into the mechanism underlying protein palmitoylation.
4 Mechanisms of S-palmitoylationAlthough 23 zDHHCs have been identified to catalyze protein S-palmitoylation, the specific process remains unclear (Yuan et al., 2024). Previous studies have found that in most cases, zDHHCs catalyze substrate S-palmitoylation through a two-step process, known as the ping-pong kinetic mechanism (Rana et al., 2019; Roth et al., 2002). First, zDHHC proteins undergo auto-palmitoylation, where the cysteine residue in the DHHC-CRD domain of zDHHC protein covalently binds with palmitoyl coenzyme A to form an acyl-enzyme intermediate (Malgapo and Linder, 2021). Subsequently, the crucial second step reaction occurs, where zDHHC proteins catalyze the transfer of the palmitoyl group from their own binding site to the cysteine thiol group of the protein substrate. Simultaneously, zDHHC proteins revert to their initial state, and the protein substrate forms an unstable thioester bond, completing the palmitoylation modification (Rana et al., 2018; Jennings and Linder, 2012; Chamberlain and Shipston, 2015; Salaun et al., 2020). The aforementioned reaction mechanisms apply to the catalytic processes of most zDHHC molecules (Stix et al., 2020). However, there is still insufficient evidence to determine whether zDHHC13, 17, and 19 can catalyze auto-palmitoylation or not. Further investigation may be needed to explore common mechanisms underlying zDHHC catalyzed palmitoylation modification and whether auto-palmitoylation does not occur in all zDHHCs (Lemonidis et al., 2014; Verardi et al., 2017).
Most proteins undergo palmitoylation on multiple amino acid residues, with reports suggesting up to 5-6 individual palmitoylated cysteine residues in some proteins (Zhang et al., 2018). While the above two-step kinetic mechanism applies to the majority of proteins, it has been observed that some proteins undergo auto-palmitoylation independently of the zDHHC protein family. Examples include yeast proteins (Swf1 and Pfa4) (Das et al., 2021), myelin protein P0 (Bharadwaj and Bizzozero, 1995) and others, which possess acyl-CoA within their structures. Due to the unique characteristics of their structures, some of these proteins exhibit intrinsic “enzyme-like” activities and can directly bind with palmitoyl-CoA to undergo auto-palmitoylation (Smotrys and Linder, 2004; Chan et al., 2016).
In conclusion, our comprehension of the mechanisms by which ZDHHC and APT enlist their protein substrates remains incomplete (Rana et al., 2018; Verardi et al., 2017; Lee et al., 2022). Data from the SwissPalm database reveals that palmitoylation encompasses over 2,400 mammalian proteins, as evidenced by findings from more than 100 proteomics and biochemical studies (Blanc et al., 2015). These proteins encompass integral membrane proteins, peripheral membrane proteins, cytoplasmic signaling proteins, and transcription factors. Oncoproteins such as NRAS, KRAS4A, HRAS, epidermal growth factor receptor (EGFR), and p53 depend on S-palmitoylation cycling to modulate their localization, activity, or interaction partners (Tate et al., 2019).
5 Functions of S-palmitoylationFrom a biochemical perspective, the binding of the lipid molecule palmitic acid can enhance the hydrophobicity of specific domains of target proteins (Jiang et al., 2018). Additionally, due to the instability of thioester bonds, palmitoylation and depalmitoylation can rapidly switch, acting as a protein switch or modulator similar to protein ubiquitination or phosphorylation (Blaskovic et al., 2013). Changes in hydrophobicity/hydrophilicity may affect various properties and functions of proteins. It can modulate the affinity of protein molecules for membrane structures, thereby determining protein localization (Levental and Lyman, 2023). Through different localizations, protein-protein interactions (PPI) may be altered, leading to activation or inhibition of downstream signaling pathways, affecting processes such as gene replication, transcription, expression, or protein secretion (Zhang Z. et al., 2021; Pei et al., 2022; Lee et al., 2021).
Palmitoylation modification plays a crucial role in the pathological and physiological functions of key proteins in signaling pathways such as RAS, MET, STING, EGFR and Hedgehog, etc. (Runkle et al., 2016; Lin et al., 2017). Taking the Ras protein family as an example, newly synthesized Ras proteins undergo palmitoylation modification during transport to the Golgi apparatus to enter the secretory pathway and transfer to the PM After activation at the PM, depalmitoylation occurs, weakening the protein’s binding to the membrane, leading to its transport back to the Golgi apparatus (Shahinian and Silvius, 1995; Busquets-Hernández and Triola, 2021). The dynamic palmitoylation of Ras regulates its cycling between the PM and Golgi apparatus, preventing nonspecific residence on the PM and facilitating the transmission of activated Ras signals downstream (Rocks et al., 2005). Moreover, there is evidence suggesting that EGFR signaling can be both promoted and inhibited by its palmitoylation. This might be due to the presence of multiple palmitoylation sites, which are regulated by different ZDHHC proteins and have distinct functional effects (Bollu et al., 2015). Palmitoylation also regulates the activity of G protein-coupled receptors, including the melanocortin-1 receptor (MC1R), which drives melanin production and DNA repair following UV exposure (Chen S. et al., 2017). Therefore, impaired palmitoylation of MC1R increases the risk of melanoma. Additionally, palmitoylation is involved in regulating the tumor microenvironment, including crucial processes such as angiogenesis and immune evasion (Lee et al., 2021; Pan and Chen, 2022). S-palmitoylation imparts different characteristics to proteins by attaching palmitic acid molecules, thereby affecting protein hydrophobicity, structural stability, localization, migration between membrane regions, and interactions with effectors, enzyme activity, protein storage, and more.
S-palmitoylation is also associated with resistance to cancer treatments. For example, ZDHHC2-mediated palmitoylation of mitochondrial acylglycerol kinase (AGK) increases sunitinib resistance in renal cell carcinoma by activating the AKT–mTOR signaling pathway (Sun et al., 2023). Similarly, ZDHHC16-mediated palmitoylation of PCSK9 induces sorafenib resistance in cancer through activation of the PI3K–AKT pathway (Sun et al., 2022).
6 GlioblastomaGlioblastoma (GBM) is a common malignant tumor originating from the central nervous system, which remains essentially incurable. Arising from neural glial cells, GBM can occur in various parts of the brain, accounting for approximately 51% of all malignant tumors of the brain. According to statistics, the incidence of GBM is 5 per 100,000 individuals, with a rising trend annually. While the affected population primarily comprises middle-aged and elderly individuals, GBM can occur across all age groups, including children (Xiao et al., 2023). Despite the standard treatment regimen for GBM, which involves maximal safe surgical resection followed by adjuvant therapies such as temozolomide (TMZ) chemotherapy, adjunctive radiotherapy, and tumor-treating fields (TTF) therapy, the median survival period post-diagnosis remains merely 8 months, with a 5-year survival rate of less than 7% (Zhong et al., 2021; Ostrom et al., 2022). Additionally, the majority of GBM patients experience tumor recurrence (Jiang et al., 2020; Daniel et al., 2022). The high recurrence rate may be attributed to the presence of dormant tumor-seeding cells located distant from the initial tumor or tumor cells infiltrating the normal brain parenchyma at the tumor’s periphery (Darmanis et al., 2017; Johnson et al., 2014).
GBM, also known as glioblastoma multiforme, was classified by the World Health Organization (WHO) in 2016 into IDH-wildtype and IDH-mutant subtypes based on the mutation status of isocitrate dehydrogenase (IDH) (Louis et al., 2016). Due to the closer resemblance of IDH-mutant GBM to anaplastic astrocytoma, some scholars have proposed renaming IDH-mutant GBM as astrocytoma, IDH-mutant, grade 4 (White et al., 2020). The 2021 WHO classification defines GBM as lacking IDH mutation, along with alterations related to telomerase reverse transcriptase (TERT) promoter mutation, epidermal growth factor receptor (EGFR) amplification, chromosome 10 copy number loss, and chromosome 7 copy number gain (Louis et al., 2021).
As a typical solid tumor, GBM exhibits characteristics of sustained cell proliferation, resistance to cell death, continuous angiogenesis, increased cellular invasion and metastasis. These features are accompanied by genomic instability and mutations, altered cellular metabolism, replicative immortality, sustained inflammation, evasion of growth suppression, and immune suppression (Hanahan, 2022; Verdugo et al., 2022). The mechanisms underlying its occurrence involve multiple aspects such as genetics, epigenetics, and transcriptomics, ultimately leading to significant alterations in crucial signaling pathways. The entire process can be summarized as follows: (Tabaczar et al., 2017): External signaling molecules activate signaling pathways via membrane proteins; (Zhang Y. et al., 2021); Protein kinases activate signals from the membrane to the nucleus; (Sobocińska et al., 2018); Transcription factors activate effector genes, resulting in cellular biological effects (Blanc et al., 2015; Lothrop et al., 2013; Nakada et al., 2011).
Current research has revealed several key factors contributing to the occurrence and development of GBM, including gene mutations (Brennan et al., 2013), tumor microenvironment (TME) (Quail and Joyce, 2017; Erices et al., 2023) and aberrant signaling pathways (Anido et al., 2010; Stommel et al., 2007). These processes are typically controlled by various oncogenes and/or tumor suppressor genes, many of which undergo post-translational modifications (PTMs) such as phosphorylation (Huang et al., 2021; Wang et al., 2021), methylation (Li et al., 2023; Leske et al., 2023), acetylation (Li et al., 2024; Liu X. et al., 2023), ubiquitination (Chang et al., 2023) and so on, which to some extent affect protein localization, stability, and function (Fhu and Ali, 2021). Protein lipidation is a significant and diverse class of post-translational modifications (PTMs) that involves the covalent attachment of specific lipid molecules to various amino acid residues on target proteins (Jiang et al., 2018; Liu et al., 2021). Lipidated proteins typically exhibit a stronger affinity for non-polar structures, such as lipid bilayers, influencing the localization, diffusion, and interactions of these modified proteins (Chen et al., 2018; Lanyon-Hogg et al., 2017; Burnaevskiy et al., 2015). It is widely recognized that this modification can regulate various biological processes in eukaryotic cells, including cell division, differentiation, and immune responses (Resh, 2006b). Most protein lipidation modifications are considered irreversible, including N-myristoylation (Lin et al., 2012), S-farnesylation (Kmiec et al., 2021), O-palmitoylation (Zou et al., 2011), and N-palmitoylation (Chen et al., 2004), etc. In contrast, protein S-palmitoylation is a reversible post-translational modification (PTM) (Jiang et al., 2018; Linder and Jennings, 2013). This characteristic allows the modified proteins to cycle between palmitoylated and depalmitoylated forms, making it essential for understanding how protein palmitoylation affects the function of individual proteins in both normal and cancer cells (Martin et al., 2011; Won and Martin, 2018).
7 zDHHC and GBMSimilar to lipid imbalance situations, palmitoylation not only participates in numerous physiological processes but also associates with various diseases, including neurological disorders, viral infections, cardiovascular diseases, immune system disorders, and the onset and progression of tumors (Mesquita et al., 2021; Ramos et al., 2023; Chong et al., 2023; Zhou et al., 2022). The importance of protein palmitoylation in tumorigenesis has been a subject of attention and research over the past decade. Palmitoylation influences multiple aspects of cancer, including cancer cell proliferation and survival, invasion and metastasis, and anti-tumor immunity (Ko and Dixon, 2018; Jin et al., 2021; Tang et al., 2022a). The crucial role of zDHHC-mediated palmitoylation in GBM is also increasingly recognized, particularly in key proteins associated with its occurrence and progression (Chen et al., 2020a). In our preliminary study, we compared the expression of various zDHHC molecules in normal brain tissue and brain tumors by querying the GENT database (Figure 2). Accumulating evidence suggests that palmitoylation or palmitoyltransferases may serve as novel targets for cancer therapy (Liu et al., 2020).
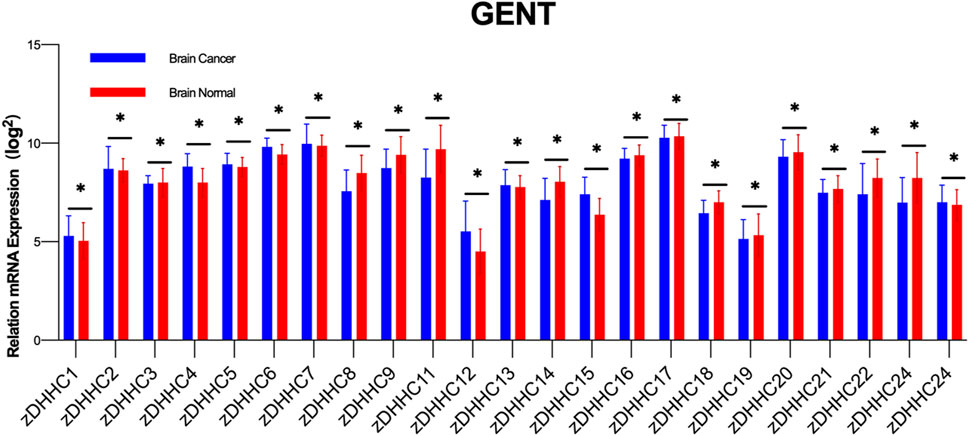
Figure 2. Differential Expression of zDHHC Genes in Normal Brain Tissue and Glioblastoma. The figure shows the differential expression of various zDHHC genes between normal brain tissues (n = 873) and glioblastoma tumor tissues (n = 3439) as analyzed from the GENT database. Multiple unpaired t-tests were conducted to compare the expression levels of each zDHHC gene in the two types of tissues. The bar graph shows the mean values with error bars representing the standard deviation (SD). The results indicate significant statistical differences in the expression of zDHHC genes between normal and tumor tissues, highlighting their potential role in the pathogenesis of glioblastoma. Each group showed significant p-values, denoting strong evidence against the null hypothesis of no difference in expression levels.
Understanding the cellular signaling and molecular mechanisms of palmitoylation modification, as well as its pathological effects, is crucial. Here, we will review the latest research to unveil the mechanisms by which zDHHC-mediated S-palmitoylation modification regulates protein function in the occurrence and progression of GBM. Finally, we aim to explore opportunities and new strategies for therapeutic intervention targeting protein palmitoylation (Table 1).
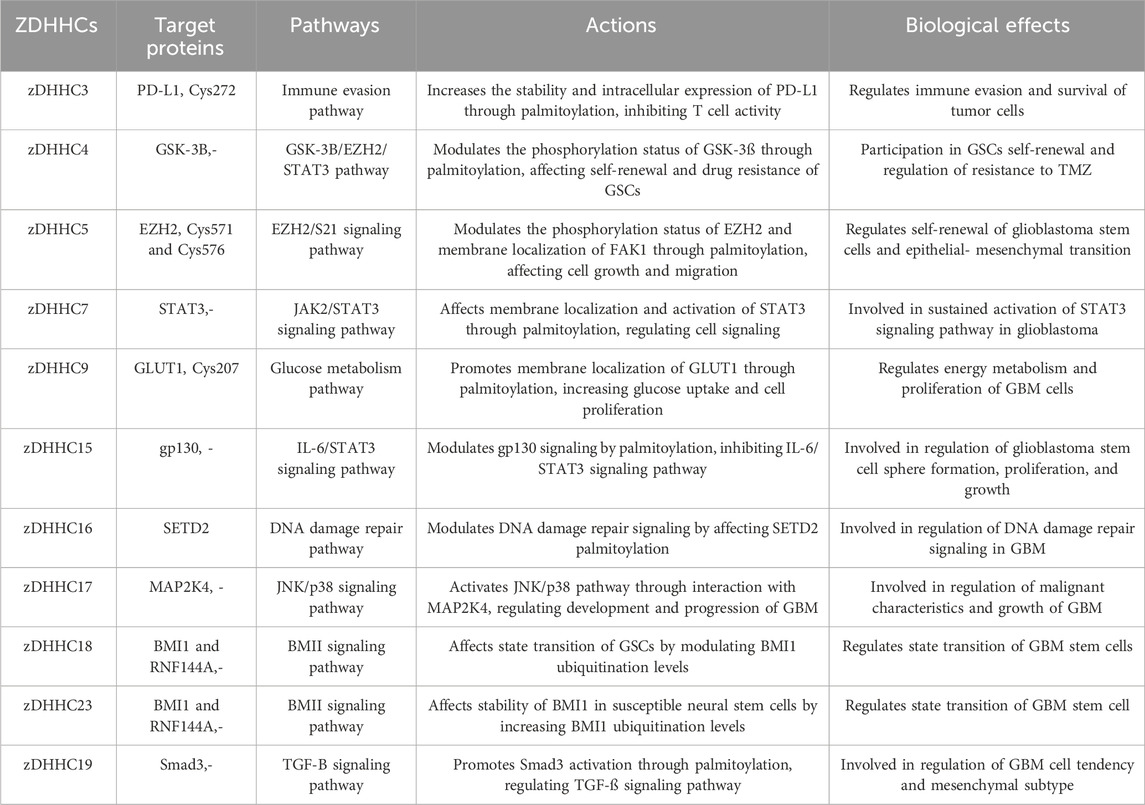
Table 1. Target proteins, pathways and biological roles ZDHHC protein-mediated protein palmiylation
7.1 zDHHC3GBM is considered a “cold tumor,” where tumor cells, microglia, macrophages, T cells, and myeloid-derived suppressor cells (MDSCs) collectively form an immunosuppressive microenvironment (Da Ros et al., 2018; Lim et al., 2018). Programmed Death Ligand-1 (PD-L1) is expressed on the surface of GBM cells, which, upon binding to Programmed Death-1 (PD-1) on T cell surfaces, resists T cell cytotoxicity, ultimately leading to tumor immune evasion (Yao et al., 2021; Yang Y. et al., 2019; Freeman et al., 2000; Feng et al., 2023). Tang et al. demonstrated the colocalization and physical interaction between ZDHH3 and PD-L1 in GBM cell lines using immunofluorescence and co-immunoprecipitation in their glioma research (Tang W. et al., 2022). zDHHC3-mediated palmitoylation modification plays a crucial role in regulating tumor immune evasion and tumor cell survival. Through palmitoylation modification of PD-L1 protein (Cys272), zDHHC3 inhibits PD-L1 monoubiquitination, preventing its delivery to lysosomes for degradation, thereby increasing PD-L1 expression levels within cells. This results in increased surface expression of PD-L1 on tumor cells, which, by binding to PD-1, inhibits T cell activity, reducing tumor cell immune clearance (Yao et al., 2019). Blocking PD-L1 palmitoylation modification pharmacologically promotes PD-L1 lysosomal degradation, thereby activating anti-tumor immune responses and enhancing T cell cytotoxicity. Additionally, palmitoylation modification by zDHHC3 affects the intracellular distribution of PD-L1, depleting its intracellular pool, making anti-PD-L1 therapy more effective (Tang W. et al., 2022). Therefore, zDHHC3-mediated palmitoylation modification regulates the stability and function of PD-L1, influencing tumor immune evasion and survival. Current antibody therapies mainly target surface expression of PD-L1, while targeting PD-L1 palmitoylation simultaneously reduces its expression levels on the cell membrane and within cells. This strategy sensitizes tumor cells to T cell cytotoxicity more effectively, thereby more efficiently inhibiting tumor growth (Kinney et al., 2017).
7.2 zDHHC4Glycogen Synthase Kinase-3β (GSK-3β) belongs to the serine/threonine protein kinase family and is primarily localized in the cytoplasm, with distribution in the nucleus and mitochondria as well. Apart from its initial discovery in regulating the activity of glycogen synthase (GS), GSK-3β also influences the structure and function of various signaling proteins and transcription factors, contributing to tumor formation and progression. Depending on the different molecular modifications it undergoes, GSK-3β exhibits diverse effects on tumor cells (T et al., 2016; He et al., 2020). Zhao et al. (C et al., 2022) found that zDHHC4 catalyzes the palmitoylation modification of GSK-3β, followed by phosphorylation of EZH2 at S21. This process further regulates the phosphorylation and methylation of STAT3. Through the GSK-3β-EZH2-STAT3 axis, it is involved in the self-renewal of Glioblastoma Stem Cells (GSCs) and the development of resistance to TMZ (a chemotherapy drug) in GBM. Additionally, this modification affects the interaction between GSK3β and AKT and p70S6K, thereby regulating their phosphorylation status. This discovery reveals the critical role of GSK-3β palmitoylation modification in the regulation of tumor stem cells, providing a new theoretical basis for understanding and intervening in tumor development.
7.3 zDHHC5The ZDHHC5 gene is located in an unstable chromosomal region and is upregulated in breast cancer and lung adenocarcinoma (Tian et al., 2015). Chen et al. (Chen X. et al., 2017) found that the p53 gene controls the transcriptional activation of the ZDHHC5 promoter in GBM, suggesting an association between ZDHHC5 expression, p53 regulation, and tumorigenesis. Furthermore, it was discovered for the first time that in GBM, both the mRNA and protein levels of ZDHHC5 increase with tumor grading, correlating with increased p53 mutation frequency. Further functional studies revealed that mutant p53 can physically interact with the transcription factor NF-Y, forming an enhanced functional complex independent of the type of p53 mutation, leading to aberrant upregulation of ZDHHC5. Additionally, overexpression of ZDHHC5, along with mutations in KRAS, TERT, and p53 oncogenes, is sufficient to trigger comprehensive and rapid malignant transformation of GBM. EZH2 is a histone methyltransferase targeting H3K27 and is thus considered a suppressor of tumor suppressor genes. When ZDHHC5 catalyzes the palmitoylation modification of EZH2 at Cys571 and Cys576, it affects the phosphorylation level of EZH2 at the S21 position, and the levels of these two modifications are inversely correlated. Palmitoylation is a necessary condition for EZH2 localization to the Golgi apparatus, while phosphorylation occurs at the Golgi apparatus. Decreasing the levels of ZDHHC5 and EZH2 palmitoyltransferase significantly inhibits the growth of glioma tumors (Tang et al., 2022c). ZDHHC5 can inhibit the expression of other pluripotency-related transcription factors by inhibiting EZH2 activation, thus preventing the self-renewal of GBM stem cells. In a recent study, Wang et al. (Wang et al., 2024) discovered that ZDHHC5 can also catalyze the S-palmitoylation of the tumor-related protein Focal Adhesion Kinase 1 (FAK1), thereby enhancing FAK’s localization on the cell membrane and promoting epithelial-mesenchymal transition (EMT) of cells. EMT is a crucial process in tumor progression, enabling tumor cells to acquire invasive and migratory capabilities (Pastushenko and Blanpain, 2019; Thiery et al., 2009). Therefore, ZDHHC5-mediated palmitoylation modification of FAK may promote the development and metastasis of GBM by promoting the EMT process. This discovery provides important clues for understanding the molecular mechanisms of tumor development and offers new targets for therapeutic strategies targeting this modification process. Overall, the upregulation of ZDHHC5 in GBM is associated with p53 mutations and plays a role in influencing the self-renewal of GSCs and tumorigenesis. These findings suggest that ZDHHC5 may be a potential therapeutic target for treating p53-mutant GBM.
7.4 zDHHC7Signal transducer and activator of transcription 3 (STAT3) is a transcription factor located in the cytoplasm, whose activation by JAK, MAPK, or mTOR kinases can lead to phosphorylation of tyrosine or serine residues in the C-terminal domain of the STAT3 protein and its dimerization, thereby activating STAT3 dimers to enter the nucleus and initiate transcription of target genes (Becker and Wilting, 2019). Typically, STAT3 activation is transient and modulated. However, in tumor cells, 50%–90% of tumor cells exhibit sustained activation (LI et al., 2019) and in 66%–83% of GBM, the STAT3 pathway is constitutively activated (Carro et al., 2010; Kim et al., 2013). Moreover, the level of STAT3 phosphorylation is closely related to tumor grading, with significant differences observed between low-grade and high-grade tumors (Mizoguchi et al., 2006).
Advancements in research on palmitoylation mediated by zDHHC7 in GBM have demonstrated its significant role in the transcription factor STAT3. Studies have shown that cysteine 108 of STAT3 is palmitoylated by zDHHC7, which promotes the membrane localization of STAT3 and phosphorylation of JAK2, thereby affecting STAT3 activation and cellular signaling (Zhang et al., 2020). Additionally, it has been found that APT2 selectively depalmitoylates p-STAT3, promoting its nuclear translocation. This research reveals the crucial role of the palmitoylation-depalmitoylation cycle in regulating STAT3 activation, providing important clues for understanding the role of palmitoylation in the pathogenesis of GBM. These findings may provide a basis for the development of new therapeutic strategies. For example, therapeutic strategies targeting zDHHC7 and APT2 may help intervene in the STAT3 signaling pathway, thereby impacting the development and treatment of GBM.
7.5 zDHHC9Glucose transporter 1 (GLUT1) belongs to the glucose transporter protein family and is responsible for the transmembrane uptake of glucose under normal conditions (Hg et al., 2002; Mueckler et al., 1985). It is widely expressed in various tissues, with the highest expression levels observed in endothelial cells of barrier tissues such as red blood cells and the blood-brain barrier (Uldry and Thorens, 2004). In the central nervous system, GLUT1 plays a crucial role in endothelial cells and astrocytes, facilitating glucose uptake in astrocytes and glucose transport across the blood-brain barrier, making it considered the primary energy transporter in the brain.
A prominent characteristic of cancer is altered metabolism (Pavlova et al., 2022) and GLUT1 is aberrantly expressed in various cancers, including lung cancer, brain cancer, breast cancer, and bladder cancer (Ganapathy et al., 2009; Macheda et al., 2005). In the case of GBM, studies have shown high levels of GLUT1 expression in GBM cells to meet their high energy demands for glucose uptake (Mg et al., 2009; Komaki et al., 2019). DHHC9-mediated S-palmitoylation of GLUT1 is crucial for maintaining its localization on the cytoplasmic membrane. This palmitoylation promotes glucose uptake, glycolysis rate, and lactate production in GBM cells, thereby facilitating cell proliferation and tumor formation (Zhang Z. et al., 2021). This discovery is correlated with patient prognosis, highlighting the importance of zDHHC9 in regulating GLUT1 function in GBM.
7.6 zDHHC15Glycoprotein 130 (gp130) is a glycoprotein located on the cell membrane and is one of the signaling receptor subunits of the interleukin-6 (IL-6) family (Cron et al., 2016; Wagener et al., 2014). It directly regulates signaling pathways such as STAT, MAPK, and PI3K/AKT and activates the SOCS negative feedback regulatory mechanism (Nogueira-Silva et al., 2013). As a crucial protein mediating cell survival, abnormal stabilization and overexpression of gp130 is closely associated with tumor progression. In normal cells, the level of gp130 protein is intricately regulated through various mechanisms, including ubiquitin-dependent degradation, endocytosis, and Caspase-induced protein cleavage. Specifically, the tetraspanin CD9 forms a complex with gp130, reducing its ubiquitination and maintaining high levels of gp130 in GSCs, ensuring sustained activation of STAT3 (Shi et al., 2017).
zDHHC15 plays a role by palmitoylating the IL-6 receptor subunit gp130, inhibiting the IL-6/STAT3 signaling pathway. Through a positive feedback mechanism, zDHHC15 effectively suppresses GSCs’ sphere formation, cell proliferation, and growth (Fan et al., 2021a). Local anesthetics such as lidocaine, bupivacaine, mepivacaine, or ropivacaine can disrupt the transcription of ZDHHC15, reducing the palmitoylation level of gp130 and its localization on the cell membrane, thereby inhibiting the activation of the IL-6/STAT3 signal. This study emphasizes the crucial role of zDHHC15 in regulating the IL-6/STAT3 signaling pathway, which has a beneficial impact on the biological behavior of GSCs. Furthermore, the expression of ZDHHC15 in GBM is positively correlated with tumor grade, and high expression levels are associated with GSC self-renewal (Liu Z-Y. et al., 2023). Therefore, ZDHHC15 is not only an important regulatory factor for GSCs but also a potentially useful biomarker for GBM diagnosis and prognosis.
7.7 zDHHC16Fan et al. (Fan et al., 2022) demonstrated that the ZDHHC16/SETD2/H3K36me3 signaling axis is inactivated in EGFR-altered GBM. Specifically, ZDHHC16 is significantly downregulated in GBM compared to normal brain tissue, which is closely associated with changes in EGFR. These events lead to the activation of p53, causing cells to arrest at the G1/S checkpoint. Additionally, in EGFR-amplified GBM, ionizing radiation-induced DNA damage affects DNA damage repair signaling, involving reduced palmitoylation of SETD2 and methylation of its target H3K36.
7.8 zDHHC17ZDHHC17 is primarily localized in the Golgi apparatus, and its deficiency leads to arrest at the G2/M transition, along with an increase in the proportion of cells with dense Golgi. Studies have shown that ZDHHC17 is upregulated in GBM and interacts with MAP2K4 through its N-terminal signal transduction and protein-protein interaction ankyrin domain, forming a signaling module that activates the JNK/p38 pathway, thereby regulating the malignant development and progression of GBM (Chen et al., 2020b). This finding suggests the importance of the ZDHHC17-MAP2K4 signaling pathway in GBM and provides clues for further understanding the pathogenesis of GBM. Importantly, the JNK/p38 activation promoted by ZDHHC17 is independent of PAT, and glioma cells with suppressed ZDHHC17 expression are insensitive to 2-BP inhibition. This discovery highlights the importance of ZDHHC17 in regulating the JNK/p38 signaling pathway through a unique mechanism in GBM development.
7.9 zDHHC18 and zDHHC23Bmi1 belongs to the Polycomb group (PcG) and is a key marker of stem cells (Xu et al., 2022). Initially discovered as an oncogene in mouse lymphomas, Bmi1 was later identified as an important regulator of hematopoietic and neural stem cell self-renewal (Wang et al., 2007). In this context, the role of zDHHC23 becomes evident as it recruits BMI1 and RNF144A (an E3 ligase involved in post-translational regulation of BMI1) to increase the ubiquitination level of BMI1 in susceptible neural stem cells, ensuring the stability of BMI1 protein in different subtypes of GSCs (Chen et al., 2019). On the other hand, ZDHHC18 affects the binding of BMI1 and RNF144A and reduces the polyubiquitination level of BMI1 in mesenchymal neural stem cells through competitive interaction with RNF144A. Overall, the relative changes in the abundance of zDHHC18 and zDHHC23 can regulate the expression pattern of BMI1, playing an important promoting role in the transition of glioblastoma stem cell states (Chen et al., 2019). These findings provide a new perspective for understanding the pathogenesis of GBM and lay the theoretical foundation for developing therapeutic strategies.
7.10 zDHHC19Due to the significant intra- and inter-tumoral heterogeneity of GBM (including cellular and molecular complexity, therapies targeting a single molecular pathway are ineffective for GBM (Balça-Silva et al., 2019; Lah et al., 2020). GBM contains glioblastoma stem cells (GSCs), which are highly resistant to radiotherapy and chemotherapy, leading to high recurrence rates (Bao et al., 2006; Sharifzad et al., 2019). Targeting GSCs and identifying new markers are crucial issues in developing innovative strategies to eradicate GBM. IDH1 mutation induces the activation of HIF-1α and reduces the expression of TGF-β1 in proneural GSCs. However, a mechanism has been identified in mesenchymal-type GSCs whereby Smad3 activation is controlled through the regulation of Smad3 palmitoylation. This palmitoylation, facilitated by membrane localization and TGF-β1/2 phosphorylation, promotes Smad3 activation (Fan et al., 2021b). Inhibiting the activity of HIF-1α and Smad3 may effectively suppress the proneural and mesenchymal subtypes of GBM. EP300, a histone acetyltransferase (HAT), is involved in the formation of transcription complexes and plays crucial roles in cell cycle regulation and DNA damage repair (Snowden and Perkins, 1998). Interaction between activated TGF-β and EP300 further enhances the expression of corresponding markers in mesenchymal-type GBM (Fan et al., 2021b). This discovery reveals the intricate regulatory relationship between IDH1 mutation, the TGF-β signaling pathway, and mesenchymal features (Figure 3).
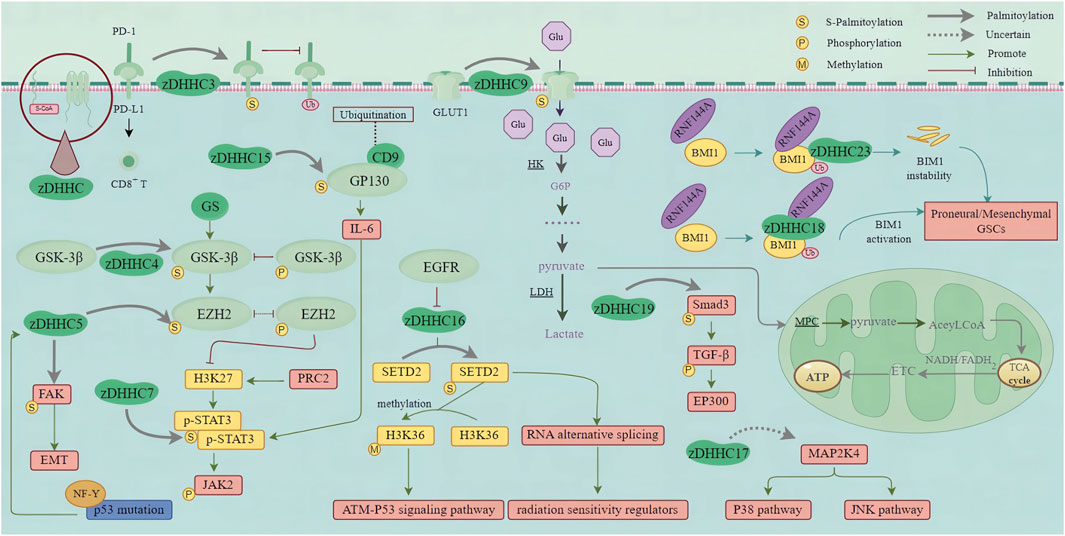
Figure 3. zDHHC-mediated S-palmitoylation in GBM Oncogenic Pathways. This figure illustrates the role of zDHHC enzymes in the S-palmitoylation of proteins involved in major oncogenic pathways in glioblastoma multiforme (GBM). zDHHC enzymes catalyze the addition of palmitoyl groups to specific proteins, such as EGFR, HRAS, and so on, which are critical in GBM pathogenesis. S-palmitoylation of these proteins affects their localization, stability, and interaction with other signaling molecules, thereby modulating key signaling pathways that promote GBM progression and malignancy. (PD-1, Programmed death receptor 1; PD-L1, Programmed death ligand 1; GSK-3β, Glycogen synthase kinase-3β; EZH2, Enhancer of Zeste homolog 2; GS, Glycogen synthase; FAK, Focal adhesion kinase; EMT, Epithelial-Mesenchymal Transition; NF-Y, Nuclear factor Y; H3K27, Histone 3 Lys 27; PRC2, Polycomb Repressive Complex 2; STAT3, Signal transducer and activator of transcription 3; JAK2, Janus kinase-2; CD9, Cluster of differentiation 9; GP130, Glycoprotein 130; IL-6, Interleukin 6; Glu, Glucose; GLUT1, Glucose transporter; HK, Hexokinase; G6P, Glucose 6-phosphate; LDH, lactate dehydrogenase; MPC, Mitochondrial pyruvate carrier; TCA cycle, Tricarboxylic acid cycle; ETC., Electron transfer chain; ATP, Adenosine triphosphate; Smad3, Small mother against decapentaplegic 3; TGF-β, Transforming growth factor-β; EP300, E1A-binding protein P300; MAP2K4, Mitogen-activated protein kinase kinase 4; JNK pathway, C-jun N-terminal kinase pathway; EGFR, Epidermal growth factor receptor; SETD2, Set2 in yeast; H3K36, Histone H3 on lysine 36; ATM, Ataxia Telangiectasia Mutated; RNA, Ribonucleic Acid; RNF144A, Ring Finger Protein 144; BMI1, B cell-specific Moloney murine leukemia virus integration site 1; GSCs, Glioma stem cells).
7.11 Other zDHHC moleculeszDHHC2 is an important gene associated with GBM. Studies have identified zDHHC2 as a significant prognostic gene for GBM, closely linked to its prognosis (Yang H. et al., 2019). High expression of zDHHC2 in GBM is strongly associated with the “surfactant metabolism” pathway and has been validated as a prognostic marker for GBM. Additionally, zDHHC2 has been found to be prognostically relevant in various cancers. For instance, in gastric cancer, its downregulation is associated with lymph node metastasis and poor prognosis (Yan et al., 2013). While the exact mechanisms of zDHHC2 in GBM remain incompletely understood, research suggests that zDHHC2 may play a pivotal role in the pathogenesis and prognosis of GBM.
Previous studies have linked zDHHC12 to neurological disorders such as Huntington’s disease, Alzheimer’s disease, and schizophrenia (Liao et al., 2024; Young et al., 2012). Knocking out zDHHC12 in ovarian cancer significantly inhibits the precise membrane localization and protein stability of CLDN3, as well as tumor occurrence in ovarian cancer cells (Yuan et al., 2020b). Lu et al. (Lu et al., 2022) demonstrated the significant role of zDHHC12 in GBM. Firstly, their comprehensive analysis revealed upregulation of ZDHHC12 in various cancers, predicting poor prognosis in GBM and low-grade glioma (LGG). Further cell experiments indicated that zDHHC12 promotes the occurrence and progression of gliomas. Moreover, the study found that zDHHC12 exhibits a hypomethylated state in GBM, potentially being a mechanism underlying its overexpression. Hypomethylation of the zDHHC12 gene is particularly significant in high-grade glioma samples and is associated with IDH wild-type samples, suggesting that the methylation status of the zDHHC12 gene may occur before the increase in zDHHC12 expression, providing a potential biomarker for early screening of gliomas. While in vitro cell experiments demonstrate the role of zDHHC12 in the occurrence and progression of gliomas, further research is needed to collect more glioma samples and relevant clinical data to assess the performance of zDHHC12 in glioma diagnosis and prognosis.
8 Advances and strategies for targeting S-palmitoylation with drugsCurrently, research on the translational aspects of S-palmitoylation is progressing relatively slowly compared to other lipidation studies. However, selectively regulating one or more of the dozens of proteins involved in palmitoylation offers a broad and promising opportunity for the future. This regulation could potentially leverage the dynamic palmitoylation cycle to upregulate or downregulate the activity of palmitoylated tumor proteins or tumor suppressors that are otherwise difficult to target with drugs. The plasticity of palmitoylation indicates that a deeper understanding of its regulatory mechanisms could aid in the discovery of novel therapeutics.
Identifying inhibitors for several ZDHHCs or APTs is similar to developing “selective hybrid” kinase inhibitors, which already have recognized therapeutic value (Morphy, 2010). Although membrane-proximal cysteine is a common modification site, the lack of a consensus sequence for palmitoylation poses significant challenges for analysis. This variable and hydrophobic PTM makes it difficult to quantify the true extent and stoichiometry of palmitoylation. Therefore, new technological innovations may be required to enable knowledge-guided selection of specific ZDHHCs for drug discovery. For instance, recently reported chemical genetics methods have linked the metabolic labeling of palmitoylation with the activity of individual ZDHHCs, identifying specific relationships between ZDHHCs and their substrates (Ocasio et al., 2024; Tomić et al., 2024; Puthenveetil et al., 2023).
The ZDHHC proteins have a conserved hydrophobic lipid-binding site, indicating that these proteins can bind to small molecule targets (Rana et al., 2018). Although there are currently no fully validated, highly selective ZDHHC inhibitors, some APT inhibitors have demonstrated varying degrees of efficacy and selectivity (Won et al., 2016; Remsberg et al., 2021; Lan et al., 2021). Unfortunately, recent studies have tended to misuse nonspecific active compounds, such as 2-BP or cerulenin, as so-called ZDHHC inhibitors. These compounds primarily target lipid biosynthetic enzymes and have highly nonspecific and pleiotropic effects on ZDHHC activity (Lanyon-Hogg et al., 2017; Ergun et al., 2019; Davda et al., 2013).
The recent high-throughput screening for ZDHHC2 inhibitors represents the first step towards developing more selective ZDHHC inhibitors. However, the disclosed compounds were initially reported as inhibitors for another class of enzymes, so their selectivity needs further optimization (Salaun et al., 2022). For example, ZDHHC9 maintains the palmitoylation and plasma membrane expression of GLUT1, which is crucial for glucose transport in glioblastoma cells. Additionally, ZDHHC9 enhances anticancer immune suppression by catalyzing the palmitoylation of PD1 and PDL1 (67).
Directly targeting palmitoylation sites is an attractive alternative strategy due to its potential for high specificity. However, in practice, this approach may be limited to a few sites with well-defined binding pockets that are relatively buried, similar to TEAD inhibitors (Hagenbeek et al., 2023).
9 Conclusion and future perspectivesGlioblastoma (GBM) is a highly recurrent primary malignant tumor of the brain, which has long been a focus of clinical and research attention. In the studies of GBM, S-palmitoylation modification, as an important protein lipid modification, plays a crucial role in the occurrence, development, and treatment of tumors. This review summarizes the biological processes of S-palmitoylation modification and its key roles in GBM. Specifically, we focus on the zDHHC family as key enzymes of S-palmitoylation modification, their research progress, and potential roles in GBM.
Firstly, S-palmitoylation modification affects various cellular functions and signaling pathways by increasing the hydrophobicity and altering the localization of proteins. This modification plays a vital role in cell cycle regulation, cell proliferation, metastasis, and signal transduction, and its abnormal expression in tumors may lead to tumor initiation and progression.
Secondly, the zDHHC family, as crucial enzymes of S-palmitoylation modification, has attracted widespread attention in GBM research. Members of the zDHHC family may play different roles in the occurrence and development of GBM. We summarize the functions of molecules such as zDHHC3, zDHHC4, zDHHC5, zDHHC7, zDHHC9, zDHHC15, zDHHC16, zDHHC17, zDHHC18, and zDHHC23 in GBM and the signaling pathways they regulate. These molecules participate in the occurrence and progression of GBM by affecting tumor cell immune evasion, stem cell self-renewal, and activation of signaling pathways. We particularly highlight the regulation of key proteins’ palmitoylation modification by these molecules and their effects on tumor biological behaviors. For example, palmitoylation modification of PD-L1 mediated by zDHHC3 affects tumor cell immune evasion; palmitoylation modification of GSK-3β mediated by zDHHC4 is involved in glioblastoma stem cell self-renewal and resistance to chemotherapy drugs; palmitoylation modification of EZH2 mediated by zDHHC5 affects the self-renewal and metastasis ability of tumor stem cells.
With further research on the mechanisms of action of the zDHHC family in GBM, we can gain a deeper understanding of the roles of these molecules in tumor initiation and progression, providing new insights and targets for developing therapeutic strategies for GBM. For example, inhibiting tumor cell growth and metastasis by interfering with palmitoylation modification mediated by zDHHC molecules may become one of the future treatment strategies. Furthermore, exploring the relationship between the expression levels of these molecules in tumor tissues and clinical features can explore their potential value as diagnostic and prognostic markers.
However, S-palmitoylation faces some challenges and difficulties in GBM research. Firstly, the complexity of this field requires interdisciplinary cooperation, integrating expertise from biology, chemistry, bioinformatics, and other fields. Secondly, as a membrane modification process, although some progress has been made in research, a comprehensive understanding of the precise mechanism of S-palmitoylation in cells involves many complex biochemical processes and interactions, including specific steps of protein modification, enzymes involved, signaling pathways, etc. Current scientific research still needs to delve deeper to reveal the detailed mechanism of S-palmitoylation. Additionally, the heterogeneity and highly malignant characteristics of GBM itself add to the complexity of research, requiring more refined experimental design and data analysis.
Given the importance of S-palmitoylation mediated by the zDHHC family in GBM, future research can focus on several aspects: firstly, in-depth exploration of the expression patterns of each zDHHC molecule in GBM and their association with tumor development; secondly, studying the mechanisms by which each zDHHC molecule affects biological behaviors such as proliferation, invasion, and metastasis of GBM cells; finally, exploring the potential role of regulating or inhibiting the zDHHC family in GBM treatment, aiming to provide a theoretical basis and clinical application prospects for developing new therapeutic strategies.
Overall, the study of S-palmitoylation in GBM has broad prospects and important clinical application value. By overcoming the corresponding challenges, we hope to provide more effective and personalized treatment strategies for GBM patients, promoting the development of the tumor research field.
Author contributionsBT: Data curation, Writing–original draft, Writing–review and editing. WK: Data curation, Writing–original draft, Writing–review and editing. QD: Funding acquisition, Methodology, Visualization, Writing–review and editing. ZQ: Supervision, Validation, Writing–review and editing. LD: Supervision, Validation, Writing–review and editing. XZ: Supervision, Validation, Writing–review and editing. GY: Supervision, Validation, Writing–review and editing. YP: Formal Analysis, Methodology, Visualization, Writing–review and editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Project of Healty and Famliy Planing Commission of Gansu (grant nos. S-62000000024-2024-047), Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (grant nos. CY2022-YB-A05) and The Gansu Provincial Natural Science Foundation Project (No. 24JRRA332).
AcknowledgmentsWe are immensely grateful to all those who contributed to the success of this research. We thank the members of the Department of Neurosurgery at the Second Hospital of Lanzhou University for their invaluable assistance and support. Special thanks go to our
留言 (0)