Soft tissue sarcomas (STS) are rare diseases and occur with an incidence of around 50 per million inhabitants per year (1). Almost 50% of extraabdominal STS are located at the thigh or gluteal region (2). Due to the complex anatomy of this region and often large tumor extensions, the resection of these sarcomas is complex and causes long operation times. The complex dissection during surgery and proximity to the large blood vessels also frequently cause major loss of blood during or after surgery. The loss of soft tissues regularly causes large wound cavities in which hematomas and seromas may develop. In addition, neoadjuvant radiotherapy and/or chemotherapy increase the risk of wound healing disorders in many cases (3, 4). All these factors lead to a significantly increased risk of infection after resection of sarcomas in the peripelvic region (5–7). This is aggravated by the particular spectrum of bacteria in this region, which differs significantly from other regions (8–10). In previous studies (also in our own patient collective (7)), high proportions of Gram-negative and anaerobic pathogens were found (5, 6). It is also known that moist skin (as seen in the groin) has significantly more pathogenic germs (10) which might be brought into the wound mechanically during surgery.
Major efforts are and have been taken to reduce the infection rate in various studies. In addition to general antiseptic measures, this includes antibiotic prophylaxis. However, in recent literature some of the recommendations in musculoskeletal surgery are based on little evidence and there are hardly any studies on this topic. The recommendations from the 2000s recommend a differentiated approach to antibiotic prophylaxis (11). Published in 2013, the Surgical Infection Society did not recommend antibiotic prophylaxis for aseptic procedures without the implantation of prostheses at all (12). Due to the general high infection rate and the personal experience of the authors, antibiotic prophylaxis nevertheless seems to be advisable for complex resections of sarcomas in the peripelvic area. Depending on the complexity of surgery, antibiotics may also be administered on a prolonged basis. However, these are clinically based individual decisions for which evidence is currently lacking, and only sparse preliminary work in the recent literature can be found.
The aim of this study therefore was to evaluate the risk factors for the development of postoperative infections after resections of sarcomas in the groin, proximal thigh, and gluteal region. Furthermore, the effectiveness of perioperative antibiotic prophylaxis should be evaluated also in cases with prolonged antibiotic administration. In addition, we evaluated the effectiveness of an adaption of antibiotics for prophylaxis, considering the particular spectrum of bacteria in this region found by previous evaluations of our cohort.
2 Patients and methodsIn a historical patient collective of a specialized sarcoma center, patients were selected and their data retrospectively evaluated who underwent STS resections of the proximal thigh (till 15 cm distal to the groin), groin or gluteal region (peripelvic region) at our center between 2003 and 2020. Patients with the following characteristics were included:
- Resection of a STS in the peripelvic region.
- Tumor resection in our hospital.
- No superficial sarcomas.
- Primary wound closure without application of vacuum-assisted closure systems (VAC).
- Clinical follow-up time of at least 2 weeks.
- Occurrence of infection in the first 90 days after surgery.
- Availability of follow-up data for the first 90 days after surgery.
Almost all tumors were diagnosed in advance by biopsy (incisional or core-needle biopsy). The only exceptions were atypical lipomatous tumors (ALT), which were only diagnosed radiologically. Treatment decision was made in an interdisciplinary tumor board. Many tumors were neoadjuvantly treated in accordance with the ESMO (European Society for Medical Oncology) guidelines (13). Depending on the entity, grading, TNM classification of the tumor, and the individual age, patients received chemotherapy and/or radiotherapy. Patients were then operated on exclusively by 2 experienced surgeons. Depending on the interdisciplinary decision, adjuvant therapy was performed if necessary. While follow-up, all patients were seen at our center at least in the first 3 months. In the event of a postoperative infection after discharge from hospital, all patients presented again at our center.
The decision for preoperative single shot antibiotic prophylaxis was based on clinical assessment and the complexity of the resection. Due to the lack of evidence and recommendations for pre-/perioperative antibiotic prophylaxis, the decision for or against antibiotic administration was made historically based on clinical experience of surgeons from the 1990s and 2000s. No antibiotic was administered for tumors with a size <10 cm and epifascial location. If antibiotic prophylaxis was desired, it was administered 30 minutes before the incision. If the operation lasted longer than 3 hours and/or blood loss exceeded 2 litres, antibiotics were administered repeatedly. Extended postoperative prophylaxis for 5-7 days was ordered, if the duration of surgery exceeded 2 hours or if intraoperative blood loss exceeded 1000 ml. Primarily, the 2nd generation cephalosporin (cefuroxime 1.5 g) was administered. In cases of known penicillin allergy, a lincosamide antibiotic substance (clindamycin 600 mg) was applied as an alternative. The interim evaluation of the bacterial spectrum in wound infections 2018 has shown a high proportion of Gram-negative and anaerobic bacteria, many of which were associated with intestinal flora (coagulase-negative staphylococci (CoNS) in 31.5%, Enterococcus spp. in 13.3% and Escherichia coli 7.7%). 30.8% microbial species were Gram-negative bacteria, 25.9% were anaerobic species (7). Adapted to this finding, the prophylaxis was changed to ampicillin/sulbactam 2 g/1 g in consecutive patients.
In the event of a deep wound infection requiring revision, a surgical revision was performed. Postoperative deep wound infection was defined following the guidelines of the Center for Disease Control and Prevention (CDC) (14). The wound was explored, at least two microbiological swabs were taken under sterile conditions, and the wound was debrided and either closed primarily or left for secondary wound healing with VAC therapy. Antibiotics were administered according to the antibiograms of the isolated pathogens.
The following parameters were evaluated: gender, age of the patient, co-morbidities (body mass index (obesity), Diabetes mellitus, active nicotine consumption, arteriosclerosis), duration of surgery, intraoperative blood loss, substitution of erythrocyte concentrates, neoadjuvant and/or adjuvant therapy, tumor entity, tumor size, application and type of perioperative antibiotic prophylaxis, occurrence of infection, type of pathogens, change of pathogens during the treatment.
For data analysis we used descriptive statistic methods (frequency, median value, spreading, standard deviation and Kaplan-Meier curve). Significance analysis was performed using the Log-Rank, Chi-Square, or the t-test, defining a 95% confidence interval. Univariate (Cox proportional hazards regression) and multivariate analysis were used to evaluate the influence of group characteristics on the infection rate. The level of significance was set at less than 0.05. The data analysis software used was IBM® SPSS® Statistics 29. This study was approved by the institutional ethics committee (Ethics Committee of the Medical Faculty; ID 17-89). Written consent was obtained from all surviving patients. For non-surviving patients, data were irreversibly anonymized as recommended by the ethics committee. For children and adolescents informed consent from their parents/guardians was obtained.
3 Results3.1 Cohort characteristicsA total of 393 patients were evaluated. 27 patients had to be excluded from the evaluation because the data was incomplete or the minimum observation period of 3 months was not reached. In total, 366 patients could be evaluated. Of those, 204 patients (55.7%) were male. The median age of all patients was 61.3 years (range between 9 and 93 years). The median time of surgery was 79.8 min (range between 15 and 620 min). The median intraoperative blood loss was 474.9 ml (range between 40 and 8300 ml). The median maximum length of the resection specimen was 16.1 cm (range between 4 and 65 cm). Other group characteristics are shown in Table 1.
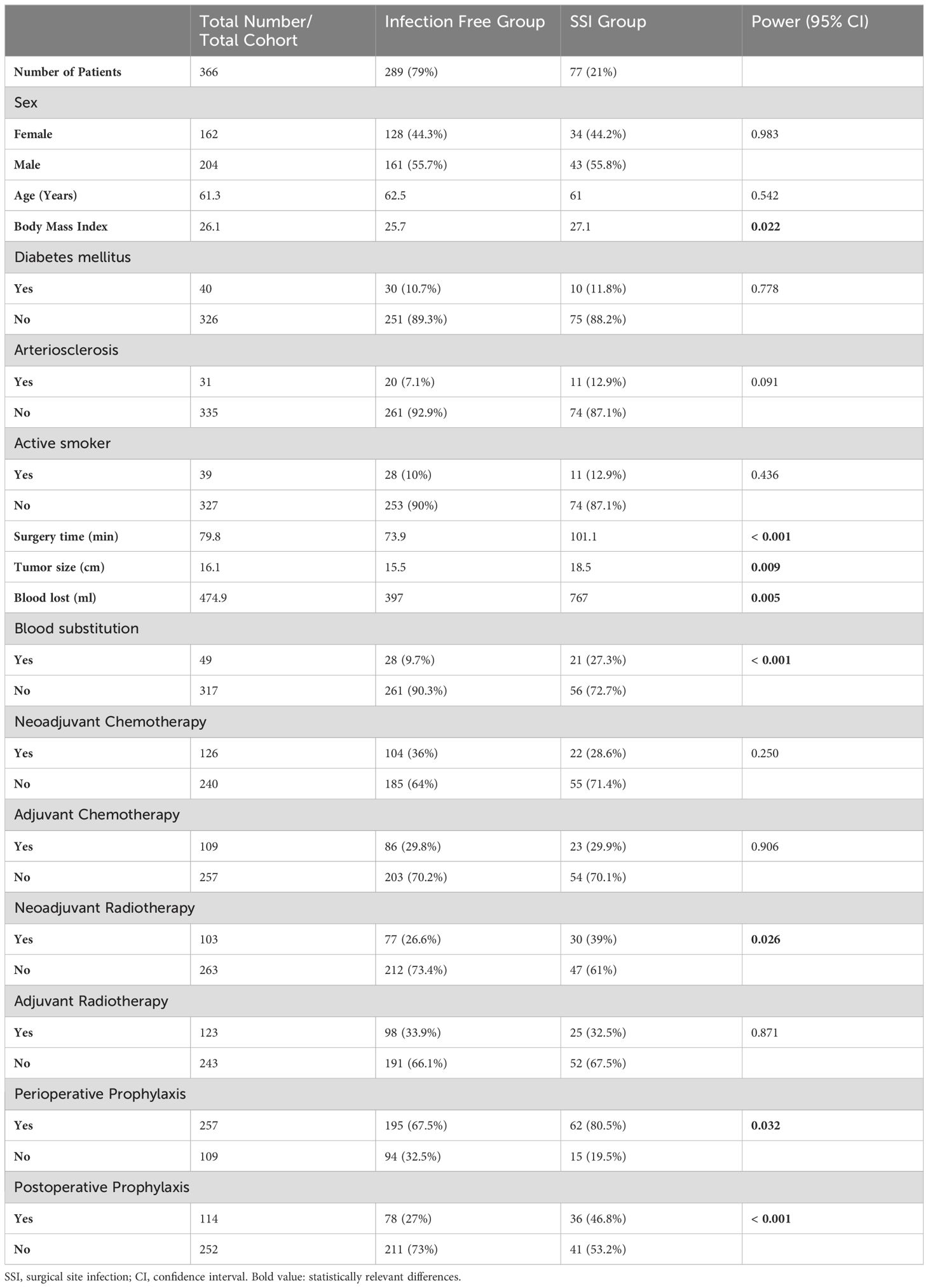
Table 1. Characteristics of patient cohort and treatment details.
Antibiotic prophylaxis was administered as a single shot perioperatively in 257 cases (70.2% of all patients), 29.8% of patients did not receive any prophylaxis because of low risk constellation. Cefuroxime or clindamycin was administered in 221 cases (56.3% of all patients), and ampicillin/sulbactam was given in 36 cases (9.8% of all patients, Figure 1). In 114 cases (31.1% of all cases or 51.6% of cases with antibiotic prophylaxis), antibiotic prophylaxis was continued postoperatively due to the complexity of surgery (Table 1).
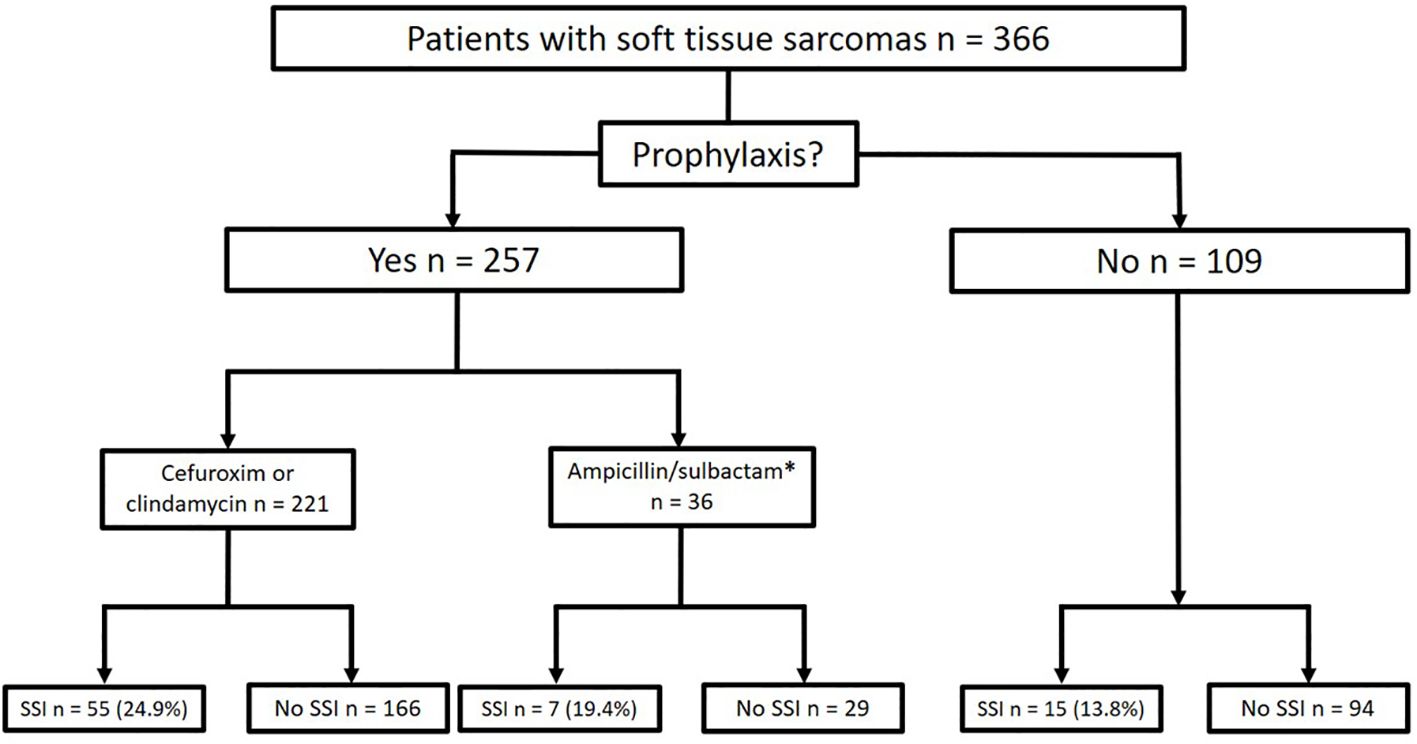
Figure 1. Frequencies of antibiotic prophylaxis and rates of postoperative infection (SSI, Surgical Site Infection; *after the interim evaluation of the pathogen spectrum in the patient cohort, antibiotic prophylaxis was switched to ampicillin/sulbactam).
3.2 Rate of SSISSI occurred in 85 cases (23.2% of all operations). Eight cases of infection occurred later than 90 days after the index surgery and were excluded from further analysis. They were considered as probable superinfections of the pre-existing seroma due to hematogenous spread. These cases were excluded for the evaluation of our study aim. In 77 cases (21% of all operations), the median time to diagnosis of infection and surgical revision was 20 days (range between 3 and 90 days). The mean time of surgery, blood loss, and tumor size differed significantly (p<0.05) between the group with and without infections (Table 1).
3.3 Risk factors for development of SSI62 infections (out of 257, 24.1%) occurred in the group with perioperative antibiotic prophylaxis. In the group without antibiotic prophylaxis 15 infections occurred in 109 cases (13.8%; p=0.032). However, both groups differed highly significantly in baseline values as tumor size (p=0.007) or blood loss and duration of surgery (both p<0.001, Table 2).
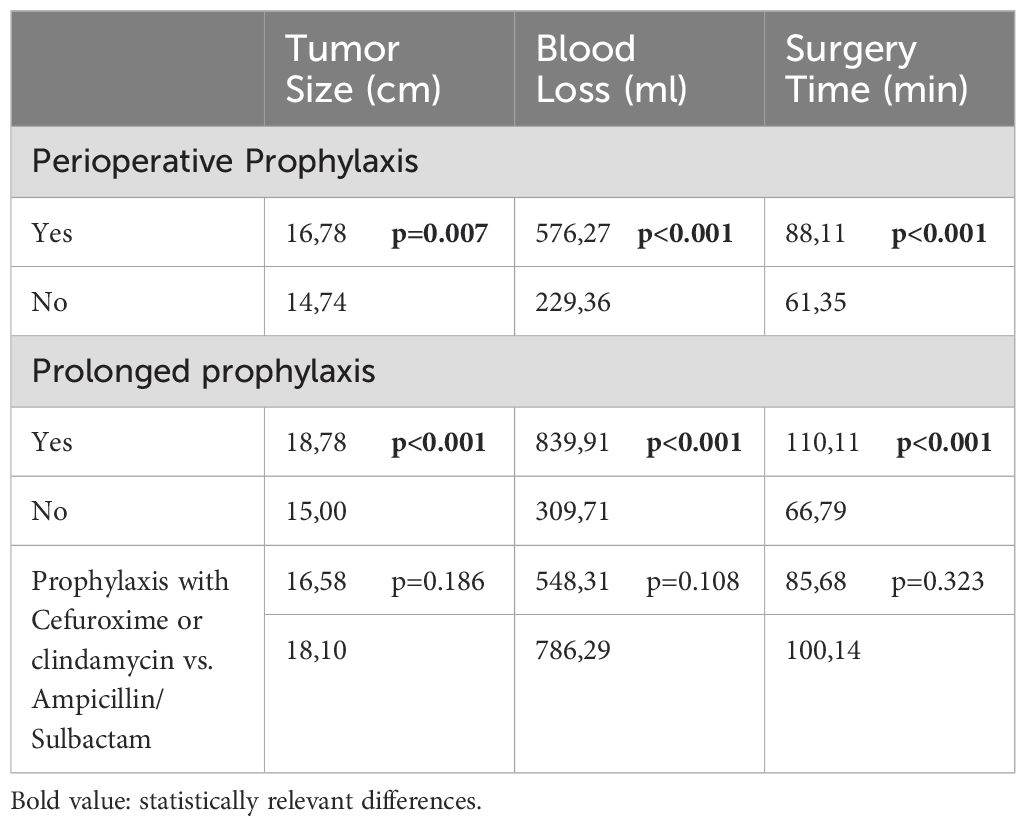
Table 2. Subgroup characteristics and it’s comparison regarding tumor size (cm, centimeter); blood loss (ml, milliliter) and surgery time (min, minutes).
In the group with single shot antibiotics, 41 infections occurred in 252 cases (16.3%; p<0.001) compared to 36 (31.6%) infections in 114 cases with prolonged antibiotic prophylaxis (Figure 1). Also here, the group characteristics (tumor size, blood loss and duration of surgery differed highly significantly (p<0.001; Table 2).
In comparison of the groups with cefuroxime (including clindamycin) and ampicillin/sulbactam, 55 infections occurred in 221 cases (24.9%) in the cefuroxime (and clindamycin) group, whereas 7 infections in 36 cases (19.4%; p=0.479) occurred in the ampicillin/sulbactam group (Figure 2, n.s.). The groups were comparable in terms of tumor size (p=0.186), blood loss (p=0.108), and time of surgery (p=0.323; Table 2).
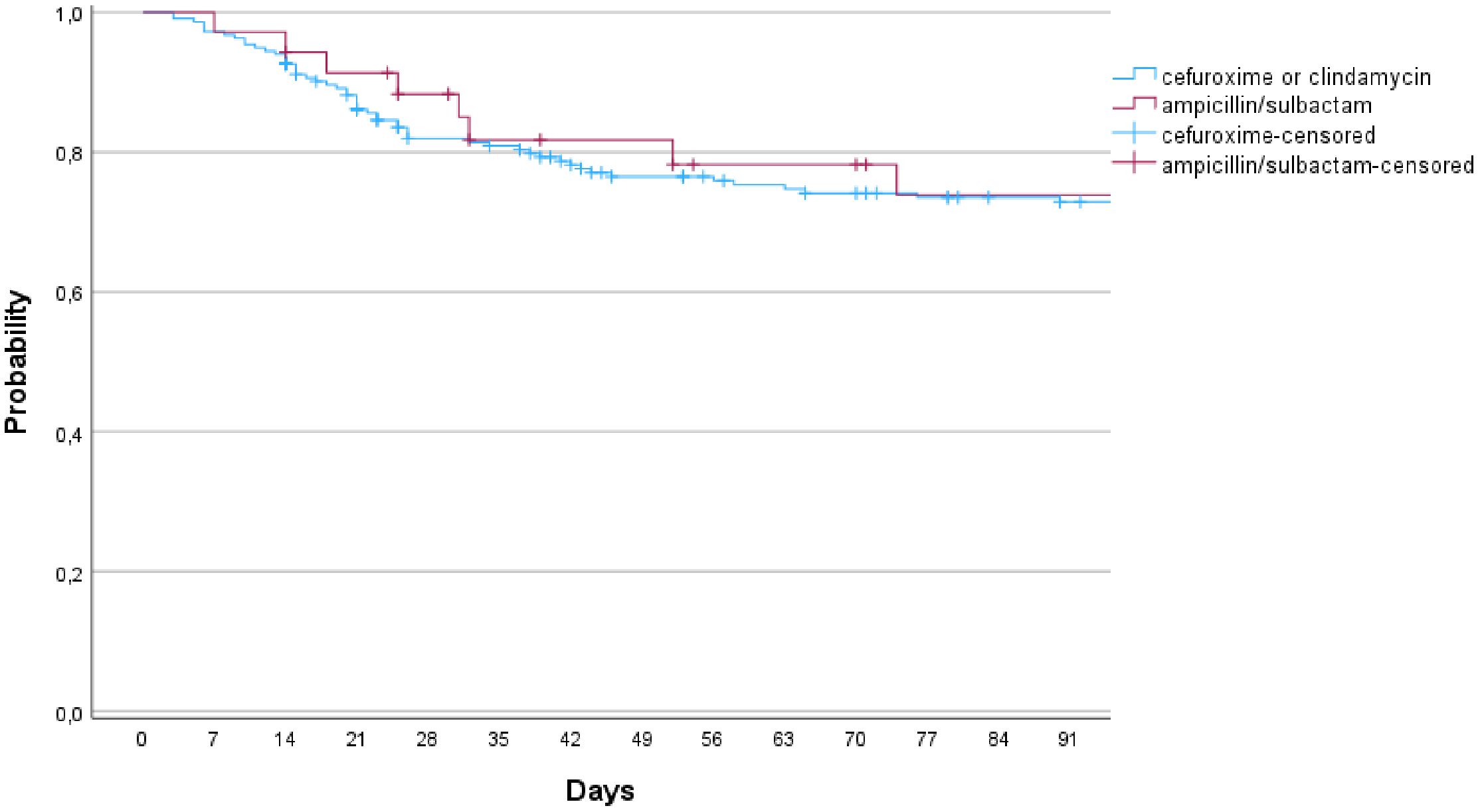
Figure 2. Infection free survival in comparison of the group of cefuroxime (or clindamycin) and ampicillin/sulbactam (p=0.775; 95% CI).
In univariate analysis, the blood loss ≥ 800 ml, substitution of erythrocyte concentrates, surgery duration longer 80 min. and neoadjuvant radiotherapy were found to be significant risk factors for the development of infection (Table 3). In multivariate analysis, the surgery duration longer 80 min, substitution of erythrocyte concentrates and neoadjuvant radiotherapy were confirmed as a risk factor. In addition, the implementation of neoadjuvant chemotherapy significantly worsened the risk for SSI (Table 3).
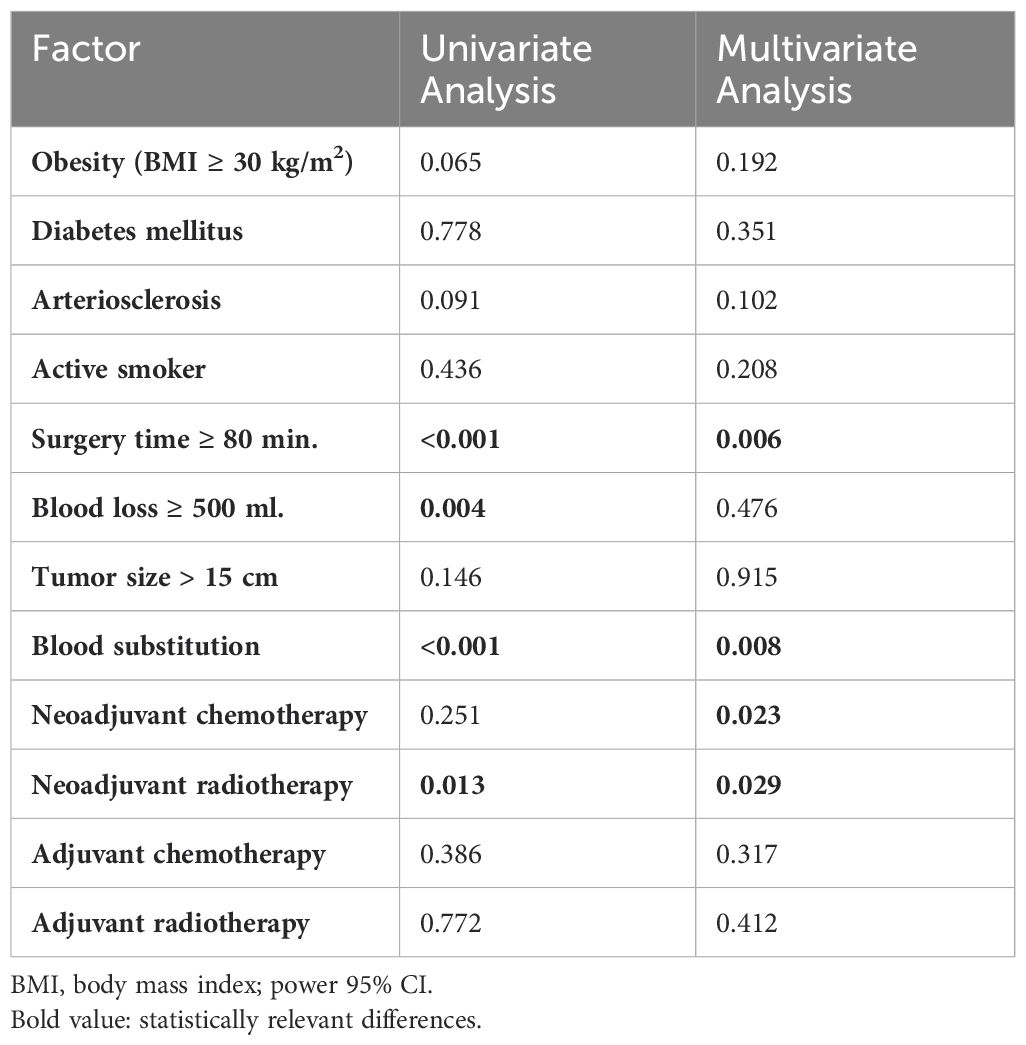
Table 3. Univariate and multivariate analysis of the risk factors, influencing the development of SSI.
4 DiscussionThe resection of STS in the peripelvic region maybe a challenge for the surgeon. Complex anatomy and a large extent of the tumor often causes long surgery durations with high blood loss. Those are known risk factors for the development of SSI. This is frequently aggravated by neoadjuvant or adjuvant chemotherapy and radiotherapy. Precisely, these risk factors are confirmed in a large meta-analysis by Slump et al. (4): The evaluation of 21 studies on STS resections at all sites showed an overall complication rate of 30.2% with a surgical revision rate of 13.4%. The risk factors identified included large tumors, neoadjuvant radiotherapy, and high intraoperative blood loss. The infection rate in patient cohorts with mixed localizations is around 15-18% (15, 16), while in pelvic bone sarcomas or soft tissue sarcomas in the peripelvic area the infection rate is about 20-25% (5, 6). In concordance with these findings, the SSI rate was 21% (7).
In current guidelines a uniform recommendation from different societies is seen: the American Surgical Infection Society recommends no antibiotic prophylaxis for aseptic orthopaedic operations without using implants (12). The recommendation of the German Paul-Ehrlich-Society is identical (17). However, risk factors for an increased risk of SSI in these procedures are also identified: Neoadjuvant radiation, surgery duration over 2 h, long anaesthesia time and high blood loss with blood transfusion. If these facts are taken into account, there is a tendency to advocate additional methods to reduce the infection rate. General aseptic measures have long been established in clinical practice: Aseptic washing, avoiding skin injuries, and using iodized films (12). However, there is no published evidence or recommendation for perioperative antibiotic prophylaxis in this cohort.
The initial hypothesis of this study was that antibiotic prophylaxis reduces the infection rate after sarcoma resection in the peripelvic area. This could not be confirmed by our evaluation. The SSI rate in all subgroups was higher with antibiotic prophylaxis compared to no prophylaxis at all. This result is certainly due to the pre-selection of patients into the respective groups (bias). The surgeons selected patients with more complex tumor resections into the groups with antibiotic prophylaxis and, if necessary, prolonged antibiotic administration based on clinical experience and assessment of the risk of infection.
However, significant differences between the groups were evident: Blood loss was higher in the group with antibiotic prophylaxis, the frequency of application of erythrocyte concentrates was higher, the duration of surgery was longer, and tumor size was more extensive. This confirms a profound selection bias regarding the administration of antibiotic prophylaxis. Other authors assume that the probability of postoperative infection in this group would even be higher without antibiotic prophylaxis. Infection rates of up to 50% have been described for these localizations (18–20). It is difficult to interpret the results in the group with prolonged antibiotic application: The infection rate was significantly higher in this group compared to a single shot (32 vs. 16%). Dadras et al. were also unable to achieve a reduction in the risk of infection through prolonged antibiotic administration (15), the Paris group showed the same results (20). Probably the only prospective and randomized study on the effectiveness of antibiotic prophylaxis in musculoskeletal oncology is the PARITY trial. Here, in a prospective double blinded and randomized trial, no benefit of prolonged antibiotic prophylaxis (1 day vs. 3 days) after bone sarcoma resection and implantation of tumor prostheses of the lower extremity could be shown (21). A study with a similar design would probably be useful to clarify this issue more precisely.
The analysis of the bacterial spectrum in postoperative infections in the peripelvic area showed a significantly higher proportion of Gram-negative and anaerobic pathogens, strongly associated with intestinal flora (7). Few studies investigated postoperative infection in sarcoma resections in this area, all showed similar results (5, 6). Because of this, antibiotic prophylaxis with cephalosporins appears inadequate. We, therefore, changed the prophylaxis to ampicillin/sulbactam. The comparison of these 2 groups from our patient cohort shows a trend in the reduction of infection rate (19% versus 24%), but the effect was not significant. In a retrospective evaluation of Ramsey et al. (22) the addition of metronidazole as an antibiotic prophylaxis to cover anaerobes reduced significantly the complication rate after sarcoma resections (27% vs. 17%; p = 0.049) underlying our own findings. In view of these results and the close relationship of the gluteal and inguinal region to the anus and, in women, to the vagina, a relation between the infectious microorganisms and the corresponding local bacterial flora can be assumed. Thus, the adaptation of antibiotic prophylaxis seems unavoidable. However, two large analyses of antibiotic prophylaxis in colorectal surgery and hysterectomy cannot clearly prove these connections (23, 24). A conclusive assessment is not possible in view of these results, but a trend is recognizable.
Resection of vessels with prosthesis reconstruction and resection of abdominal/retroperitoneal organs played no role in our cohort in terms of numbers (four in the our patient collective). In such cases, a higher SSI rate would have been expected.
The retrospective design of this study and the lack of randomization only allow limited conclusions to be drawn about the effectiveness of the prophylaxis due to the existing bias. Our results can only be interpreted as a trend. As far as our study design allows, we identified the following factors in the risk stratification for postoperative infection: Neoadjuvant radiotherapy and chemotherapy, duration of surgery, and substitution of erythrocyte concentrates as surrogate parameters for higher blood loss. The analysis of complications after surgery in nearly 1.000 STS at different locations (15) identified almost the same risk factors; further studies also confirm these results (3, 16).
There is no doubt that further studies with larger numbers of patients using the multicenter prospective approach are necessary in order to adequately answer the important question of the effectiveness of perioperative antibiotic prophylaxis.
5 ConclusionThe evaluation of treatment outcomes after resection of peripelvic STS in our patients could not demonstrate a benefit of perioperative antibiotic prophylaxis or prolonged antibiotic therapy in lowering the postoperative infection risk. We attribute that to a selection bias of antibiotic prophylaxis at all. However, in line with the published literature, we identified subgroups with an increased risk of infection: Obesety, longer duration of surgery, higher blood loss with the need for blood substitution, larger tumor extension, and the application of neoadjuvant radiotherapy. In our opinion, patients with a combination of these risk factors should receive antibiotic prophylaxis that ideally considers the increased Gram-negative and anaerobic bacterial spectrum in this body region. With that at least a trend for a lesser rate of SSI was seen.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by Ethics Committee of the Medical Faculty, University of Munich. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsAK: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. CC: Data curation, Writing – review & editing. AW: Conceptualization, Methodology, Writing – review & editing. SG: Methodology, Validation, Writing – review & editing. LB: Investigation, Methodology, Writing – review & editing. DD: Methodology, Resources, Writing – review & editing. BH: Formal analysis, Methodology, Resources, Writing – review & editing. HD: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Stiller CA, Trama A, Serraino D, Rossi S, Navarro C, Chirlaque MD, et al. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer (Oxford England: 1990). (2013) 49:684–95. doi: 10.1016/j.ejca.2012.09.011
Crossref Full Text | Google Scholar
2. Picci P, Manfrini M, Fabbri N, Gambarotti M, Vanel D. Atlas of musculoskeletal tumors and tumorlike lesions. London New York: Springer International Publishing (2014).
3. Schwartz A, Rebecca A, Smith A, Casey W, Ashman J, Gunderson L, et al. Risk factors for significant wound complications following wide resection of extremity soft tissue sarcomas. Clin Orthop Relat Res. (2013) 471:3612–7. doi: 10.1007/s11999-013-3130-4
PubMed Abstract | Crossref Full Text | Google Scholar
4. Slump J, Bastiaannet E, Halka A, Hoekstra HJ, Ferguson PC, Wunder JS, et al. Risk factors for postoperative wound complications after extremity soft tissue sarcoma resection: A systematic review and meta-analyses. J Plast Reconstr Aesthet Surg. (2019) 72:1449–64. doi: 10.1016/j.bjps.2019.05.041
PubMed Abstract | Crossref Full Text | Google Scholar
5. Sanders PTJ, Bus MPA, Scheper H, van der Wal RJP, van de Sande MAJ, Bramer JAM, et al. Multiflora and gram-negative microorganisms predominate in infections affecting pelvic endoprostheses following tumor resection. J Bone Joint Surg Am. (2019) 101:797–803. doi: 10.2106/JBJS.18.00836
PubMed Abstract | Crossref Full Text | Google Scholar
6. Angelini A, Drago G, Trovarelli G, Calabrò T, Ruggieri P. Infection after surgical resection for pelvic bone tumors: an analysis of 270 patients from one institution. Clin Orthop Relat Res. (2014) 472:349–59. doi: 10.1007/s11999-013-3250-x
PubMed Abstract | Crossref Full Text | Google Scholar
7. Klein A, Chudamani C, Wieser A, Bilgeri A, Weigert A, Arnholdt J, et al. Spectrum of pathogens in surgical site infections after sarcoma resection in the peripelvic and pelvic region. Distinct location, distinct infection? Surg Infect (Larchmt). (2024). doi: 10.1089/sur.2024.093
PubMed Abstract | Crossref Full Text | Google Scholar
8. Miller ED, Mo X, Andonian NT, Haglund KE, Martin DD, Liebner DA, et al. Patterns of major wound complications following multidisciplinary therapy for lower extremity soft tissue sarcoma. J Surg Oncol. (2016) 114:385–91. doi: 10.1002/jso.v114.3
PubMed Abstract | Crossref Full Text | Google Scholar
9. Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. (2009) 324:1190–2. doi: 10.1126/science.1171700
PubMed Abstract | Crossref Full Text | Google Scholar
12. Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). (2013) 14:73–156. doi: 10.1089/sur.2013.9999
PubMed Abstract | Crossref Full Text | Google Scholar
13. Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann Oncol. (2021) 32:1348–65. doi: 10.1016/j.annonc.2021.07.006
PubMed Abstract | Crossref Full Text | Google Scholar
15. Dadras M, Koepp P, Wagner JM, Wallner C, Sogorski A, Lehnhardt M, et al. Antibiotic prophylaxis for prevention of wound infections after soft tissue sarcoma resection: A retrospective cohort study. J Surg Oncol. (2020) 122:1685–92. doi: 10.1002/jso.v122.8
PubMed Abstract | Crossref Full Text | Google Scholar
16. Sasaki H, Nagano S, Taniguchi N, Setoguchi T. Risk factors for surgical site infection after soft-tissue sarcoma resection, including the preoperative geriatric nutritional risk index. Nutrients. (2018) 10(12):1900. doi: 10.3390/nu10121900
PubMed Abstract | Crossref Full Text | Google Scholar
17. Wacha H, Hoyme U, Isenmann R, Kujath P, Lebert C, Naber K, et al. Perioperative antibiotika-prophylaxe. Chemother J. (2010) 19:70–84.
18. Nakamura T, Nakamura K, Hagi T, Asanuma K, Sudo A. Soft tissue sarcoma at the adductor compartment of the thigh may have a greater risk of tumor-associated events and wound complications. J Orthop Surg (Hong Kong). (2019) 27:2309499019840813. doi: 10.1177/2309499019840813
PubMed Abstract | Crossref Full Text | Google Scholar
19. Kito M, Ae K, Koyanagi H, Gokita T, Furuoka H, Okamoto M, et al. Risk factor for wound complications following wide resection of soft tissue sarcoma in the adductor compartment of the thigh. Jpn J Clin Oncol. (2019) 49:932–7. doi: 10.1093/jjco/hyz101
PubMed Abstract | Crossref Full Text | Google Scholar
20. Bensaid S, Contejean A, Morand P, Enser M, Eyrolle L, Charlier C, et al. Surgical site infection after pelvic bone and soft tissue sarcoma resection: Risk factors, microbiology, and impact of extended postoperative antibiotic prophylaxis. J Surg Oncol. (2023) 128:344–9. doi: 10.1002/jso.v128.2
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ghert M, Schneider P, Guyatt G, Thabane L, Vélez R, O'Shea T, et al. Comparison of prophylactic intravenous antibiotic regimens after endoprosthetic reconstruction for lower extremity bone tumors: A randomized clinical trial. JAMA Oncol. (2022) 8:345–53. doi: 10.1001/jamaoncol.2021.6628
PubMed Abstract | Crossref Full Text | Google Scholar
22. Ramsey DC, Walker JR, Wetzel R, Gundle KR, Hayden JB, Doung YC. Is the addition of anaerobic coverage to perioperative antibiotic prophylaxis during soft tissue sarcoma resection associated with a reduction in the proportion of wound complications? Clin Orthop Relat Res. (2022) 480:2409–17. doi: 10.1097/CORR.0000000000002308
PubMed Abstract | Crossref Full Text | Google Scholar
24. Ayeleke RO, Mourad S, Marjoribanks J, Calis KA, Jordan V. Antibiotic prophylaxis for elective hysterectomy. Cochrane Database Syst Rev. (2017) 6:Cd004637. doi: 10.1002/14651858.CD004637.pub2
留言 (0)