Gut is the largest digestive and immune organ, as well as the principal interface between humans and the environment (Yang and Yu, 2021). In addition to its role in nutrient digestion and absorption, the gut also establishes a distinctive mucosal barrier that protect the host from harmful substances and pathogens (Hao et al., 2022). In general, intestinal barrier can be categorized into four components: the mechanical, immune, chemical, and biological barriers. Each component is supported by its corresponding structural foundation. An intact intestinal barrier plays a crucial role in upholding gastrointestinal function and overall human health (Di Sabatino et al., 2023). Once the intestinal barrier is damaged, it may further trigger strong inflammatory responses, autoimmune disorders, metabolic dysregulation, and deterioration of organic lesions (Albillos et al., 2020). Upon the critical importance of intestinal barrier integrity, extensive research has been conducted to investigate the causes and underlying mechanisms of intestinal barrier damage.
Previous studies have shown that intestinal barrier damage is often associated with intestinal diseases such as intestinal infection, irritable bowel syndrome, ischemia-reperfusion (I/R) injury, inflammatory diseases and intestinal malignancies (Gao et al., 2023; Zhang et al., 2020). In recent years, the interconnection between the gastrointestinal tract and extraintestinal organs has gradually been elucidated. The concepts of “gut-liver axis,” “gut-kidney axis,” and “gut-heart axis” have been proposed, unequivocally indicating the intricate communication network between the intestines and other organs (Lombardi et al., 2024; Xiao et al., 2023). Studies have demonstrated that functional impairment of certain extraintestinal organs can induce oxidative stress, provoke an inflammatory response, and disrupt metabolic homeostasis in the intestine, thereby impacting intestinal barrier function. When the integrity of the intestinal barrier is compromised, it results in increased intestinal permeability and subsequent release of gut-derived endotoxin (Nie et al., 2023). Subsequently, these endotoxins can exert their effects on the target organs via the circulatory system, leading to further deterioration of the extraintestinal organs and eventually forming a vicious cycle of “gut-organ” axis disorder, which brings greater challenges to the management of intestinal barrier injury. It can be speculated that the disturbance of these axes plays a crucial role in the occurrence and progression of intestinal barrier damage (Figure 1). It is noteworthy that during the initial phase of functional impairment in certain organs, such as liver injury, kidney injury, and cardiovascular disease, increased intestinal permeability and endotoxin release can be detected, which helps to identify some high-risk adverse events early (Lee et al., 2018). At the same time, restoring intestinal barrier function could potentially serve as a therapeutic approach to mitigate extraintestinal organs injuries.
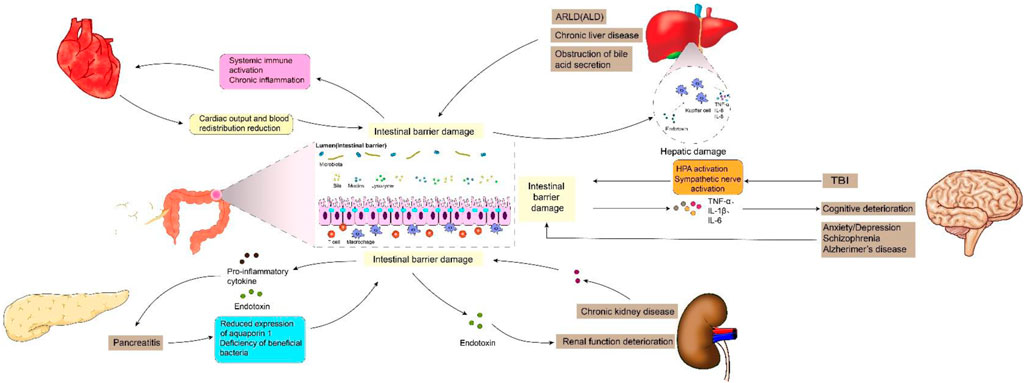
Figure 1. Intestinal barrier damage in the “gut-organ” axes.
Signal transduction pathway, serving as the foundation for various cellular functions such as proliferation, differentiation, apoptosis, metabolism, oxidative stress, and immune response, facilitates the transmission of extracellular signaling molecules to the cell via cell membrane or intracellular receptors. Its proper functioning is indispensable for maintaining cellular and organ homeostasis (Chou et al., 2022). Dysregulation of cell signaling pathways has been demonstrated to be closely associated with the disruption and restoration of the intestinal barrier in previous studies (He et al., 2022). The elucidation of the molecular mechanism underlying intestinal barrier injuries holds significant importance in preventing such injuries and enhancing intestinal barrier homeostasis. Simultaneously, a comprehensive understanding of relevant mechanisms can aid in the development of targeted drugs to reverse ectopic signaling pathways, thereby offering potential therapeutic approaches for treating intestinal barrier injury.
The exploration of space by humanity is consistently accompanied by fervent enthusiasm. Aerospace medicine, as a prominent domain in contemporary medical research, also garners significant attention. Weightlessness, being an essential and inevitable factor within the space environment, exerts a profound and intricate influence on organisms (Bharindwal et al., 2023). Studies have demonstrated that microgravity or simulated microgravity (SMG) can induce alterations in the physiopathological state of the digestive system and exert diverse effects on various intestinal barriers, encompassing mechanical, immune, chemical, and biological barriers (Yang et al., 2020). Consequently, it is imperative to summarize the modifications occurring in the intestinal barrier under microgravity conditions and to uphold gastrointestinal homeostasis among astronauts in space.
2 The key roles of intestinal barrier damage in “gut-organ” axes cross talk2.1 Gut–liver axisThe gut and liver are anatomically and physiologically closely interconnected, with this intimate association originating from a shared derivation from the ventral foregut endoderm during embryogenesis (Zorn and Wells, 2009). The gut-liver axis (GLA) is characterized by bidirectional interaction between the intestine and the liver. Bile acids and bilirubin synthesized by hepatocytes are released into the duodenum via the biliary tract, while nutrients from the intestinal lumen are transported to the liver through the portal circulation. The optimal functioning of GLA is crucial for efficient nutrient absorption and waste elimination. The liver damage, however, can lead to a decline in intestinal barrier function and the production of enterogenic endotoxins, thereby breaking the balance of GLA and exacerbating further deterioration of both the liver and intestine. The reported findings indicate a significant increase in the sensitivity of intestinal epithelial cell apoptosis and the nitration of intestinal tight junction (TJ) and adhesive junction (AJ) proteins in mice with alcohol-related liver disease (ARLD) (Rungratanawanich et al., 2023). Xiao et al. (2020) reported a decrease in the expression of ZO-1, Claudin-1, Claudin-4, and Reg3g in the intestines of mice with liver injury, leading to an increase in intestinal permeability. Furthermore, there was a negative correlation observed between the expression levels of these proteins and serum endotoxin levels (Assimakopoulos et al., 2012). Hypoxia-inducible factor 1a (HIF-1a) plays a crucial role in the transcriptional regulation of intestinal barrier integrity and inflammation, governing the expression of various genes involved in barrier protection, including intestinal trefoil factor (ITF), CD73, p-glycoprotein (P-gp), cathelicidin, claudin-1, and MUC3 (Fan et al., 2015; Saeedi et al., 2015). Shao et al. (2018) reported a significant reduction of hif-1a in the intestines of mice afflicted with alcoholic liver disease (ALD), leading to compromised intestinal barrier function and gut leakiness. Subsequent investigations demonstrated that upregulating hif-1a could serve as a potential therapeutic approach for ALD (Shao et al., 2018). In addition, the intestinal biological barrier is also affected by liver disease. Jiang et al. (2020) reported that liver injury can result in an increase of the relative abundance of potentially pathogenic Escherichia and Staphylococcus, as well as a reduction in the presence of SCFA-producing bacteria, such as Prevotella, Faecalibacterium, and Clostridium. Additionally, the presence of bacterial overgrowth in the small intestine and impaired intestinal motility have been observed in patients with chronic liver disease, leading to enterogenic endotoxemia (Voulgaris et al., 2021). The obstruction of bile acid secretion in patients with liver disease also plays a crucial role in the development of enterogenic endotoxemia. Bile acids exert direct antibacterial effects by inducing farnesol X receptor-mediated antimicrobial peptides such as angiopoietin 1, which effectively prevent bacterial overgrowth and enhance the integrity of intestinal epithelium. The bile acids and their salts, on the other hand, can also degrade endotoxin molecules into non-toxic subunits or polymerize them into colloidal molecules, thereby exhibiting antibacterial properties and regulating intestinal pH. In patients with liver disease, the impaired bile acid secretion and excretion compromise its ability to inhibit bacteria and regulate pH levels, as well as reduce the clearance of intestinal endotoxins. Moreover, elevated bile acid levels hinder the uptake and clearance of endotoxins by Kupffer cells (de Faria Ghetti et al., 2018).
The impairment of the intestinal barrier can also lead to hepatic damage via endotoxemia. The endotoxin, upon reaching the liver, binds to TLR4 and co-receptors CD14 and MD-2 in Kupffer cells, thereby initiating downstream signaling pathways that result in excessive production of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-8 (Cho et al., 2018). The mice were initially engineered by Shao et al. (2018a) to develop intestinal barrier injury through targeted knockout of intestinal epithelial HIF-1a. Subsequently, they observed a significant increase in serum alanine aminotransferase and lipopolysaccharide levels, along with the presence of liver steatosis. The biologically active lysophospholipid sphingosine 1-phosphate (S1P) plays a crucial role in various biological processes, including cell adhesion, regulation of barrier function, proliferation, differentiation, migration, and protection of the intestinal barrier (Li et al., 2023a). Chen et al. (2023) discovered that elevating the level of S1P can effectively restore intestinal barrier function, reduce lipopolysaccharide (LPS) levels in plasma and liver, mitigate inflammation and apoptosis, as well as enhance liver function. Similarly, the findings of Wu et al. (2023) also demonstrated that upregulation of rat intestinal epithelial tight junction protein expression and modulation of rat intestinal microbiota imbalance can significantly attenuate levels of endotoxin and inflammatory cytokines in rats, while inhibiting the TLR4/NF-κB signaling pathway, thereby ameliorating hepatic pathological damage and oxidative stress. In conclusion, the normalization of GLA has garnered increasing attention through repairing intestinal barrier damage and reducing enterogenic endotoxin production. The levels of serum endotoxin in the blood have also been demonstrated to exhibit a positive correlation with the severity of advanced liver diseases, such as fibrosis and cirrhosis (Rungratanawanich et al., 2023).
2.2 Gut-pancreas axisThe gut and pancreas are intricately interconnected both anatomically and functionally (Floyel et al., 2023; Svegliati-Baroni et al., 2020). Previous studies have consistently demonstrated a strong association between intestinal barrier damage and pancreatic diseases. Epithelial cells (ECs) play a crucial role in the mechanical barrier of the intestine and are indispensable for maintaining its integrity (Benede-Ubieto et al., 2024). However, pancreatitis or local pancreatic injury can induce the release of a plethora of inflammatory factors such as TNF-α and IL-1β, thereby triggering activation of the caspase-3 pathway and subsequent apoptosis of intestinal mucosal epithelial cells (Chen et al., 2018). In addition, the expression of intestinal tight junction proteins, such as occludin and ZO-1, significantly decreases in acute necrotizing pancreatitis (ANP), leading to a significant increase in intestinal permeability. This phenomenon is closely associated with high-mobility group box-1 (HMGB1) (Huang et al., 2019). Lu et al.'s study demonstrated that pancreatic tissue damage exacerbates small intestinal capillary endothelial barrier dysfunction, which may be attributed to reduced expression of aquaporin 1 (AQP1) in the small intestine (Lu et al., 2017). The integrity of the intestinal biological barrier, which is established through the close adhesion of symbiotic bacteria to the intestinal epithelial mucosa, is compromised during acute pancreatitis (Li et al., 2020). Li et al. (2023) discovered alterations in the diversity of intestinal microbiota and a deficiency of beneficial bacteria in patients with pancreatitis. Specifically, a reduction in Bacteroides uniformis abundance among these patients resulted in decreased taurine levels and increased release of IL-17 within the intestine. This subsequently triggered neutrophil extracellular trap (NET) formation, exacerbating pancreatic injury. The abundance of probiotics, such as Blautia, exhibited a inverse association with the severity of acute pancreatitis (Zhu et al., 2019). Conversely, the presence of detrimental bacteria, including Escherichia coli and Shigella, amplified the NF-κB-mediated inflammatory response while concurrently suppressing protein expression (e.g., MUC2), thereby compromising intestinal barrier integrity (Zheng et al., 2019).
Following an increase in intestinal permeability, enterogenic endotoxins and inflammatory mediators can be transported to the pancreas via systemic circulation and mesenteric lymphatic pathways, ultimately exacerbating acute pancreatic inflammation. The results of pancreatic studies have demonstrated that promoting apoptosis in intestinal inflammatory cells to inhibit the activation of the NF-κB signaling pathway, reducing endotoxin secretion, decreasing phosphorylated-p65 (p-p65) expression, and increasing IκBα expression can effectively enhance the pathological outcome of both pancreatic and intestinal tissues (Pan et al., 2024). Similarly, Mei et al. (2021) discovered that the mitigation of pro-inflammatory cytokine production (TNF-α, IL-1β, CXCL2 and MCP1) and endotoxin in the ileum, along with activation of the Nrf2/HO-1 pathway, could effectively restore ileum injury and barrier dysfunction associated with severe pancreatitis. Furthermore, there was a significant reduction observed in serum amylase levels, lipase levels, and pancreatic pulp peroxidase activity. The team led by Li et al. demonstrated enhanced intestinal barrier function and reduced intestinal inflammation in mice through microbiota transplantation (MT) and NLRP3 knockout. Subsequently, they observed a decrease in pancreatic neutrophil infiltration and necrosis (Li et al., 2020). In fact, there are limited therapeutic interventions for intestinal damage associated with acute pancreatitis, and the majority of treatments focus on hormone-mediated inhibition of pancreatic enzyme secretion, which does not effectively address intestinal injury. However, during the early stages of acute pancreatitis, enterogenic endotoxins and inflammation can propagate to the pancreas and other organs through the gut-pancreas axis, leading to severe consequences (Pasari et al., 2019). Therefore, it is imperative to address intestinal injury in association with AP by conducting comprehensive research on the gut-pancreas axis.
2.3 Gut-kidney axisThe correlation between the gut and renal is gradually being elucidated. Simultaneously, multiple studies have demonstrated the potential involvement of kidneys in the progression of intestinal barrier impairment. Clinical trials have demonstrated that patients with stage IIIb-IV chronic kidney disease (CKD) exhibit the accumulation of enterogenous uremic toxins (UT), such as indoxyl sulfate (IS) and p-toluene sulfate (PCS), along with increased intestinal permeability and constipation (Cosola et al., 2021). The presence of chronic kidney disease (CKD) has been associated with intestinal villi shortening, elongation of crypts, and infiltration of the lamina propria (Georgopoulou et al., 2024). In addition, serum levels of endotoxin, IL-6, IL-8, and IL-10 were significantly elevated in patients with stage I-IV CKD, while intestinal occludin and claudin-1 exhibited significant reduction in expression. Furthermore, their expression showed a negative correlation with systemic endotoxemia (Georgopoulou et al., 2024). The impairment of the intestinal barrier is partially facilitated by urea (Lau et al., 2015). With an increase in intestinal barrier permeability, endotoxins, bacteria, and toxins are able to enter the circulatory system, leading to further deterioration of renal function. Additionally, kidney dysfunction can also impact the gut microbiome by causing a reduction in the population of proteolytic bacteria, altering the ratio of aerobic and anaerobic bacteria, and compromising intestinal epithelial barrier integrity. Simultaneously, changes in the microbiome can lead to the generation of potentially toxic compounds that are typically eliminated by renal excretion (Mertowska et al., 2021; Jaglin et al., 2018; Alexeev et al., 2018). Microorganisms such as Enterobacterium, Enterococcus, Bifidobacterium, and Bacteroides, which are responsible for the production of short-chain fatty acids, were found to exhibit reduced abundance in blood and fecal samples from patients with CKD (50). Furthermore, there was a negative correlation observed between the concentration of short-chain fatty acids in the bloodstream and the severity of renal insufficiency (Wang et al., 2019).
The discovery of the gut-kidney axis has also established that disruption of intestinal homeostasis can promote the onset and progression of renal disease. Several recent studies have initiated the development of therapies for renal dysfunction by focusing on restoring intestinal barrier function and microbial balance. The study conducted by Yang et al. (2024) demonstrated that the renal toxicity induced by zinc could be mitigated through rectifying colon injury, restoring ZO-1 protein expression, and reestablishing the structure of intestinal flora. Wang et al. (2024) demonstrated that oral administration of Lactococcus cremoris D2022 can enhance the production of short-chain fatty acids (SCFA) in cecum samples, ameliorate intestinal barrier function, and ultimately mitigate kidney inflammation. Additionally, in the study conducted by Yang et al. (2024), it was observed that oral administration of the probiotic Lactobacillus reuteri exhibited significant enhancement in intestinal barrier function impairment associated with AKI, while also regulating the composition of intestinal microbiota and its related metabolites. Consequently, there was a reduction in serum creatinine and urea nitrogen concentrations, along with protection against renal cell necrosis and apoptosis (Yang et al., 2024). The findings suggest that the preservation of gut barrier integrity and regulation of gut microbiota and associated metabolites should not be overlooked in the treatment of kidney disease. The significance of the kidney in maintaining proper physiological functioning of the human body goes without saying (Li et al., 2024); however, it remains one of the most overlooked organs. This can be attributed not only to insufficient knowledge and action in the field of prevention but also to the fact that most kidney diseases are asymptomatic during their initial stages. Early detection and diagnosis of kidney disease are severely limited due to a lack of sensitive and specific molecular markers indicating the progression towards a particular disease entity. Therefore, it is crucial to explore novel disease markers for predicting and tailoring personalized treatments for kidney diseases. Evaluation of gastrointestinal function through comprehensive analysis of gut microbiota composition holds potential for enhancing diagnostic capabilities, necessitating further investigation into the enterorenal axis.
2.4 Gut-heart axisThe concept of the gut-heart axis elucidates the intricate connection between intestinal pathology, the intestinal microbiome, and cardiovascular disease. Disorders of the gut-heart axis are characterized by alterations in intestinal permeability and disruption of the microbiome. Then, the gastrointestinal microbiota or its derivatives traverse the intestinal epithelial barrier in a non-physiological manner, leading to systemic immune activation and chronic inflammation, which subsequently impact cardiovascular function (Witkowski et al., 2020). Additionally, the contribution of cardiovascular disease to intestinal barrier damage is significant. In patients with heart failure, reduced cardiac output and blood redistribution result in decreased intestinal perfusion, leading to ischemia and hypoxia in the mucous membrane of the villous structure of the intestinal wall. This alteration in the intestines increases permeability of the intestinal wall, causing disturbances in fluid metabolism, disruptions in intestinal microbial balance, translocation of bacteria from the intestines into the circulatory system, and a increase in endotoxins, thereby promoting a characteristic inflammatory state (Xu et al., 2024). The lactulose/mannitol test revealed a 35% elevation in small intestine permeability, while the sucralose test demonstrated a significant increase of 210% in large intestine permeability among patients with chronic heart failure (CHF) (Sandek et al., 2007). The disruption of intestinal barrier integrity and the downregulation of tight junction protein expression have been reported to be associated with neutrophil extracellular trap (NET) (Wang et al., 2018). Hoel et al. (2020) discovered that COVID-19 patients with cardiac involvement exhibited elevated levels of intestinal leakage markers (LPS-binding protein, LBP) and intestinal epithelial cell damage markers (intestinal fatty acid-binding protein, IFABP), indicating the potential existence of a Gut-heart axis in COVID-19. Recently, Blöbaum et al. (2023) conducted a groundbreaking study where they successfully diagnosed intestinal barrier dysfunction in patients with atrial fibrillation (AF). Their research findings revealed an elevation in circulating biomarkers associated with intestinal mucosal inflammation, such as mucosal adhesion molecule MAdCAM-1, and indicators of intestinal epithelial damage like intestinal fatty acid binding protein (IFABP) present in plasma among individuals experiencing early stages of atrial fibrillation (AF). Additionally, surrogate plasma markers indicating increased intestinal permeability were also detected, including LPS, CD14, and LPS-binding proteins. Moreover, Du et al. (2020) observed alterations in the composition of intestinal microbiota (particularly firmicutes and Bacteroidetes) as well as significant elevations in metabolites associated with the microbiome, including short/medium chain fatty acids, arginine, and tryptophan derivatives, in rats exhibiting cardiac hypertrophy. These changes are implicated in the impairment of intestinal barrier integrity induced by heart failure.
In recent years, the restoration of intestinal barrier integrity has emerged as a novel approach for the treatment of heart dysfunction by researchers. The correlation between intestinal permeability and markers of heart injury was found to be positive (Wu et al., 2024). Cui et al. (2023) demonstrated that enhancing the expression of tight junction proteins (ZO-1, occludin) and reducing intestinal permeability and inflammation can lead to improved cardiac function, as well as decreased serum CK-MB and LDH expression. The microbiota, as a crucial component of the intestinal biological barrier, is increasingly being targeted for heart failure treatment (Violi et al., 2023; Bianchi et al., 2022). Regulating gut microbiota through dietary interventions, probiotics administration, antibiotic therapy, fecal transplants, and microbial enzyme inhibitors can effectively enhance cardiac function and reduce mortality associated with heart failure. These interventions involve strategies such as optimizing the firmicutes/Bacteroidetes ratios and promoting the growth of beneficial microbiota (bacteroidetes and heterobacteroidetes) (Jia et al., 2019). The recognition of intestinal barrier damage as a risk factor for cardiovascular disease is increasingly growing. In certain cases of early heart disease, gut-derived low-grade endotoxemia may be present (Violi et al., 2023). Therefore, it is imperative to conduct early assessment of intestinal permeability and detection of endotoxins in high-risk groups for cardiovascular disease to facilitate timely intervention.
2.5 Gut-brain axisThe bidirectional communication between the brain and gut occurs via systemic immune pathways, neural networks, endocrine hormones, and microbiota axes (Morais et al., 2021). Studies have demonstrated that traumatic brain injury (TBI) exerts detrimental effects on the gastrointestinal tract through hormonal regulation. TBI triggers activation of the hypothalamic-pituitary-adrenal (HPA) axis, leading to an elevation in cortisol levels (Li et al., 2023). This surge in cortisol enhances intestinal barrier permeability, ultimately resulting in intestinal leakage (Morais et al., 2021). Consequently, enteric pathogens infiltrate the bloodstream and induce systemic inflammatory response syndrome, thereby releasing a plethora of cytokines and chemical mediators into the systemic circulation. The sharp escalation of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 accelerates cognitive function deterioration (Milleville et al., 2021). In addition, gut can be influenced by brain injury through neuroregulation. TBI can induce sympathetic overactivity, leading to an elevation in circulating catecholamine levels, subsequently resulting in reduced blood flow within the gastrointestinal tract and causing gut dysmotility as well as damage to the intestinal barrier (Montgomery et al., 2016). There is also a correlation between anxiety and intestinal disorders. A clinical study investigation revealed that patients with depression/anxiety disorder exhibited dysregulation of the gut microbiota and significantly elevated levels of LPS, zonulin, and FABP2 in comparison to healthy subjects. Assessing markers of intestinal permeability can aid in interventions for depression and anxiety (Stevens et al., 2018). Furthermore, studies have demonstrated increased intestinal permeability in individuals with schizophrenia, characterized by tight junctions disruption, adhesive junctions dysfunction, and heightened bacterial translocations (Maes et al., 2019). The assessment of the lactulose to mannitol ratio revealed a significantly higher index in patients with schizophrenia compared to the control group (Ishida et al., 2022). There is a strong correlation between gut flora and the central nervous system. The microbiota has the ability to regulate the synthesis of neurotransmitters that impact the gut-brain axis, such as tryptophan. Tryptophan serves as a crucial precursor for aminergic neurotransmitters and cannot be synthesized by the human body; however, it is produced by gut microbes through the shikimate pathway. Notably, Alzheimer’s disease onset is associated with tryptophan secretion in intestinal flora. Although the gut and brain are physiologically and anatomically distant, neuroimmune links between the two are gradually being discovered, which could help in the development of more intestinal biomarkers to make the prevention and diagnosis of neurological diseases more effective.
2.6 Gut-liver-brain axisGut-liver-brain axis serves as a tripartite communication pathway, garnering increasing attention due to its paramount significance (Muhammad et al., 2022). Maintenance of homeostasis within the gut-hepato-brain axis is imperative for optimal brain functionality, contingent upon the intestinal barrier’s integrity and hepatic filtration efficacy. Intestinal tract is the primary reservoir of bacteria within the human body, and its barrier function effectively prevents the translocation of intestinal toxins into capillaries. The liver functions as a prominent “detoxification factory,” efficiently metabolizing and filtering exogenous and endogenous metabolites, bacterial products, and toxins (Wiest et al., 2017). Impairment of either the liver or intestinal barrier can lead to an exacerbation of abnormal gut-liver-brain axis states. Specifically, the impairment of the intestinal barrier facilitates the translocation of bacteria or their metabolites into the liver, thereby precipitating various hepatic disorders (Bauer et al., 2022). In addition, the gut can also affect nerve signals between the gut and the brain by influencing the production of various peptides or hormones. Subsequently, the brain innervates liver and intestinal activity through neuroimmunomodulation, exacerbating the disorder of the enteroliver-brain axis (Socala et al., 2021). Studies have demonstrated that alterations in the composition of intestinal microbiota and its metabolites, along with increased intestinal permeability, play a pivotal role in the pathogenesis of hepatic encephalopathy (Aguirre Valadez et al., 2016). In individuals with liver dysfunction, compromised integrity of the intestinal barrier can further exacerbate hepatic failure. In patients with cirrhosis, impaired liver function hampers ammonia metabolism, leading to elevated blood ammonia levels. Subsequently, ammonia traverses the blood-brain barrier and accumulates within the brain parenchyma, thereby triggering hepatic encephalopathy and cognitive impairment (Milosevic et al., 2019). Hyperammonemia can induce microglial activation, indicating the generation of inflammation in the central nervous system (Aitbaev et al., 2017). Furthermore, the gut microbiota also plays a pivotal role in hepatic encephalopathy development. Research has demonstrated that alterations in intestinal motility and decreased bile acid levels among cirrhosis patients contribute to an overgrowth of intestinal bacteria, resulting in dysbiosis of the gut flora. Bajaj et al. demonstrated that, in comparison to the control group, patients with hepatic encephalopathy exhibited an elevated proportion of enterobacteriaceae, Fusobacteriaceae, and Veillonaceae in their intestinal mucosal flora. Furthermore, these microbial groups were positively correlated with intestinal inflammation. Additionally, enterobacteriaceae was found to be associated with astrocyte changes linked to hyperammonemia (Ahluwalia et al., 2016). These findings suggest a potential association between specific gut microbiota and cirrhosis-related brain dysfunction.
3 Causes of intestinal barrier damageThe occurrence of intestinal inflammation is a pathological process triggered by external stimuli or pathogen invasion in the intestinal tissues (Medzhitov, 2010). In the early stages of inflammatory diseases, such as acute intestinal infections, pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) are released in large quantities and cause an inflammatory cascade. Conversely, anti-inflammatory cytokines (IL-4, IL-10, and IL-13) can suppress the inflammatory response and mitigate damage caused by excessive inflammation. The dysregulation of inflammatory/anti-inflammatory factors results in the infiltration of immune cells, concurrently activating signaling targets such as NF-κB and MAPK, thereby influencing the expression of downstream proteins (intestinal barrier proteins, immune receptors, and related enzymes) (Dong et al., 2019), ultimately leading to an elevation in intestinal permeability (Wells et al., 2017). In addition, intestinal infection can induce apoptosis of intestinal epithelial cells, resulting in the subsequent release of damage-associated molecular patterns. This leads to a persistent state of heightened inflammation within the intestinal mucosa and impairs the functionality of goblet cells and mucosal epithelial regeneration, thereby compromising the repair process of the damaged intestinal barrier (Jiang et al., 2010). However, the presence of ulcers, bleeding, and an imbalance in the mucosal environment can further compromise the integrity of the intestinal mucosal barrier, ultimately resulting in a detrimental cycle of ongoing mucosal damage and destruction, impaired reconstruction capacity, and exacerbated homeostatic imbalance (Chang et al., 2013).
Studies have demonstrated that cigarette smoking exerts both direct and indirect effects on the gastrointestinal tract, encompassing oxidative damage, compromised immune cell functionality, alterations in epigenetic patterns, as well as changes in microbial composition that collectively contribute to intestinal barrier dysfunction (Papoutsopoulou et al., 2020). The study conducted by Berkowitz et al. (2019) demonstrated that smoking adversely affects the integrity of the intestinal barrier in the small intestine through its impact on Pan’s cells, specialized epithelial cells located in this region responsible for secreting antimicrobial peptides crucial for regulating microbial growth. When mice were exposed to cigarette smoke condensate (CSC), specific alterations in Paneth cell granules in the ileum were observed, leading to a decrease in antimicrobial peptide production and bactericidal capacity. Furthermore, CSC induced an imbalance in the gut bacterial community and heightened susceptibility to bacterial infection-induced ileal damage in mice. The potential risk of intestinal injury due to chronic smoke exposure may be attributed to modifications in mucin distribution within the intestinal epithelium and alterations in flora composition (Allais et al., 2016).
In addition, other irritants including alcohol, radiation, and drug abuse are important triggers for intestinal barrier damage. The activation of pro-oxidases/genes and the inhibition of antioxidant levels, including glutathione, have been reported as key factors contributing to alcohol-induced intestinal damage, leading to heightened oxidative and nitrification (nitrosation) stress. Similarly, factors such as physical stimulation (radiation) and bacterial infection can also impact intestinal barrier damage by inducing imbalances in intestinal oxidative stress. When there is an excessive oxidation of intestinal tissues, it can hinder the regeneration of intestinal epithelial cells (IECs), increase the disruption of tight junction integrity, and reduce the secretion of antioxidants and other physiological processes that affect intestinal barrier function (Gao et al., 2023). Oxidative stress has the ability to directly impair IECs and activate various pathways related to oxidative and anti-oxidative stress, thereby regulating the extent of intestinal damage.
The occurrence of intestinal barrier damage is often not limited to a single cause. In addition to complex infection, inflammation, oxidative stress, apoptosis, and other pathological processes, intestinal ischemia/hypoxia and genetic factors are also closely associated with intestinal barrier damage, involving intricate mechanisms of interaction among them. To achieve a more accurate understanding of the etiology of intestinal injury, comprehensive and in-depth research on the molecular mechanism and injury mechanism needs to be conducted.
4 Molecular mechanism of intestinal barrier damage4.1 TLR signaling pathwayToll-like receptor (TLR) is an innate immune pattern recognition receptor, promptly triggering intracellular signaling cascades upon detection of pathogen-associated molecular patterns (PAMPs), including proteins, nucleic acids, and lipids derived from invading pathogenic microorganisms. Ultimately, this activation leads to the initiation of both non-specific and specific immune responses aimed at eliminating pathogens (Liu et al., 2024). LPS/TLR4 signaling pathway can be classified into MyD88-dependent and MyD88-independent pathways, both of which activate the NF-κB and MAPK pathways that regulate inflammation (Izadparast et al., 2022). In intestinal inflammatory diseases, the activation of the LPS/TLR signaling pathway induces a robust inflammatory response, resulting in increased apoptosis of intestinal epithelial cells (IECs) and downregulation of tight junction protein expression, ultimately leading to impaired intestinal barrier function (Figure 2). Recent studies have demonstrated that neutrophil extracellular traps (NETs) induce the activation of Toll-like receptor 9 (TLR9) signaling pathway in septic patients, thereby triggering endoplasmic reticulum (ER) stress in intestinal epithelial cells (IECs), leading to increased intestinal inflammation, apoptosis of IECs, tight junction injury, and ultimately compromising the mechanical barrier of the intestinal mucosal epithelium. Inhibition of TLR9-ER stress signaling can significantly ameliorate NETs-induced apoptosis of IECs and improve intestinal function (Sun et al., 2021). As custodians of small intestine crypts, stem cell-derived Panes cells not only enhance the function of stem cells and promote epithelial regeneration but also secrete highly potent antimicrobial peptides such as alpha-defensin and lysin.
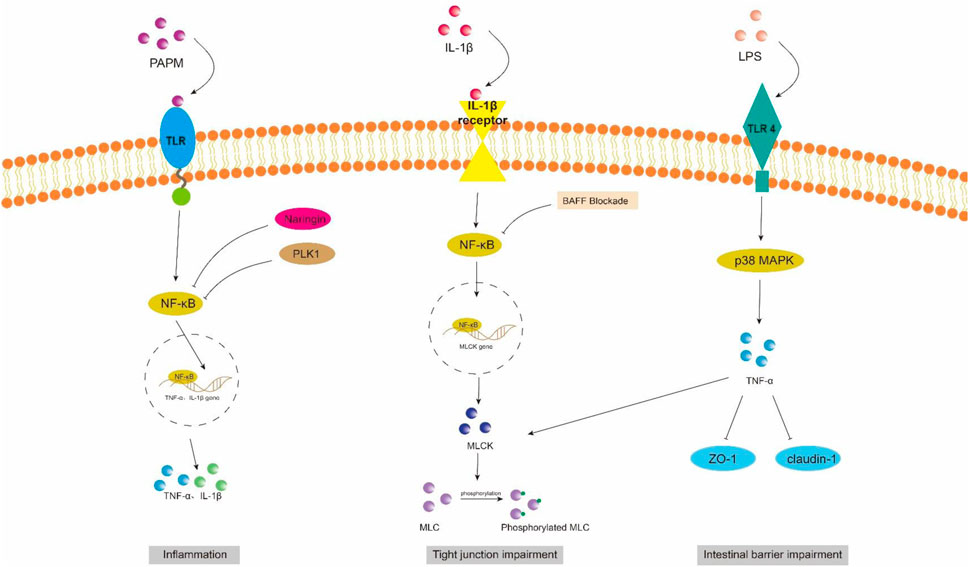
Figure 2. NF-κB and MAPK signaling pathway in intestinal barrier damage. PAPM, pathogen-associated molecular pattern; LPS, Lipopolysaccharides; TLR, Toll-like receptors; PLK1, Polo-like Kinase 1; ZO-1, zonula occluden-1.
However, in sepsis mice, it has been observed that the activation of the TLR4/ATF/CHOP signaling pathway by ER stress can induce apoptosis or dysfunction in Panzer cells, leading to exhaustion and subsequently impairing intestinal stem cell mobility, reducing secretion of antimicrobial peptides, exacerbating intestinal injury, and ultimately increasing mortality (Wang et al., 2022). Scholars have also discovered that the overexpression of pentosan-3 (PTX3) can inhibit the TLR signaling pathway, thereby reducing levels of inflammatory factors such as TNF-α, IL-1β, and interferon (INF)-γ. This inhibition helps alleviate apoptosis in intestinal epithelial cells (IECs) and promotes the expression of tight junction proteins ZO-1 and occludin between these cells, ultimately leading to a reduction in damage to the intestinal epithelium (Li et al., 2022).
4.1.1 NF-κB signaling pathwayNF-κB is a crucial nuclear transcription factor involved in inflammation and immune response, while also regulating apoptosis and stress response. In sepsis, the activation of Transforming growth factor kinase 1 (TAK1) can be mediated by inflammatory factors such as TNF-α and IL-6. Upon activation, TAK1 phosphorylates the downstream inhibitory protein IκBα, leading to dissociation of NF-κBp65 from IκBα and subsequent translocation into the nucleus for transcriptional regulation of related genes. It has been observed that Polo-like kinase 1 (PLK 1) is suppressed in septic rats, leading to reduced expression of IκB-α and enhanced nuclear translocation of NF-κB p65. The combined downregulation of PLK1 and activation of NF-κB result in apoptosis of intestinal epithelial cells, thereby compromising the integrity of the intestinal mechanical mucosal barrier (Cao et al., 2020; Cao et al., 2018). At the same time, the expression levels of pro-caspase-3 and IκB-a were significantly upregulated upon pretreatment of human colon cells with NF-κB activity-inhibiting drugs, suggesting that inhibition of NF-κB can reduce the apoptosis of intestinal epithelial cells (Cao et al., 2020). Additionally, NF-κB signaling pathway can impair the integrity of the intestinal barrier by influencing the junctions between intestinal epithelial cells. The activated NF-κB p65 interacts with the promoter region of myosin light chain kinase (MLCK) and regulates the transcription of MLCK (Al-Sadi et al., 2008). Consequently, phosphorylation of MLC by MLCK triggers actin-myosin filament contraction, leading to downregulation of tight junction protein expression and increased intestinal permeability (Gatica-Andrades et al., 2017). Quan et al. (2020) discovered that rats treated with LPS exhibited significant increases in the phosphorylation levels of NF-κB p65 and IκBa, as well as MLCK and MLC, leading to a decrease in ZO-1 and occludin expression which compromised intestinal epithelial cell integrity and increased permeability. However, B-cell activator (BAFF) can inhibit the NF-κB/MLCK/MLC signaling pathway while increasing ZO-1 and occludin expression. Furthermore, a study conducted on animals demonstrated that the administration of gadolinium chloride (GdCl3) to sepsis rats resulted in a reduction of both systemic and intestinal inflammatory responses. This was primarily achieved through inhibition of NF-κB activation, leading to decreased MLCK expression, while promoting the expression of atrexin and ZO-1 (Zhao et al., 2021). Therefore, the restoration of the intestinal TJ barrier in endotoxemia can potentially be achieved by employing chemical compounds that possess inhibitory effects on NFκB and MLCK.
4.1.2 MAPK signaling pathwayMitogen-activated protein kinase (MAPK) is a cytoplasmic serine/threonine protein kinase that plays a crucial role in various physiological and pathological processes, including cell proliferation, differentiation, apoptosis, and survival. MAPK signaling network comprises three distinct pathways: the p38 mitogen-activated protein kinase (p38MAPK) pathway, the extracellular signal-regulated protein kinase 1/2 (ERK 1/2) pathway, and the c-Jun amino-terminal kinase (JNK/SAPK) pathway (Yang et al., 2013). ERK1/2 regulates cell survival, differentiation, and proliferation. The involvement of JNK and p38MAPK in inflammation is characterized by their ability to impede cell cycle progression and facilitate apoptosis. Moreover, these signaling pathways may exert crucial pro-apoptotic functions in damaged intestinal epithelial cells (He et al., 2015). Luo et al. (2023) demonstrated that LPS treatment markedly upregulated the mRNA expression of p38 MAPK in the ileum of mice. Upon activation, the p38 MAPK signaling pathway induces robust oxidative stress response and triggers the release of a plethora of inflammatory factors, including TNF-α, IL-1β, and IL-6. Consequently, this leads to an increase in crypt depth and a decrease in villus height and the ratio of villus height to crypt depth (V/C) of the intestine. Simultaneously, there was a significant reduction observed in the expression levels of intestinal barrier proteins such as zonula occludens 1 (ZO-1), occludin, claudin, mucin 2 (MUC2), and junctional adhesion molecule 2 (JAM2). The activation of the MAPK pathway has been demonstrated to be closely associated with the impairment of intestinal barrier function, which is attributed to the phosphorylation of myosin light chain (MLC) (Raj et al., 2023). Phosphorylation of MLC by myosin light chain kinase (MLCK) regulates cellular actomyosin contraction, a crucial step in maintaining barrier integrity through the opening of paracellular pathways (Turner et al., 1997). After MAPK activation, the phosphorylation of MLCK is increased by a significant number of inflammatory factors (such as TNF-α), subsequently leading to MLC phosphorylation and ultimately resulting in the disruption of intestinal tight junctions and an increase in intestinal permeability. Moreover, inhibition of MLCK enhances the barrier function of TNF-α-stimulated intestinal epithelial cells (Zolotarevsky et al., 2002). In addition, TNF-α can also exert an inhibitory effect on the promoter activity of occludin, leading to the rearrangement of ZO-1 and claudin-1 (Wang et al., 2005). Wang et al. (2017) demonstrated that IL-1β induces phosphorylation of p38 MAPK, upregulates MLCK expression, and enhances paracellular permeability in the intestinal epithelial cells. Additionally, they discovered that curcumin inhibits IL-1β-induced activation of p38 MAPK, thereby attenuating the increased expression of MLCK and safeguarding the integrity of the intestinal epithelial barrier against translocation of bacterial LPS from the intestine into systemic circulation. It has been reported that the JNK inhibitor SP600125 has been shown to reduce intestinal inflammatory response and prevent intestinal barrier breakdown by increasing ZO-1 and closure protein expression (Samak et al., 2015). Similarly, Sotetsuflavone exhibited protective effects on the intestinal barrier by suppressing the JNK and p38 signaling pathways in inflammatory conditions (Ge et al., 2023). Xu et al. (2024b) demonstrated that through the inhibition of inflammatory macrophages, regulation of extracellular redox homeostasis, and downregulation of the MAPK/ERK signaling pathway, it is possible to suppress over-activated inflammation and restore cell-tight junction proteins. This leads to a reshaping of the intestinal microenvironment and achieves the purpose of treating endotoxemia.
4.2 ApoM/S1P signaling pathwayGut vascular barrier (GVB), a novel anatomical structure in the mouse and human gut described by Spadoni et al., in 2015, is composed of vascular endothelial cells, pericellular fibroblasts, enteric glial cells, as well as junction complexes including tight junctions (TJs) and adhesion junctions (AJs) (Ge et al., 2020). GVB serves to prevent the entry of microorganisms into the bloodstream and regulate antigen translocation (Sorribas et al., 2019). As an intestinal barrier, GVB has gained increasing attention in recent years. Studies have found that GVB may be impaired in sepsis and its permeability increases (Liu et al., 2020). Sphingosine-1-phosphate (S1P) is a sphingomyelin metabolite that exhibits diverse biological activities, including involvement in cell growth, apoptosis, and regulation of the immune and clotting systems through binding to G protein-coupled receptors. Within the intestinal epithelium, S1P serves as a crucial regulator of epithelial cell barrier function by activating the receptor S1PR1 located in intestinal blood vessels. This activation leads to an increase in trans-monolayer electric resistance of endothelial cells and helps maintain vascular integrity during inflammatory bowel disease (Karuppuchamy et al., 2017). The primary carrier of S1P in plasma is ApoM, with approximately 5% of HDL particles containing ApoM (Frej et al., 2016). In conclusion, the ApoM/S1P axis plays a crucial role in safeguarding the integrity of the intestinal barrier (Li et al., 2023a). Studies have demonstrated that LPS, TNF-α, and IL-1 exert inhibitory effects on hepatic ApoM production (Jiang et al., 2015). Kumaraswamy et al. (2012) observed a significant reduction in plasma ApoM levels among patients with varying degrees of sepsis, septic shock, and SIRS compared to healthy controls, with the extent of reduction reflecting the severity of SIRS/sepsis. Christensen et al. (2016) confirmed an increased intestinal permeability in ApoM (−/−) mice as opposed to ApoM (+/+) mice, suggesting that disruption of the ApoM/S1P axis is responsible for heightened intestinal leakage and damage to the gut-vascular barrier. Sphingosine kinase (SPHK) is a rate-limiting enzyme involved in the synthesis of S1P and plays a pivotal role in maintaining the integrity of the intestinal epithelial barrier (Li et al., 2023d). Chen et al. (2023) discovered an elevation in plasma lipopolysaccharide (LPS) levels in mice with alcoholic liver disease, accompanied by a reduction in SPHK2 protein expression and its regulated S1P, ultimately leading to disruption of the intestinal barrier. Their study substantiated that targeting SPHK2 and increasing S1P levels can ameliorate gut microbiota, reduce plasma LPS levels, and restore intestinal barrier function. In addition, Li et al. (2020) discovered that berberine can augment plasma ApoM and APOM-binding S1P levels during sepsis by inhibiting gluconeogenesis, insulin resistance, and secretion of pro-inflammatory molecules. Subsequent investigations revealed that APOM-bound S1P reduced the expression of PV1, an endothelial permeability marker induced by LPS. Furthermore, the levels of occludin and β-catenin suppressed by LPS were elevated (Li et al., 2020; Spadoni et al., 2015). It is postulated that targeting the ApoM/S1P axis could be a novel approach for treating LPS-induced intestinal barrier damage (Figure 3).
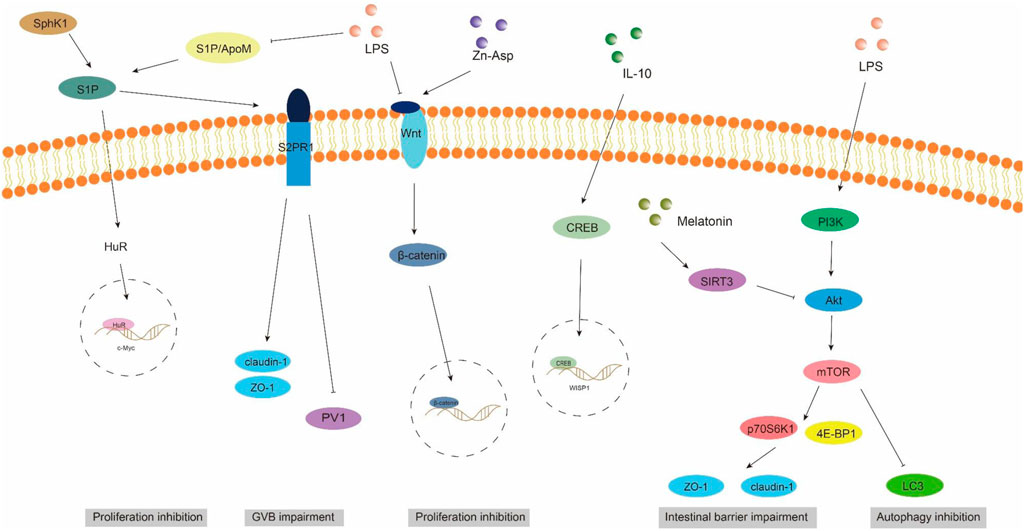
Figure 3. ApoM/S1P, Wnt/beta-catenin, and PI3K/AKT/mTOR signaling pathways involved in intestinal barrier damage. S1P, sphingosine 1-phosphate; S2PR1, sphingosine 2-phosphate receptor 1; SPHK1, sphingosine kinase 1; PV1, plasmalemmal vesicle-associated protein-1.
4.3 Wnt/beta-catenin signaling pathwayThe WNT pathway is a complex signaling network that encompasses the classical WNT/β-catenin pathway and the non-classical WNT pathway. It plays a pivotal role in various biological processes, including cell proliferation, apoptosis, and migration (Moparthi and Koch, 2019). The translocation of β-catenin to the nucleus occurs following activation by the Wnt ligand, thereby facilitating transcription of downstream gene transcription factors that play a pivotal role in shaping the morphology, growth, and regeneration of intestinal epithelial cells (Jung and Park, 2020). Within the gastrointestinal tract, activation of the Wnt/β-catenin signaling pathway is indispensable for maintaining epithelial homeostasis and holds dominance in recognizing and sustaining epithelial stem cells (Kuhnert et al., 2004) (Figure 2). Moreover, this pathway exhibits close association with inflammatory signaling pathways such as NF-κB and MAPK signaling, exerting influence on epithelial homeostasis and tissue regeneration; inhibition of this pathway results in loss of intestinal crypts and tissue denaturation (Moparthi and Koch, 2019). Xie et al. (2020) reported an over-activation of the Wnt/β-catenin signaling pathway following intestinal infection, resulting in aberrant proliferation of intestinal stem cells and crypts, leading to the disruption of the intestinal mucosal barrier and subsequent onset of diarrhea. Mouries et al. (2019) confirmed that fatty liver disease development is accompanied by impairment of GVB and the intestinal barrier, which is closely associated with perturbations in the WNT/β-catenin signaling pathway. Additionally, driving β-catenin activation in endothelial cells prevents damage to GVB and inhibits NASH progression. Studies have demonstrated that during the onset of weaning stress, piglets experience a range of complications including impairment to the integrity of the intestinal epithelial barrier, transient intestinal inflammation, and diarrhea (Wang et al., 2024), which are closely associated with perturbations in the wnt/β-catenin signaling pathway (Wang et al., 2022). It has been reported that upregulation of β-catenin expression can impede the proliferation of pathogenic bacteria, thereby mitigating weaning stress-induced intestinal inflammation and damage to the intestinal barrier (Tong et al., 2023). In addition, the repair of the intestinal mucosal barrier by the anti-inflammatory factor IL-10 has been demonstrated to partially rely on the activation of the Wnt pathway in epithelial cells (Quiros et al., 2017). Regulating the Wnt/β-catenin pathway can also confer protection on GVB function in sepsis (He et al., 2018). The findings of Zhou et al. (2020) demonstrated that zinc L-aspartate (Zn-Asp) effectively augmented the renewal and regeneration of intestinal stem cells (ISCs) through activation of the Wnt/β-catenin signaling pathway. Additionally, it successfully mitigated inflammation in jejunal epithelial cells and preserved intestinal barrier integrity against deoxynivalenol (DON)-induced damage. The preservation of intestinal stem cells (ISCs) is crucial for the sustained regeneration and repair of the intestinal mucosal epithelium following injury, as ISCs possess the capacity to generate multiple cell lineages within the intestinal epithelium, which relies on the proper functioning of the Wnt/beta-catenin signaling pathway (Ma et al., 2023).
4.4 PI3K/AKT/mTOR signaling pathwayAutophagy exerts beneficial effects on cellular, tissue, and organ homeostasis, while also playing a crucial role in maintaining intestinal barrier function (Wu et al., 2019). The phosphatidylinositol 3-kinase (PI3K)/Protein kinase B (AKT)/Mammalian target of rapamycin (mTOR) signaling pathway serves as a pivotal transduction factor in autophagy and is involved in the regulation of diverse cellular functions such as cell survival, growth, proliferation, and metabolis (Guo et al., 2019). It has been documented that LPS induces a significant upregulation of mRNA and phosphorylation levels within the AKT/PI3K/mTOR signaling pathway in mice, leading to intestinal inflammatory response and impairment of barrier function. Simultaneously, inhibition of the AKT/PI3K/mTOR signaling pathway can effectively safeguard the intestine against LPS-induced damage to its barrier integrity (Cheng et al., 2024). Wang et al. (2024) demonstrated that LPS significantly attenuated autophagosome formation and suppressed the expression of LC3, a key protein involved in autophagy, within the gastrointestinal tract. Consequently, this impaired elimination of damaged cellular components, leading to heightened intestinal oxidative stress and an exaggerated immune response. Subsequently, they discovered that inhibition of the PI3K/Akt/mTOR signaling pathway reversed LPS-induced suppression of autophagy, thereby mitigating damage to the intestinal barrier. The study conducted by Xu et al. (2021) showed that melatonin induces the upregulation of Sirtuins3 (SIRT3), which in turn modulates the AMPK/mTOR pathway and enhances autophagy, thereby mitigating small intestine damage in sepsis. In addition to affecting autophagy, the activation of the PI3K/Akt/mTOR signaling pathway in intestinal epithelial cells is also closely associated with the expression of tight junction proteins (Wang et al., 2015). Akt activation facilitates mTOR phosphorylation, leading to downstream substrate activation including p70S6K1 and 4E-BP1, which subsequently promote protein synthesis (Manning and Cantley, 2007). Yan and Ajuwon (2017) discovered that stimulation with LPS results in a reduction in the levels of phosphorylated Akt and total Akt, subsequently leading to a decrease in the abundance of phosphorylated 4E-BP1. This cascade effect ultimately causes a decline in the expression of tight junction proteins such as occludin, claudin-4, ZO1and 2. Furthermore, they observed that upregulation of Akt signaling counteracts the LPS-induced decrease in tight junction protein synthesis. These findings suggest that disruption of the epithelial barrier induced by LPS may be achieved through inhibition of the Akt/mTOR signaling pathway. It can be speculated that the PI3K/Akt/mTOR signaling pathway may lead to LPS-induced intestinal barrier damage through autophagy and regulatory protein synthesis. Inhibition of PI3K/Akt/mTOR may enhance autophagy while potentially reducing protein synthesis, thus emphasizing the criticality of maintaining a balanced state within the PI3K/Akt/mTOR signaling pathway to safeguard intestinal barrier integrity (Figure 3).
5 Effects of microgravity on intestinal barrierMicrogravity is the predominant characteristic of the space environment, which can induce physiological adaptation and pathophysiological alterations in multiple systems, such as muscle atrophy, bone demineralization, and immune system dysregulation (Nie et al., 2024). As a vital interface with the external milieu, the intestinal barrier function plays a crucial role in safeguarding the internal homeostasis by effectively impeding the entry of harmful substances. In recent years, increasing attention has been devoted to investigating the impact of microgravity on gastrointestinal physiology and function, particularly pertaining to the intestine (Figure 4). Moreover, despite its close interconnection with other extraintestinal organs, our understanding of gut-organ axis under microgravity conditions remains limited. Future comprehensive exploration into the intricate interactions between gut and extraintestinal organs within a microgravity environment will significantly contribute to unraveling the mechanisms underlying intestinal barrier fun
留言 (0)