The global incidence of thyroid cancer, the most common malignancy in the endocrine system, has continued to rise in recent years (1). The majority of thyroid cancers originate from follicular epithelial cells, with differentiated thyroid cancer (DTC) accounting for about 90% of lesions. DTC retains some of the physiological functions of thyroid follicular epithelial cells, such as the expression of the sodium iodine transporter (NIS) and the capacity to uptake iodine. As a consequence of these actions, most DTCs are sensitive to radioactive iodine (RAI) and can be cured by surgery, radioactive iodine therapy, and thyroid hormone suppression, with a 20-year survival rate of > 95% (2). However, following the development of the disease, the expression of the NIS in some recurrent or metastatic lesions is decreased or the targeting of the NIS to the cell membrane is diminished (3). This results in decreased uptake of RAI by the lesions, resulting in rapid progression of radio-iodine refractory thyroid cancer (RAIR-DTC), with a 10-year survival rate of < 10% (4–6).
At present, localized, targeted, or redifferentiation therapies are mainly recommended for the treatment of RAIR-DTC. The primary objective of localized treatment is to relieve local compression symptoms. The tyrosine kinase inhibitors (TKIs) drugs approved by the FDA to treat progressive RAIR-DTC have been studied extensively in recent years and the results of clinical trials have been impressive. However, these TKIs mainly target receptors for vascular endothelial growth factor but not the special RAIR drugs, and therefore need to be used continuously. The side effects of these drugs also reduce the quality-of life of patients and increase the risk of death (5). RAIR-DTC often occurs in case of thyroid dedifferentiation, so a potential treatment option is to induce the lesions to restore radioactive iodine uptake after redifferentiation (7, 8). However, the majority of drugs currently being tested for induction of redifferentiation are in the research stage or have been shown to have limited clinical value (4, 9, 10). Predictions in published literature are that the incidence and crude death rate of thyroid cancer will continue to rise in the future (11). In view of this apparent lack of effective treatment, some studies have investigated how to predict the occurrence of RAIR-DTC. In this paper, we review studies on how to predict RAIR-DTC, and examine the factors involved in cancer development and feasible methods for forecasting this development. The original and review literature were searched in the Pubmed and Wanfang databases using the search terms “iodine refractory differentiated thyroid cancer” and “prediction”. The references in the relevant articles were also searched to identify any other relevant articles.
The value of demographic characteristicsSeveral studies have identified the cut-off age for predicting RAIR-DTC, with one study reporting the cut-off point was ≥ 55 years old (12), while other studies used >45 (13), >48 (14), or >55 years when the thyroglobulin antibody (TgAb) was positive and >40 years when TgAb was negative (15). Although a meta-analysis of 13 studies (16) showed that age was not an influential factor in the development of RAIR-DTC, the authors considered that this result may have been due to age-matching in the majority of the studies and therefore suggested that young age may be a protective factor. It is speculated that age is a possible predictive factor for RAIR-DTC, but the influence of age may be related to the fact that elderly people are more likely to have an advanced tumor grade and stage (17). Whether or not age influences the development and prognosis of RAIR-DTC, patients with tumor are generally significantly older than those without RAIR-DTC (18) that the probability of developing RAIR is up to 4.5 times greater in patients older than 46 years (19). Elderly patients have a poor prognosis due to a number of factors, such as clinic-pathologic factors and selection of therapy (17). Therefore, the possible predictive value of age on RAIR should be taken into account when choosing therapeutic options for elderly patients.
The incidence of thyroid cancer in females is higher than that in males, while for DTC, the prognosis of males is worse than that of females (17). In one study, the proportion of males in the RAIR patient group was higher than that in the non-RAIR group, with logistic regression analysis showing that male gender was a predictive factor for RAIR (18). However, a study by WL (20) showed that the ratio of females in the RAIR group was higher than that of males, although this difference was not statistically significant. Meanwhile, other studies also reported that gender was not a factor in the occurrence of RAIR-DTC (12, 14, 15). Considering the small number of RAIR-DTC cases in the literature (18), a study on a large number of patients should be carried out in the future to verify whether sex is a predictive factor.
White race ethnicity has been shown to be associated with a reduced odds ratio of RAIR disease (19). A study on the germline genetic landscape of African American and Caucasian patients showed some RAIR risk haplotypes occurred only in African American patients with >80% of African ancestry (21). These results indicate that race/ethnicity may be a factor in RAIR.
In addition, smoking and body mass index (BMI) ≥24 kg/m2 (12) were reported to increase the incidence of RAIR-DTC, Although most of the above studies were a retrospective design, the demographic characteristics may have influenced the course of RAIR, and prospective studies are therefore required in the future to analyze the predictive value of these demographic factors in the occurrence of RAIR-DTC.
The value of clinicopathological featuresPapillary carcinoma is the most common subtype in thyroid cancers, and also in most patients with RAIR-DTC (12, 20, 22). Multivariate analysis confirmed that follicular thyroid cancer (12) was one of the independent factors that predicted the prevalence of RAIR-DTC. Binary logistic regression analysis showed that a high-risk histological subtype was one of the independent risk factors for RAIR-DTC (20), while a meta-analysis (16) also showed high-risk pathological subtypes significantly increased the risk of RAIR-DTC.
In addition to the pathological classification, other clinicopathological characteristics of the tumor can also predict the development of RAIR-DTC. The probability of RAIR is increased significantly by the occurrence of vascular invasion (23) and extrathyroid invasion (12, 15, 16). As the primary tumor size is either >10 mm or >20 mm the risk of RIAR is increased significantly (12), with a maximum tumor diameter ≥12.5mm shown to be an independent risk factor for RAIR (20). In addition to the pT stages, the risk of RAIR is also increased in the pN or pM stages (12). The specificity of RAIR predicted by the combination of distant metastasis, high-risk histological subtype and maximum tumor diameter ≥12.5 mm was 98% (20). Therefore, it can be seen that the risk of developing RAIR-DTC increases gradually with the progression of the tumor. From this perspective, vigorous treatments for DTC patients should be carried out in order to determine whether this reduces the chance of developing RAIR. However, other studies reached different conclusions. For example, when the groups were divided according to TgAb positive or negative, tumor size, multiple focal points, TNM stage, lung metastasis size and lymph node metastasis size this did not increase the risk of RAIR in the TgAb negative group (15). Aamna et al. (24) also demonstrated there was no correlation between TNM stage and the occurrence of RAIR, while there was no significant correlation between tumor size and RAIR in a meta-analysis (16). Taken together, these studies indicate that it is controversial to perform a total thyroidectomy or lobectomy in low-risk thyroid cancer patients, and therefore we consider that it would be of value to clarify whether there is a correlation between TNM stage and RAIR in surgical protocols.
BRAFV600E is the most effective activator of the mitogen activated protein kinase (MAPK) bypass which plays a key role in the regulation of cell growth and proliferation (25). It is controversial whether there is a correlation between BRAFV600E and RAIR. Some studies have reported that BRAFV600E is an independent predictive factor for RAIR (13), independent of the setting of TgAb positivity or negativity (15), and that the BRAFV600E mutation is related closely to the occurrence of RAIR in papillary carcinoma (22). However, the study by Shobab reported that the prevalence of BRAFV600E mutation in RAIR patients was lower than that in general DTC patients (19). Based on the result that lower iodine avidity was observed in BRAFV600E-mutated lymph node metastases, we speculate that BRAFV600E may affect the occurrence of RAIR. In addition to BRAFV600E gene mutation, TERT mutation has also been shown to be an independent factor for predicting RAIR and significantly increases the risk of RAIR (16, 26). Compared with DTC with only BRAF gene mutation, patients with DTC and TERT mutation accompanied by distant metastasis are more likely to develop RAIR during initial iodine therapy, which indicates that TERT mutation predicts the occurrence of RAIR earlier than that of BRAFV600E mutation (27). In contrast, the incidence of lower iodine avidity in cases of lymph node metastatic DTC was less with the TERT mutation than that observed in the BRAFV600E group (28). Regardless of which mutation is more likely to be associated with the development of RAIR, the ratio of RAIR occurrence was reported to be significantly higher with the coexistence of the BRAFV600E and TERT mutations (26).
Expression of several proteins such as thyroid peroxidase (TPO) has been shown to have a significant correlation with iodine avidity in the thyroid (28), with iodine avidity decreasing with decreased expression of TPO. Consistent with this, the absence of TPO expression in the thyroid cancer lesions showed by immunohistochemical analysis predicts the failure of iodine therapy and the occurrence of RAIR (29). When the proportion of thyroglobulin (Tg) positive cells in metastatic lesions is < 56%, it effectively monitors the occurrence of RAIR. The expression level of anexelekto (AXL), a tyrosine kinase receptor, has also been shown to be a predictor of RAIR and significantly reduces the expression of NIS and RAI uptake (30). These results confirm that the occurrence of RAIR can be predicted based on the expression of these proteins in thyroid lesions thereby guiding effective individual treatment after surgery.
Taken together, these results suggest that the clinicopathological characteristics of thyroid lesions deserve attention, which may allow treatment plans to be adjusted in the early clinical stage.
Predictive value of serological markersThe prognosis of patients with thyroid cancer after surgery and radioactive iodine therapy can be assessed by monitoring Tg levels when the TgAb is negative in the clinic. Based on the finding of a retrospective study that showed preoperative serum Tg was significantly higher in patients with repeated iodine therapy than in those who did not receive this therapy or only received one iodine administration of iodine, an increase in serum Tg could be used as an independent predictor of RAIR (31). It has been suggested that if Tg changes greatly during follow-up, the possibility of RAIR should be considered, especially when the level doubles within a short period of time (< one year) (5). Some studies used ROC curve analysis to determine the cut-off value for changes in Tg levels that predicted RAIR. In pulmonary metastatic DTC patients, the cut-off value for detecting RAIR was 0.544 for stimulated Tg ratio before the first and second RAI therapy and 0.564 for a suppressed Tg ratio before and after the second RAI therapy (13). Based on the ratio of stimulating or inhibiting Tg between two RAI therapies, 57% and 81% were shown to be the cut-off values for predicting RAIR-DTC, respectively (32). When TgAb was positive, the TgAb level was 14.8 times higher than the upper limit level, or when the TgAb level decreased by < 46.4% between two RAI treatments this indicated the occurrence of RAIR (15). These studies showed that predicting RAIR was feasible using the changes in Tg or TgAb levels, especially when the Tg levels increased significantly in a short period of time or alternatively when the TgAb level did not decrease significantly between the two RAI treatments.
It has also been reported in the literature that conventional blood biomarkers can also be used to predict early-stage RAIR. The low density lipoprotein cholesterol-to-total cholesterol ratio (LDL-Ch/Tch) correlated positively with the incidence of RAIR, while the white blood cell (WBC) count at surgery was shown to be inversely associated with RAIR (33). However, it is important to note that the level of these two indices may be influenced by other factors. Therefore, the predictive value of conventional blood biomarkers in RAIR-DTC needs to be verified.
Predictive value of positron emission tomography/computed tomography imagingThe definition of RAIR in the 2015 American Thyroid Association (ATA) thyroid cancer guidelines focused on the uptake of RAI in thyroid cancer-related lesions (34). It was shown that more than 90% of recurrences or metastatic DTC with negative radioiodine uptake could take up Fluorine 18(18F) fluorodeoxyglucose (18F-FDG) (35). When 18F-FDG was taken up by DTC metastases, this indicated that the lesions were not sensitive to iodine therapy, with the degree of 18F-FDG uptake correlating negatively with the iodine therapeutic effect. This suggested that 18F-FDG imaging predicted the occurrence of RAIR (36, 37). Regardless of the degree of RAI uptake, lesions with 18F-FDG uptake are an independent predictor of the occurrence of RAIR, and of great value in the diagnosis and prediction of prognosis of RAIR (14, 37, 38). The diagnostic efficacy of RAIR was highest when the ratio of the maximum standardized uptake value (SUVmax) of the lesion and the standardized uptake value (SUV) of the liver was 3.01 (39). In view of these findings, the possibility of RAIR should be considered when thyroid cancer-related non-primary lesions show 18F-FDG avidity. At this point, clinicians should consider treatment options other than iodine therapy when the lesion is FDG positive either with or without the presence of iodine uptake.
With the advent of new novel molecular probes, some have been used to detect the lesions in RAIR, such as fibroblast activation protein inhibitor (FAPI) (40, 41) and prostate specific membrane antigen (PSMA) (42). A study by Martina (43) also showed that the expression of PSMA correlated strongly with the aggressiveness of DTC. In addition, several other articles reported that lesions in RAIR patients exhibited higher FAPI uptake. However, there is no evidence for the relationship between FAPI uptake in thyroid lesions and the risk of developing RAIR. It is hoped in the future that some new molecular probes can be used to predict RAIR at an earlier stage than that achieved by 18F-FDG.
Predictive value of radio iodine therapy characteristicsOne of the definitions for RAIR in the European Thyroid Association Guidelines (44) is reaching a cumulative radioactive activity of iodine > 22.2 GBq. A retrospective study also showed that the cumulative radioactive iodine dose and treatment times of RAIR patients were increased significantly compared with those in non-RAIR patients (19, 33). Therefore, when the frequency of radioactive iodine treatment and cumulative dose of radioactive iodine are used as variables, the frequency of radioactive iodine treatment ≥ 3 times is a predictor of RAIR, and the specificity and sensitivity of the occurrence of RAIR is increased significantly (18). The recurrence between the operation and iodine-131 treatment was also reported to have a good ability to predict RAIR (14). An interval between the initial diagnosis and the diagnosis of RAIR metastatic disease < 3 years has also been shown to predict poor survival (22). In conclusion, the rate of RAIR increases with the increase of iodine therapy. Given the indications for iodine therapy, it may be more likely that the risk of RAIR increases in cases with more severe forms of cancer.
Multi-parameter modelInformation on the patient was assigned to multiple variables and the predictive potential of these variables analyzed using a parametric model. Table 1 lists the studies that used a nomogram model to predict RAIR. Of the five studies, the independent predictors of RAIR in three were mainly the clinicopathological characteristics of the patients. There were some differences in the risk factors and cut-off values in these three models, due possibly to differences in the inclusion criteria for patients, such as the number of iodine treatments or the proportion of FTC. The sensitivity, specificity, and area under the curve (AUC) of these scoring systems were: 77.7%, 81.2%, 0.795 (12), 76%, 93%, 0.898 (14) and 86%, 92% and 0.95 (23), respectively. Of the three models, the model of Li et al (12) is suitable for postoperative use, with the prediction period being slightly earlier than that of the other two models (14, 23), and the AUC being slightly lower than that reported for the other two models which are suitable for use after follow-up or in patients who have undergone 18F-FDG examinations. However, regardless of their differences, the three models are easy to operate.
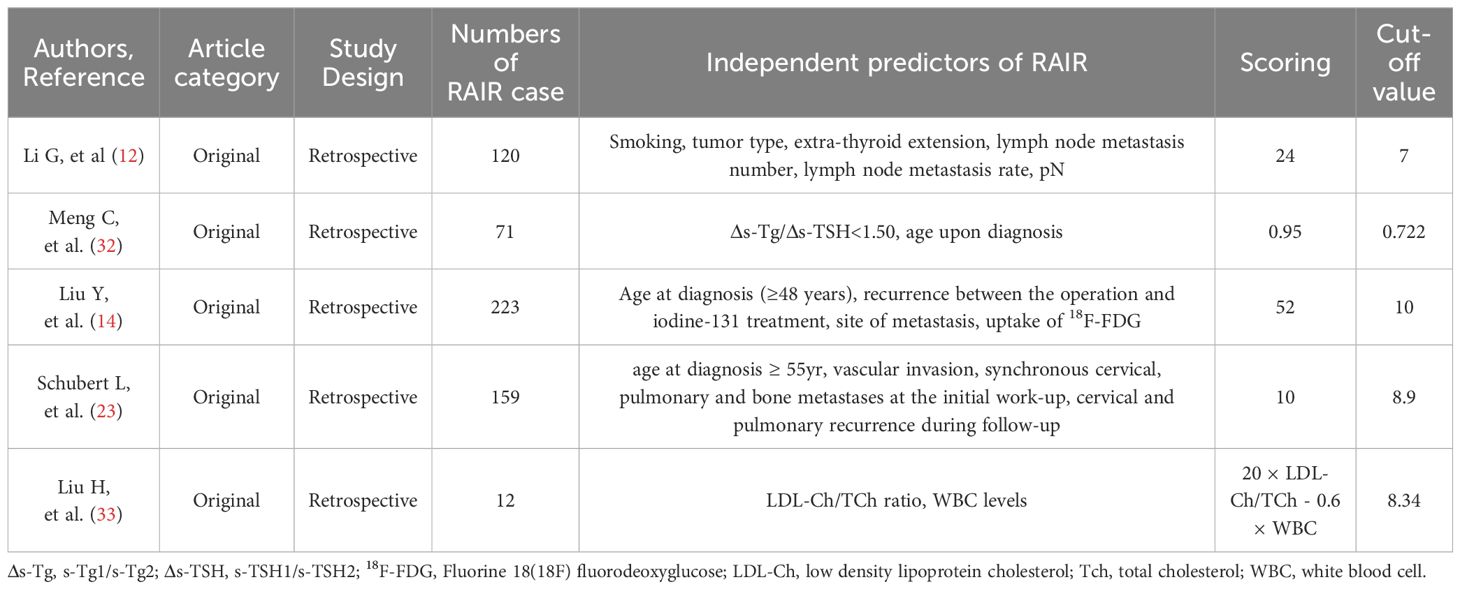
Table 1. Key data of studies that used a nomogram model to predict the development of RAIR.
The other two models mainly focused on serological markers. The study of Meng et al. (32) used a prediction model combining changes of the stimulated Tg between the first and second RAI treatments with the age at diagnosis, with the model having a specificity of 0.830, sensitivity of 0.755, and AUC of 0.830. It was obvious that this model was suitable for patients who needed repeated RAI treatment. Another prediction model for RAIR (33) used preoperative serological indicators with a low LDL-Ch/TCh and WBC count and was reported to have a sensitivity, specificity, and AUC of 0.833, 0.875, and 0.861 respectively. This model greatly advanced the prediction time of RAIR and could be carried out before the operation and was very easy to perform. However, there are many factors influencing these serological indicators and therefore the practicability of this model needs to be further investigated.
Taken together, these current multi-parameter model studies confirm that the multi-parameter model analysis is effective for predicting RAIR. However, all the above studies were a retrospective design and therefore prospective studies are needed to confirm the predictive value of these models.
This paper reviewed and summarized the relevant factors that predicted the development of RAIR thereby providing the basis for effective clinical treatment. A limitation of the paper was that most of the references were for retrospective studies, with a lower number of studies reporting the use of multi-parameter prediction models. Therefore, a larger number of prospective studies are needed in the future to confirm the clinical value of relevant predictors.
ConclusionsCurrent studies have shown that the occurrence of RAIR can be predicted by analyzing the clinicopathological characteristics of thyroid cancer lesions and that more attention should be paid to these characteristics. RAIR should be taken into account when the lesions are positive in 18F-FDG images or when Tg levels increase significantly over a short period of time. The multi-parameter model which combines the information of multiple patients has a good predictive efficacy, is easy to carry out and could be used to identify the risk of developing RAIR thereby assisting in the development of effective treatment measures.
Author contributionsYW: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Conceptualization. XL: Writing – review & editing, Methodology, Investigation, Formal analysis. HL: Writing – review & editing, Visualization, Validation, Supervision, Resources, Funding acquisition.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the National Natural Science Foundation of China (Grant number: 82372009).
AcknowledgmentsThe authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsDTC, differentiated thyroid cancer; NIS, sodium iodine transporter; RAI, radioactive iodine; RAIR, radio-iodine refractory thyroid cancer; TKI: tyrosine kinase inhibitor; BMI, body mass index; MAPK, mitogen activated protein kinase; TSH, Thyroid Stimulating Hormone; Tg, Thyroglobulin; TgAb, Thyroglobulin Antibody; TPO, thyroid peroxidase; AXL, anexelekto; 18F-FDG, Fluorine 18(18F) fluorodeoxyglucose; SUV, Standardized uptake value; PET/CT, Positron Emission Tomography/Computed Tomography; ROC, receiver operating characteristic; LDL-Ch/TCh, low density lipoproteincholesterol-total cholesterol ratio; WBC, white blood cell; ATA, American Thyroid Association.
References1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
2. Mohamed AF, Gonzalez JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. (2015) 2015:438235. doi: 10.1155/2015/438235
PubMed Abstract | Crossref Full Text | Google Scholar
3. Oh JM, Ahn BC. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics. (2021) 11:6251–77. doi: 10.7150/thno.57689
PubMed Abstract | Crossref Full Text | Google Scholar
4. Liu J, Liu Y, Lin Y, Liang J. Radioactive iodine-refractory differentiated thyroid cancer and redifferentiation therapy. Endocrinol Metab (Seoul). (2019) 34:215–25. doi: 10.3803/EnM.2019.34.3.215
PubMed Abstract | Crossref Full Text | Google Scholar
5. Jin Y, Van Nostrand D, Cheng L, Liu M, Chen L. Radioiodine refractory differentiated thyroid cancer. Crit Rev Oncol Hematol. (2018) 125:111–20. doi: 10.1016/j.critrevonc.2018.03.012
PubMed Abstract | Crossref Full Text | Google Scholar
6. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. (2006) 91:2892–9. doi: 10.1210/jc.2005-2838
PubMed Abstract | Crossref Full Text | Google Scholar
7. Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. (2014) 2:830–42. doi: 10.1016/S2213-8587(14)70051-8
PubMed Abstract | Crossref Full Text | Google Scholar
8. Toro-Tobon D, Morris JC, Hilger C, Peskey C, Durski JM, Ryder M. Clinical outcomes of radioactive iodine redifferentiation therapy in previously iodine refractory differentiated thyroid cancers. Thyroid: Off J Am Thyroid Assoc. (2024) 34(1):70–81. doi: 10.1089/thy.2023.0456
Crossref Full Text | Google Scholar
9. Yaqi Z, Xiqun Z, Qianyu F, Jian C. Progress in re-differentiating therapy of radioiodine-refractory differentiated thyroid cancer. Cancer Res Prev Treat. (2022) 49:1086–92. doi: 10.3971/j.issn.1000-8578.2022.22.0011
Crossref Full Text | Google Scholar
10. Genco MT, Yaqub A, Jana S. Challenges in the evaluation and management of radioactive iodine-refractory differentiated thyroid cancer. Nucl Med Commun. (2022) 43:743–5. doi: 10.1097/MNM.0000000000001568
PubMed Abstract | Crossref Full Text | Google Scholar
11. Chengzhi Y, Min Z, Yuke Z, Yiyun Z, Xia W, Wenjing X, et al. Analysis and prediction of thyroid cancer morbidity and mortality trends in China. Chin J Epidemiol. (2023) 44:917–23. doi: 10.3760/cma.j.cn112338-20221010-00869
Crossref Full Text | Google Scholar
12. Li G, Lei J, Song L, Jiang K, Wei T, Li Z, et al. Radioiodine refractoriness score: A multivariable prediction model for postoperative radioiodine-refractory differentiated thyroid carcinomas. Cancer Med. (2018) 7:5448–56. doi: 10.1002/cam4.1794
PubMed Abstract | Crossref Full Text | Google Scholar
13. Wang C, Zhang X, Li H, Li X, Lin Y. Quantitative thyroglobulin response to radioactive iodine treatment in predicting radioactive iodine-refractory thyroid cancer with pulmonary metastasis. PloS One. (2017) 12:e0179664. doi: 10.1371/journal.pone.0179664
PubMed Abstract | Crossref Full Text | Google Scholar
14. Liu Y, Wang Y, Zhang W. Scoring system and a simple nomogram for predicting radioiodine refractory differentiated thyroid cancer: a retrospective study. EJNMMI Res. (2022) 12:45. doi: 10.1186/s13550-022-00917-8
PubMed Abstract | Crossref Full Text | Google Scholar
15. Chai J, Zhang R, Zheng W, Zhang G, Jia Q, Tan J, et al. Predictive value of clinical and pathological characteristics for metastatic radioactive iodine-refractory differentiated thyroid carcinoma: A 16-year retrospective study. Front Endocrinol. (2022) 13:930180. doi: 10.3389/fendo.2022.930180
Crossref Full Text | Google Scholar
16. Luo Y, Jiang H, Xu W, Wang X, Ma B, Liao T, et al. Clinical, pathological, and molecular characteristics correlating to the occurrence of radioiodine refractory differentiated thyroid carcinoma: A systematic review and meta-analysis. Front Oncol. (2020) 10:549882. doi: 10.3389/fonc.2020.549882
PubMed Abstract | Crossref Full Text | Google Scholar
17. Shi L-Y, Liu J, Yu L-J, Lei Y-M, Leng SX, Zhang H-Y. Clinic-pathologic features and prognostic analysis of thyroid cancer in the older adult: A SEER based study. J Cancer. (2018) 9:2744–50. doi: 10.7150/jca.24625
PubMed Abstract | Crossref Full Text | Google Scholar
18. Ying L, Sha Z, Li X, Wei L, Xianghua M. The analysis of prognostic factors influencing the radioiodine refractory ⁃ differentiated thyroid cancer. J Nanjing Medicial Univ. (2020) 40:846–51,57. doi: 10.7655/nydxbns20200613
Crossref Full Text | Google Scholar
19. Shobab L, Gomes-Lima C, Zeymo A, Feldman R, Jonklaas J, Wartofsky L, et al. Clinical, pathological, and molecular profiling of radioactive iodine refractory differentiated thyroid cancer. Thyroid: Off J Am Thyroid Assoc. (2019) 29:1262–8. doi: 10.1089/thy.2019.0075
Crossref Full Text | Google Scholar
20. Liu W, Jiang B, Xue J, Liu R, Wei Y, Li P. Clinicopathological features of differentiated thyroid carcinoma as predictors of the effects of radioactive iodine therapy. Ann Diagn Pathol. (2023) 69:152243. doi: 10.1016/j.anndiagpath.2023.152243
PubMed Abstract | Crossref Full Text | Google Scholar
21. Hurst Z, Liyanarachchi S, He H, Brock P, Sipos J, Nabhan F, et al. Risk haplotypes uniquely associated with radioiodine-refractory thyroid cancer patients of high african ancestry. Thyroid: Off J Am Thyroid Assoc. (2019) 29:530–9. doi: 10.1089/thy.2018.0687
Crossref Full Text | Google Scholar
22. Saïe C, Wassermann J, Mathy E, Chereau N, Leenhardt L, Montcel STD, et al. Impact of age on survival in radioiodine refractory differentiated thyroid cancer patients. Eur J Endocrinol. (2021) 184:667–76. doi: 10.1530/eje-20-1073
PubMed Abstract | Crossref Full Text | Google Scholar
23. Schubert L, Mbekwe-Yepnang AM, Wassermann J, Braik-Djellas Y, Jaffrelot L, Pani F, et al. Clinico-pathological factors associated with radioiodine refractory differentiated thyroid carcinoma status. J endocrinological Invest. (2024) 47:1573–81. doi: 10.1007/s40618-024-02352-z
Crossref Full Text | Google Scholar
24. Hassan A, Riaz S, Bashir H, Nawaz MK, Hussain R. Can the american thyroid association risk of recurrence predict radioiodine refractory disease in differentiated thyroid cancer? Eur Thyroid J. (2016) 5:261–7. doi: 10.1159/000448920
PubMed Abstract | Crossref Full Text | Google Scholar
25. Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. (2005) 90:6373–9. doi: 10.1210/jc.2005-0987
PubMed Abstract | Crossref Full Text | Google Scholar
26. Cao J, Zhu X, Sun Y, Li X, Yun C, Zhang W. The genetic duet of BRAF V600E and TERT promoter mutations predicts the poor curative effect of radioiodine therapy in papillary thyroid cancer. Eur J Nucl Med Mol Imaging. (2022) 49:3470–81. doi: 10.1007/s00259-022-05820-x
PubMed Abstract | Crossref Full Text | Google Scholar
27. Yang X, Li J, Li X, Liang Z, Gao W, Liang J, et al. TERT promoter mutation predicts radioiodine-refractory character in distant metastatic differentiated thyroid cancer. J Nucl medicine: Off publication Soc Nucl Med. (2017) 58:258–65. doi: 10.2967/jnumed.116.180240
Crossref Full Text | Google Scholar
28. Nilsson JN, Siikanen J, Condello V, Jatta K, Saini R, Hedman C, et al. Iodine avidity in papillary and poorly differentiated thyroid cancer is predicted by immunohistochemical and molecular work-up. Eur Thyroid J. (2023) 12(4):e230099. doi: 10.1530/etj-23-0099
PubMed Abstract | Crossref Full Text | Google Scholar
29. Zelinskaya A. Immunocytochemical characteristics of thyrocytes in radioiodine refractory metastases of papillary thyroid cancer. Exp Oncol. (2019) 41:342–5. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-4.13705
PubMed Abstract | Crossref Full Text | Google Scholar
30. Collina F, Sala LL, Liotti F, Prevete N, Mantia EL, Chiofalo MG, et al. AXL is a novel predictive factor and therapeutic target for radioactive iodine refractory thyroid cancer. Cancers (Basel). (2019) 11(6):785. doi: 10.3390/cancers11060785
PubMed Abstract | Crossref Full Text | Google Scholar
31. Cheng X, Xu S, Zhu Y, Wu J, Bao J, Yu H, et al. Markedly elevated serum preoperative thyroglobulin predicts radioiodine-refractory thyroid cancer. Eur J Clin Invest. (2022) 52:e13721. doi: 10.1111/eci.13721
PubMed Abstract | Crossref Full Text | Google Scholar
32. Meng C, Song J, Long W, Mu Z, Sun Y, Liang J, et al. A user-friendly nomogram for predicting radioiodine refractory differentiated thyroid cancer. Front Endocrinol. (2023) 14:1109439. doi: 10.3389/fendo.2023.1109439
Crossref Full Text | Google Scholar
33. Liu H, Chen Q, Liu B, Wang J, Chen C, Sun S. Blood profiles in the prediction of radioiodine refractory papillary thyroid cancer: A case-control study. J Multidiscip healthcare. (2023) 16:535–46. doi: 10.2147/jmdh.S403045
Crossref Full Text | Google Scholar
34. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 american thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid: Off J Am Thyroid Assoc. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
Crossref Full Text | Google Scholar
35. Ha LN, Iravani A, Nhung NT, Hanh NTM, Hutomo F, Son MH. Relationship between clinicopathologic factors and FDG avidity in radioiodine-negative recurrent or metastatic differentiated thyroid carcinoma. Cancer imaging: Off Publ Int Cancer Imaging Soc. (2021) 21:8. doi: 10.1186/s40644-020-00378-z
Crossref Full Text | Google Scholar
36. Roy M, Edet-Sanson A, Lefebvre H, Vera P, Decazes P. Using 18F-FDG-PET/CT metrics to predict survival in ra-dio-iodine refractory thyroid cancers. Diagnostics. (2022) 12(10):2381. doi: 10.3390/diagnostics12102381
PubMed Abstract | Crossref Full Text | Google Scholar
37. Kang SY, Bang J-I, Kang KW, Lee H-Y, Chung J-K. FDG PET/CT for the early prediction of RAI therapy response in patients with metastatic differentiated thyroid carcinoma. PloS One. (2019) 14:e0218416. doi: 10.1371/journal.pone.0218416
PubMed Abstract | Crossref Full Text | Google Scholar
38. Matsuo M, Baba S, Hashimoto K, Isoda T, Kitamura Y, Kogo R, et al. Utility of FDG PET at the initial radioiodine therapy in differentiated thyroid cancer. Anticancer Res. (2023) 43:183–90. doi: 10.21873/anticanres.16148
PubMed Abstract | Crossref Full Text | Google Scholar
39. Xiaowei T, Liang S, Jun W, Zhenyu Z, Ting B, Feng W. Differential diagnosis of 18F-FDG PET/CT in high risk and radioiodine-refractory differentiated thyroid carcinoma. Chin J Med Imaging. (2022) 30:210–4,29. doi: 10.3969/j.issn.1005-5185.2022.03.004
Crossref Full Text | Google Scholar
40. Chen Y, Zheng S, Zhang J, Yao S, Miao W. (68)Ga-DOTA-FAPI-04 PET/CT imaging in radioiodine-refractory differentiated thyroid cancer (RR-DTC) patients. Ann Nucl Med. (2022) 36:610–22. doi: 10.1007/s12149-022-01742-8
留言 (0)