According to the latest estimate of the Global Cancer (GLOBOCAN) statistical report, the annual incidence of gastric cancer in the world in 2020 is 1.089 million people (corresponding to the age-standardized incidence of 11.1 per 100,000 people), ranking fifth among all malignant tumors (1). A study published in 2022 estimated that new cases of stomach cancer worldwide will increase by 62% to 1.77 million cases by 2040 (2). Clinical evidence shows that most gastric cancer patients have serious nutrient absorption disorders and lower immune function than normal patients (3). Continuous malnutrition will reduce the tolerance of gastric cancer patients to chemotherapy, seriously hinder the completion of chemotherapy plan, and affect the therapeutic effect of cancer.
There are two main ways of clinical nutrition support therapy: enteral nutrition (EN) and parenteral nutrition. After surgical operation, patients will have high metabolism and negative nitrogen balance, which will affect their absorption of nutrients and easily lead to malnutrition (4). EN refers to the nutritional support mode that provides nutrients required for human metabolism through the gastrointestinal tract (5). Enteral immune nutrition preparations refer to the addition of glutamine, arginine, ω-3 unsaturated fatty acids, nucleotides and other immunomodulatory nutrients into standard enteral nutrition formulations to improve cellular immune function by affecting the production and release of local or systemic inflammatory factors (6). Studies have shown that immune-enhanced EN preparations containing immune regulatory components of ω-3 polyunsaturated fatty acids are beneficial to patients undergoing surgery for head and neck tumors and upper digestive tract tumors by reducing the risk of infection, improving immune and inflammation-related indicators, maintaining body mass and reducing hospital stay (7, 8).
The systematic evaluation of the clinical value of ω-3 polyunsaturated fatty acid nutrition drugs is an urgent requirement to promote the rational use of nutrition drugs in clinic, and it is also an effective way to enhance the therapeutic effect of diseases and improve the health of people. However, up to now, there is still a lack of high-quality systematic reviews on the efficacy evaluation and analysis of ω-3 polyunsaturated fatty acids, especially the lack of systematic reviews on the safety and effectiveness of perioperative use of ω-3 polyunsaturated fatty acids in patients with gastric cancer. Therefore, through systematic review, this study analyzed the clinical value of EN preparations containing ω-3 polyunsaturated fatty acids compared with conventional EN preparations in perioperative nutritional support therapy for gastric cancer patients at home and abroad. On the one hand, it provided high-quality grade evidence for clinical nutritional support therapy for gastric cancer patients, and on the other hand, it provided theoretical basis for clinical standardized application of gastric cancer patients. Enteral immunonutrition technology is one of the effective ways to improve this predicament.
2 Materials and methods2.1 Literature search strategyThe databases of Pubmed, EMbase, The Cochrane Library, CNKI and Wanfang Medical were searched by computer from the establishment of the database to August 1, 2024. The English search terms included “fatty acids”, “ω-3”, “n-3” and “gastric cancer”, while the Chinese search terms included “enteral immune nutrition”, “polyunsaturated fatty acids”, “ω-3 polyunsaturated fatty acids”, “n-3 polyunsaturated fatty acids” and “gastric cancer”.
2.2 Study selection2.2.1 Inclusion criteria① The study type was randomized non-placebo controlled trial. ② The study subjects were patients with gastric cancer who received EN containing ω-3 polyunsaturated fatty acids or conventional EN during perioperative period. ③ The outcome measures included safety indicators and efficacy indicators, among which safety indicators included postoperative infection complications; The efficacy indexes included perioperative mortality, immune indexes, inflammatory indexes, and nutritional indexes.
2.2.2 Exclusion criteriaFull text not available; Incomplete data; Single-arm study; Duplicate publications; The surgical site does not meet the requirements; No clinical outcome measures or intermediate measures were reported; Subjective qualitative research.
2.3 Data extraction and quality assessmentContributions were screened independently by two researchers according to inclusion and exclusion criteria. Information, including study author, time, and general characteristics of participants, was extracted using a pre-designed data extraction form and submitted one by one Cross check. If there are differences, they are resolved through discussion. If the data reported in the study is incomplete, further contact the author of the study to obtain; If no relevant data is obtained, the study is excluded.
The Cochrane risk bias assessment tool was used to evaluate literature quality. The tool evaluated risk bias in seven areas, including random sequence generation, assignment hiding, investigator and subject blinding, blind evaluation of study results, outcome data integrity, selective reporting of risk, and other sources of bias. For each indicator, “low bias risk”, “uncertain bias risk” and “high bias risk” are used for judgment (9).
3 Statistical analysisThe methods described in the Cochrane Collaborative network meta-analysis guidelines were used for meta-analysis of data using Review Manager 5.4 statistical software. The P-value of the forest map in the meta-analysis was used to detect the differences among different trials. When the P-value was < 0.05, the results were considered to be significantly different. Chi-square test was used to test the heterogeneity of all included studies. If the heterogeneity was small (P > 0.1 and I2 ≤ 50%), the fixed-effect model was used for meta-analysis. If the heterogeneity is large (P ≤ 0.1 and I2 > 50%), the random effects model is used for analysis. If the data provided by the trial cannot be meta-analyzed, only descriptive qualitative analysis will be performed. According to the recommendations of the Cochrane Manual for the production of systematic reviews, publication bias was tested by funnel plot.
4 Results4.1 Search resultsAfter initial search, title, abstract preliminary screening, full-text screening and final screening, a total of 20 studies were finally included in the literature search results. Literature search and inclusion process are shown in Figure 1.
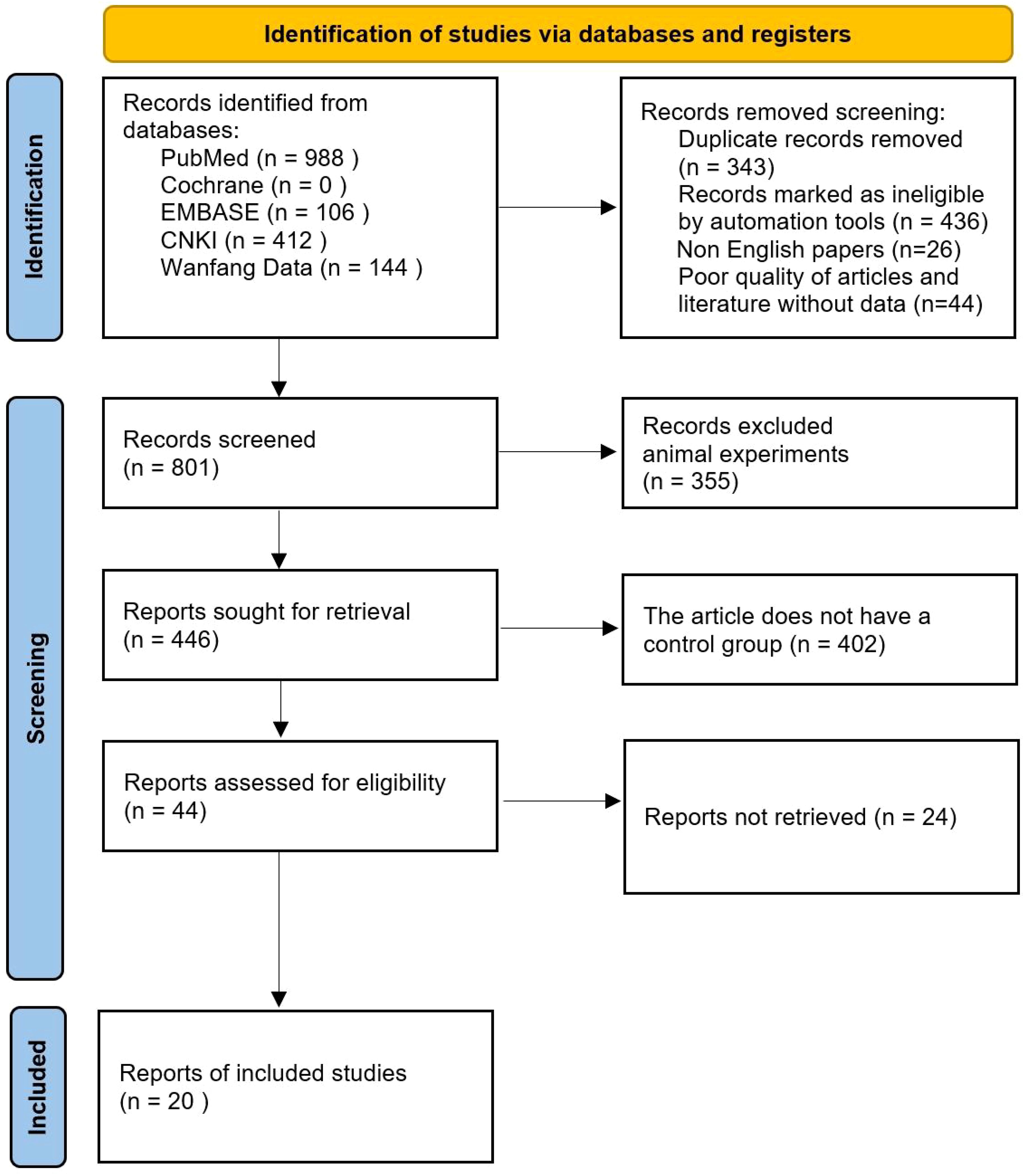
Figure 1. PRISMA Flow chart of article selection.
4.2 Study characteristicsThe authors, time, number of cases and other information of the 20 studies included in the characteristics table are shown in Table 1 (10–29). Of the 20 articles included, 10 were conducted in China. The results of bias risk assessment are shown in Figure 2.
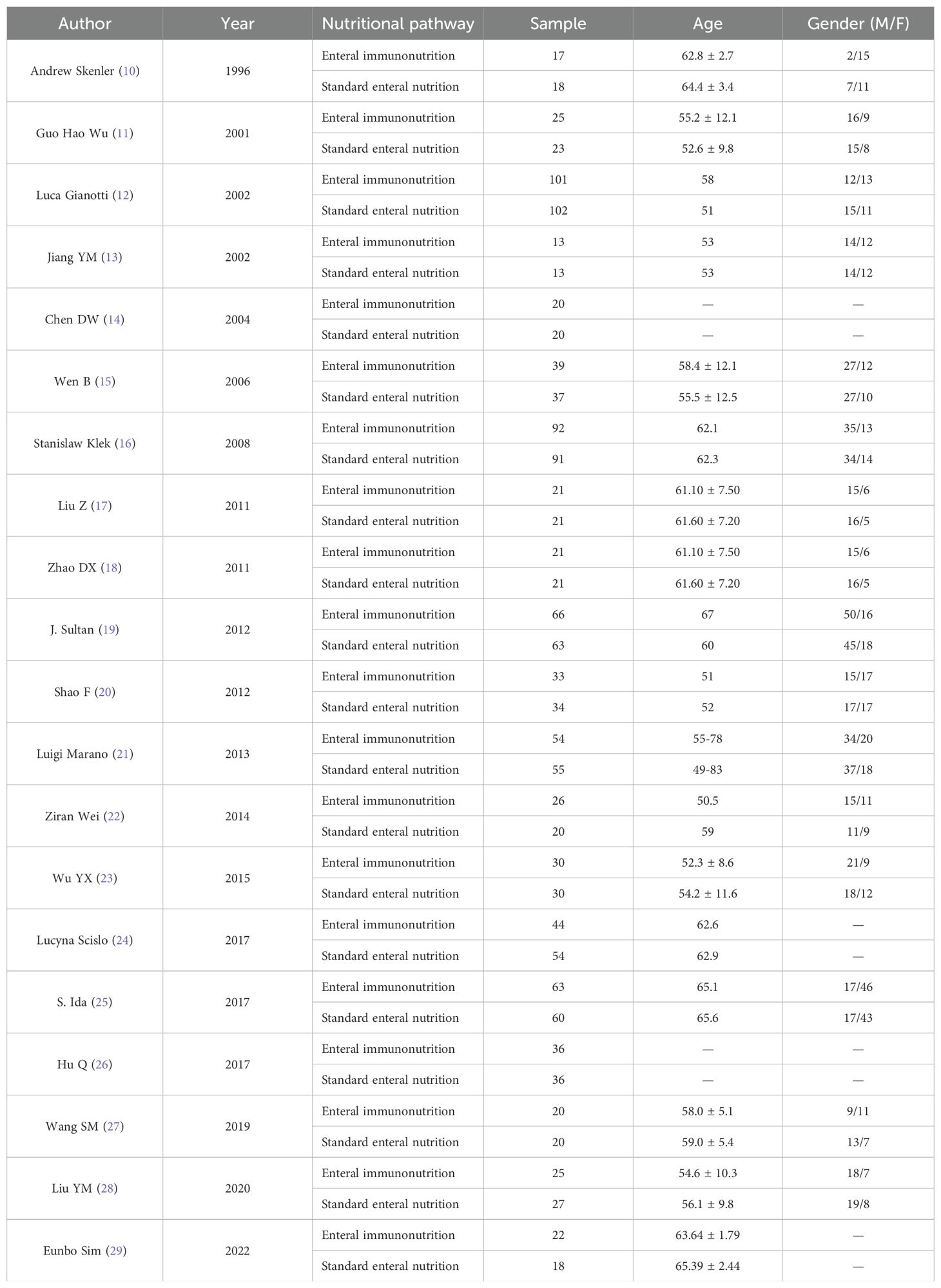
Table 1. Basic information of literatures (10–29).
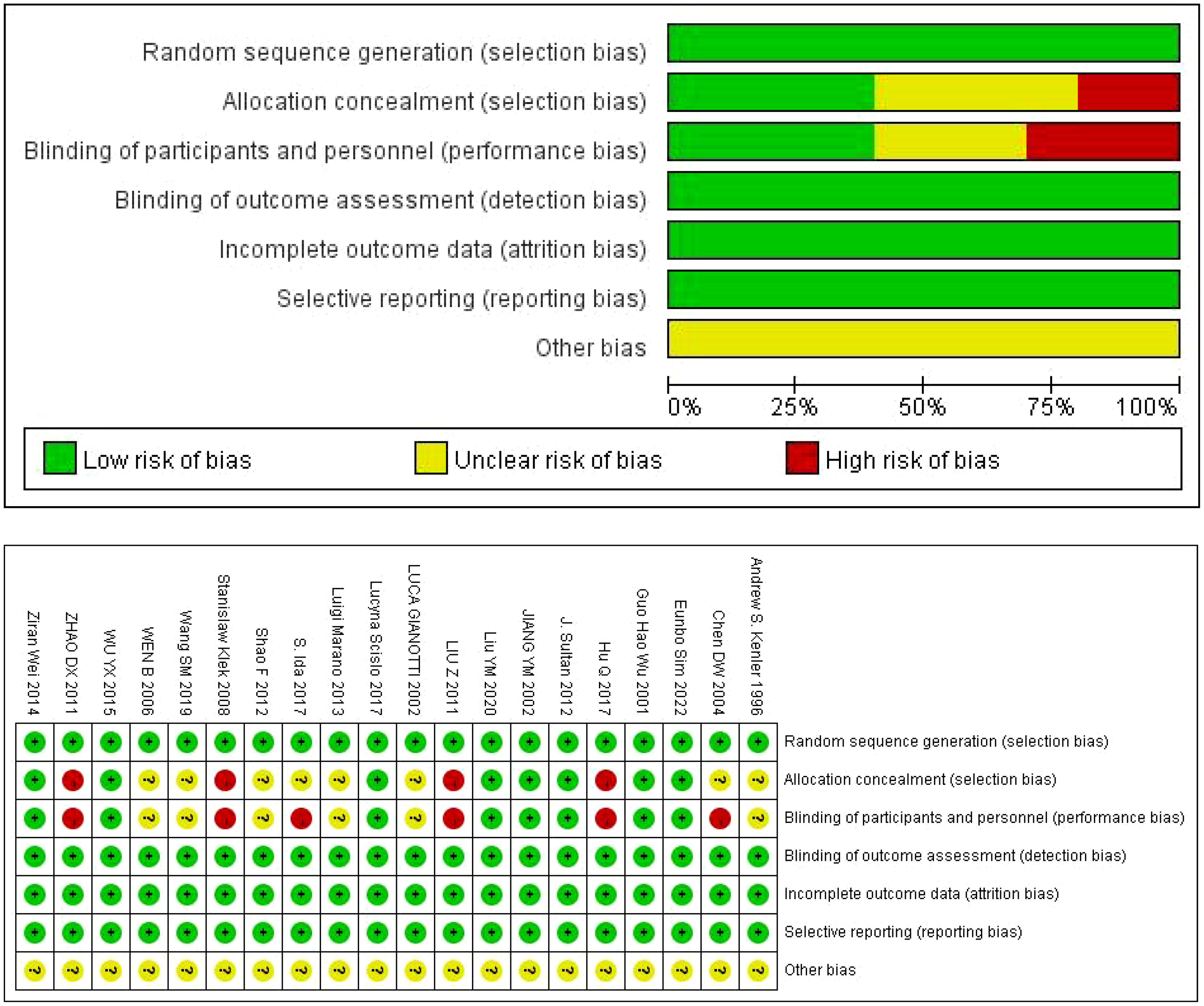
Figure 2. Evaluation results of methodology quality of included studies.
4.3 Meta-analysis outcomes4.3.1 Mortality rateA total of 4 studies reported mortality results, including 265 cases of ω-3 polyunsaturated fatty acids and 274 cases in the control group. I2 = 0%, P = 0.50 indicated that there was no heterogeneity. Statistical results showed that compared with the control group, the mortality rate in the enteral nutrition with ω-3 polyunsaturated fatty acid preparation group was 0.46 times higher than that in the control group (RR = 0.46, P = 0.17), but there was no statistical difference, as shown in Figure 3.
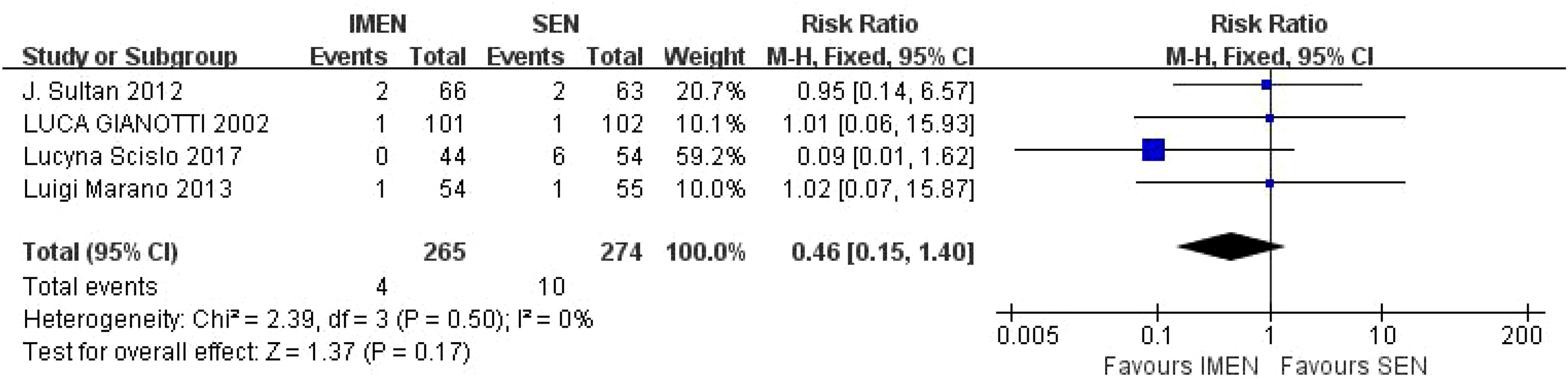
Figure 3. Meta-analysis of mortality rate.
4.3.2 Postoperative infectious complications4.3.2.1 Infectious complicationsResults of Infectious Complications were reported in 7 papers, including 362 cases of ω-3 polyunsaturated fatty acids and 361 cases of control. I2 = 7%, P = 0.37 indicated that there was no heterogeneity. Statistical results showed that compared with the control group, the risk of Infectious Complications in the enteral nutrition with ω-3 polyunsaturated fatty acid preparation group was 0.81 times higher than that in the control group (RR = 0.81, P = 0.05), showing a statistical difference, as shown in Table 2.
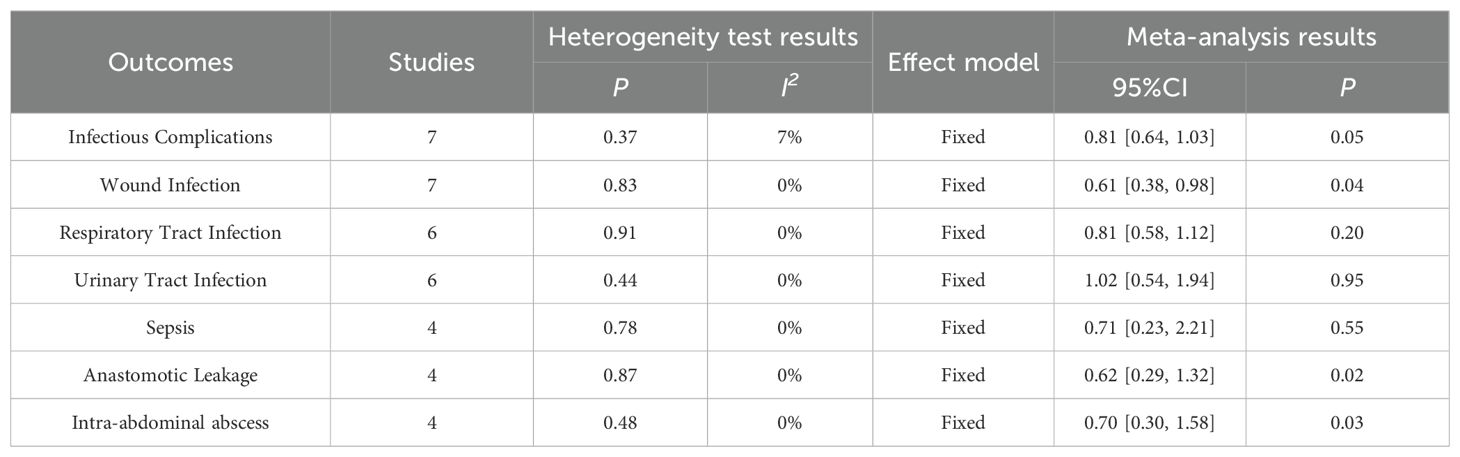
Table 2. Meta-analysis of postoperative infectious complications.
4.3.2.2 Wound infectionA total of 7 literatures reported the results of Wound Infection, including 400 cases of ω-3 polyunsaturated fatty acids and 403 cases of control group. I2 = 0%, P = 0.83 indicated that there was no heterogeneity. Statistical results showed that compared with the control group, the risk of Wound Infection in the enteral nutrition with ω-3 polyunsaturated fatty acid preparation group was 0.61 times that of the control group (RR = 0.61, P = 0.04), showing a statistical difference, as shown in Table 2.
4.3.2.3 Respiratory tract infectionA total of 6 articles reported the results of Respiratory Tract Infection, including 383 cases of ω-3 polyunsaturated fatty acids and 385 cases of control group. I2 = 0%, P = 0.91 indicated that there was no heterogeneity. The statistical results indicated that compared to the control group, the risk of Respiratory Tract Infection in the enteral nutrition with ω-3 polyunsaturated fatty acid group is 0.81 times that of the control group (RR = 0.81, P = 0.20), but there is no statistical significance. as shown in Table 2.
4.3.2.4 Urinary tract infectionA total of 6 literatures reported the results of Urinary Tract Infection, including 339 cases of ω-3 polyunsaturated fatty acids and 331 cases of control group. I2 = 0%, P = 0.44 indicated that there was no heterogeneity. Statistical results showed that compared with the control group, the risk of Urinary Tract Infection in the enteral nutrition with ω-3 polyunsaturated fatty acid preparation group was marginally elevated by 1.02 times compared to the control group, however, this increase did not reach statistical significance (RR = 1.02, P = 0.95), but there was no statistical difference, as shown in Table 2.
4.3.2.5 SepsisA total of 4 literatures reported Sepsis results, including 238 cases of ω-3 polyunsaturated fatty acids and 238 cases of control group. I2 = 0%, P = 0.78, indicated that there was no heterogeneity. Statistical results showed that compared with the control group, the risk of Sepsis in the enteral nutrition with ω-3 polyunsaturated fatty acid preparation group is 0.71 times that of the control group (RR = 0.71, P = 0.55), but there is no statistical difference, as shown in Table 2.
4.3.2.6 Anastomotic leakageA total of 4 literatures reported the results of Anastomotic Leakage, including 275 cases of ω-3 polyunsaturated fatty acids and 269 cases of control group. I2 = 0%, P = 0.87 indicated that there was no heterogeneity. The statistical results showed that compared with the control group, the risk of Anastomotic Leakage in the enteral nutrition with ω-3 polyunsaturated fatty acid preparation group was 0.62 times that of the control group (RR = 0.62, P = 0.02), and there was a statistical difference, as shown in Table 2.
4.3.2.7 Intra-abdominal abscessA total of 4 literatures reported the results of Intra-abdominal abscess, including 322 cases of ω-3 polyunsaturated fatty acids and 316 cases of control group. I2 = 0%, P = 0.48, indicated that there was no heterogeneity. The statistical results showed that compared with the control group, the risk of Intra-abdominal ESS in the enteral nutrition with ω-3 polyunsaturated fatty acid preparation group was 0.70 times higher than that in the control group (RR = 0.70, P = 0.03), as shown in Table 2.
4.3.3 Postoperative nutritional variablesThe results of the meta-analysis indicated that the perioperative nutritional indicators of patients with gastric cancer undergoing surgery in the enteral nutrition with ω-3 polyunsaturated fatty acid preparation group were superior to those in the conventional EN preparation group, specifically for Total protein (WMD = 0.68, P = 0.05), Albumin (WMD = 0.34, P = 0.03), and Transferrin (WMD = 0.07, P = 0.03). However, no statistically significant difference was observed in Pre-albumin (WMD = -0.48, P = 0.59). For detailed information, please refer to Table 3.
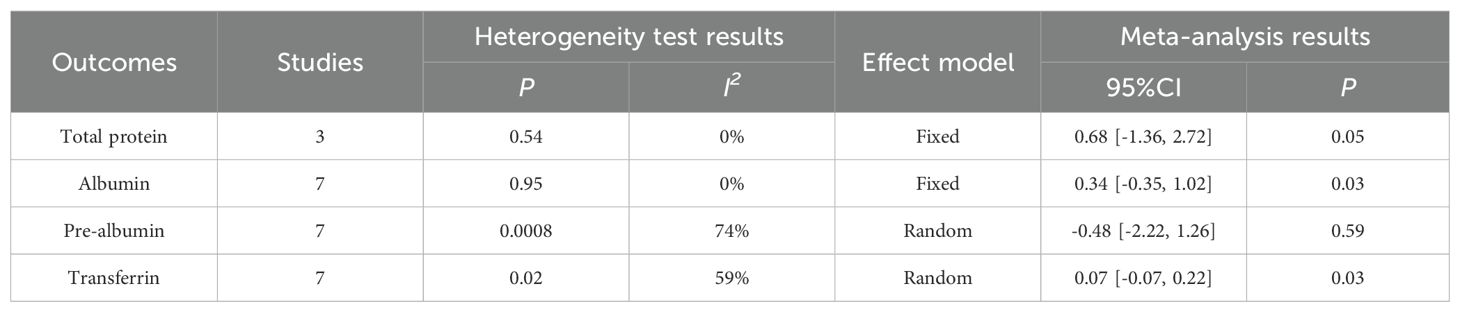
Table 3. Meta-analysis of postoperative nutritional variables.
4.3.4 Postoperative immune indicesThe meta-analysis results showed that there was no statistical difference between CD3 (WMD = 4.85, P = 0.06) and Total T lymphocyte cells (WMD = -0.17, P = 0.66). Perioperative immune indexes of patients with gastric cancer treated with enteral nutrition with ω-3 polyunsaturated fatty acid preparations during surgery (including IgG [WMD = 1.14, P < 0.0001], IgA [WMD = 0.36, P = 0.0008], IgM [WMD = 0.22, P < 0.0001], CD4 [WMD =6.81, P = 0.0001] and CD4/CD8 [WMD = 0.46, P = 0.002]) were superior to conventional EN preparations. In terms of CD8 (WMD = -1.56, P = 0.002), conventional EN preparations were superior to enteral nutrition with ω-3 polyunsaturated fatty acid preparations, and the difference was statistically significant, as shown in Table 4.
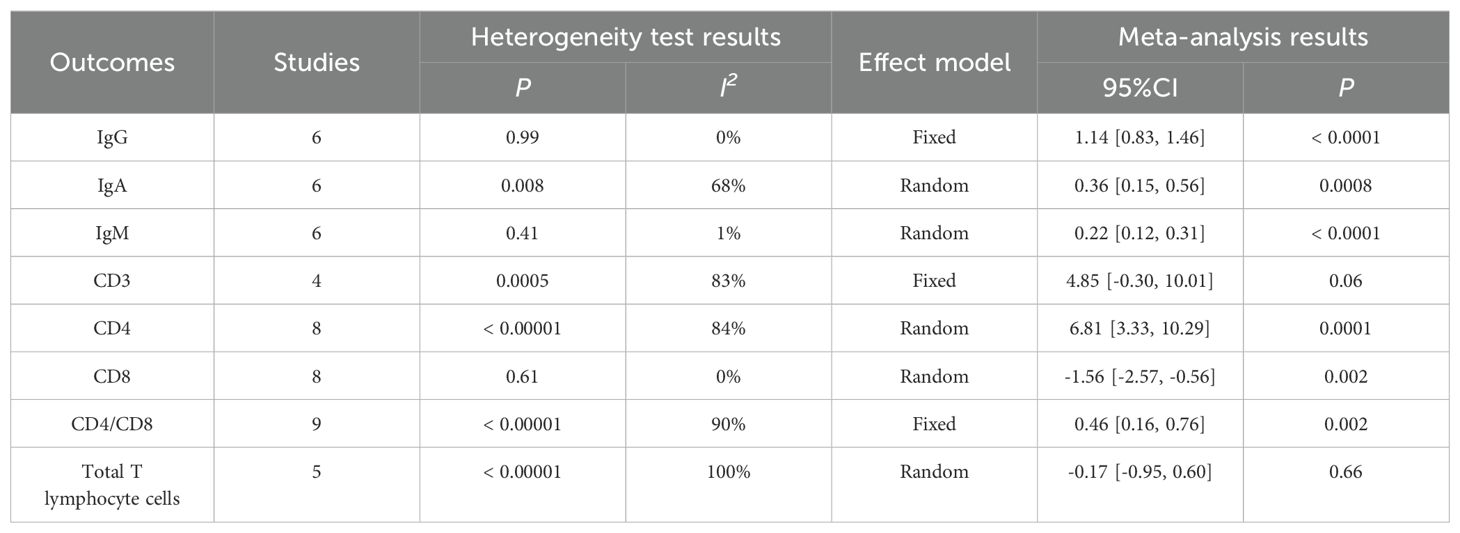
Table 4. Meta-analysis of postoperative immune indices.
4.3.5 Postoperative inflammatory indicesThe Meta analysis results showed that, except for the results of WBC (WMD = 0.68, P = 0.59) and TNF-α (WMD = -11.03, P = 0.46), the perioperative inflammatory indexes [including CRP(WMD = -1.51, P = 0.04), IL-1β (WMD = -26.34, P = 0.0005), IL-6 (WMD =- 5.38, P < 0.00001)] of gastric cancer patients treated with enteral nutrition with ω-3 polyunsaturated fatty acid preparations during surgery were better than those of conventional EN preparations, and the difference was statistically significant, as shown in Table 5.
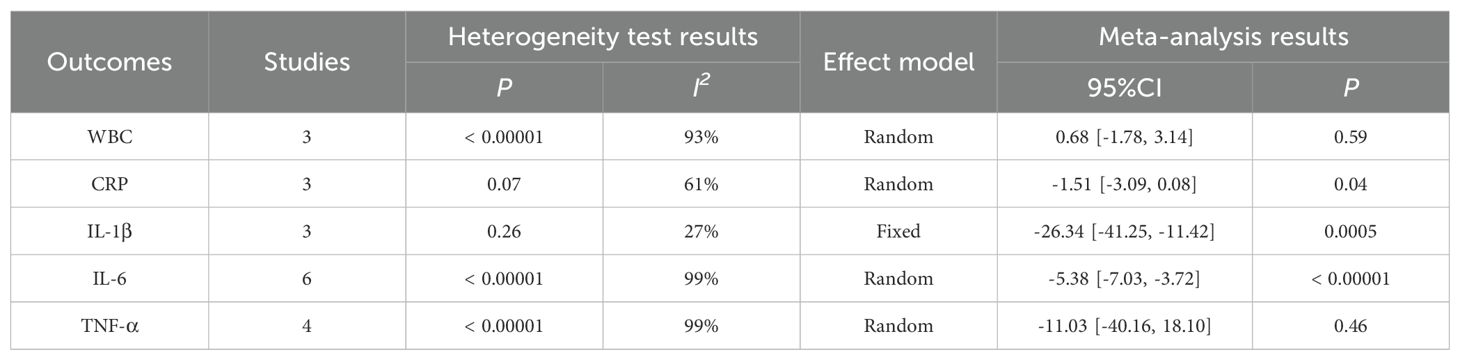
Table 5. Meta-analysis of postoperative inflammatory indices.
4.4 Risk of biasFunnel plot was used to analyze the effect of publication bias on meta-analysis results. Taking Infectious Complications as an example, it was shown that the included studies were evenly distributed on both sides of the funnel plot, suggesting that publication bias had little effect on the results in this study, as shown in Figure 4.
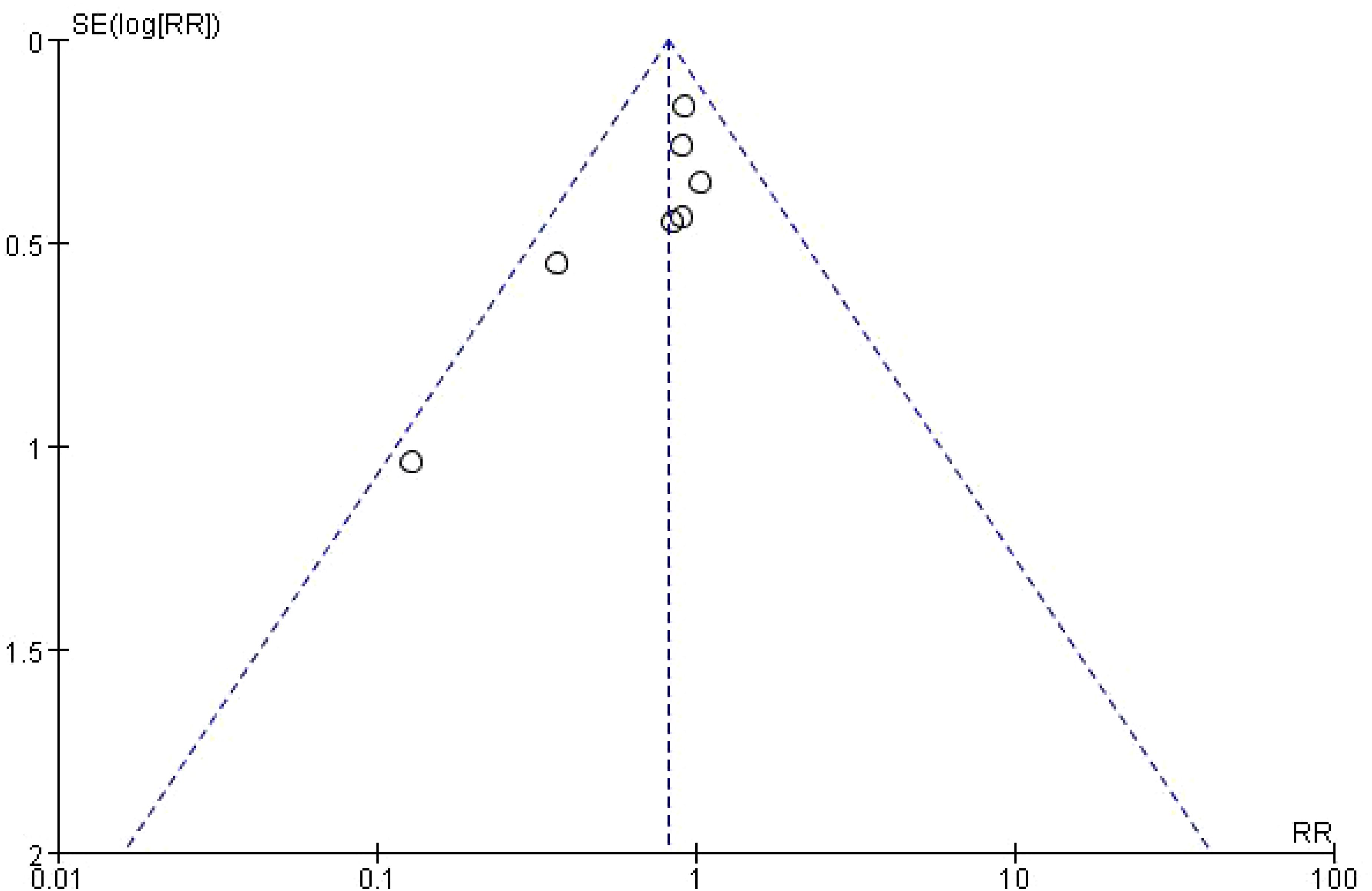
Figure 4. Funnel analysis of Infectious Complications.
5 DiscussionMalnutrition is a clinical condition in which the lack of energy, protein and other nutrients in the body leads to adverse outcomes in the body and in the clinic. With the improvement of people’s living standards, the probability of malnutrition in normal adults has decreased significantly compared with the past. In recent years, the incidence of malignant tumors has increased year by year, the proportion of nutritional risk in patients has exceeded 50%, and the incidence of malnutrition has reached over 30% (30–32). Studies have shown that systemic inflammation is common in patients with malignant tumors. Systemic inflammation occurs at the early stage of the tumor and worsens with the progression of the disease. Inflammation promotes tumor progression through multiple ways and affects nutrient metabolism, resulting in an imbalance in energy intake and utilization (33–36). Dewys et al. (37) reported that 87% of gastric cancer patients were undernourished, and the incidence of cachexia was as high as 65%-85%, ranking first among malignant tumors. Clinical evidence shows that the occurrence of malnutrition in tumor patients has a certain impact on chemotherapy efficacy and tolerance to chemotherapy drugs (38–41). Therefore, in order to ensure the therapeutic effect of malignant tumors, it is necessary to take nutritional support therapy for patients to improve body function, improve clinical efficacy, and reduce the incidence of complications and mortality.
The early diagnosis rate of early gastric cancer is low due to its inconspicuous clinical manifestations or non-specific characteristics, and most patients are already in the advanced stage when diagnosed. Clinical data show that patients with advanced gastric cancer are often accompanied by severe malnutrition, which is mainly caused by the following three factors (42): (1) anorexia and fullness caused by tumor; (2) Mechanical obstruction of digestive tract caused by protrusion of cancer; (3) Adverse reactions occurred during tumor treatment, such as gastrointestinal mucosal injury and abnormal secretion of digestive fluid. Patients with advanced gastric cancer often lose the chance of surgery, and chemotherapy is an important treatment method for them, while the incidence of malnutrition in patients with advanced gastric cancer is 15%-55% (43). Studies have reported that patients with advanced gastric cancer accompanied by malnutrition have a significant decline in chemotherapy tolerance, and it is difficult to complete chemotherapy as planned (24).Therefore, for advanced gastric cancer patients with malnutrition, their nutritional status should be improved at the same time of chemotherapy to eliminate the negative effects of malnutrition as far as possible.
The commonly used nutritional support methods in clinic include enteral nutrition and parenteral nutrition. Compared with parenteral nutrition, enteral nutrition can not only supplement the nutrients required by the body, but also protect the integrity of the intestinal mucosal barrier structure and reduce the damage to gastrointestinal function. Enteral immune nutrition preparation is a method used in clinical application in recent years to improve the immune function of patients. It refers to adding glutamine, arginine, ω-3 unsaturated fatty acids, nucleotides and other immunomodulatory nutrients to the standard enteral nutrition formula. These immunomodulatory components can stimulate the immune cells of the body and improve the immunity of the body after being absorbed by the body. ω-3 polyunsaturated fatty acids mainly include α-linolenic acid, eicosapentaenoic acid and docosahexaenoic acid. Studies have shown that ω-3 polyunsaturated fatty acids can play a role in mediating the inflammatory response of patients with malignant tumors by changing the structure, function and fluidity of cell membranes and reducing the production of pro-inflammatory mediators through competitive inhibition (7). In vitro cell tests showed that the secretion levels of pro-inflammatory factors TNF-α and IL-6 in macrophages were changed after the intervention of ω-3 polyunsaturated fatty acids, which further confirmed the direct anti-inflammatory effect of ω-3 polyunsaturated fatty acids on macrophages (44). Numerous clinical studies have shown that ω-3 polyunsaturated fatty acids, as an immune nutrient, play an important pharmacological role in the prevention and treatment of diabetes, hypertension, arthritis, tumor and other inflammatory and autoimmune diseases (45).
The research on the relationship between ω-3 unsaturated fatty acids and tumor prevention began 30 years ago, but there is still controversy to this day. Brasky TM et al. (46) believed that dietary supplementation of ω-3 unsaturated fatty acids could reduce the risk of breast cancer (HR = 0.68, 95%CI = 0.50-0.92). In 1982, Chavarro JE et al. tracked the blood levels of ω-3 unsaturated fatty acids in 14916 healthy men for a period of 13 years. Finally, 476 subjects were diagnosed with prostate cancer and matched with a control group. It was found that those with lower blood levels of ω-3 unsaturated fatty acids had an increased risk of developing prostate cancer (RR = 0.59, 95%CI = 0.38-0.93) (47). Hall MN et al. tracked the concentration of blood ω-3 unsaturated fatty acids in 21406 healthy individuals for a period of 22 years. Finally, 500 cases were diagnosed with colorectal cancer (388 cases of colon cancer and 112 cases of rectal cancer). They believed that as the intake of ω-3 unsaturated fatty acids increased, the risk of colorectal cancer decreased (RR = 0.74, 95%CI = 0.57-0.95) (48). The possible mechanism of ω-3 unsaturated fatty acids on tumor development is related to their function in regulating inflammatory response. Inflammatory response plays an important role in the occurrence and progression of tumors, so a diet rich in ω-3 unsaturated fatty acids A may have a preventive effect on tumors. Omega-6 unsaturated fatty acids are another type of polyunsaturated fatty acid in the human body. The first unsaturated bond in their molecule appears at the sixth position of the methyl end of the carbon chain, including linoleic acid and arachidonic acid. ω-3 unsaturated fatty acids and omega-6 unsaturated fatty acids share the same metabolic enzymes, namely cyclooxygenase and lipoxygenase. EPA and AA can both produce inflammatory mediators through the action of cyclooxygenase and lipoxygenase, but the pro-inflammatory and pro proliferative activity caused by EPA’s production of inflammatory mediators is relatively weak (49). ω-3 unsaturated fatty acids have a stronger affinity for this type of enzyme and can competitively inhibit the conversion of ω-6 unsaturated fatty acids, reducing the synthesis of arachidonic acid and thus alleviating inflammatory reactions. Therefore, a diet rich in ω-3 unsaturated fatty acids can negatively regulate the inflammatory cascade response and reduce the risk of tumor development (50).
This study investigated the therapeutic efficacy of enteral nutrition with ω-3 polyunsaturated fatty acid preparations versus standard enteral nutrition (SEN) emulsions in perioperative management of patients with advanced gastric cancer experiencing malnutrition. Although no statistically significant disparity was noted in mortality rates between the two groups, notable differences emerged in indicators pertaining to infection, immunity, inflammation, and nutrition. Specifically, the incidence of infection-related complications, such as wound infections, abdominal abscesses, and stoma complications, was significantly lower in the IEN group compared to the SEN group. Furthermore, the IEN group demonstrated enhanced recovery of immune markers (IgG, IgA, IgM, CD4, and CD4/CD8 ratios) and nutritional indices (Total protein, Albumin, and Transferrin), suggesting a positive impact on overall immune status and nutritional replenishment. Regarding inflammation, the IEN group exhibited reduced levels of CRP, IL-1β, and IL-6, indicating an attenuation of the inflammatory response compared to the SEN group. These findings underscore the significant value of enteral nutrition with ω-3 polyunsaturated fatty acid in ameliorating perioperative nutritional status and potentially improving clinical outcomes for patients with advanced gastric cancer. The results of this study are also consistent with the aforementioned research findings.
6 ConclusionIn summary, enteral nutrition preparations containing ω-3 polyunsaturated fatty acids have more advantages than standard nutrition preparations in the perioperative nutritional treatment of gastric cancer patients in terms of effectiveness and safety, and can be used as the preferred clinical drug. Due to the limited number and quality of included studies, the above conclusions need to be verified by more high-quality studies.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributionsTF: Formal Analysis, Software, Writing – original draft. WH: Data curation, Formal Analysis, Project administration, Writing – review & editing. LC: Formal Analysis, Methodology, Software, Writing – review & editing. JD: Data curation, Software, Writing – review & editing, Formal Analysis, Methodology.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe thank all teams and individuals who were involved in this work.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
2. Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. (2023) 20:338–49. doi: 10.1038/s41571-023-00747-0
PubMed Abstract | Crossref Full Text | Google Scholar
3. López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. (2023) 181:103841. doi: 10.1016/j.critrevonc.2022.103841
PubMed Abstract | Crossref Full Text | Google Scholar
5. Mohamed Elfadil O, Velapati SR, Patel J, Hurt RT, Mundi MS. Enteral nutrition therapy: historical perspective, utilization, and complications. Curr Gastroenterol Rep. (2024) 26:200–10. doi: 10.1007/s11894-024-00934-8
PubMed Abstract | Crossref Full Text | Google Scholar
6. Zhang G, Zou J. Clinical application of enteral immune nutrition for chronic obstructive pulmonary disease patients. Zhonghua Yi Xue Za Zhi. (2015) 95:1501–4.
PubMed Abstract | Google Scholar
7. Shahidi F, Ambigaipalan P. [amp]]omega;-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. (2018) 9:345–81. doi: 10.1146/annurev-food-111317-095850
PubMed Abstract | Crossref Full Text | Google Scholar
9. Gan Y, Li L, Zhang L, Yan S, Gao C, Hu S, et al. Association between shift work and risk of prostate cancer: a systematic review and meta-analysis of observational studies. Carcinogenesis. (2018) 39:87–97. doi: 10.1093/carcin/bgx129
PubMed Abstract | Crossref Full Text | Google Scholar
10. Kenler AS, Swails WS, Driscoll DF, DeMichele SJ, Daley B, Babineau TJ, et al. Early enteral feeding in postsurgical cancer patients. Fish oil structured lipid-based polymeric formula versus a standard polymeric formula. Ann Surg. (1996) 223:316–33. doi: 10.1097/00000658-199603000-00013
PubMed Abstract | Crossref Full Text | Google Scholar
11. Wu GH, Zhang YW, Wu ZH. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J Gastroenterol. (2001) 7:357–62. doi: 10.3748/wjg.v7.i3.357
PubMed Abstract | Crossref Full Text | Google Scholar
12. Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. (2002) 122:1763–70. doi: 10.1053/gast.2002.33587
PubMed Abstract | Crossref Full Text | Google Scholar
13. Jiang Y, Cao W, Yan M. Provide different formulations of enteral nutrition in the early postoperative period Clinical significance of gastric cancer patients. Surg Theory Pract. (2002) 7:291–4.
14. Chen D, Fei Z, Zhang Y. Immune enteral nutrition for gastric cancer patients undergoing surgery The impact of post nutrition and immune function. Chin J Clin Nutr. (2004) 12:228–31.
15. Bing W. The impact of different nutritional pathways on the postoperative recovery of gastric cancer patients. Med Sci Res J. (2006) 35(5):59–61.
16. Klek S, Kulig J, Sierzega M, Szczepanek K, Szybiński P, Scislo L, et al. Standard and immunomodulating enteral nutrition in patients after extended gastrointestinal surgery–a prospective, randomized, controlled clinical trial. Clin Nutr. (2008) 27:504–12. doi: 10.1016/j.clnu.2008.04.010
PubMed Abstract | Crossref Full Text | Google Scholar
17. Liu Z, Yu J. Immune enhanced enteral nutrition for postoperative immunity in patients undergoing radical gastrectomy for gastric cancer The impact of epidemic function and inflammatory response. Chin J Cancer Prev Treat. (2011) 18:66–7.
18. Zhao D, Zhu J, Chen Y. Immune enhanced enteral nutrition for radical treatment of gastric cancer The impact of postoperative immune function and inflammatory response on patients. Chin Exp Diagnosis. (2011) 15:428–30.
19. Sultan J, Griffin SM, Di Franco F, Kirby JA, Shenton BK, Seal CJ, et al. Randomized clinical trial of ω-3 fatty acid-supplemented enteral nutrition versus standard enteral nutrition in patients undergoing oesophagogastric cancer surgery. Br J Surg. (2012) 99:346–55. doi: 10.1002/bjs.7799
PubMed Abstract | Crossref Full Text | Google Scholar
20. Shao F, Yang C, Liu X. Immune microecological enteral nutrition in patients with diabetes Application in patients with gastrointestinal tumors. Chin J Gastrointestinal Surg. (2012) 15:476–9.
21. Marano L, Porfidia R, Pezzella M, Grassia M, Petrillo M, Esposito G, et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol. (2013) 20:3912–8. doi: 10.1245/s10434-013-3088-1
PubMed Abstract | Crossref Full Text | Google Scholar
22. Wei Z, Wang W, Chen J, Yang D, Yan R, Cai Q. A prospective, randomized, controlled study of ω-3 fish oil fat emulsion-based parenteral nutrition for patients following surgical resection of gastric tumors. Nutr J. (2014) 13:25. doi: 10.1186/1475-2891-13-25
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wu Y, Xia L, Wang Y. Preoperative application of ω-3 unsaturated lipids in gastric cancer patients Observation of the clinical efficacy of enteral nutrition preparations containing fatty acids. Chin Gen Foreign Stud Foundation Clin J. (2015) 22:312–6.
24. Scislo L, Pach R, Nowak A, Walewska E, Gadek M, Brandt P, et al. The impact of postoperative enteral immunonutrition on postoperative complications and survival in gastric cancer patients - randomized clinical trial. Nutr Cancer. (2018) 70:453–9. doi: 10.1080/01635581.2018.1445770
PubMed Abstract | Crossref Full Text | Google Scholar
25. Ida S, Hiki N, Cho H, Sakamaki K, Ito S, Fujitani K, et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br J Surg. (2017) 104:377–83. doi: 10.1002/bjs.10417
PubMed Abstract | Crossref Full Text | Google Scholar
26. Hu Z, Sun Y, Wang F. [amp]]omega;-3 unsaturated fatty acid enteral nutrition and general nutrition The effect of enteral nutrition on the immune function of postoperative gastric cancer patients. Chin Clin Pract Nutr J. (2017) 25:189–90.
27. Wang S, Jia J, Ping M. Enteral immune nutritional support combined with chemotherapy for delayed treatment Research on stage gastric cancer. Electronic J Compr Cancer Ther. (2019) 5:36–9.
28. Liu Y, Zhang X, Liu Z. Intestinal nutrition containing ω-3 polyunsaturated fatty acids The impact of nutrition on the nutritional status and quality of life of gastric cancer patients. Chin Clin Camp Nourishing Magazine. (2020) 28:151–7.
29. Sim E, Kim JM, Lee SM, Chung MJ, Song SY, Kim ES, et al. The effect of ω-3 enriched oral nutrition supplement on nutritional indices and quality of life in gastrointestinal cancer patients: A randomized clinical trial. Asian Pac J Cancer Prev.
留言 (0)