The family Anaplasmataceae consists of a group of Gram-negative, obligate intracellular alpha-proteobacteria that cause nonspecific febrile diseases in susceptible animals and humans (Rikihisa, 1991; Dumler et al., 2001; Rikihisa, 2010). Although the diseases are usually self-limiting in humans, severe illness can occur upon infection with Ehrlichia chaffeensis or Anaplasma phagocytophilum, with some cases proven fatal (Bakken and Dumler, 2000). Notably, the Centers for Disease Control and Prevention must be notified for every infection case, which shows that disease incidence has increased significantly in the past two decades (CDC, 2024). E. chaffeensis causes the systemic disease human monocytic ehrlichiosis (HME), for which doxycycline is the only treatment (CDC, 2024). To facilitate current efforts to develop an HME vaccine (McGill et al., 2016; Thomas, 2016; Budachetri et al., 2020, Budachetri et al., 2022), more research is needed to understand HME pathogenesis and to discover effective therapeutic and vaccine targets.
An ehrlichial species was recently characterized by whole-genome sequencing and classified as Ehrlichia japonica sp. nov (Lin et al., 2021; Oren and Garrity, 2022). This species was originally isolated from Ixodes ovatus ticks in Japan and was previously known as an I. ovatus ehrlichia (IOE) agent or Ehrlichia sp. HF strain. Phylogenetic analysis revealed that E. japonica is closely related to E. chaffeensis based on 16S rRNA and GroEL protein sequences (Shibata et al., 2000). Laboratory mice challenged with E. japonica develop an acute systemic infection followed by death that resembles severe HME (Shibata et al., 2000; Sotomayor et al., 2001; Zhang et al., 2024). Thus, over the past several years, E. japonica infection in immunocompetent mice has been increasingly used as a model for studying HME (Shibata et al., 2000; Okada et al., 2001, Okada et al., 2003; Winslow et al., 2005; Ismail et al., 2006; Habib et al., 2016; Haloul et al., 2019; Ahmed and Ismail, 2020; Ismail et al., 2022; Sharma et al., 2023). As E. japonica causes a dose-dependent, full-spectrum disease with an LD50 of approximately 100 bacteria (Bekebrede et al., 2020), this mouse model provides an excellent way to investigate ehrlichiosis pathogenesis in vivo and to bridge the knowledge gap between E. chaffeensis infection and severe HME.
To overcome the mammalian immune system, establish infection, and cause disease within the host, Ehrlichia must utilize additional strategies, such as in vivo virulence factors, beyond those required for infection of eukaryotic cells in culture (Rikihisa, 2021). However, the vast majority of Ehrlichia virulence factors and their functions in vivo remain undetermined. According to recently published whole-genome sequencing data, E. japonica contains 866 protein-coding genes (Lin et al., 2021). Besides those genes essential for protein or nucleotide biosynthesis and key metabolic pathways, the functions of 244 proteins remain unknown and hence such proteins are annotated as hypothetical proteins. Because Ehrlichia spp. and other members of the family Anaplasmataceae have evolved to have a relatively small genome (Dunning Hotopp et al., 2006; Rikihisa, 2015), it is very likely that at least some, if not all, of these hypothetical proteins could have played key roles in the evolutionary success of Ehrlichia spp. To investigate the functions of these unknown Ehrlichia genes, we used a Himar1 transposon random mutagenesis system (Felsheim et al., 2006) to create a library of E. japonica that comprises at least 158 distinct mutants, carrying genetic mutations at different loci (Bekebrede et al., 2020). Among the mutants that can replicate well in cell culture but are attenuated in mouse virulence is the H59, a clone of a Himar1 insertional mutant disrupting EHF_0962 gene (EHF_0962::Himar1), which caused significantly lower bacterial loads in the blood, liver, and spleen with reduced clinical signs at day 7 post-infection (pi) (Bekebrede et al., 2020). EHF_0962 encodes a previously uncharacterized hypothetical protein with 120 amino acid residues (molecular mass, ~13.5 kDa, pI 5.78, GenBank accession no. WP_052349286.1) (Lin et al., 2021), which was named in this study as resistance-inducing protein of Ehrlichia (RipE). Here, we characterized RipE functions in E. japonica pathogenesis in mice. Our results suggest that RipE helps bacteria survival during the extracellular stage of Ehrlichia, thereby facilitating in vivo infection.
ResultsCharacteristics of the ΔripE mutant and RipETo study the functions of RipE in E. japonica pathogenesis, we first confirmed the stable clonality of the ΔripE mutant H59 (Bekebrede et al., 2020). Himar1 insertion site-specific flanking PCR for ripE (Supplementary Table S1) (Bekebrede et al., 2020) showed that the ΔripE mutant produced a single, larger PCR product containing the Himar1 insert (2,254 bp) compared with WT E. japonica (421 bp, Figures 1A, B). Himar1 insertions occur mostly once and rarely twice per genome (Cartman and Minton, 2010; Cain et al., 2020); therefore, to verify that there were no additional Himar1 insertions in the ΔripE mutant genome, quantitative PCR (qPCR) was performed, and the results showed a ratio of 1:1 for the copy number of the mCherry gene vs. the 16S rRNA gene, which is a single copy per Ehrlichia genome (Figure 1C). Wild-type (WT) E. japonica and the ΔripE mutant had similar growth curves when cultured in DH82 canine macrophages or ISE6 tick cells (Bekebrede et al., 2020). The intracellular micro-colonies (morulae) formed by WT and ΔripE E. japonica are essentially indistinguishable by HEMA3 staining (Bekebrede et al., 2020) (Figure 1D), whereas the ΔripE mutant could be easily distinguished from WT E. japonica owing to its mCherry fluorescence under a fluorescence microscope (Figure 1D). To investigate whether the ripE gene is involved in mammalian endothelial tropism and infection, we also compared the growth curves of WT and ΔripE E. japonica in the RF/6A rhesus monkey endothelial cell line, but no significant difference was observed (Supplementary Figure S1).
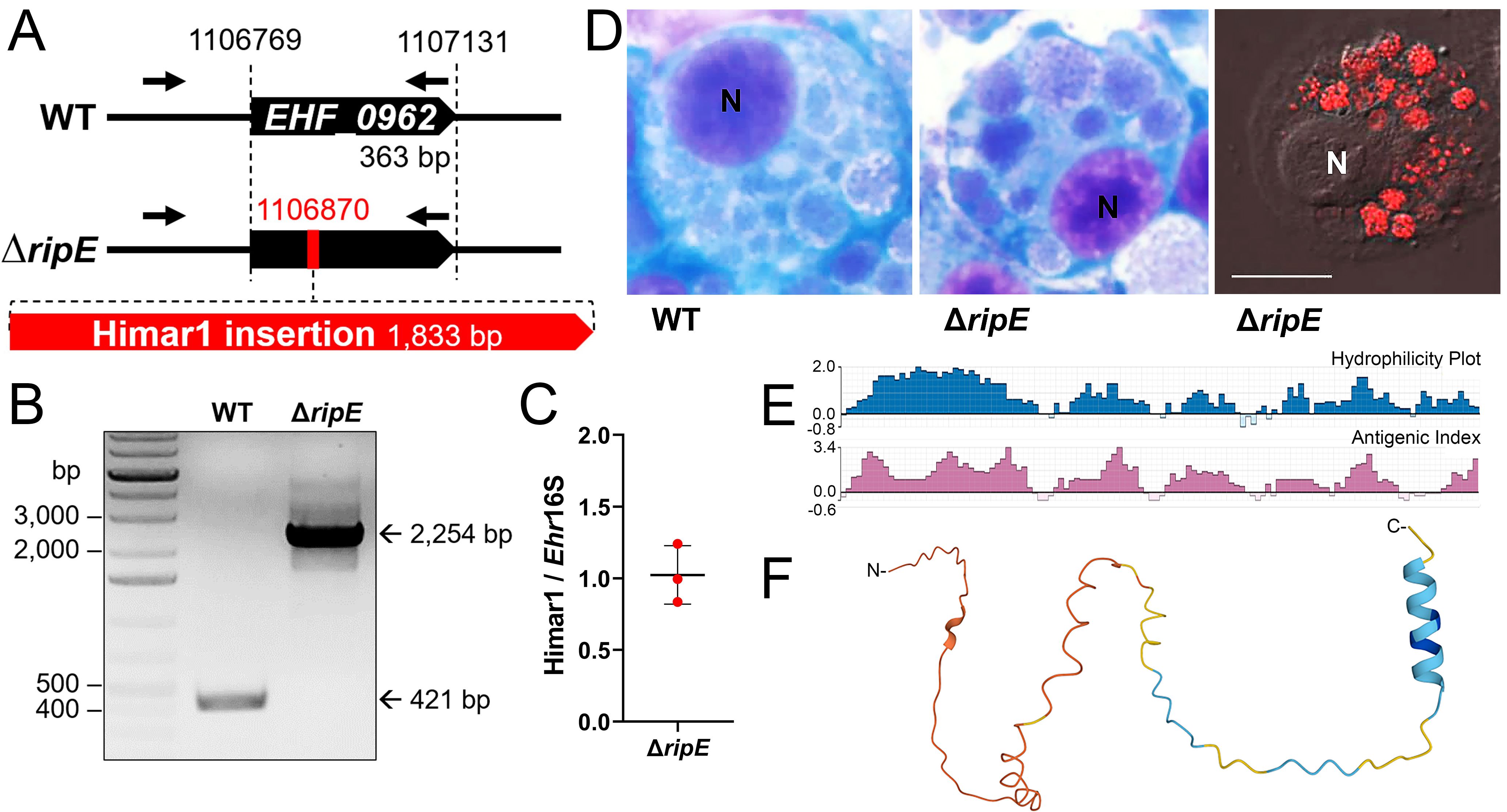
Figure 1. Clonality of ΔripE E. japonica and bioinformatic analysis of RipE protein. (A) Diagram of E. japonica genome showing ΔripE with Himar1 transposon insertion in locus EHF_0962 (ripE). GenBank Reference Sequence number: NZ_CP007474.1. ORFs (black pentagon arrows). Himar1 insertion containing spectinomycin/streptomycin resistance gene and mCherry (red pentagon arrow). ripE-specific flanking PCR primers (arrows). (B) ripE-specific flanking PCR for ΔripE E. japonica clonality verification. (C) Copy numbers of the mCherry gene vs. the single-copy Ehrlichia 16S rRNA gene in ΔripE E. japonica genome determined by qPCR from three different batches of cultures. (D) WT and ΔripE E. japonica cultured in DH82 cells. Note the characteristic Ehrlichia micro-colonies (morulae) by HEMA3 stain in the cytoplasm of infected DH82 cells, and ΔripE mutants were also detected by mCherry fluorescence using a Leica Thunder fluorescence microscope. N, nucleus. Scale bar, 10 µm. (E) Antigenic index and hydrophilicity plot of RipE by Protean prediction. (F) RipE protein 3D structure prediction by AlphaFold.
Protein Blast search revealed that RipE homologs were identified in all sequenced Ehrlichia spp., including E. muris, E. chaffeensis, E. canis, and E. ruminantium (Table 1). However, no RipE homologs could be found beyond the genus Ehrlichia, even in other closely related members in the family Anaplasmataceae such as Anaplasma and Neorickettsia spp., suggesting that RipE is a unique protein that evolved among Ehrlichia spp. As RipE lacks any known motifs cataloged in the NCBI database, bioinformatic analyses were performed to determine its secondary-structure characteristics. Analysis using Protean (DNASTAR Lasergene) revealed that RipE consists of six hydrophilic regions each with a high antigenic index intervened with short hydrophobic linkers (Figure 1E). Three-dimensional structure prediction based on AlphaFold (Jumper et al., 2021) indicated that RipE contains mostly intrinsically disordered regions, which are flexible and lack well-defined three-dimensional structures except for one α-helix at the C-terminus and a short β-sheet near the N-terminus (Figure 1F).
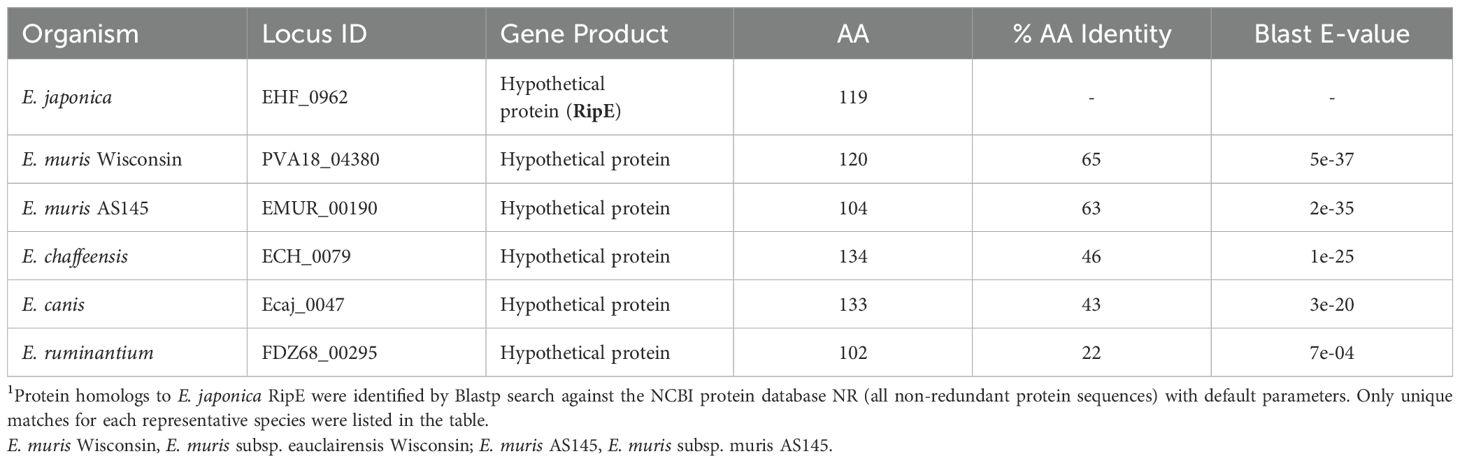
Table 1. Homologous proteins of E. japonica RipE in Ehrlichia species1.
RipE expression by E. japonica in DH82 cellsWestern blotting with a mouse antiserum developed against full-length recombinant RipE (rRipE) demonstrated that native RipE was expressed by WT E. japonica but was indeed absent in the ΔripE mutant (Figure 2A). However, in agreement with previous growth curve analysis by PCR based on Ehrlichia 16S rRNA (Bekebrede et al., 2020), the growth of WT and ΔripE E. japonica in DH82 cells was also similar as determined by Western blotting with an antibody (Ohashi et al., 1998) against the recombinant Ehrlichia major outer-membrane protein P28 (rP28) (Figure 2A). Inside host cells, Ehrlichia spp. undergo a biphasic developmental cycle (Zhang et al., 2007; Rikihisa, 2010), i.e., a nonreplicating infectious dense-core (DC) cell form (0.4–0.6 µm) and a replicating noninfectious reticulate cell (RC) form (>0.8 µm) (Popov et al., 1995). To determine the expression of ripE mRNA by E. japonica, synchronized cultures of WT E. japonica-infected DH82 cells were established as previously described (Liu et al., 2012). The E. japonica growth curve was first determined based on reverse transcription-qPCR (RT-qPCR) analysis comparing the levels of Ehrlichia 16S rRNA with dog GAPDH (Figure 2B). The results revealed a lengthy lag phase of Ehrlichia growth up to 36 h pi, followed by slow growth between 36 and 48 h and exponential growth between 60 and 84 h pi (Figure 2B). RT-qPCR using ripE-specific primers (Supplementary Table S1) demonstrated that the expression of ripE mRNA peaked at 48–60 h pi in synchronous cultures prior to the exponential growth (Figure 2B). Immunofluorescence microscopy showed that, at 3 days pi, the majority of E. japonica-containing morulae were positive for RipE (Figure 2C). To further characterize RipE expression among individual Ehrlichia of various sizes, host cell-free E. japonica was purified and immunofluorescence staining was performed using antibodies against RipE and P28 or CtrA, a transcription factor of the two-component system of Ehrlichia, which is primarily expressed at the DC stage (Cheng et al., 2011). Quantitation of RipE-expressing E. japonica (RipE+) among all P28-positive Ehrlichia showed that the majority (~80%) were in DC form, with a diameter of <0.5 μm (Figures 2D, E). Similarly, there was a significant correlation between RipE- and CtrA-positive Ehrlichia morulae (Figures 2F, G).
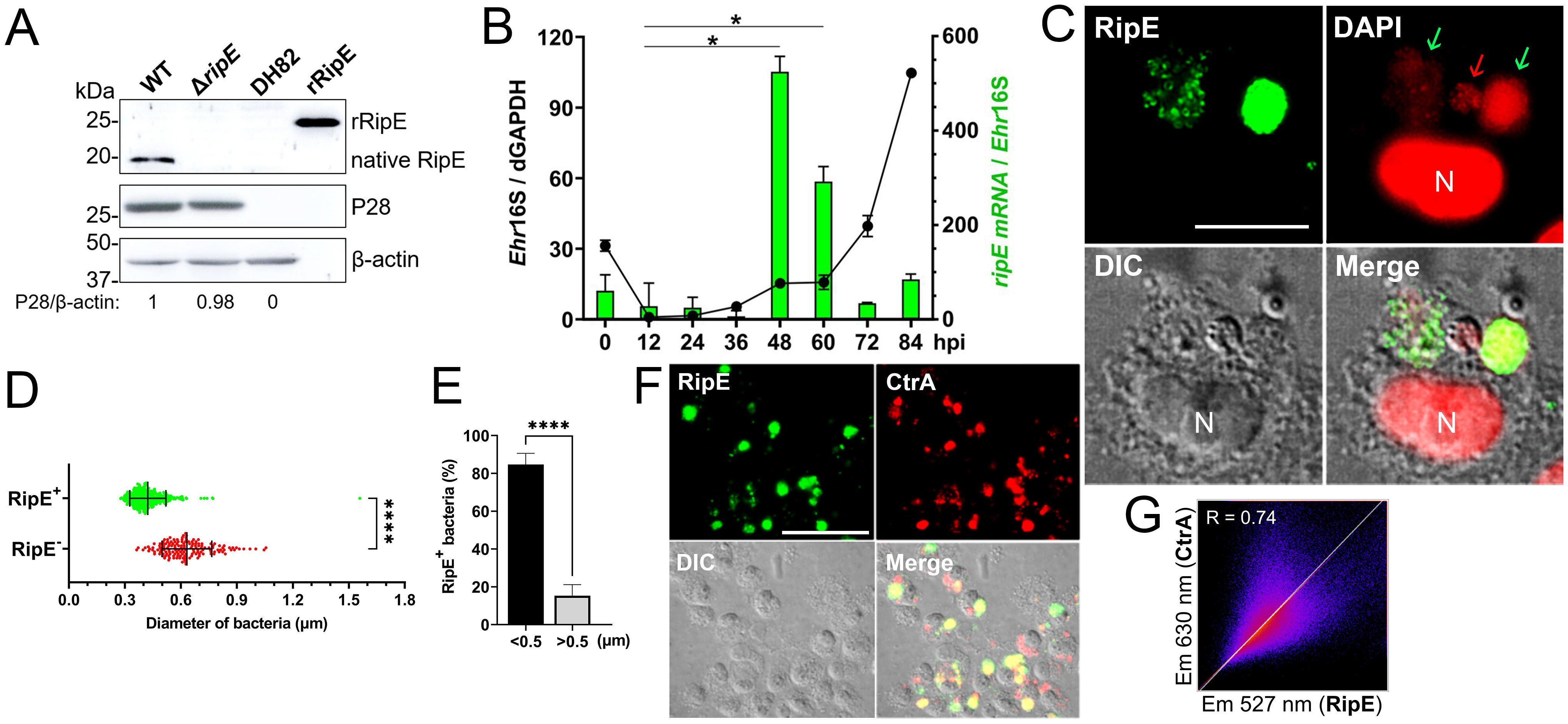
Figure 2. RipE is not expressed by the ΔripE mutant and differentially expressed by WT E. japonica. (A) Lysates of WT and ΔripE E. japonica-infected and uninfected DH82 cells and rRipE were analyzed by Western blotting using antibodies against rRipE, Ehrlichia P28, and β-actin. (B) Growth curve of Ehrlichia (black line) and expression of RipE mRNA (green bar) by WT E. japonica in DH82 cells were analyzed by RT-qPCR. E. japonica 16S rRNA (Ehr16S) expression was normalized by dog GAPDH mRNA (dGAPDH). hpi, hours post infection. RipE mRNA/Ehr16S with the ratio at 12 hpi set as 1. Data are displayed by the 2−ΔΔCT method and shown as means ± standard deviations (n = 3). The result is a representative of two independent experiments. *p < 0.05 by ANOVA. (C) WT E. japonica-infected DH82 cells at 3 dpi were fixed and labeled with mouse anti-rRipE and goat anti-mouse IgG (AF488, green). DNA was stained with DAPI (pseudo-colored in red). N, nucleus. Scale bar, 10 µm. (D, E) Host cell-free WT E. japonica was fixed and double labeled with mouse anti-rRipE (AF488, green) and rabbit anti-P28 (AF555, red). The diameter of individual WT bacteria was measured using ImageJ software. (D) Size distributions of P28-labeled E. japonica with or without RipE labeling. RipE+, RipE-positive, and P28-positive (green) bacteria. RipE−, RipE-negative and P28-positive bacteria. Vertical bars indicate the mean and standard deviation. ****, Significantly different (p < 0.0001) by two-tailed Student’s t-test (total bacteria, n = 565). (E) The percentage of RipE+ bacteria in two size groups: >0.5 µm and <0.5 µm. Data indicate the mean ± standard deviation of immunofluorescence images (n = 6) from Panel (D) ****, Significantly different (p < 0.0001) by two-tailed Student’s t-test. (F) Double immunofluorescence labeling of WT E. japonica-infected DH82 cells with mouse anti-rRipE (AF488, green) and rabbit anti-Ehrlichia CtrA (AF555, red). Merge, fluorescence images merged with differential interference contrast (DIC) image. Scale bar, 50 µm. (G) Pearson’s correlation coefficient (R = 0.74) of RipE and CtrA in WT E. japonica morulae in DH82 cells from panel (F) as determined by using ImageJ software with the Coloc 2 plugin.
RipE is expressed by E. japonica in mice, and ΔripE proliferates less in the blood and tissues than WTUpon intraperitoneal (i.p.) inoculation, E. japonica expressed ripE mRNA in various tissues of mice and levels were higher in the blood and spleen than in the liver and peritoneal lavage at day 7 pi (Figure 3A). To investigate the time course of proliferation of ΔripE bacteria in vivo, mice were inoculated i.p. with ΔripE or WT E. japonica, and bacterial load in blood samples was determined by qPCR during the early infection period. Up to day 5 pi, bacterial load did not differ significantly between the ΔripE- and WT-infected mouse groups (Figure 3B). From days 5 to 7 pi, however, the bacterial load of WT E. japonica was significantly greater than that of ΔripE E. japonica (Figure 3B). E. japonica infection in mouse blood via i.p. injection involves multiple steps, including the infection of resident macrophages in the peritoneal cavity and the subsequent spread to circulating blood. To examine E. japonica proliferation directly in the blood, mice were inoculated intravenously (i.v.) through the retro-orbital venous plexus with host cell-free ΔripE or WT E. japonica. RT-qPCR data showed that, as early as 6 h pi, the abundance of WT E. japonica in the blood was significantly greater than that of ΔripE, which remained higher throughout the 2-day infection time course (Figure 3C). In addition, mice inoculated i.v. with WT E. japonica showed signs of severe illness at day 3 pi and were moribund or had died by day 4, whereas mice inoculated with ΔripE did not exhibit any signs of illness at day 4 pi.
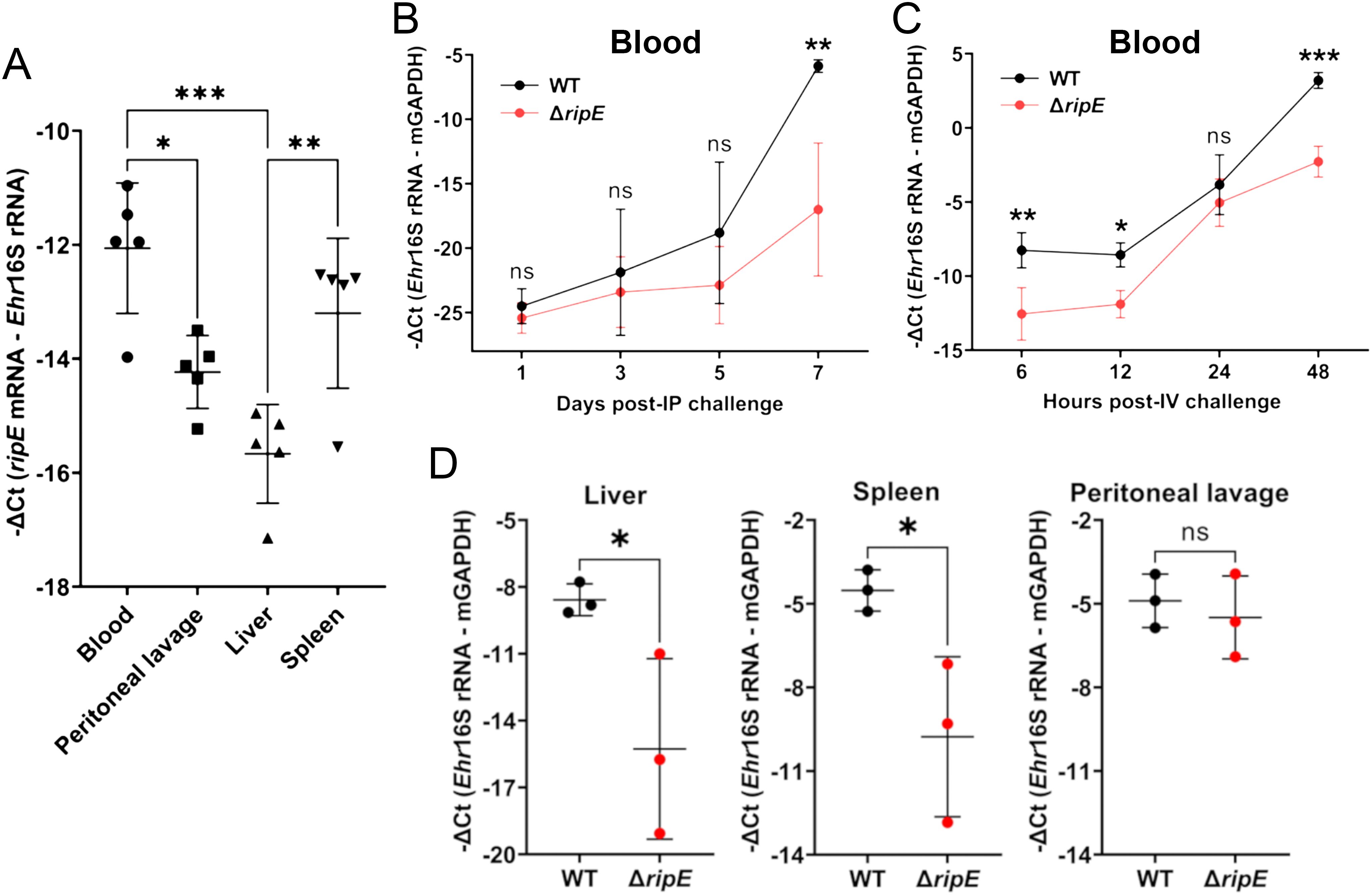
Figure 3. RipE is expressed by E. japonica in mice and the ΔripE mutant has reduced infection in the blood and tissues compared to WT. (A) ripE mRNA expression of WT E. japonica in mouse tissues at 7 days pi. ICR mice were inoculated i.p. with infected DH82 cells containing ~24,000 WT E. japonica. Relative amount of ripE mRNA was analyzed by RT-qPCR and normalized against Ehrlichia (Ehr) 16S rRNA. Data indicate the mean ± standard deviation (n = 5). Results were analyzed with one-way ANOVA followed by Tukey’s multiple comparisons. ***p < 0.001, **p < 0.01, *p < 0.05. (B) Temporal bacteria loads (Ehr 16S rRNA) in the blood of C57BL/6 mice i.p. inoculated with infected DH82 cells containing ~1,000 WT or ΔripE E. japonica by qPCR. (C) Temporal bacteria loads (Ehr 16S rRNA) in the blood of C57BL/6 mice i.v. inoculated with 1 × 107 host cell-free WT or ΔripE E. japonica by RT-qPCR. (B, C) Input DNA or RNA was normalized by mouse GAPDH. Data indicate the mean ± standard deviation (n = 3). Results were analyzed with repeated-measures ANOVA followed by Šídák multiple comparisons. ***p < 0.001, **p < 0.01, *p < 0.05, ns, not significantly different. (D) Bacteria loads in the tissues of C57BL/6 mice i.p. inoculated with infected DH82 cells containing ~100,000 WT or ΔripE E. japonica. Ehr 16S rRNA was analyzed by RT-qPCR and normalized by mouse GAPDH mRNA. Data indicate the mean ± standard deviation (n = 3). *p < 0.05, ns, not significantly different by the Student’s t-test.
To better understand the role of RipE during the early phase of infection, we inoculated mice (i.p.) with ~1,000-fold greater numbers of ΔripE or WT E. japonica than used for the experiment shown in Figure 2B, followed by euthanasia and tissue sampling for subsequent RT-qPCR analysis. As early as 3 days pi, the bacterial load of the ΔripE mutant was significantly lower in the liver and spleen compared with WT E. japonica (Figure 3D); for peritoneal lavage, however, the difference was not statistically significant (Figure 3D). Taken together, these data suggested that RipE may be involved in systemic infection and bacterial spread to various organs via blood.
Extracellular ΔripE E. japonica loses infectivity more rapidly and has lower ATP content than WT E. japonicaAs an obligatory intracellular bacterium, E. japonica must bind and enter host cells (Rikihisa, 2010). We therefore examined whether RipE plays a role in this process. Using two-step fluorescent labeling, we quantified host cell surface-bound (non-internalized) vs. internalized Ehrlichia upon incubation with DH82 and RF/6A cells as previously described (Mohan Kumar et al., 2013), revealing no significant difference in bacterial binding or entry between ΔripE and WT E. japonica (Figure 4).
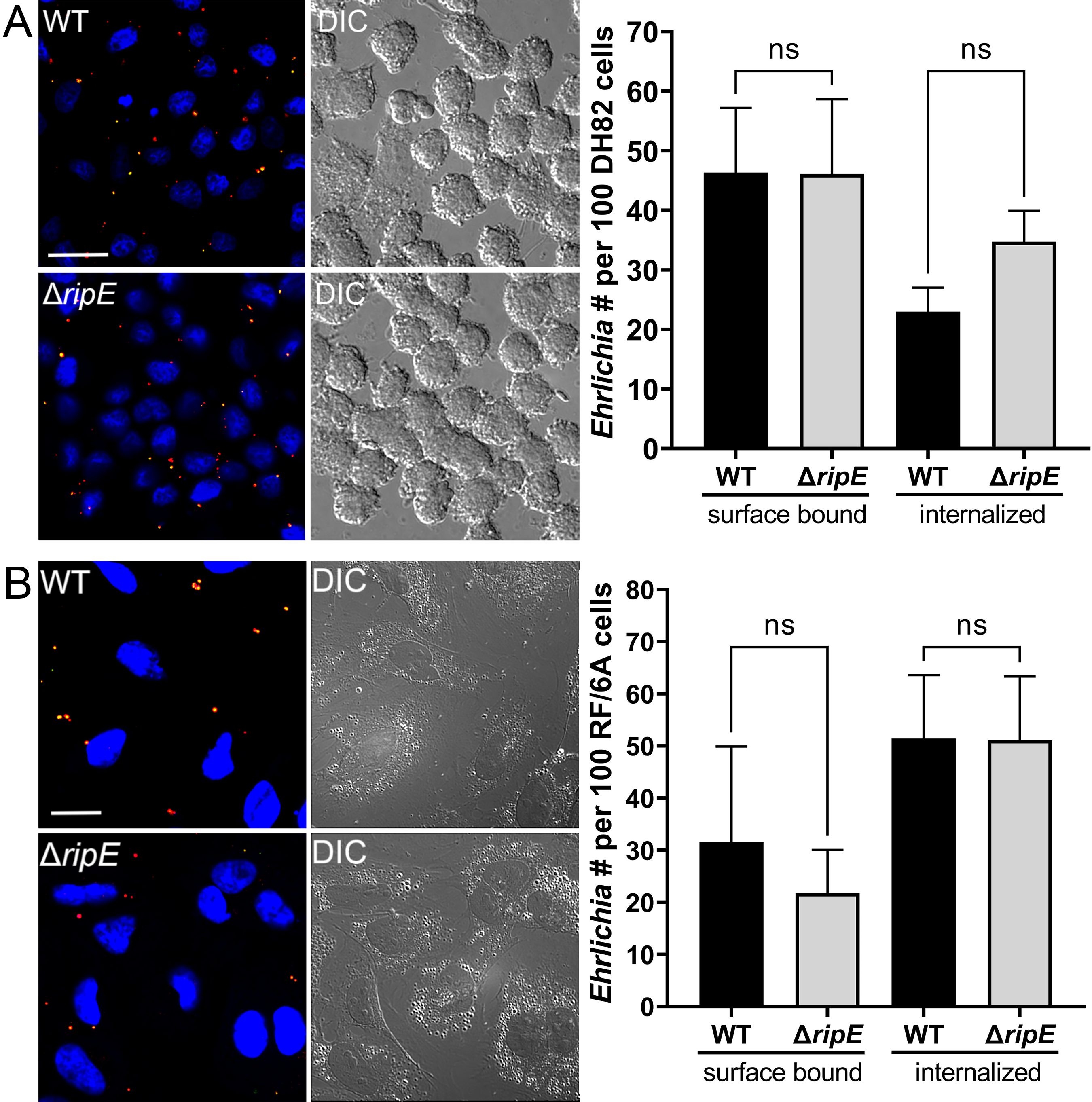
Figure 4. Host cell-free WT and ΔripE E. japonica showed no difference in binding and entering mammalian cells in culture. (Left) Immunofluorescence image showing host cell-free WT and ΔripE E. japonica incubated with DH82 for 30 min (A) or RF/6A cells for 60 min (B). Cells were fixed and stained with anti-P28 (AF488, green), and then stained with anti-P28 (AF555, red) with saponin permeabilization. Scale bar, 20 µm. (Right) The numbers of bound bacteria (yellow) and internalized bacteria (red) in 100 cells were scored in ImageJ software. ns, not significantly different by the Student’s t-test (n = 3).
Ehrlichia must be released from infected host monocytes/macrophages and survive the extracellular stage to infect new host cells and spread to other tissues/organs. Therefore, we next compared the infectivity of host cell-free Ehrlichia that had been briefly preincubated in culture medium up to 60 min. Without preincubation, freshly isolated ΔripE and WT E. japonica had the same infectivity on DH82 cells (Figures 5A, B). Both ΔripE and WT E. japonica progressively lost their infectivity in the host cell-free medium, but ΔripE lost its infectivity significantly faster than WT did (Figure 5A).
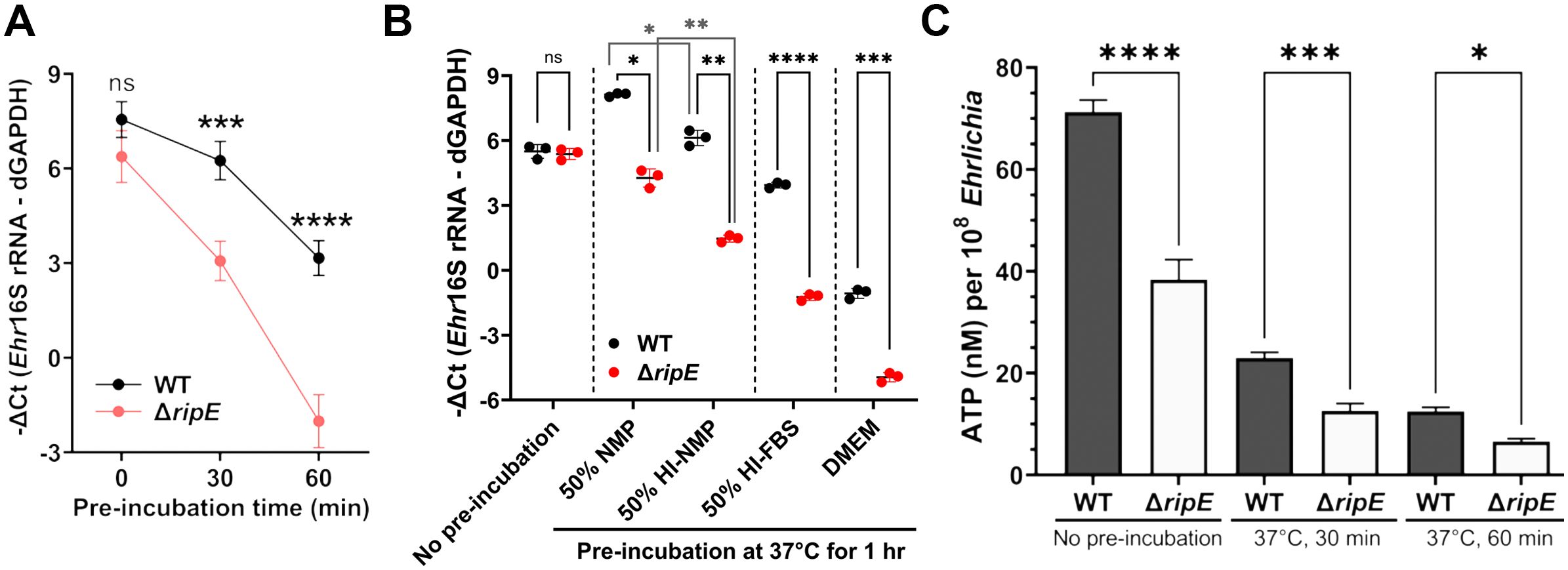
Figure 5. Host cell-free ΔripE E. japonica more rapidly loses infectivity and ATP reserve in culture than WT E. japonica. (A) Temporal loss of infectivity of host cell-free WT or ΔripE E. japonica preincubated at 37°C in culture medium (DMEM with 4% heat-inactivated FBS) in DH82 cells (MOI 2,500–3,000). (B) Infectivity of host cell-free WT and ΔripE E. japonica preincubated at 37°C for 1 h in different media in DH82 cells (MOI 500–1,000). NMP, normal mouse serum; HI, heat inactivated at 56°C for 30 min. (A, B) The infected DH82 cells were harvested at 2 dpi for Ehrlichia 16S rRNA-specific RT-qPCR. Input RNA was normalized by dog GAPDH (dGAPDH). Data indicate the mean ± standard deviation (n = 3). Results were analyzed with repeated-measures ANOVA followed by Šídák multiple comparisons (A) or one-way ANOVA followed by Tukey’s multiple comparisons (B). ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns, not significant different. (C) Levels of ATP in host cell-free WT or ΔripE E. japonica incubated in 4% heat-inactivated FBS for 0, 30, and 60 min. ATP levels were determined using the Luminescent ATP Detection Assay Kit normalized with the number of Ehrlichia determined by Ehr16S rRNA gene-specific qPCR. Data indicate the mean ± standard deviation of triplicate samples. ****p < 0.0001, ***p < 0.001, *p < 0.05, significantly different based on one-way ANOVA followed by Tukey’s multiple comparisons.
Extracellular Ehrlichia is exposed to the serum, and serum complement resistance has been reported for certain Rickettsia spp (Riley et al., 2018). Thus, to determine the effects of serum on extracellular Ehrlichia, and whether E. japonica is complement-resistant, we preincubated host cell-free Ehrlichia (WT or ΔripE) in 50% normal mouse plasma (NMP), which mimics mouse blood, and 50% heat-inactivated NMP; negative controls include 50% heat-inactivated fetal bovine serum (FBS, used in cell culture of E. japonica) and serum-free Dulbecco’s modified Eagle medium (DMEM). The presence of serum in the culture media profoundly protected the infectivity of extracellular Ehrlichia regardless of the type of serum in both WT and ΔripE mutant (Figure 5B). The infectivity of E. japonica was significantly lower by heat inactivation of serum components (Figure 5B), suggesting that E. japonica is complement-resistant, and heat-labile plasma components are rather beneficial for extracellular Ehrlichia. The ΔripE mutant lost its infectivity significantly more than WT E. japonica in each medium tested, including in the absence of serum (Figure 5B), implying that the presence of RipE helps maintain the infectivity of extracellular Ehrlichia via a mechanism that is independent of factors in the serum.
Freshly isolated Ehrlichia can transiently produce the essential chemical-energy component adenosine triphosphate (ATP) (Weiss et al., 1989). We therefore quantified the time course of the total ATP in host cell-free Ehrlichia using the Luminescent ATP Detection Assay Kit (see Materials and Methods). The total ATP levels in host cell-free Ehrlichia were significantly higher in WT than in the ΔripE mutant regardless of the incubation time in the culture medium containing 4% heat-inactivated FBS (Figure 5C).
Genomic complementation of the ΔripE mutant partially restores the Ehrlichia ATP level, and overexpression of ripE increases the ATP level and in vivo virulenceTo test if RipE produced within Ehrlichia counteracts reduced ATP levels in the extracellular ΔripE mutant and increases Ehrlichia virulence in mice, we performed genomic complementation of the ΔripE mutant with ripE or overexpression of ripE in WT E. japonica bacteria by Himar1 mutagenesis with the newly constructed pCis-FLAG-RipE-Gent-Himar plasmid, which encodes a gentamicin resistance gene and FLAG-RipE (Figure 6A). A total of three transformed WT (WT+; genomic insertion of Himar 1, including Eja+0814, Eja+1020, and Eja+1231) and one transformed ΔripE (ΔripE+; genomic insertion of Himar1, H59+1014) E. japonica were obtained and stably cultured in DH82 cells (Bekebrede et al., 2020) (Table 2). In subsequent experiments, WT+ (Eja+0814) was used as a representative of E. japonica overexpressing RipE, whereas ΔripE+ (H59+1014) was used to represent rescued ΔripE E. japonica. ripE-gene-specific flanking PCR confirmed the original Himar1 insertion in ΔripE and ΔripE+, whereas the ripE gene was intact in WT and WT+ (Figure 6B).
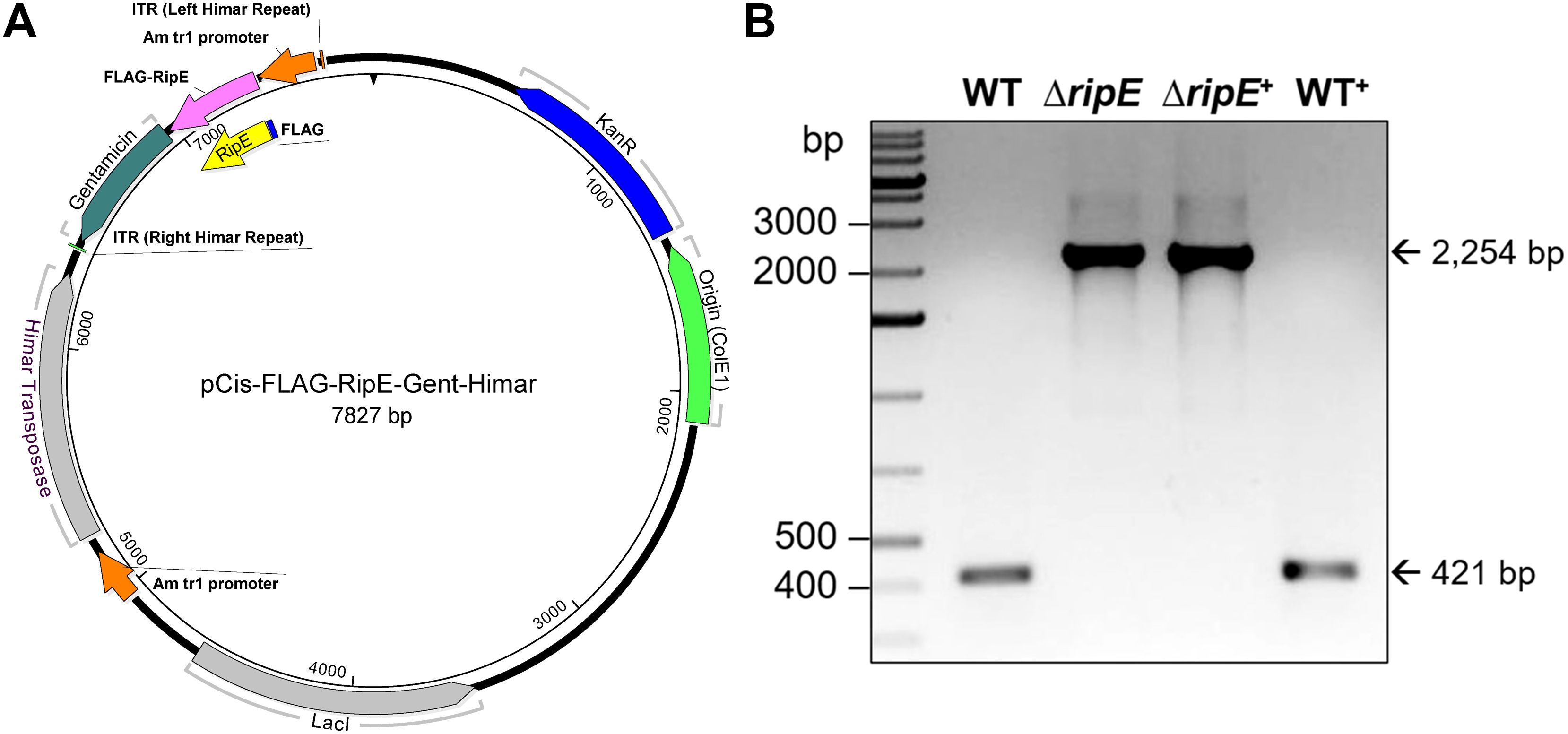
Figure 6. Plasmid construction for genomic complementation of ripE in ΔripE E. japonica and overexpression of ripE in WT E. japonica. (A) pCis-FLAG-RipE-Gent-Himar plasmid map. (B) ripE gene-specific flank PCR for ΔripE and ΔripE+ clonality verification. Corresponding amplicon sizes for WT (421 bp) and the original Himar1 insertion disrupted ripE gene (2,254 bp) are indicated by arrows.
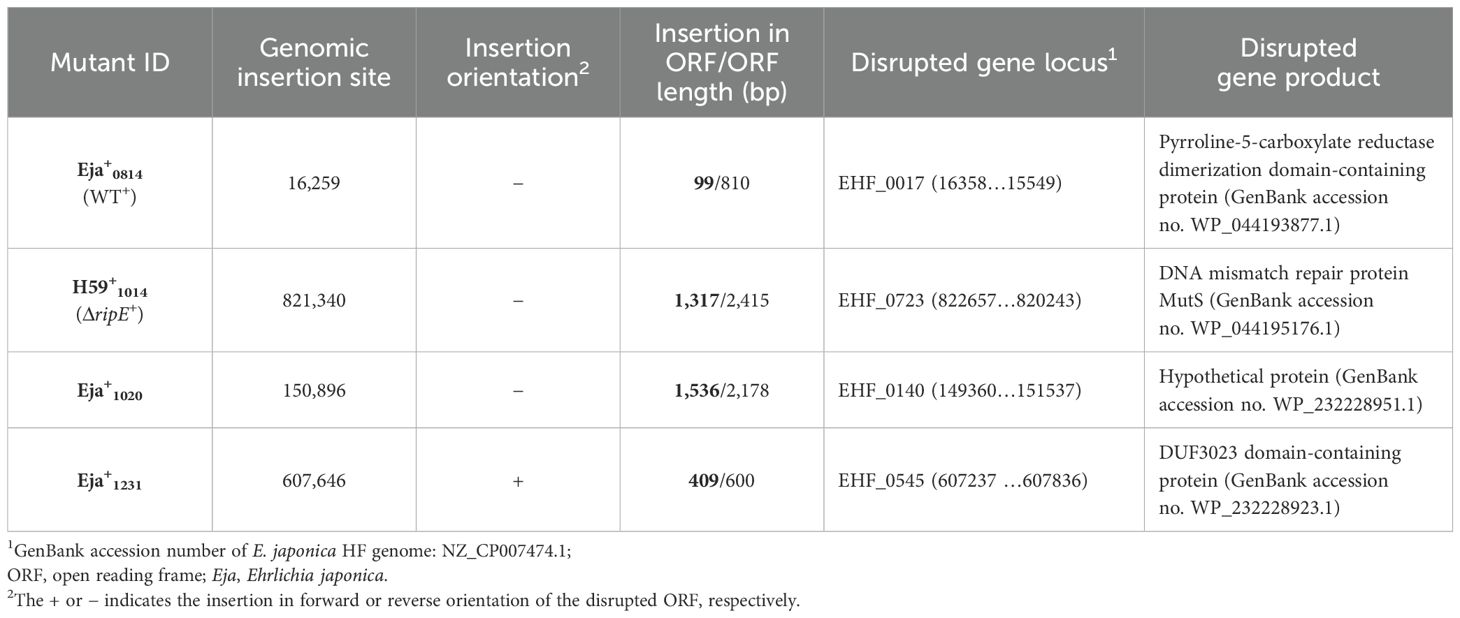
Table 2. Mutants of E. japonica transformed with pCis-FLAG-EHF0962-Gent-Himar A7 plasmid1.
Immunofluorescence staining revealed that WT+E. japonica overexpressed RipE within more than 80% of the Ehrlichia morulae, much higher than what was observed for WT E. japonica, which typically contained 20-30% RipE-positive morulae (Figure 7A). Genomic complementation of the ΔripE mutant partially restored ripE expression in ΔripE+E. japonica, although RipE abundance was relatively low, i.e., less than 5% RipE-positive morulae (Figure 7A). Western blotting confirmed that WT+E. japonica overexpressed approximately 9-fold or 60-fold more RipE protein than WT or ΔripE+E. japonica in culture, respectively (Figure 7B).
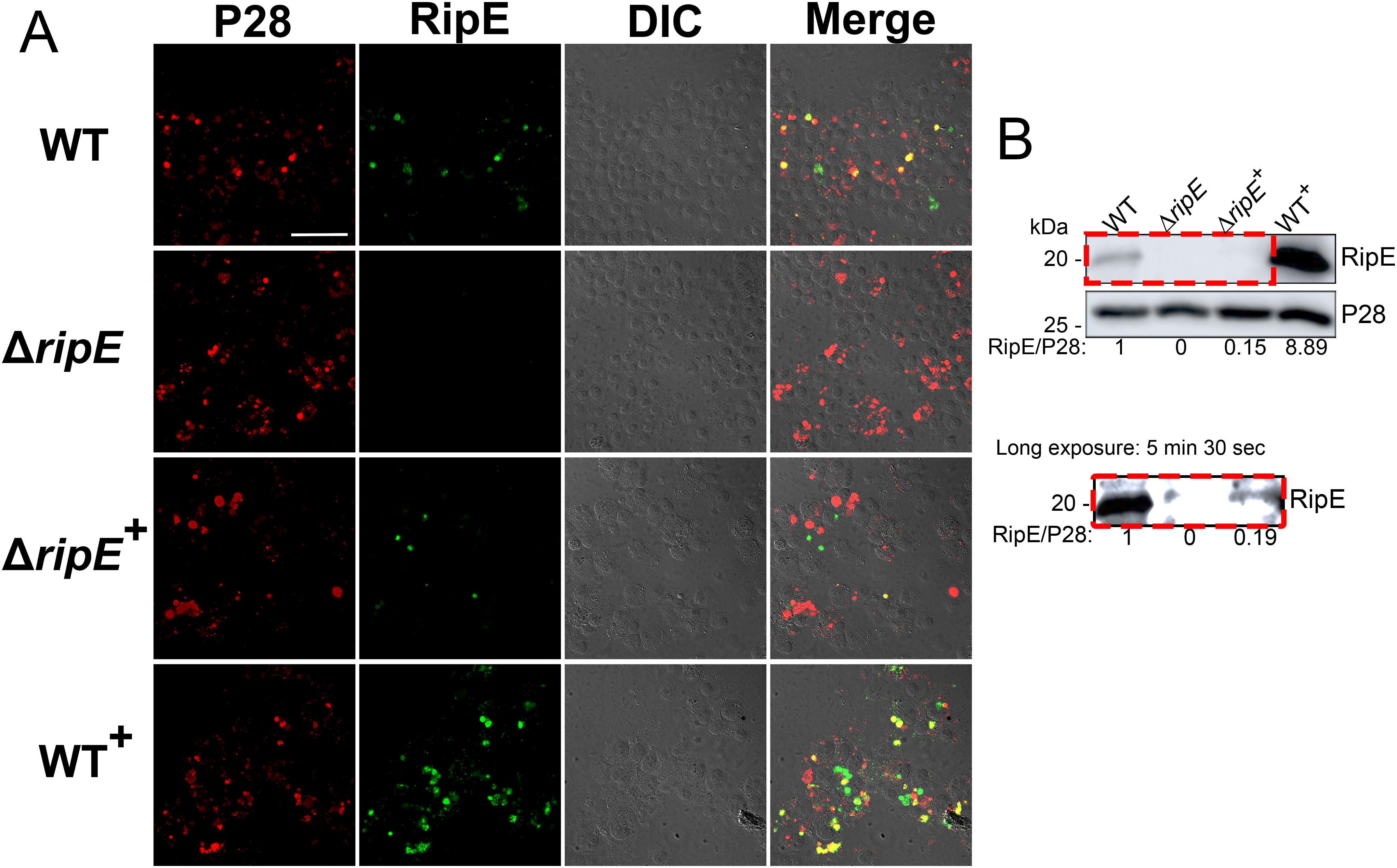
Figure 7. Genomic complementation or overexpression of ripE. (A) Double immunofluorescence labeling of WT, ΔripE, RipE-complemented ΔripE (ΔripE+), and RipE-overexpressing WT (WT+) E. japonica-infected DH82 cells with rabbit anti-Ehrlichia P28 (AF555, red) and mouse anti-rRipE (AF488, green). Merge, fluorescence image merged with differential interference contrast (DIC) image. Scale bar, 50 µm. (B) Lysates of WT, ΔripE, ΔripE+, and WT+E. japonica-infected DH82 cells were analyzed by Western blotting using anti-rRipE and anti-P28. Numbers below each band are relative ratios of RipE band intensities normalized by P28, with the ratio of WT set as 1. Red dashed box on the lower panel showed the image under long exposure time (5 min and 30 s).
To test if RipE amounts within Ehrlichia correlate with ATP levels in extracellular Ehrlichia, the time course of ATP loss in extracellular WT, ΔripE, and the transformed E. japonica in the culture medium that contains 4% heat-inactivated FBS was examined. Without incubation at 37°C, WT+E. japonica had the highest average ATP level, followed by WT, ΔripE+, and ΔripE E. japonica (Figure 8A). After incubation at 37°C for 30 or 60 min, however, the ATP levels dropped greatly in all groups, although WT+E. japonica maintained a significantly higher ATP level compared to WT, ΔripE+, and ΔripE E. japonica (Figure 8A).
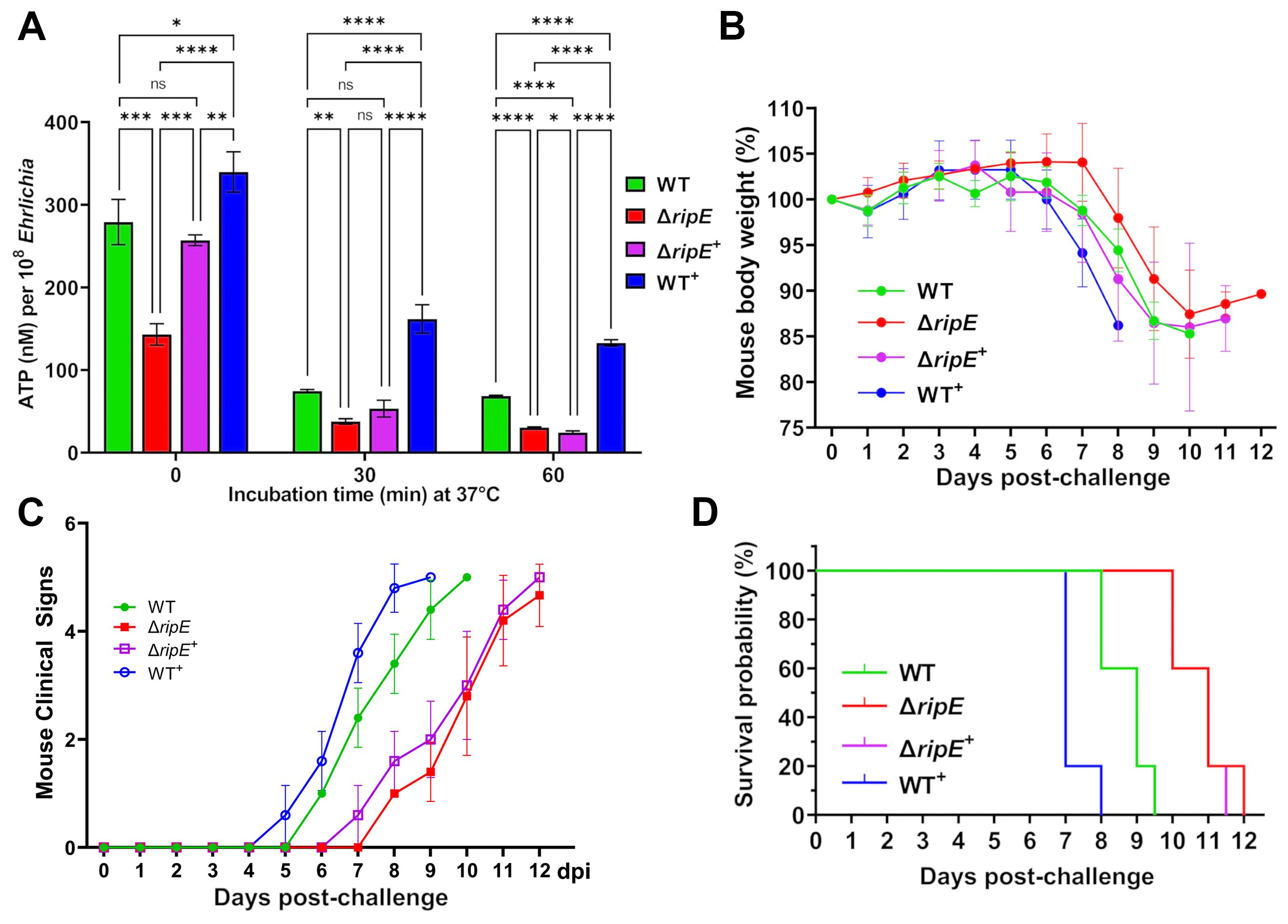
Figure 8. RipE expression increases Ehrlichia ATP reserve and in vivo virulence. (A) Levels of ATP in host cell-free WT, ΔripE, ΔripE+, and WT+E. japonica incubated in 4% heat-inactivated FBS for 0, 30, and 60 min. ATP levels were determined using the Luminescent ATP Detection Assay Kit and normalized with bacterial numbers determined by Ehrlichia 16S rRNA gene-specific qPCR. Data indicate mean ± standard deviation of triplicate samples. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; significantly different, ns, not significantly different based on one-way ANOVA followed by Tukey’s multiple comparisons. (B–D) ICR mice (4-week-old male, five mice per group) were i.p. inoculated with WT, ΔripE, ΔripE+, and WT+E. japonica-infected DH82 cells. The number of Ehrlichia inoculated was determined by Ehrlichia 16S rRNA-specific qPCR: WT, 5,195 bacteria/mouse; ΔripE, 5,902; ΔripE+, 4,654; and WT+, 9,490. (B) Body weight changes. (C) Signs of illness (ruffled fur coat, hunched back, and squinty eyes, and reluctance to move) were assigned the rating of 0 to 4 for severity and 5 for death. Statistical analysis was performed using two-way repeated-measures ANOVA and showed that clinical signs of the infected mice were significantly more severe in those challenged with E. japonica expressing RipE in the order of WT+ > WT >> ΔRipE+ than the ΔRipE mutant that does not express RipE (p < 0.01). Data indicate mean ± standard deviation of five mice. (D) Kaplan–Meier survival curves. Compared with mice inoculated with WT, mice inoculated with ΔripE and ΔripE+ survived significantly longer (p < 0.01), whereas mice inoculated with WT+ died significantly earlier (p < 0.01) analyzed by log-rank (Mantel–Cox) test (n = 5). There was no significant difference between ΔripE and ΔripE+ (p = 0.663).
To test whether intrinsic RipE levels correlate with Ehrlichia virulence in vivo, ICR mice were inoculated i.p. with WT, ΔripE, ΔripE+, or WT+E. japonica (five mice per group). Mice were weighed daily and monitored for clinical signs. Although the body weight changes were not dramatically different among the groups (Figure 8B), the onset and severity of clinical signs of the infected mice were much earlier and significantly more severe in those challenged with E. japonica expressing RipE in the order of WT+ > WT >> ΔripE+ than the ΔripE mutant that does not express RipE (Figure 8C). Kaplan–Meier survival curves showed that WT+E. japonica was significantly more virulent than WT and ΔripE E. japonica (Figure 8D); however, loss of virulence in ΔripE E. japonica was not restored in ΔripE+E. japonica (Figure 8D), in agreement with the observed low level of RipE expression (Figures 7A, B).
RipE is a bacterial outer-membrane proteinTo study mechanisms by which RipE is involved in maintaining the infectivity and ATP levels in extracellular E. japonica, we analyzed the subcellular localization of RipE in E. japonica using DeepLocPro, an AI-based prokaryotic deep-learning algorithm (Izadi-Pruneyre et al., 1999; Moreno et al., 2024). By this analysis, RipE protein was predicted to localize to the bacterial membranes or be secreted. Immunofluorescence labeling using RipE- and outer-membrane protein P28-specific antibodies demonstrated that RipE colocalized with P28, showing ring-like labeling patterns in both E. japonica-infected DH82 cells that had been permeabilized with saponin (Figure 9A) and in host cell-free WT E. japonica without permeabilization (Figure 9B). These results suggested that RipE was expressed on the Ehrlichia surface.
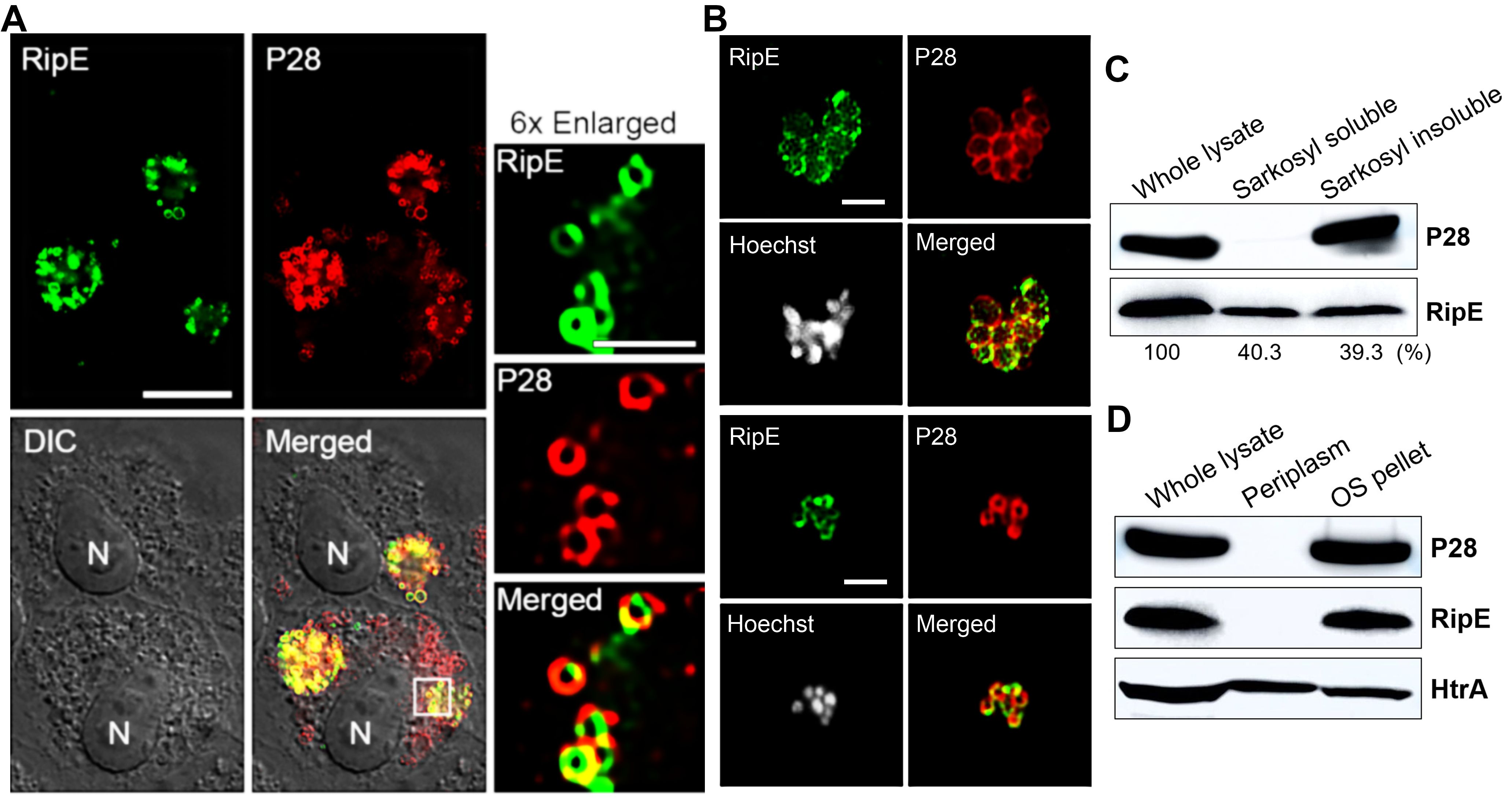
Figure 9. RipE is present on the outer membrane of Ehrlichia. (A) Immunofluorescence labeling of E. japonica overexpressing RipE (WT+) in DH82 cells with mouse anti-rRipE (AF488, green) and rabbit anti-P28 IgG (AF555, red) with membrane permeabilization by saponin. Scale bar, 10 µm. Merged, fluorescence images merged with DIC image. N, nucleus. Boxed area was enlarged 6× on the right. Scale bar, 2 µm. (B) Immunofluorescence labeling of spontaneously released WT E. japonica with mouse anti-rRipE (AF488, green) and rabbit anti-P28 IgG (AF555, red) without membrane permeabilization. DNA was stained by Hoechst 33342 and pseudocolored gray. Scale bar, 2 µm. (C) Western blot analysis of the whole lysate, and Sarkosyl-soluble and -insoluble (outer membrane) fractions of purified WT Ehrlichia with mouse anti-rRipE and rabbit anti-P28. Numbers below each band are percentages of RipE protein relative to the whole lysate (set as 100%). (D) Western blot analysis of the whole lysate, soluble (periplasm), and insoluble fractions by osmotic shock (OS) of purified WT E. japonica with mouse anti-rRipE and rabbit anti-Ehrlichia P28 and anti-HtrA sera.
To confirm the surface localization of RipE, E. japonica was fractionated by the sodium dodecyl sarcosine (Sarkosyl) solubilization method, which is an amphiphilic, ionic detergent that has been used to isolate the E. chaffeensis outer-membrane fraction (Ohashi et al., 1998). Sarkosyl fractionation of purified WT E. japonica resulted in soluble (cytosol, inner membrane, and periplasm) and insoluble (outer membrane) fractions. Using Ehrlichia P28 as a control, the results showed that native RipE of WT E. japonica was present in both Sarkosyl-soluble and Sarkosyl-insoluble fractions at a ratio of approximately 1:1 (Figure 9C), confirming that ~50% of RipE localizes to the outer membrane. To test if the remaining 50% of RipE is present in the periplasm of E. japonica, the osmotic shock method (Cheon et al., 2021) was adapted for isolating Ehrlichia periplasmic proteins (Kumagai et al., 2010). The results showed that, unlike the control E. japonica HtrA protein, which is a serine protease normally present in the E. chaffeensis periplasm and outer surface (Kumagai et al., 2010), RipE was absent in the periplasmic fraction (Figure 9D), suggesting that ~50% RipE is localized in the inner membrane or cytoplasm of E. japonica.
rRipE induces a robust humoral response with the production of an E. japonica-neutralizing antibody and is a vaccine candidate for ehrlichiosisAnti-RipE antibody produced using rRipE recognized denatured protein by Western blotting (Figures 2A, 7B, 9C, D) and native protein by immunofluorescence staining (Figures 2C, F, 7A, 9A, B), indicating that rRipE is antigenic and capable of inducing a robust humoral response in mice. Indeed, in vitro neutralization experiments revealed that mouse anti-rRipE antisera could significantly reduce E. japonica infection in vitro in a dose-dependent manner compared to NMP (Figure 10). The neutralizing effect of the mouse anti-RipE antiserum for reducing E. japonica infection in vitro was similar to that of anti-rP28 (Figure 10), which can neutralize E. chaffeensis infection in immunocompetent mice (Ohashi et al., 1998) and protect severe combined immunodeficiency mice from fatal E. chaffeensis infection (Li et al., 2001).
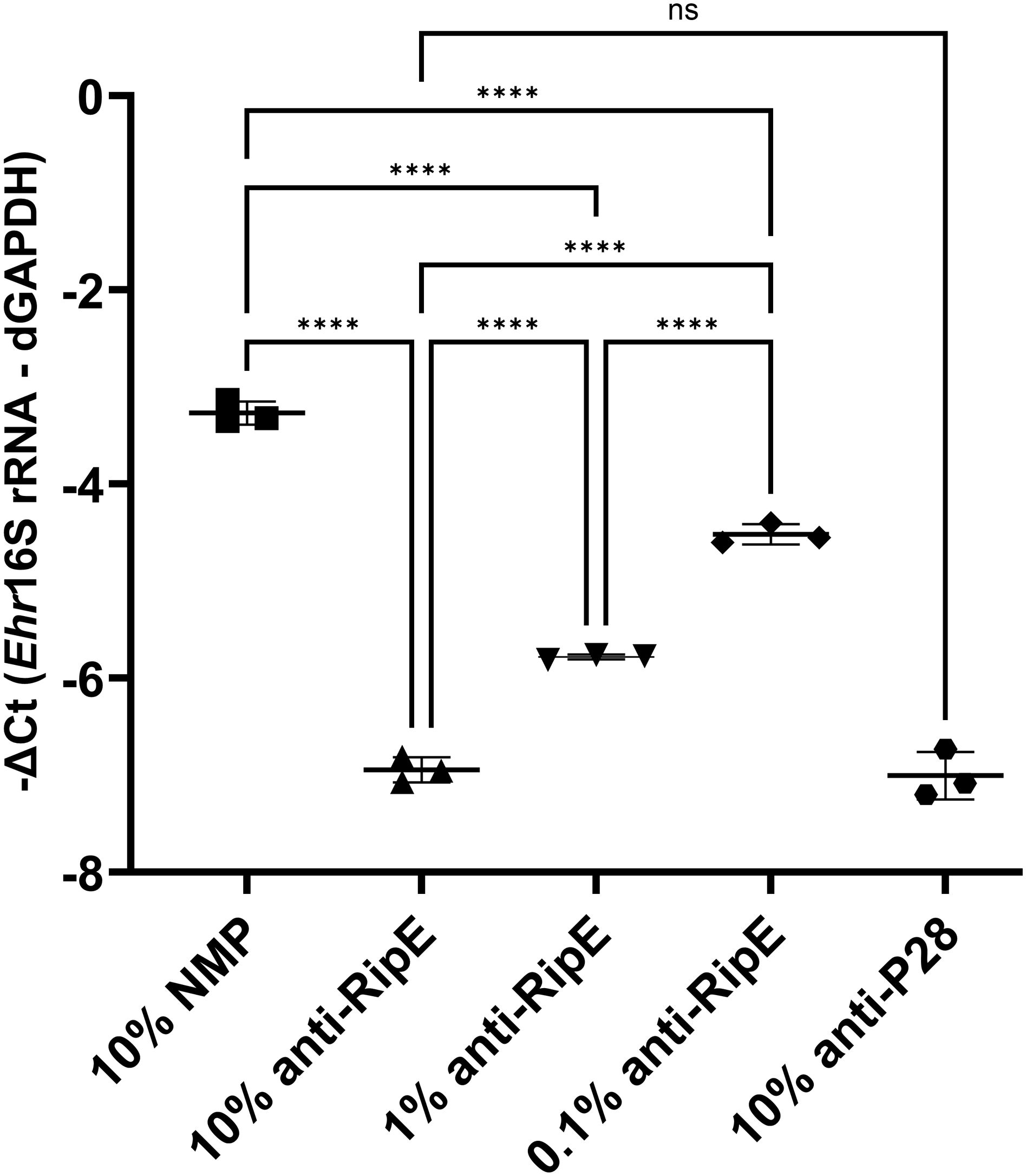
Figure 10. In vitro neutralization of E. japonica by anti-rRipE. Freshly isolated host cell-free WT E. japonica (~1,000 MOI) was preincubated with 10% normal mouse plasma (NMP), 10%, 1%, or 0.1% mouse anti-rRipE serum, or 10% rabbit anti-P28 serum at 37°C for 30 min. Sera and plasma were all diluted in the culture medium. The mixtures of Ehrlichia and antisera were added to DH82 cells and incubated at 37°C, and infection was determined at 48 hpi by Ehrlichia 16S rRNA gene-specific qPCR. Input DNA was normalized by dog GAPDH (dGAPDH). ****, significantly different (p < 0.0001); ns, not significantly different based on one-way ANOVA followed by Tukey’s multiple comparisons (n = 3). The result is representative of two independent experiments.
To test whether the rRipE-induced Ehrlichia-neutralizing antibody could prevent fatal ehrlichiosis in vivo, we immunized five mice three times with rRipE at 2-week intervals followed by a lethal challenge with WT E. japonica through i.p. injection. An enzyme-linked immunosorbent assay (ELISA) demonstrated that all rRipE-immunized mice generated a significant rRipE-specific IgG after the third immunization (Figure 11A), whereas the sham-immunized controls did not. At day 7 post-WT E. japonica challenge, all sham control mice had lost more than 10% of body weight (Figure 11B) and appeared severely ill or moribund, characterized by ruffled haircoat, hunched back, squinty eyes, and reluctance to move, whereas rRipE-immunized mice experienced lesser body weight loss, with mice being relatively normal, alert, and responsive. Bacterial load in the blood of rRipE-immunized mice was significantly lower than in sham controls at 5 days pi (Figure 11C). At 7–8 days pi, however, bacterial load did not differ between the two groups (Figure 11C). Kaplan–Meier curves showed that rRipE-immunized mice survived significantly longer than the sham controls, although all mice eventually succumbed to fatal ehrlichiosis up to 11 days pi (Figure 11D).
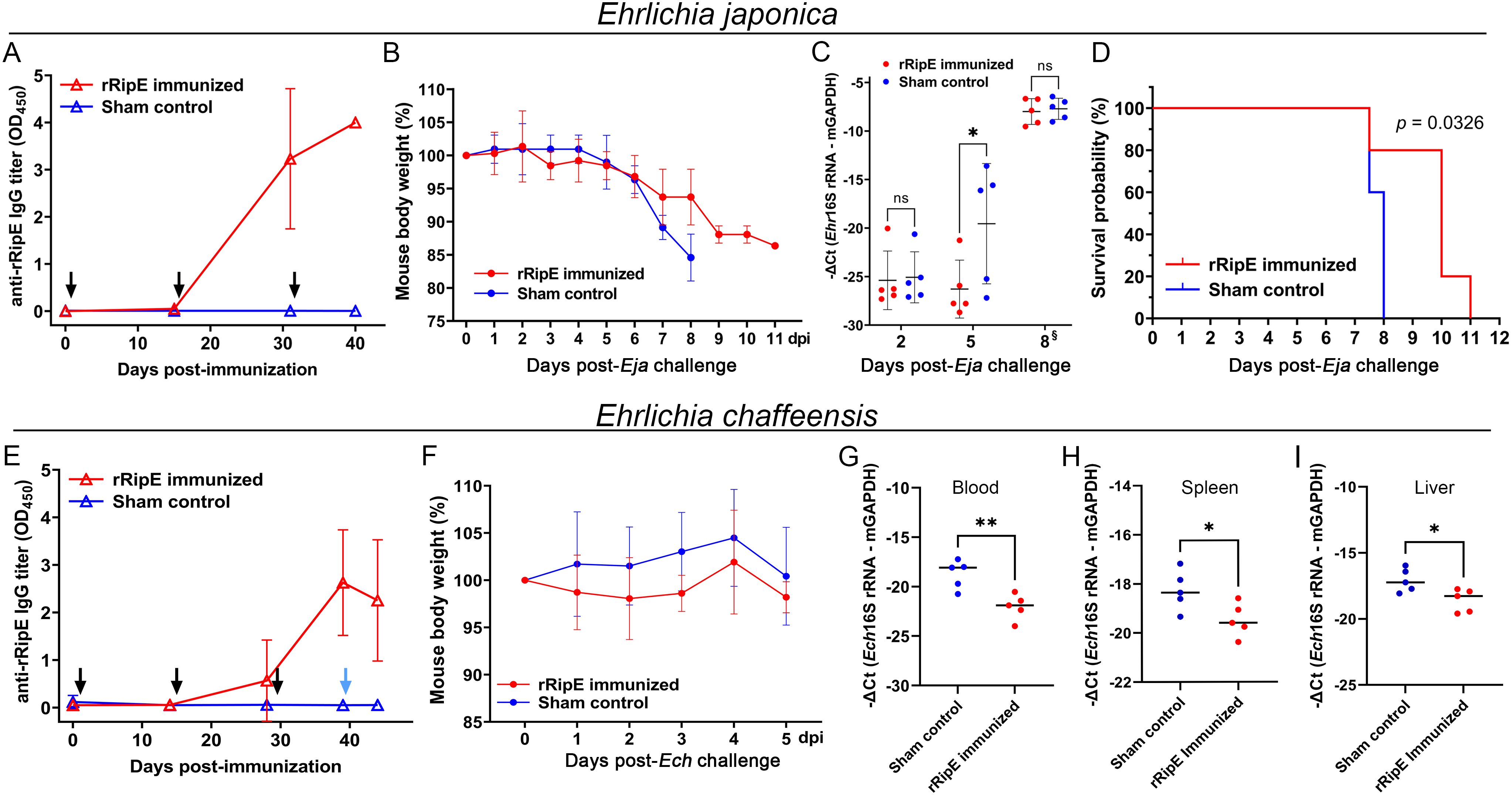
Figure 11. rRipE immunization increased the survival time of mice challenged with a lethal dose of E. japonica. ICR (A–D) or C57BL/6 (E–I) mice were subcutaneously injected with rRipE plus QuilA adjuvant (rRipE immunized) or QuilA alone (Sham control), and challenged by i.p. inoculation of infected DH82 cells containing a lethal dose of WT E. japonica (11,750) at 9 days post third immunization [Eja, (A–D)], or infected THP-1 cells containing E. chaffeensis (~120,000) at 11 days post third immunization [Ech, (E–I)], respectively. (A, E) Titers of rRipE-specific antibody in mice sera were measured by ELISA using rRipE as the antigen. Black arrows indicate days on which mice were vaccinated, and blue arrow denotes the day on which mice were challenged with E. chaffeensis. Data indicate the mean ± standard deviation (n = 5). (B, F) Mouse body weight changes in rRipE-immunized and sham-immunized mice upon challenge with WT E. japonica (B) or E. chaffeensis (F) (n = 5). (C) Bacterial loads of E. japonica in the blood of rRipE-immunized and control mice by Ehrlichia 16S rRNA gene-specific qPCR normalized by mouse GAPDH (mGAPDH). Data indicate the mean ± standard deviation (n = 5). Results were analyzed with repeated-measures ANOVA followed by Šídák multiple comparisons. *p < 0.05; **p < 0.01; ns, not significantly different. §Mouse blood samples were collected at 8 days pi or at the time of death (two control mice and one rRipE immunized mice were found dead a few hours before 8 days pi). (D) Kaplan–Meier survival curves for rRipE- and sham-immunized mice following lethal challenge with WT E. japonica. Compared with control mice, rRipE-immunized mice survived significantly longer (p < 0.05) by log-rank (Mantel–Cox) test (n = 5). (G–I) Bacterial loads of E. chaffeensis in the rRipE-immunized and sham-immunized mice on day 5 post-challenge were determined using Ehrlichia 16S rRNA gene normalized by mouse GAPDH (mGAPDH). (G) Blood samples, qPCR. (H) Spleen and (I) liver samples, RT-qPCR. The scatter plot shows the normalized Ehrlichia levels in individual mice, with the horizontal bar representing the mean value. **p < 0.01; *p < 0.05 by Student’s t-test.
As E. japonica and E. chaffeensis RipE share 46% amino acid identities (Table 1), we tested whether mice vaccinated with E. japonica rRipE could protect infection by the human ehrlichiosis agent E. chaffeensis. Mice immunization with E. japonica rRipE or sham control as described above was challenged i.p. with E. chaffeensis-infected DH82 cells (~1.2 × 105 bacteria per mouse). Similarly, all rRipE-immunized mice generated an abundance of rRipE-specific IgG after the third immunization (Figure 11E). Mouse infection with E. chaffeensis did not exhibit any apparent clinical signs (weight loss, lethargy, anorexia, squinting eyes, or ruffled fur) throughout the experimental course (Figure 11F). However, E. chaffeensis loads in the blood, spleen, and liver samples of rRipE-immunized mice were significantly lower than those of sham controls at 5 days post-challenge (Figur
留言 (0)