Renal cell carcinoma (RCC) was reported to cause approximately 179,000 deaths worldwide in 2020, accounted for 2.2% of the total new-diagnosed cancer cases and 1.8% of the cancer deaths globally.[1] Among all subtypes of RCC, tubulocystic renal cell carcinoma (TRCC) is a kind of rare variant and was not clarified as a pathologically independent entity by the World Health Organization until 2016.[2] According to limited data, TRCC accounted for 1% of all RCC,[3] and approximately 100 confirmed-TRCC cases have been documented up to now.[4] Majority of TRCC patients were asymptomatic owing of its typically indolent character and were diagnosed incidentally, so could receive early treatment. TRCC patients usually exhibit a good prognosis and rarely experience recurrence or metastasis after surgery although a very few patients will develop metastatic sites.[5] The formal identification of the disease has not significantly enhanced our knowledge on this pathological variant due to its low incidence, which mainly concentrates on histopathological profiles and differentiation.[6] The multiple radiological examinations can provide complementary information for imaging manifestations and cross-validate the results to improve the reliability of the diagnosis. There are no reports summarizing the multimodal radiological characters about TRCC currently. Herein, we report the multimodal imaging findings of a case with TRCC admitted in our hospital. This case report was prepared following the CARE guidelines and the patient has provided informed consent.
CASE REPORTA 71-year-old male was admitted after a urological ultrasound revealed a cystic nodule (Bosniak III, malignancy not excluded) on the middle pole of the right kidney during a routine physical examination. The patient was asymptomatic and he claimed no hematuria on admission. Physical examination showed no dilatations in bilateral renal areas, negative for percussion pain in bilateral kidney. Following urine and blood tests found no abnormalities, and the patient denied the family history of RCC.
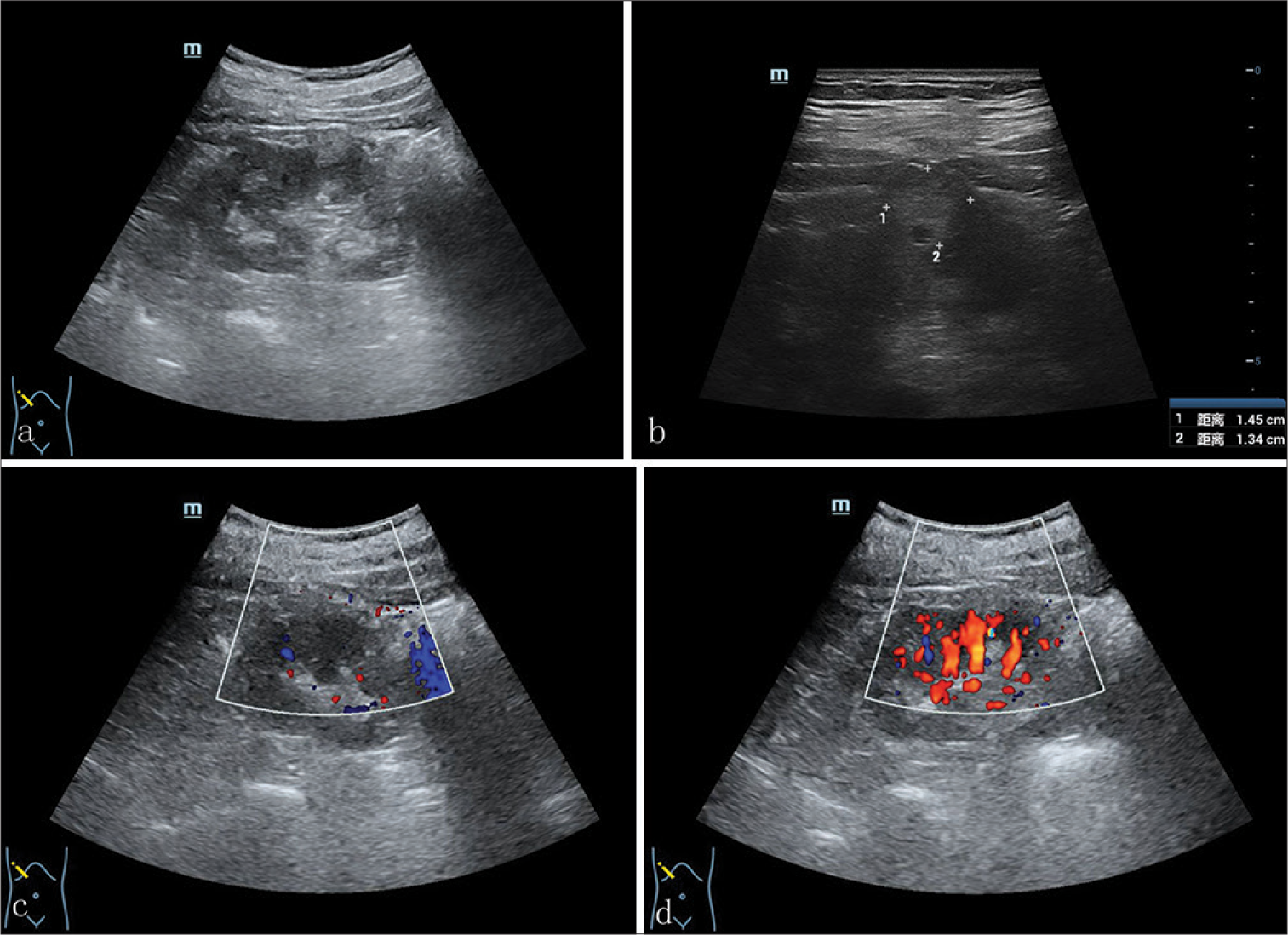
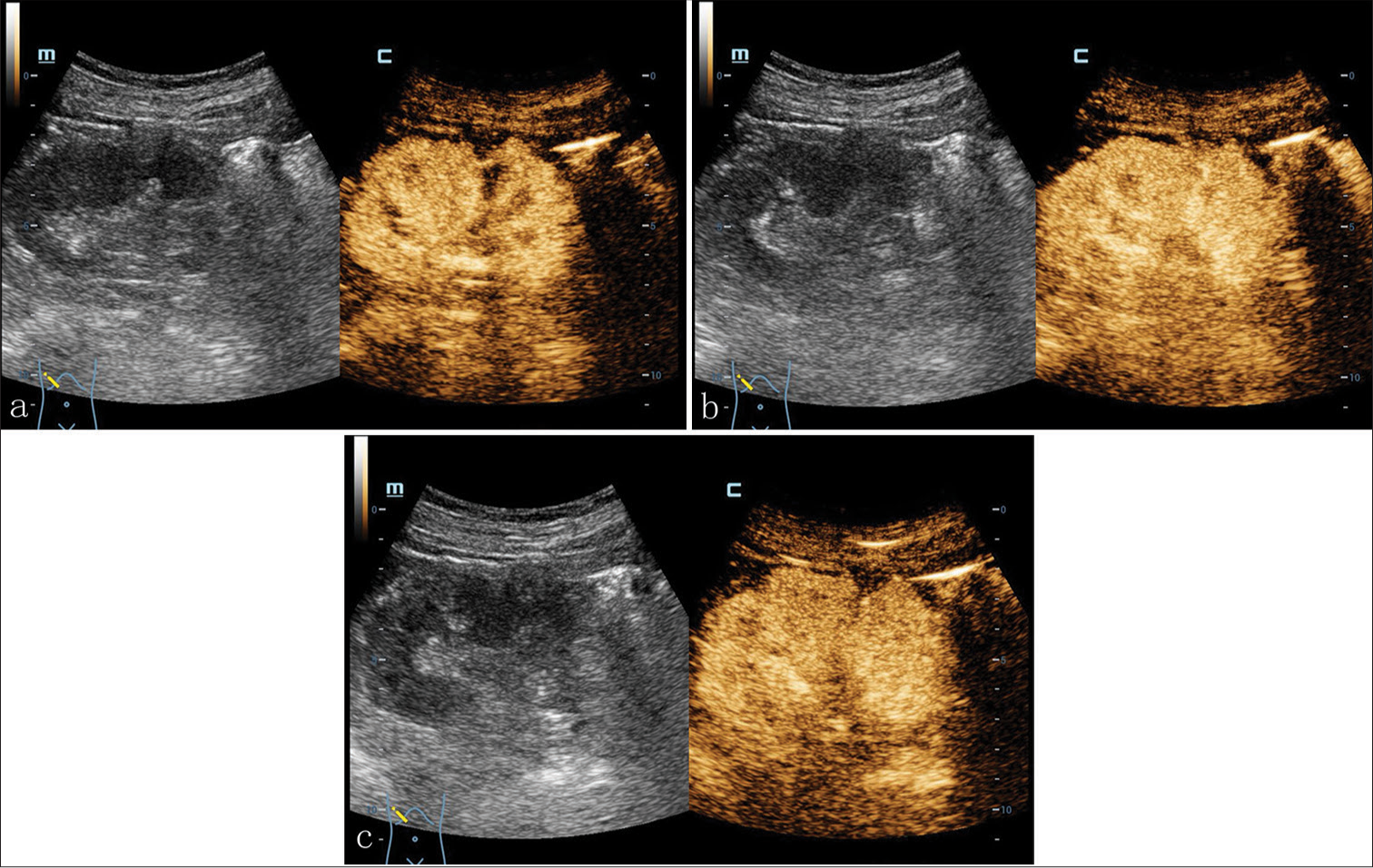
Conventional ultrasound with a probe frequency of 3.5–10.0 MHz revealed a 1.4 × 1.3 × 1.1-cm hyperechoic nodule in the middle part of the right renal parenchyma. The nodule exhibited a regular shape and clear boundaries. No obvious septum was observed within the nodule. An internal small anechoic area with a range of 0.2 × 0.3-cm could be observed [Figure 1a and b]. Color Doppler flowing imaging (CDFI) exhibited no obvious blood flow signal within the nodule. However, the blood flow signal in the renal parenchyma around the nodule increased, showing a pattern of branching detour [Figure 1c and d]. Contrast-enhanced ultrasound (CEUS) was performed targeting at the nodule in the middle part of the right renal parenchyma. The contrast agent of 1.2 mL SonoVue (Bracco SpA, Milan, Italy) was administered intravenous bolus. Based on the imaging findings, the right renal cortex started to enhance at 11 s after injection of contrast agent and reached a peak at 18 s. The contrast agent was noted to diffuse into the nodule at 12 s, exhibiting nodular uneven enhancement with a range of 0.2 × 0.6-cm, and the peak of enhancing reached at 26 s. Non-enhancing was observed in the remaining parts of the middle pole of the right renal parenchyma, presenting the profile consistent with a cystic nodule. The infiltration of contrast agent could be seen at margin of nodule and the enhancing portion was cystic wall connected to the renal parenchyma. The enhancing intensity of thicken cystic wall at peak phase approached to that of renal parenchyma, and the boundary between the nodule and the peripheral renal parenchyma was poorly defined. Similar to the conventional ultrasound, the measured size of the nodule was 1.5 × 1.4 × 1.1-cm. The contrast agent began to fade at 39 s, and the nodule and its surrounding renal parenchyma were cleared simultaneously [Figure 2a-c].
Export to PPT
Export to PPT
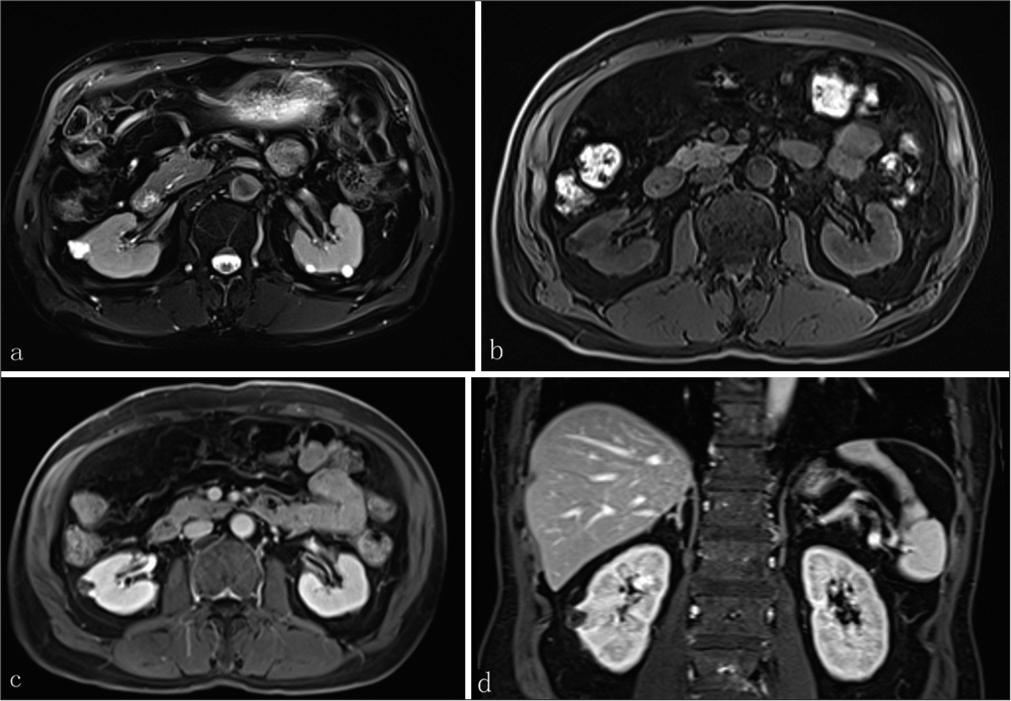
The patient underwent the examinations of enhanced computed tomography angiography (CTA) and magnetic resonance imaging (MRI). Based on the enhanced CTA using the single bolus protocol, a 1.5 × 1.4 × 1.2-cm, slightly low-density, multilocular cystic-dominant lesion was present in the right kidney consistent with a Bosniak class III renal cyst (average cycle threshold [CT] value: 21.17 HU) [Figure 3a]. The cystic lesion was well-circumscribed, presenting part of lesion protruded from the renal contour. No signal enhancement was observed in the enhanced computed tomography (CT) renal cortex phase (average CT value: 28.54 HU). Minimally enhancement appeared in the renal medulla phase (average CT value: 45.62 HU). Enhancement weakened eventually in the renal excretory phase (average CT value: 26.80 HU) [Figure 3b-d]. There was no formation of tumor thrombus in inferior vena cava or renal vein. A standardized magnetic resonance (MR) urography protocol was initiated. The images were obtained with true fast imaging with steady-state free precession vascular imaging, transverse sectional T2-weighted images (WI), transverse T1WI, in-phase and outof-phase, and d iffusion-weighted imaging (DWI) sequences. Transverse dynamic T1WI scanning was performed after the intravenous bolus injection of gadoliniumdiethylenetriaminepentaacetic acid (30 mL, Bayer Yakuhin, Osaka, Japan) at 3 mL/s, with imaging time for cortical phase at 20 s, medullary phase at 45 s, and parenchymal phase at 120 s. Coronary T1WI imaging was performed simultaneously during the parenchymal phase. MR imaging revealed a 1.2 × 1.0-cm polycystic mass in the middle pole of the right kidney that exhibited hypointense on T1WI, hyperintense on T2WI, and no definite hyperintense on DWI. On T2WI, irregular tumor was observed consisting of multiple small cysts with thickened septa and showed heterogeneous water-like signal of cystic fluids [Figure 4a]. No-enhancing in cystic parts of tumor was observed in MR axial plain scan [Figure 4b], axial and coronal contrast-enhanced scan [Figure 4c and d]. Furthermore, contrast-enhanced scanning showed enhancement of cyst wall and septa in nephrographic phase.
![A 71-year-old male presented with a case with TRCC underwent (a) Plain computed tomography (CT) scan which showed slightly low-density, multilocular cystic-dominant lesion (average cycle threshold [CT] value: 21.17 hounsfield unit (HU), range of CT value: −16.65HU). (b-d) Contrast-enhanced CT showed the degree of enhancement decreased gradually in renal cortex phase (range of CT value: −30.89 HU), nephrographic phase (range of CT value: −17.104 HU), and excretory phase (range of CT value: −23.98 HU), presenting the profile of heterogeneously mild contrast enhancing. (TRCC: Tubulocystic renal cell carcinoma)](https://clinicalimagingscience.org/content/12/2024/14/1/img/JCIS-14-37-g003.png)
Export to PPT
Export to PPT
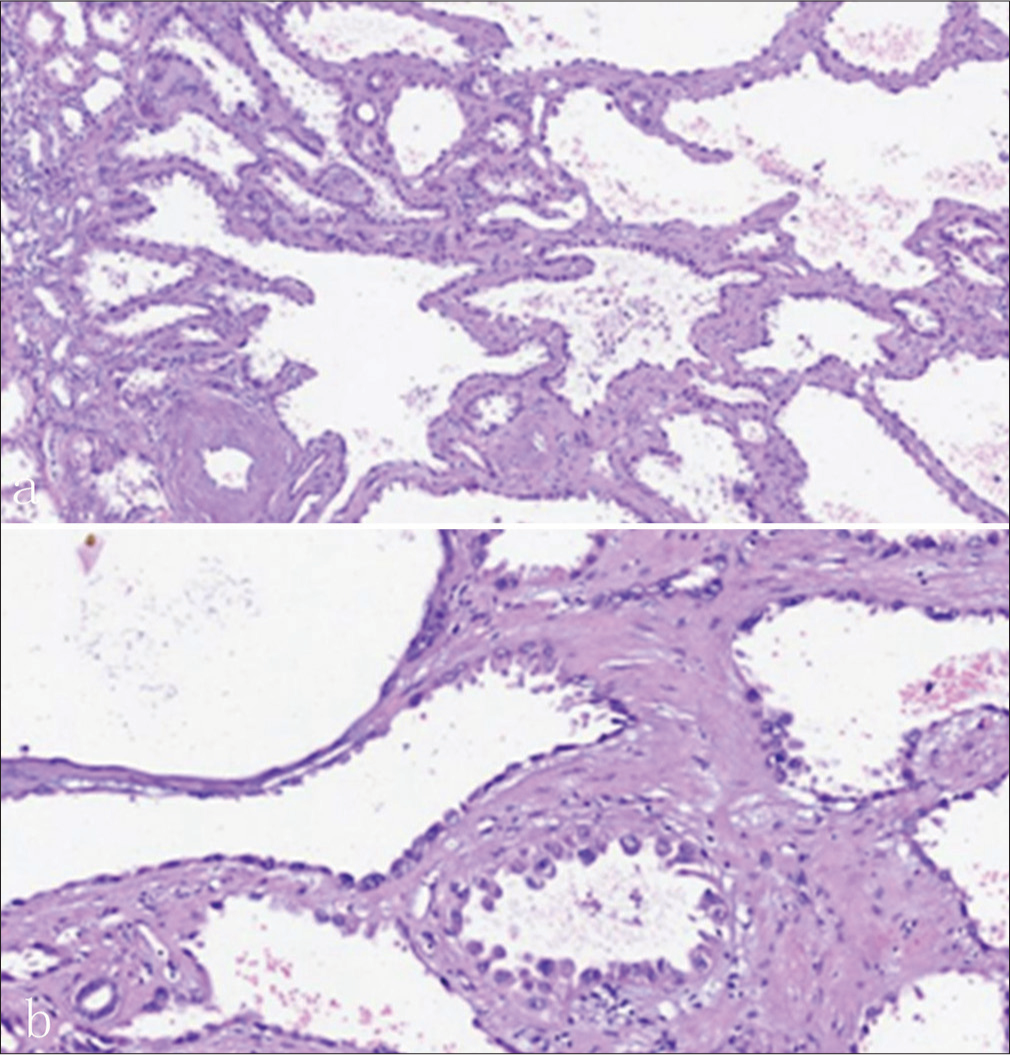
The uneventful laparoscopic right radical nephrectomy was conducted. Macroscopic evaluation showed a well-circumscribed lesion with a diameter of approximately 1.2 cm in the middle superior pole of the right kidney, and peri-lesion fat invasion was present. The pathological examination reported a 1.3 × 1.1 × 0.7-cm, well-demarcated lesion in the cut surface, presenting a pale spongy appearance with clear serous fluid inside. This sponge-like structure was composed of the typical tubular or multiloculated cystic components lined by a single layer of epithelial cells, and the nuclei were irregularly enlarged with prominent nucleoli [Figure 5a and b]. Immunohistochemical analysis of the mass was positive for PAX8, CD10, P504s and CAM5.2, and focal positive staining with PAX2 and Ki-67, as well as negative for CK7, HCK, CA9, TTF-1, CD117, WT-1, ER, PR, ERG, and Des. The final pathological diagnosis was TRCC. This patient was followed up for 12 months after discharge and no signs of local or distant metastasis were found.
Export to PPT
DISCUSSIONTRCC was initially recognized as a low-grade collecting duct carcinoma due to its similar multiloculated cystic growth pattern. Accumulated evidences have demonstrated that TRCC is more related to papillary renal cell carcinoma (PRCC), and about 10% of TRCC patients were documented to coexist with PRCC.[4] To date, approximately 100 patients have been reported in literature reviews. TRCC usually occurs in adults in their 50s and 60s and predominantly affects male (female: male ratio of 1:7). About 60% lesions involve in left kidney.[7] TRCC cases usually manifest an optimistic prognosis due to its indolent behavior.
As a renal epithelial-derived tumor with cystic predominance, TRCC is characterized by a polycystic lesion with sponge-like grossly pathological appearance. Noticeably, numerous cysts and tubules are lined by a single layer of flat or cuboidal epithelial cells. The microscopical morphology shows obviously atypical nucleus with irregular nuclear membrane and enlarged nucleoli.[8] Immunohistochemically, TRCC has been found to be positive for CK7, PAX8, AMACR, probably focal positive with PAX2, and strongly responsive to CD10 and P504s.[9,10] As presented in our report, biomarkers such as PAX8, CD10, and P504s were positive and PAX2 was focal positive, which was consistent with previous reports.
Imaging characteristics of TRCC have not been adequately summarized at present due to its rarity. The multiple radiological examinations can provide the comprehensive assessment, such as ultrasound and CDFI providing real-time images and blood flow signal, and CT and MRI presenting more detailed anatomical and functional information, ensuring an accurate diagnosis, detailed assessment and optimal treatment planning. To the best of our knowledge, the multimodal imaging findings of TRCC have not been reported. Typically, imaging reports have suggested that most of TRCCs harbored multiloculated cystic mass categorizing as the Bosniak classification II, III, or IV.[11] It can also appear as well-circumscribed, intrarenal, or extrarenal-protruded solid mass, with various degrees of enhancement after contrast-enhancement. In our study, the cyst was classified as Bosniak III. Guidelines from standard practice recommend no medical intervention for Bosniak II and surgery for Bosniak III and IV.[12]
In this case, the nodule was reported to show high echogenicity on conventional ultrasound and tended to be solid-cystic, which may be caused by the poor acoustical permeability of intracystic hemorrhage or the formation of clots. While in CEUS, most areas within the hyperechoic solid nodule did not show the signs of contrast agent entry, indicating a cystic structure. Only the margin of nodule exhibited the entry of contrast agent and the enhancing portion was cystic wall connected to the renal parenchyma. The imaging intensity of thicken cystic wall in the peak phase was similar to that of the renal parenchyma, confirming the rich blood supply. However, no blood flow signal in this part was detected in the CDFI. When there are complex cystic lesions in the kidney, the conventional CDFI can only detect some coarse blood flow signals, but it is helpless in revealing microvessels with tiny lesions. As far as the images of enhanced CT, they are collected only in the third phase, which is not dynamic and real-time, and cannot show the continuously changed characteristics of whole enhancement process with some risks resulting in omission of lesion. CEUS has improved the diagnosis of cystic renal carcinoma to the microvascular level. It was fully demonstrated that CEUS could detect the subtle blood flow signal that could not exhibit in CDFI, and had huge advantage in determining the presence of capillaries in lesion. The contrast agent can enter capillaries through microbubbles, but will not penetrate into the tissue space through vascular wall, thus more sensitively display the profiles of imperceptible blood perfusion in the lesion. CEUS has gradually become a new-fashioned tool for evaluating microcirculation in cystic renal cell carcinoma and can be used as an important basis for the qualitative diagnosis of complex cystic renal tumors.[13]
TRCC lesions mostly appear as multilocular, unilocular cystic, or solid-cystic. TRCC with a solid part tends to be associated with aggression.[14] Due to the slowly expansive growth of the tumor, the lesion is well-demarcated radiologically. The tumor lacks blood supply, most lesions exhibit no enhancement or subtle enhancement after enhancement scanning. In our case, CTA showed minimal contrast enhancement in renal cortex and medulla phase, and contrast enhancement declined in renal parenchymal and excretion stage. Clinically, CT is preferential and more common used in characterizing polycystic lesion. However, it has been publicly recognized that MRI is superior to CT in identifying the non-fluid parts such as septum and solids or mild enhancement of septum, and classifying the lesion according to the Bosniak standards.[11] On the other hand, though up-to-date radiological techniques are sufficient to diagnose malignant tumors so as to contribute to timely interventions, differentiating the TRCC from other cystic renal tumors with similar imaging finding is still up against challenging. In consideration of TRCC mimicking a series of cystic renal mass, the adult cystic nephroma, mixed epithelial and stromal tumor, papillary renal cell carcinoma, and multilocular cystic renal cell carcinoma should be taken into the differential diagnosis.[4,11,15]
CONCLUSIONWe described a TRCC case from multimodal imaging findings. TRCC is a distinct renal cell carcinoma and is characterized by multilocular cyst with inner serous fluid. The indolent behavior of tumor suggests that patients can have better prognosis and low risk for metastasis after surgery. Further studies are necessary for better understanding this rare tumor.
留言 (0)