In the last three years, the SARS-CoV-2 pandemic has prompted numerous studies investigating the neuropsychiatric and systemic consequences of infection with this virus. Evidence indicates that the risk of developing anxiety disorders (AD), depression, or substance abuse disorders is higher in patients after COVID-19 compared to those hospitalized for other infections or reasons (Xie et al., 2022). The emergence of such an aggressive pathogen and its associated complications, including mental health issues, has revitalized discussions on the etiology of mental disorders, providing an opportunity to refine existing knowledge. However, SARS-CoV-2 is not the only virus that can potentially influence the development of neuropsychiatric disorders, and this concept is relatively old. Emil Kraepelin advocated the theory of autoinfection and focal infections as etiological factors for dementia praecox, now called schizophrenia (SCZ) (Noll, 2004). In 1893, after the Asian flu pandemic, cases of post-influenza delirium were documented, distinguished from febrile delirium by their persistence after the fever subsided. Some cases also exhibited ‘delirium’ prior to the rise in temperature. Additionally, psychosis associated with influenza was noted to be significantly more frequent compared to other febrile illnesses of similar severity (Althaus, 1893). After the Spanish flu epidemic, Karl Menninger described numerous cases of ‘dementia praecox’ occurring during acute infection. Some patients experienced symptom resolution, while others exhibited symptoms persisting for up to five years post-infection (Influenza and schizophrenia, 1994). Menninger’s theory of ‘reversible dementia praecox’ proposed that dementia praecox was a somatopsychosis—a mental manifestation of encephalitis, with reversibility dependent on the inflammation’s duration and severity (Menninger, 1922). Contrastingly, some later studies negated the association between viral infections and mental disorders. Mid-20th century research into post-influenza depression and the correlation between other mental illnesses and antibody titers against various viruses found no significant relationships (Pokorny et al., 1973; Sinanan and Hillary, 1981; King et al., 1985). Modern researchers investigating the impact of infections on the development of mental disorders consider potential etiopathogenetic factors including influenza, HSV-1, HSV-2, EBV, CMV, measles, rubella (RV), mumps, polio, Coxsackie virus B4, HIV, BoDV, T. gondii, and Treponema pallidum (Yolken and Torrey, 2008). Questions have also arisen regarding whether, in considering the impact of viruses on mental illness development, we should exclude viruses with relatively recent human infection histories, such as HIV, or those with declining infection rates, such as RV, mumps, and measles. The issue of seasonality in mental illnesses also warrants consideration, necessitating scrutiny of viruses exhibiting seasonal patterns, such as influenza. However, a recent meta-analysis did not substantiate the proposed influence of birth season on the risk of developing BD or SCZ, as hypothesized 25 years ago (Torrey et al., 1997; Saleh et al., 2021). Additionally, urban birth and upbringing have been shown to increase SCZ risk, which some authors suggest may be related to accelerated and facilitated pathogen transmission due to high population density and a large number of interpersonal contacts (Eaton et al., 2000; Pedersen and Mortensen, 2001; Harrison et al., 2003). Exploration of viral impacts on mental illnesses is grounded in immunology, pathophysiology, and biochemistry. Numerous mechanisms exist through which viruses could influence the brain and mental disorder development. Viruses may enter the body via various routes, establish systemic or localized infections, penetrate the brain via the bloodstream or neuronal routes, and traverse or breach the blood-brain barrier. They may cause acute, persistent, or latent infections—potentially lifelong and reactivating. Viral replication can be cytopathic, destroying CNS cells, or non-cytopathic (Koyuncu et al., 2013; Nath and Johnson, 2022). Direct viral impacts on the brain include cellular damage during replication or interaction of viral proteins with brain cell receptors, while indirect impacts involve cytokine and chemokine induction, neurotransmission alterations, increased excitotoxicity, and oxidative stress (Chhatbar and Prinz, 2021; Rocamonde et al., 2023). Viral infections within the CNS elicit robust immune responses, paralleling those observed in mental illnesses. Given the multitude of mechanisms by which viruses can affect the brain, and considering the high frequency of infections and the diversity of viruses, including those yet undiscovered, it is imperative to contemplate viral impacts on mental disorder development. The SARS-CoV-2 pandemic has underscored this relevance, reigniting discussions on virus-mental illness connections. Despite the pandemic is in retreat, its mental health repercussions may persist, particularly in generations born or in early development during its peak. Thus, it is crucial to elucidate whether viruses contribute to mental illness, and if so, whether the risk is influenced solely by systemic responses or also by the type of virus and its mechanism of action.
1.1 Objectives of the reviewThis work aims to enhance our understanding of the intersection between viral infections and mental health, ultimately aiding in better clinical assessments and preparedness for future epidemics as well as new or mutated viruses. We also aim to expand and enrich the existing knowledge concerning the etiology of mental disorders and to identify possible future research directions. This goal encompasses:
• presenting the inflammatory basis of mental disorders and current knowledge on cytokine disturbances in mental illnesses
• creating cytokine profiles of mental disorders
• concisely and critically presenting the current knowledge on the connections between specific, common viral infections and the occurrence of mental disorders
• creating cytokine profiles of viral infections and comparing them to the cytokine profiles of mental disorders
• identifying other possible mechanisms of viral impact on the CNS and other factors increasing the risk of developing mental disorders
• critically discussing the gathered information, interpreting it, and drawing conclusions from it
1.2 Methods of this review articleSince this work is a narrative review, we did not establish strict inclusion criteria for the articles. We selected articles that met just several key criteria: they were meta-analyses, original research papers, or large systematic reviews, and their topics were related to mental disorders associated with viral diseases (or negated such an association), cytokine disturbances in mental disorders and viral diseases, and other mechanisms of viral impact on the CNS. Additionally, we included relevant supporting literature. Exclusion criteria included articles not available in English due to concerns about errors from incorrect translation and incomplete understanding of the text, as well as to ensure that most readers could read the source literature in its original form if desired. We also excluded studies focusing on the occurrence of viral diseases in populations with already diagnosed mental disorders, as this data was not directly relevant to assessing the first-time occurrence of a mental illness and its association with prior viral infection. A literature search was conducted in PubMed, Scopus, and Google Scholar databases. The search strategy consisted of the following keywords: ‘cytokine’, ‘inflammation’, ‘biomarkers’, ‘psychiatric sequel’, ‘mental disorder’, ‘psychosis’, ‘mental health’, ‘COVID-19’. ‘SARS-CoV-2’, ‘Influenza’, ‘HIV’, ‘HSV’, ‘CMV’, ‘EBV’, ‘BoDV’, ‘ Rubella’ as well as variations and combinations of these terms. The strategy for creating cytokine profiles is described later in the work, in the footnotes to the relevant tables.
2 InflammationTo elucidate the role of viruses in the etiopathogenesis of mental disorders, it is essential to explain the rationale behind this hypothesis. The proposed mechanism by which viruses could induce psychiatric disorders is multifaceted, with inflammation being the most well-studied and likely pathway. Extensive research has demonstrated the impact of inflammation on major depressive disorder (MDD) (Gałecki and Talarowska, 2018; Lee and Giuliani, 2019; Beurel et al., 2020; Roohi et al., 2021), BD (Brietzke et al., 2009; Berk et al., 2011; Modabbernia et al., 2013), SCZ (Müller et al., 2015; Müller, 2018; Mongan et al., 2020), and to a lesser extent, obsessive-compulsive disorder (OCD) (Rodríguez et al., 2017; Cosco et al., 2019; Westwell-Roper et al., 2022). Notably, in SCZ, there is considerable discussion regarding the effects of pre- or perinatal exposure to infections or generalized inflammation on immune reactivity throughout life, thereby increasing the risk of developing SCZ (Brown, 2006; Brown, 2011; Müller, 2018). Inflammation involves the production of pro- and anti-inflammatory cytokines. Proinflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-2 (IL-2), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and interferon gamma (IFN-γ), are primarily produced by Th1 lymphocytes and M1 macrophages, as well as microglial cells within the central nervous system (CNS). Conversely, anti-inflammatory cytokines, including interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-10 (IL-10), interleukin-13 (IL-13), and transforming growth factor β (TGF-β), are secreted by Th2 lymphocytes, M2 macrophages, and astroglial cells in the CNS (Berger, 2000; Raphael et al., 2015). Th1 cells are crucial in the immune response to intracellular bacteria and viruses, with M1 macrophages activating lymphocytes at inflammation sites. Th2 cells are primarily involved in humoral and allergic responses, while M2 macrophages facilitate wound healing, tissue repair, and remodeling post-inflammation (Romagnani, 1999; Raphael et al., 2015). Pro-inflammatory cytokines render the brain more susceptible to stress and disrupt its functions by adversely affecting neuroplasticity, hippocampal neurogenesis, synaptogenesis, and synaptic plasticity. These cytokines also induce cellular apoptosis, promote free radical production, and act as neuromodulators, disrupting neuroendocrine and neurochemical pathways (Fuchs et al., 2004; Khairova et al., 2009; Cattaneo et al., 2015). Although cytokines can be produced and released within the CNS itself (Ja S et al., 2012; Choi et al., 2014; Marisa et al., 2018), peripheral cytokines also reach and influence the CNS, increasing blood-brain barrier (BBB) permeability, affecting astrocytes and microglia, which in turn produce more cytokines, perpetuating CNS inflammation (Miller et al., 2009; Miller et al., 2013; Cheng et al., 2018; Sun et al., 2022). The condition characterized by the most severe cytokine disturbances is a cytokine storm, a systemic pathological immune response in which a positive feedback loop occurs between cytokines and immune cells, leading to an uncontrolled elevation of cytokine levels. A peripheral cytokine storm does not necessarily correlate with a similar phenomenon in the brain. During systemic inflammation, increased BBB permeability allows more cytokines to enter the CNS. If the brain’s anti-inflammatory systems promptly balance this influx, an inflammatory cascade and cytokine storm may be averted. Cytokine abnormalities in the brain can lead to various forms of neuroinflammation. Acute and severe neuroinflammation manifests as encephalitis, but milder, chronic neuroinflammation can occur, termed mild neuroinflammation (MNI) or mild encephalitis (ME). These milder forms may primarily cause psychiatric symptoms, with neurological symptoms emerging as the condition progresses. Another complication is inflammatory encephalopathy, characterized by lasting brain dysfunction due to severe systemic inflammation, without direct brain inflammation. Extreme cytokine disorders, such as cytokine storms within the brain, typically lead to severe neuroinflammation or encephalitis, although this is not always the case (Bechter, 2019; Tetsuka et al., 2021; Vengalil et al., 2023).
The complexity of biochemical alterations during inflammation poses significant challenges in identifying specific patterns that increase the risk of mental disorders. Consequently, this study concentrates on cytokine changes, which can be systematically analyzed. Each cytokine exerts multidirectional effects and interacts with other cytokines, inflammatory factors, chemokines, and oxidative stress factors, resulting in a specific cytokine “mixture” that can differentially impact the central nervous system (CNS). This has led to efforts to establish cytokine profiles for specific mental disorders. Table 1 provides an overview of the direction of cytokine and chemokine concentration changes in patients with specific mental disorders, based on a review of the literature. It is noticeable that these changes are not consistent in every case. These differences may be due to factors such as disease progression, comorbidities, and treatment methods. However, in all cases, it is evident that cytokine disturbances are present to a greater or lesser extent, and that there is most often an increase in the concentration of pro-inflammatory cytokines—particularly IL-6 and TNF-alpha. This finding, though nonspecific, supports the inflammatory theory of mental disorders. Despite the fact that the cytokine disturbances described in the literature are not entirely consistent with each other, it is possible to identify which cytokines most frequently undergo changes in their concentration and the direction of these changes, i.e., increase or decrease. The changes determined in this way create a ‘cytokine profile’ that occurs in a given disorder. In our work, we have attempted to create such profiles, and the results are illustrated in Table 2. Constructing these profiles is crucial and holds potential future value, as understanding the physical diseases or infections that elicit cytokine secretion patterns akin to those observed in mental disorders may facilitate the prediction of psychiatric disorder development in patients.
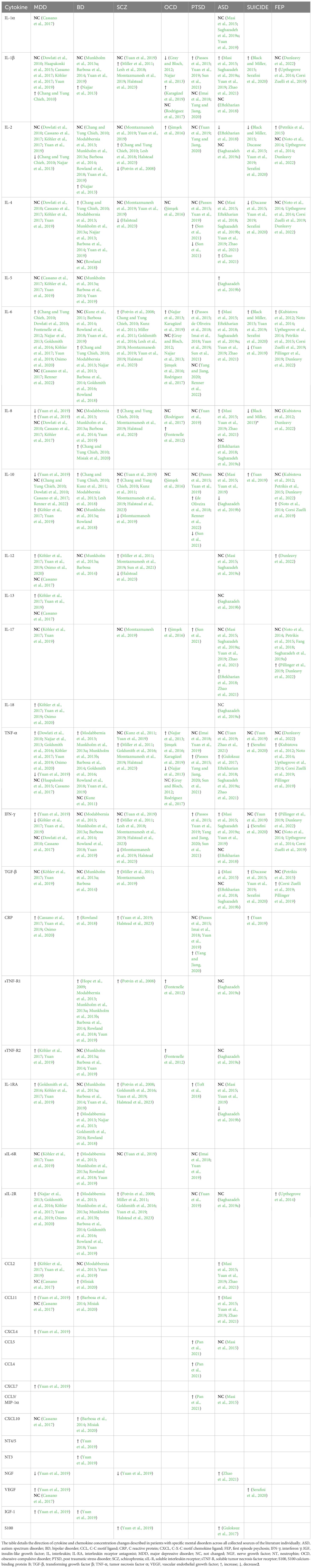
Table 1. Cytokine changes in mental disorders.
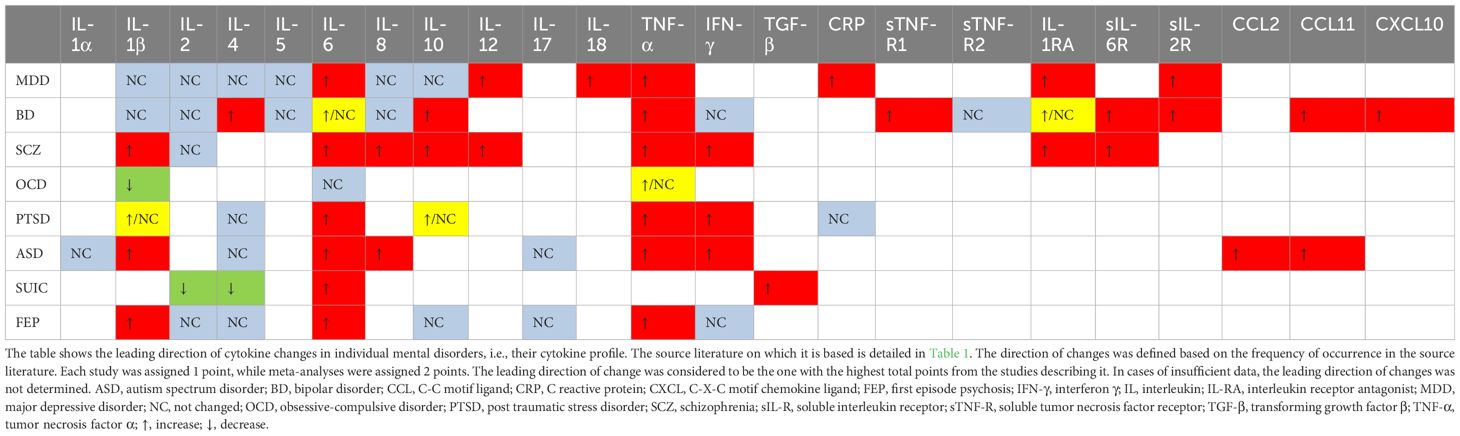
Table 2. Cytokine profiles in mental disorders.
3 Viruses involved in neuropsychiatric illness3.1 Influenza virusInfluenza viruses, classified under the Orthomyxoviridae family, are single-stranded RNA viruses comprising three types: A, B, and C. It replicates in a cytolytic cycle, causing apoptosis in many types of cells. In humans, it usually causes symptoms such as fever, chills, joint and muscle pain, weakness, cough, nasal congestion, and light sensitivity. It can also lead to more serious complications, such as pneumonia or bronchitis, and sometimes even death. These more serious complications are mainly associated with influenza A and B. Of these, influenza A virus exhibits the highest genetic variability, with subtypes distinguished based on their glycoproteins, hemagglutinins (H) and neuraminidases (N). There are 18 hemagglutinin and 11 neuraminidase subtypes. The most prevalent subtypes of influenza A virus affecting humans are H1N1 and H3N2. Notably, influenza A was responsible for the 1918 “Spanish flu” pandemic and the 1957 “Asian flu” epidemic (Bouvier and Palese, 2008; Centers for Disease Control and Prevention, 2023). A limited number of influenza virus strains, primarily influenza A virus, are neurotropic, meaning they can infect and replicate in nervous system cells. A few autopsy studies have documented the presence of influenza virus and its antigens in the cerebrospinal fluid and brain tissue of patients who succumbed to severe influenza. The regions of the brain where the virus has been found include the mesencephalon, thalamus, cortex, and hippocampus (Franková et al., 1977; Ishigami et al., 2004; Hosseini et al., 2018). Non-neurotropic subtypes, like H1N1 and H3N2, can also cause CNS complications, likely through indirect activation of microglial cells by inflammatory factors entering the CNS from the periphery (Barbosa-Silva et al., 2018).
3.1.1 Psychiatric complications of influenzaThe psychiatric complications of influenza remain a topic of ongoing research and debate. Early 20th-century studies and more recent research from the early 21st century suggest an association between maternal influenza infection during the first trimester of pregnancy and a sevenfold increase in the risk of SCZ in offspring compared to the general population. However, these findings necessitate further validation in larger cohorts (Brown et al., 2004). The same researcher observed a twofold increased risk of SCZ in adult offspring of mothers who experienced upper respiratory tract infections during the second trimester, an association not found for infections in the first and third trimesters (Brown et al., 2000b). Conversely, no increased SCZ risk was reported in offspring of mothers infected during the 1957 influenza epidemic, although this study relied on self-reported data and did not measure antibody levels (Selten et al., 2010). Influenza infection during pregnancy has also been implicated in a nearly fourfold increased risk of BD in offspring (Parboosing et al., 2013). Moreover, maternal influenza infection has been linked to a fivefold higher risk of developing BD with psychotic features in offspring, without a corresponding increase in BD without psychotic features (Canetta et al., 2014). Patients with a history of mood disorders are significantly more likely to be seropositive for influenza A or B. Notably, seropositivity for influenza B virus is associated with a more than 2.5-fold increased risk of suicide attempts and psychotic symptoms during affective episodes, a relationship not observed for influenza A virus (Okusaga et al., 2011). Contrarily, a 1981 study involving 400 participants found no differences in influenza A or B antibody levels between patients with and without depression (Sinanan and Hillary, 1981). The impact of influenza infection on psychiatric disease risk remains inconclusive. Research focusing on maternal influenza infection during pregnancy suggests a potential link to SCZ, though the virus rarely crosses the placenta (Irving et al., 2000).
3.1.2 Cytokine profile of influenza and other mechanisms of its influence on the CNSThe virus’s effects on the fetal CNS are likely indirect, possibly mediated by maternal IgG antibodies crossing the placenta and cross-reacting with fetal brain antigens via molecular mimicry (Brown et al., 2004). Another potential mechanism involves elevated maternal cytokine levels, their transplacental passage, and subsequent impacts on fetal brain development (Gilmore and Jarskog, 1997; Brown et al., 2004; Ratnayake et al., 2013). However, studies on cytokine transfer across the placenta are mixed. Some indicate limited transfer for cytokines such as IL-1, IL-4, IL-8, IL-10, IL-13, and TNF-α (Reisenberger et al., 1996; Zaretsky et al., 2004; Aaltonen et al., 2005; Lim and Kobzik, 2009), whereas IL-6 and IL-2 have been shown to cross the placenta (Zaretsky et al., 2004; Dahlgren et al., 2006; Ponzio et al., 2007). Additionally, the placenta itself may produce cytokines in response to maternal infection or elevated cytokine levels (Shimoya et al., 1999; Thiex et al., 2009). Placental immune cells continuously produce cytokines like IL-11, IL-17A, IL-17F, TGF-β, and VEGF, and in response to endotoxin or polyinosinic:polycytidylic acid (poly I:C), they produce IL-1, IL-6, IL-8, IL-10, and TNF-α (Urakubo et al., 2001; Gilmore et al., 2005; Ashdown et al., 2006; Pavlov et al., 2020). Influenza may also influence the development of affective disorders in adults through cytokines released during the immune response, such as interferons, TNF-α, IL-1β, and IL-6, which activate the kynurenine pathway, producing neurotoxic metabolites. Elevated levels of 3-hydroxykynurenine, quinolinic acid, kynurenine, and kynurenic acid are associated with depressive disorders and mania (Correia and Vale, 2022; Karimi et al., 2022; Lorkiewicz and Waszkiewicz, 2022). A rare complication of influenza in adults is influenza-associated encephalitis (IAE), which can be acute, subacute, or late-onset. Psychiatric complications of IAE, similar to other causes of encephalitis, may include AD, insomnia, mood disorders, and psychotic symptoms (Ishigami et al., 2004; Meijer et al., 2016; Butler et al., 2024).
The cytokine profile commonly described in influenza studies includes increased levels of C-C motif ligand 2 (CCL2), IL-8, C-X-C motif ligand 10 (CXCL10), IFN-α, -β, -γ, IL-1β, IL-6, TNF-α, and IL-18 (Chan et al., 2005; Liu et al., 2016; Betakova et al., 2017; Fiore-Gartland et al., 2017; Guo X zhi and Thomas, 2017; Gu et al., 2019; Turianová et al., 2019; Gu et al., 2021). Studies in mice have shown that higher, lethal doses of the virus, unlike lower doses, induce IL-4, IL-7, IL-10, IL-11, IL-12p40, IL-13, IL-15, and shift Th1 cell polarization towards Th2, with increased expression of M2 macrophages (Turianová et al., 2019). The most common cytokine changes in viral infections mentioned above, i.e., the cytokine profile, are visualized in Table 3. From this table, it can be observed that the cytokine profile during mild to moderate influenza infection closely resembles those found in SCZ, autism spectrum disorder (ASD), and first-episode psychosis (FEP). Severe infections shift the profile more towards SCZ and BD. While ASD is not typically associated with influenza in scientific research, SCZ and BD are, particularly in offspring of mothers infected during pregnancy. Although the passage of cytokines through the placenta is unlikely, they may induce changes in the hypothalamic-pituitary-adrenal (HPA) axis (Dunn et al., 1989; Dunn, 2000), metabolic pathways (tryptophan) (Holtze et al., 2008; Campbell et al., 2014) and immunological responses, potentially increasing the risk of these mental disorders.
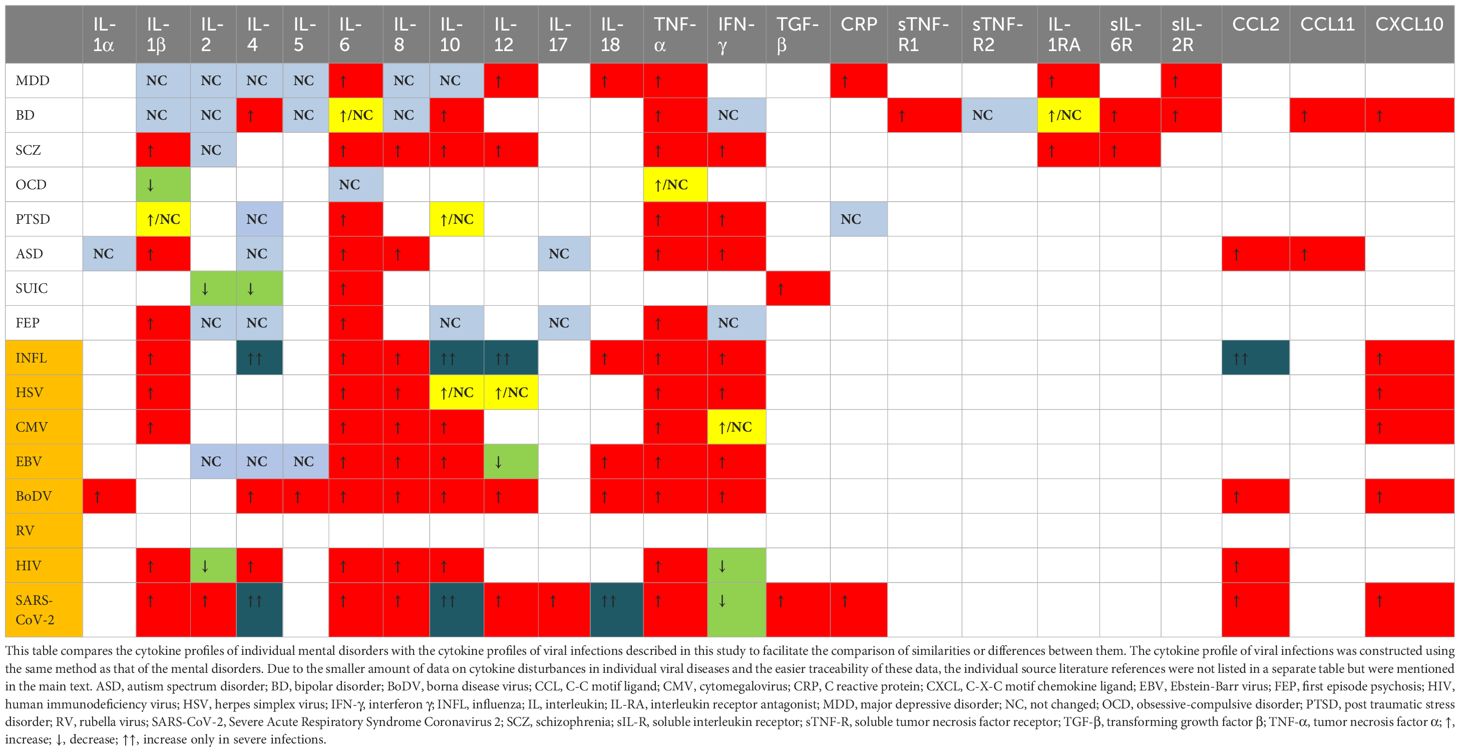
Table 3. Cytokine profiles in mental disorders and viral infections.
3.1.3 ConclusionsDespite historical reports suggesting a link between influenza infection and the development of mental illnesses in adults, more recent research does not clearly support this association if the infection occurred during adulthood. However, prenatal exposure to influenza may influence the development of psychiatric conditions such as SCZ and BD. The cytokine profile observed during infection is broader than those associated with these specific diseases.
3.2 Herpes simplex virusHSV, a member of the Herpesviridae family of double-stranded DNA viruses, is classified into two subtypes: HSV-1 and HSV-2. According to the World Health Organization (WHO), over 3.7 billion individuals under the age of 50 are infected with HSV-1, and nearly 500 million individuals aged 15-49 are infected with HSV-2 (Herpes simplex virus, 2023). While HSV-1 and HSV-2 are primarily responsible for labial and genital herpes, they are also implicated in encephalitis, meningitis, and neonatal HSV infection (Xu et al., 2019). Postmortem studies have detected HSV DNA in various brain regions, particularly in the frontal and temporal cortex (Olsson et al., 2016; Marcocci et al., 2020). The virus typically infects via oral-labial or sexual routes and replicates primarily in a cytolytic manner. Following the acute phase, HSV establishes latent infection in the dorsal root ganglia, with potential reactivation triggered by factors such as excessive sunlight exposure, psychological and physical stress, menstruation, fever, and surgical procedures (Steiner et al., 2007).
3.2.1 Psychiatric complications of HSVA 2009 study revealed a significantly higher prevalence of IgM anti-HSV antibodies in children with ASD compared to healthy children (65% vs. 17.5%). The levels of other antibodies, such as anti-EBV, -CMV, -measles, or -RV, remained unchanged across groups. Additionally, 96% of children with ASD and IgM anti-HSV exhibited IgG antibodies against brain tissue (including anti -amygdala, -caudate, -cerebellum, -medulla, -hippocampus, -commissure magnum, and -cortex) in their serum, compared to only 17% of children with ASD without IgM anti-HSV (Mora et al., 2009). The presence and titers of these antibodies correlated directly with ASD severity, suggesting a potential influence of HSV on ASD severity, independent of causation. Furthermore, elevated titers of anti-HSV-2 IgG antibodies in mid-pregnancy maternal plasma have been associated with an increased risk of ASD in male offspring. This association was not observed for HSV-1, CMV, or RV (Mahic et al., 2017). However, the methodology and reporting of these findings have been questioned (Magaret and Wald, 2017). Other studies have not corroborated higher seropositivity for HSV-1 or HSV-2 in children with ASD compared to healthy controls, irrespective of congenital or later-acquired infection (Gentile et al., 2014; Zappulo et al., 2018). HSV’s impact on depression has also been documented. HSV-2 exposure, confirmed by anti-HSV-2 IgG antibodies, was associated with a twofold increased risk of depression, a finding not observed for HSV-1 (Simanek et al., 2014; Gale et al., 2018). In contrast, patients with BD did not exhibit increased seropositivity for HSV-1 or HSV-2 (Tedla et al., 2011; Snijders et al., 2019). Interestingly, studies have demonstrated a strong association between HSV and the development of SCZ. Elevated levels of anti-HSV-2 IgG and IgM in pregnant mothers are significantly associated with an increased risk of psychotic disorders, including SCZ, in their offspring, whereas this association was not observed for HSV-1 (Buka et al., 2001; Buka et al., 2008; Mortensen et al., 2010; Arias et al., 2012). Herpesviruses may also be linked to cognitive disorders and the development of late-onset Alzheimer’s disease. HSV-1 infection has been associated with amnesia, semantic memory impairment, visual agnosia, and impaired executive function, primarily following HSV-induced encephalitis (Lin et al., 2002; Damiano et al., 2022).
3.2.2 Cytokine profile of HSV and other mechanisms of its influence on the CNSThe data suggest that HSV’s potential impact is most pronounced concerning SCZ and, to a lesser extent, ASD and depression. The risk appears to be subtype-dependent, with HSV-2 being more significant than HSV-1, despite the latter’s higher detection rate in the brain. The exact mechanism by which HSV influences these disorders remains unclear, though its neurotropic properties and detection of its DNA in the CNS of asymptomatic patients are noteworthy (Lin et al., 2002; Marcocci et al., 2020). This implies that HSV may exert effects on the CNS even in the absence of overt infection signs. Table 3 illustrates the cytokines most commonly associated with HSV infection, which include TNF-α, IL-6, IL-1β, IL-8, IFN-α, IFN-β, IFN-γ, CCL5, and CXCL10, with IL-10 and IL-12 appearing in later infection stages (Mikloska et al., 1998; Li et al., 2006; Melchjorsen et al., 2006; Stefanidou et al., 2013; Feng et al., 2021; Smith et al., 2022). This cytokine profile resembles those observed in SCZ and BD, although research on HSV-induced cytokines is limited and often fails to differentiate between HSV-1 and HSV-2, potentially leading to erroneous conclusions. If HSV is assumed to increase SCZ risk, one probable pathway involves maternal IL-8 elevation during pregnancy, previously linked to increased SCZ risk and CNS developmental anomalies associated with SCZ, combined with HSV’s direct CNS effects (Buka et al., 2001; Marcocci et al., 2020).
3.2.3 ConclusionsSome studies suggest a potential link between HSV-2 infection during pregnancy and ASD occurrence in offspring, although this remains uncertain and warrants further investigation. HSV-2 may also be associated with depression and SCZ in adults, with prenatal virus exposure elevating the risk. The cytokine profile of HSV infection partially aligns with those in SCZ and BD, although discrepancies exist regarding the associated mental illnesses. Maternal IL-8 elevation during pregnancy could be a significant factor influencing SCZ and psychosis development in offspring.
3.3 CytomegalovirusThe CMV belongs to the Herpesviridae family and primarily infects humans. In industrialized nations, 60-70% of adults may carry the virus, while in developing countries, infection rates can reach nearly 100%. Transmission occurs via contact with bodily fluids from an infected individual. Similar to HSV, replication occurs in a cytolytic cycle, and after the primary infection, the virus enters a latency phase. In immunocompetent adults, CMV rarely leads to severe complications. Most often, the infection is asymptomatic or can resemble mononucleosis, causing fever, fatigue, muscle aches, and enlarged lymph nodes. However, in immunocompromised individuals, particularly those with AIDS, it can cause severe complications, including encephalitis, as confirmed by the detection of its DNA in the autopsy examinations of the brains of deceased patients (Morgello et al., 1987; Achim et al., 1994; Nangle et al., 2018; Balegamire et al., 2022). CMV can also be transmitted congenitally. According to the Centers for Disease Control and Prevention (CDC), vertical transmission of CMV occurs in approximately 1 in 200 births in the United States and is the leading infectious cause of birth defects. Congenital CMV (cCMV) is asymptomatic in up to 95% of cases at birth but can later result in severe complications, such as deafness, delayed psychomotor development, vision loss, and epilepsy. These complications occur in 15-18% of infected children and result in mortality in 5-10% of affected newborns (Yow et al., 1988; Centers for Disease Control and Prevention, 2022). Thus, CMV-related complications are closely linked to the central nervous system (CNS) and may be associated with the development of mental disorders.
3.3.1 Psychiatric complications of CMVStudies investigating the impact of CMV on depression indicate that CMV infection alone does not increase its risk. However, among those infected, a higher anti-CMV titer is significantly correlated with an increased risk of developing depression (Phillips et al., 2008; Gale et al., 2018). Among seropositive individuals, an increase in anti-CMV antibody concentration by one unit raises the risk of depression by 26%, with those having the highest antibody levels exhibiting nearly a fourfold increased risk. The link between CMV and other affective disorders is less clear. While a large meta-analysis did not find an association between CMV seropositivity and BD, a case-control study of 495 individuals noted that BD patients had significantly higher anti-CMV IgG titers compared to healthy controls (Tedla et al., 2011). Moreover, BD patients often test seropositive for CMV and that group of patients have a higher incidence of mania with psychotic symptoms (Frye et al., 2019). Furthermore, BD patients with elevated mood display higher anti-CMV antibody titers comparing to euthymic patients, and the higher antibody titres are, the greater impulsivity is (Prossin et al., 2013; Prossin et al., 2015). Despite the pathogenetic similarities between BD and SCZ, the impact of CMV on SCZ is less definitive and requires more research. A 2012 meta-analysis found no association between CMV and SCZ, and a systematic review the same year reported no effect of maternal CMV infection on the risk of developing SCZ in offspring (Arias et al., 2012; Khandaker et al., 2013).
3.3.2 Cytokine profile of CMV and other mechanisms of its influence on the CNSCMV has the greatest impact on increasing the risk of affective disorders. This virus is neurotropic and appears to have an affinity for the limbic system, which dysfunction plays a major role in affective disorders. In cases of intrauterine infection, the virus seems to favor the ventricular regions of the brain. Changes are also found in the hippocampus and olfactory bulb (van den Pol et al., 1999; Krstanović et al., 2021). The immune response to CMV is also significant. Reactivation of CMV can lead to the production of pro-inflammatory cytokines and subsequent blocking of type I and II interferons, which are essential for inflammation induction and defense against viruses (Abate et al., 2004; Marshall and Geballe, 2009). CMV seropositivity is associated with an inversion of the CD4:CD8 cell ratio due to the accumulation of low-diversity CD8 clones (Pawelec et al., 2009). The transfer of maternal CD8+ T cells through the placenta contributes to perinatal brain damage due to intrauterine infection, so the increase in CD8+ cells associated with CMV infection may be crucial for the neurological and psychiatric complications of this infection (Lei et al., 2018). CMV also induces a strong immune response from glial cells, resulting in the production and release of TNF-α, IL-6, CCL3, CCL5, and CXCL10 from microglia, and IL-8 and MCP-1 from astrocytes (Lokensgard et al., 2002). Chronic CMV infection and viral reactivation can elicit a sustained immune response, evidenced by elevated titers of anti-CMV antibodies. This persistent immune activation, in conjunction with the virus’s affinity for limbic structures, particularly the hippocampus, and localized cytokine secretion, may contribute to the pathological damage observed in these neural regions. The most frequently described peripheral cytokines produced during CMV infection are IL-1β, IL-6, IL-8, IL-10, TNF-α, CXCL10, and CCL5 (Humar et al., 1999; Lokensgard et al., 2002; Compton et al., 2003; Zhang et al., 2008; Clement and Humphreys, 2019; Chinta et al., 2020; Bourgon et al., 2022). This cytokine profile aligns most closely with those of SCZ and BD (as shown in Table 3), although the primary mechanism of CMV’s action may be more related to the previously mentioned cytokines secreted during glial activation.
3.3.3 ConclusionsThe relationship between CMV and depression appears promising, with some evidence suggesting a link to BD. The cytokine profile of CMV infection aligns with BD, but not depression. Further research is needed to clarify CMV’s role in affective disorders and to identify any non-inflammatory mechanisms contributing to these diseases.
3.4 Ebstein-Barr virusEBV, a representative of the Herpesviridae family, targets B lymphocytes and epithelial cells, replicating within them in a lytic cycle before establishing latent infections. The most common disease caused by EBV is infectious mononucleosis, which is characterized by symptoms such as fever, sore throat, swollen lymph nodes, fatigue, muscle and joint pain, and sometimes an enlarged spleen and a skin rash that occurs after the administration of ampicillin. However, the virus is also associated with more severe conditions, such as Hodgkin’s lymphoma and Burkitt’s lymphoma. It is estimated that approximately 90% of the human population is infected with this virus (Gequelin et al., 2011; Shi et al., 2021). During the transition from the latent to the lytic phase of replication, the virus can penetrate the CNS alongside infected lymphocytes, damage the blood-brain barrier (BBB), thereby increasing its permeability, and replicate within the CNS (Jha et al., 2015; Lupia et al., 2020). EBV also has the ability to directly infect nerve cells, which some authors link to neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (MS) (Jha et al., 2015; Lee et al., 2021; Zhang et al., 2022). In the context of MS, autopsy examinations of 101 patients revealed the presence of EBV in the brain in up to 90% of cases; however, these changes were not associated with any specific region of the brain (Hassani et al., 2018).
3.4.1 Psychiatric complications of EBVDespite the potential for EBV to influence the CNS, there is no conclusive evidence linking it to mental illnesses. A 2012 meta-analysis did not confirm an association between EBV and SCZ, but a 2019 study involving over 400 SCZ patients found that they had higher levels of antibodies to some, but not all, EBV proteins compared to a healthy control group (Arias et al., 2012; Dickerson et al., 2019). Studies on the association of EBV with depression are also inconclusive. A meta-analysis on the relationship between infectious agents and depression showed that EBV-positive patients are almost twice as likely to develop depression (Wang et al., 2014). Other studies failed to confirm these results, but the authors of the aforementioned meta-analysis point out that only a sufficiently large sample can reveal a relationship between EBV and depression (Amsterdam et al., 1986; Conejero-Goldberg et al., 2003). Interestingly, pregnant women with symptoms of depression are significantly more likely to show immunological markers of EBV reactivation compared to healthy pregnant women. In this context, the question arises as to whether the reactivation of EBV precipitates depression during pregnancy, or conversely, whether depression and the concomitant immunosuppression facilitate EBV reactivation (Haeri et al., 2011; Johnson et al., 2011). Evidence for the latter may be the fact that depressive symptoms in pregnant women at 33-34 weeks of gestation were associated with a three-fold increase in EBV reactivation, as assessed one week before delivery (Zhu et al., 2013). BD has also been investigated for an association with EBV, including cerebrospinal fluid testing for anti-EBV antibodies, but no association has been observed (Stich et al., 2015; Barichello et al., 2016; Snijders et al., 2019). Therefore, the relationship between BD and EBV seems unlikely at this point.
3.4.2 Cytokine profile of EBV and other mechanisms of its influence on the CNSThe potential mechanism by which EBV might cause MDD-like disorders involves its ability to migrate to the CNS with infected B lymphocytes. The virus can replicate in the CNS and infect neuronal cells, leading to chronic inflammation and cytokine production within CNS structures, contributing to the development of mental disorders. Moreover, EBV attacks immune system, causing its disorders and dysregulation. It sensitizes the infected individual to other infections, activates the stress axis, and disrupts the cytokine balance, which collectively could lead to the development of MDD. Cytokine imbalances most commonly described in EBV infection include increased concentrations of TNF-α, IL-6, IL-8, IL-10, IFN-γ, CCL3, CCL4, and sometimes IL-18 and IL-1β. Some authors also note a lack of increase or a decrease in the concentration of IL-2, IL-5, IL-12, and TGF-β (Mosialos, 2001; Attarbaschi et al., 2003; Miyauchi et al., 2011; Wada et al., 2013; Coleman et al., 2015; Draborg et al., 2016; cui et al., 2017; Kianfar et al., 2022). The cytokine profile of EBV infection aligns most closely with the cytokine profile of SCZ, but studies of the relationship between these two diseases do not show any correlation (see Table 3).
3.4.3 ConclusionsAlthough EBV is a widely prevalent virus with potentially serious health implications, its direct association with most mental disorders remains inconclusive, except for a possible link with depression that requires further investigation.
3.5 Borna disease virusBoDV, a single-stranded RNA virus belonging to the Bornaviridae family, primarily infects horses, sheep, and cattle. This neurotropic virus induces Bornean disease in animals, colloquially referred to as sad horse disease. In animals, BoDV can cause fatal encephalitis and symptoms such as ataxia, anxiety, hyperactivity, aggression, and depressive-like behaviors (Carbone, 2001). The primary route of infection is through the nasal and olfactory epithelium, where the virus targets nerve cells and travels towards the central nervous system (CNS). BoDV replicates in a non-cytolytic cycle and does not exhibit classical cytopathic properties, allowing it to evade detection and establish latent infection in the CNS. In adults and adolescents, although rare, BoDV can cause severe encephalitis with high mortality. Several autopsy studies have detected BoDV RNA and antigens in the brains of infected patients (De La Torre et al., 1996; Klaus et al., 2018; Riccò et al., 2023).
3.5.1 Psychiatric complications of BoDVUntil the mid-1980s, it was believed that BoDV only infected animals. However, in 1985, antibodies against BoDV were detected in 16 out of 979 examined psychiatric patients (Rott et al., 1985). Subsequent studies revealed the presence of anti-BoDV antibodies in 5% of psychiatric patients compared to 3% of surgical patients (Bechter, 2013). A 2014 meta-analysis, encompassing 15 studies on BoDV and depression, indicated that individuals with depression are 3.25 times more likely to be infected with BoDV (Wang et al., 2014). Additionally, a case-control study in Iranian patients with affective disorders found anti-BoDV antibodies in 29.5% of controls and 40.04% of patients, with the highest seropositivity observed in patients with BD at 45.03% (Mazaheri-Tehrani et al., 2014). Recent research also demonstrated that antiretroviral therapy with amantadine significantly alleviated depressive symptoms and reduced suicidal behavior in BoDV-seropositive patients (Dietrich et al., 2020). According to a 2012 meta-analysis, there is a notable association between BoDV infection and the incidence of SCZ (Arias et al., 2012). However, the retrospective nature of most studies complicates the assessment of whether BoDV infection genuinely increases the risk of developing mental disorders, as the infection may precede or coincide with the onset of these disorders.
3.5.2 Cytokine profile of BoDV and other mechanisms of its influence on the CNSThe neurotropic nature of BoDV and the symptomatic manifestations in animals suggest a potential role for BoDV in the pathogenesis of human mental disorders. BoDV exhibits a high affinity for the limbic system, potentially damaging its structures, which is characteristic of affective disorders (Gonzalez–Dunia et al., 1997; Rubin et al., 1999; Ovanesov et al., 2008). Infection during the developmental stages of the CNS may lead to neuroanatomical damage and impairments in neuroplasticity and neural conduction (Carbone, 2001). Neuronal infection by BoDV induces significant increases in DNA double-strand breaks (DSBs), adversely affecting neurotransmission regulation and cognitive functions. Infected cells exhibit reduced expression of surface NMDAR receptors and damage to the GluN2A subunit, resulting in disrupted glutamatergic transmission and decreased spontaneous electrical activity in neuronal networks (Marty et al., 2021). Glutamatergic pathway disturbances are critical in the pathophysiology of SCZ, MDD, and BD. Furthermore, cytokines such as IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-13, IL-18, TNF-α, and IL-1α are expressed in BoDV-infected brains, with their concentrations correlating with neurological symptom severity. BoDV also induces IFN-γ production in astrocytes and microglia and the secretion of chemokines such as CCL-2, CCL-5, CXCL-10, and IL-8 (Rauch et al., [[NoYear]]; Gonzalez–Dunia et al., 1997). During the transition from acute to chronic infection, a shift from a Th1 to a Th2 immune response occurs, accompanied by increased IL-4 secretion (Hatalski et al., 1998). The cytokine profile associated with BoDV infection is broad and non-specific, with several changes overlapping with cytokine alterations observed in SCZ and MDD, though some do not align with the cytokine profiles of these disorders, which may be seen in Table 3.
3.5.3 ConclusionsThere is evidence suggesting that BoDV may play a role in the pathogenesis of certain mental disorders. Given the current state of knowledge, its association with affective disorders appears most likely, which aligns with the symptoms it causes in animals. However, the cytokine profile of the infection is quite non-specific, although its mechanism of action in the brain would support the occurrence of such diseases. Further research is necessary to definitively confirm these associations.
3.6 Rubella virusThe RV is a single-stranded RNA virus belonging to the Matonaviridae family, with humans as its sole host. Transmission occurs via respiratory droplets and transplacental routes. Regarding its replication, RV is typically described as a non-cytolytic virus, but it is cytopathic, inducing apoptosis in cells or inhibiting cell division. Postnatal infections are predominantly asymptomatic or result in a mild clinical course, presenting with symptoms such as rash, fever, swollen lymph nodes, headache, sore throat, joint and muscle pain, and less commonly, conjunctivitis. However, prenatal infections can lead to congenital rubella syndrome (CRS), characterized by significant anomalies including the classical CRS triad of cataracts, cardiac defects, and sensorineural deafness. There are limited post-mortem studies on RV; however, several autopsy reports of fatal CRS cases have identified RV antigens in brain tissues (Lazar et al., 2016). Infection may also result in stillbirth or spontaneous miscarriage, with the highest risk of complications occurring during the first trimester (Parkman, 1996; Popova et al., 2022).
3.6.1 Psychiatric complications of RVHistorical data from 1971 revealed that 125 out of 243 children with CRS exhibited mental disorders, predominantly intellectual disability. Notably, 10 children were diagnosed with ASD, and 8 exhibited partial autistic symptoms, indicating a 200 times higher incidence compared to the general population. This led to the hypothesis that ASD may have organic etiologies, including RV infection (Chess, 1971; Chess, 1977). A 2017 study assessing anti-RV IgG levels in the mid-pregnancy blood samples of 442 mothers of children with ASD found no significant association between antibody levels and the occurrence of ASD in their offspring (Mahic et al., 2017). Mathematical modeling has estimated that RV vaccination prevented approximately 16,000 CRS cases between 2001 and 2010, which corresponds to the prevention of around 1,228 ASD cases (Berger et al., 2011). In developed countries, RV vaccination is widespread; however, in certain regions, vaccination is not yet standardized, with RV affecting about 5% of pregnant women globally. The RV may also contribute to other mental disorders beyond ASD. Intrauterine RV exposure, particularly during the first trimester, is associated with a five-fold increased risk of non-affective psychosis in adulthood. Additionally, individuals prenatally exposed to RV have a higher likelihood of developing SCZ spectrum disorders later in life (Brown et al., 2000a; Brown et al., 2001). Reports of psychiatric symptoms linked to adult RV infections are scarce. Nevertheless, CRS can lead to the development of panencephalitis in later life, even adulthood, potentially exacerbating neurological deficits and resulting in epileptic seizures, intellectual decline, ataxia, and spasticity (Townsend et al., 1975).
3.6.2 Cytokine profile of RV and other mechanisms of its influence on the CNSThe mechanism through which RV may contribute to the development of these disorders involves its ability to cross the placenta and directly affect fetal brain tissue. The virus primarily targets microglial cells, which become susceptible to infection due to factors released by other brain cells. Microglial cells cannot be infected in the absence of those factors. Infected microglia secrete interferon, inducing a response from other cells and leading to chronic inflammation that disrupts CNS development (Popova et al., 2022). In the developing brain, this inflammation causes circulatory disturbances and subsequent ischemia, resulting in damage. The RV also inhibits mitosis, leading to reduced brain growth, hypocellularity, and delayed myelination due to impaired oligodendrocyte replication (Brown et al., 2001). While comprehensive studies on RV-related cytokine disturbances are limited, which makes the analysis of the cytokine profile impossible (which is the reason why the cytokine profile of RV infection is missing in Table 3), it is known that the primary cellular response involves interferon production. RV infection induces the secretion of type I and III interferons (e.g., IFN-α, IFN-β, IFN-λ) (Schilling et al., 2021). Overproduction of IFN-α/β in the brain leads to dendritic loss, reduced neurogenesis, decreased neurotrophic signaling, and impaired glutamatergic signaling in hippocampal and pyramidal neurons. These effects contribute to deficits in long-term potentiation and inhibition of angiogenesis (Viengkhou and Hofer, 2023). Chronic elevation of IFN-I levels in the CNS, referred to as cerebral interferonopathy, may also occur. Neuropsychiatric complications associated with interferon are well-documented, with patients often developing depression, fatigue, personality changes, cognitive disorders, psychosis, or mania, which typically resolve upon discontinuation of interferon therapy (Onyike et al., 2004; Cheng et al., 2009; Hoyo-Becerra et al., 2014; Pinto and Andrade, 2016; Su et al., 2019). Most of the available studies on interferon in mental disorders described the level of type II interferon, i.e., IFN-γ, as detailed in Tables 1, 2. All interferons have similar effects; however, IFN-γ most strongly activates macrophages.
3.6.3 ConclusionsThere is evidence to suggest that CRS may be associated with mental disorders, although such evidence is scarce. Mentions of psychiatric complications from RV infection in adults are also rare, making such a connection doubtful. However, the RV’s life cycle and mechanisms of action in the brain seems conducive to the occurrence of mental disorders. Therefore, further research is needed to confirm or refute the association of this infection with mental illnesses.
3.7 Human immunodeficiency virusHIV is a single-stranded RNA virus belonging to the Retroviridae family. According to World Health Organization (WHO) statistics, at the end of 2022, approximately 39 million people globally were living with HIV, and approximately 40 million have died as a result of the infection since its identification (HIV and AIDS, 2023). The virus is transmitted through human body fluids and attacks the host’s immune system, particularly cells expressing the CD4 receptor, including T helper cells, macrophages, dendritic cells, and astrocytes. It is not classically cytolytic, but it has cytopathic properties and slowly destroys immune system cells (Seitz, 2016). Until recently, HIV infection was nearly synonymous with premature death; however, the advent of effective antiretroviral therapies has significantly altered this prognosis (Eggleton and Nagalli, 2023). Untreated HIV infection leads to a gradual decrease in CD4+ cell counts, culminating in the development of acquired immunodeficiency syndrome (AIDS). The progression to AIDS is marked by numerous non-specific symptoms, diseases, and opportunistic infections, such as aseptic meningitis, encephalitis, peripheral neuropathy, herpes zoster, and HIV-associated encephalopathy (Seitz, 2016; Justiz Vaillant and Naik, 2023). HIV exhibits neuroinvasiveness by replicating in the brain’s microglia and macrophages. It is also described as neurotropic, although this property is contested by some researchers. Nevertheless, it has been shown to have a predilection for specific brain regions such as the fronto-cortical regions, basal ganglia, caudate, putamen, globus pallidus, and substantia nigra, as observed in autopsy studies (Ghorpade et al., 1998; Kumar et al., 2011). Neurological and neuropsychiatric disorders can manifest at any stage of infection, with approximately 50% of adults with AIDS experiencing neurological complications. These complications are collectively referred to as HIV-associated neurocognitive disorders (HAND), encompassing asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD). Despite the effectiveness of highly active antiretroviral therapy (HAART) in reducing the incidence of HAD, the prevalence of ANI and MND continues to increase, potentially due to prolonged survival, accumulated neurological damage, and chronic inflammation. It is noteworthy that the positive effects of HAART on the peripheral immune system may not fully extend to the CNS (Rao et al., 2014).
3.7.1 Psychiatric complications of HIVA meta-analysis conducted in 2014 indicated that 39.1% of individuals living with HIV experience depression (Uthman et al., 2014). HIV-positive individuals are 2 to 7 times more likely to meet the criteria for MDD compared to uninfected populations (Satz et al., 1997; Ciesla and Roberts, 2001). The stigma and fear associated with HIV infection, alongside the activation of the stress axis, may further exacerbate the risk of depression. Moreover, the viral mechanism, particularly the exposure of the brain to the viral Tat protein, has been shown to initiate a cytokine signaling pathway leading to depression-like sickness behavior in mouse models (Lawson et al., 2011). Additionally, certain antiretroviral medications, such as efavirenz, have been linked to an increased incidence of depressive symptoms, which may be due to efavirenz enhancing the activity of tryptophan-2-3-dioxygenase and redirecting tryptophan to the kynurenine pathway, thereby increasing the levels of its neurotoxic metabolites and decreasing serotonin levels in the brain (Munjal et al., 2017; Checa et al., 2020).
In addition to MDD, HIV-positive patients exhibit a higher prevalence of psychosis. HIV-related psychosis can be categorized as primary or secondary. Primary psychosis occurs in the absence of any HIV-related neurological disorder or acute metabolic dysfunction, directly attributed to the viral infection itself. Conversely, secondary psychosis is associated with opportunistic brain infections or metabolic encephalopathy related to respiratory, hepatic, or renal failure (
留言 (0)