Hepatocellular carcinoma (HCC), which accounts for the majority of liver cancers, is a malignancy with high recurrence and mortality rates and is projected to cause 1 million related deaths worldwide by 2030.[1] Transarterial chemoembolization (TACE) is the standard of care for patients with HCC in the intermediate stages of the barcelona clinic liver cancer (BCLC) staging system.[2,3]
Conventional TACE (cTACE) and drug-eluting bead TACE (DEB-TACE) are two different chemotherapeutic methods that use lipiodol and DEBs, respectively, as carriers for chemotherapeutic drugs.[4,5] DEB-TACE was introduced more recently than cTACE and is based on the theory that beads can release drugs locally and continuously for a long time into the tumor.[6,7] Since the clinical introduction of DEB-TACE in around 2004, many clinical studies and meta-analyses have shown that DEB-TACE has lower drug toxicity or post-embolization syndrome than cTACE.[8,9] On the other hand, the disadvantage of DEB-TACE is that it has lower local tumor control than cTACE. Further, in DEB-TACE cases, we should be aware of the occurrence of the vascular lake phenomenon (VLP), which is thought to be caused by the rupture of fragile microvessels in the tumor.[8,10]
VLPs are assumed to result from the accumulation of DEBs in blood-rich tumor regions, altering the tumor’s internal pressure gradient. This change, in turn, impacts the fragile microvasculature of the tumor, leading to rupture.[10] The occurrence rate of VLP is 12–26% and can occur at any time during the embolization of DEB-TACE and after the DEB-TACE procedure.[11,12] Once VLP has occurred, additional embolization with gelatin sponge particles is necessary to prevent subsequent tumor rupture in some cases with steadily increasing size of VLP during DEB-TACE.[10] Patients with VLP have been observed to have a better treatment response than those without VLP.[13,14] Several studies reported that the risk factors for VLP include tumor size >3 cm in diameter, small bead size, presence of an enhancing capsule, and high alpha-fetoprotein (AFP).[14,15] However, these previous reports have been conducted in patients with newly diagnosed HCC who received DEBTACE as primary treatment, and there are no reports that consider the effect of previous interventional treatments, such as the number of transarterial infusion chemotherapy (TAI) or cTACE before the DEB-TACE to our knowledge.
In clinical practice, TACE is performed repeatedly, and the choice of treatment is required during the long clinical course of HCC. Several studies showed that DEB-TACE could be a therapeutic option for advanced HCC patients with cTACE refractory and decreased liver function.[16-19] Therefore, the initial introduction of DEB-TACE is sometimes performed after multiple sessions of TAI or cTACE, and it is necessary to identify the risk factors for VLP, considering the number of previous interventional treatments. The purpose of our study was to evaluate the factors associated with the occurrence of VLP during DEB-TACE, considering the number of previous interventional treatments.
MATERIAL AND METHODS Patient selectionThe Institutional Review Board approved this retrospective study and waived the requirement for informed consent. Between November 2010 and April 2024, 50 consecutive patients underwent a total of 109 DEB-TACE procedures for the treatment of HCCs at our institution. The exclusion criteria were as follows: (a) discontinued DEB-TACE procedure due to a shock (n = 1) and (b) non-initial DEB-TACE (n = 59). Finally, 49 initial DEB-TACE procedures in 49 patients were included in the present study [Figure 1]. Gender and age at the time of the DEB-TACE procedure were obtained from the electronic medical record. The pre-treatment evaluation of background factors included laboratory data to evaluate the etiology of background chronic liver disease or cirrhosis, Child-Pugh score, AFP, and BCLC staging classification.[20]
Export to PPT
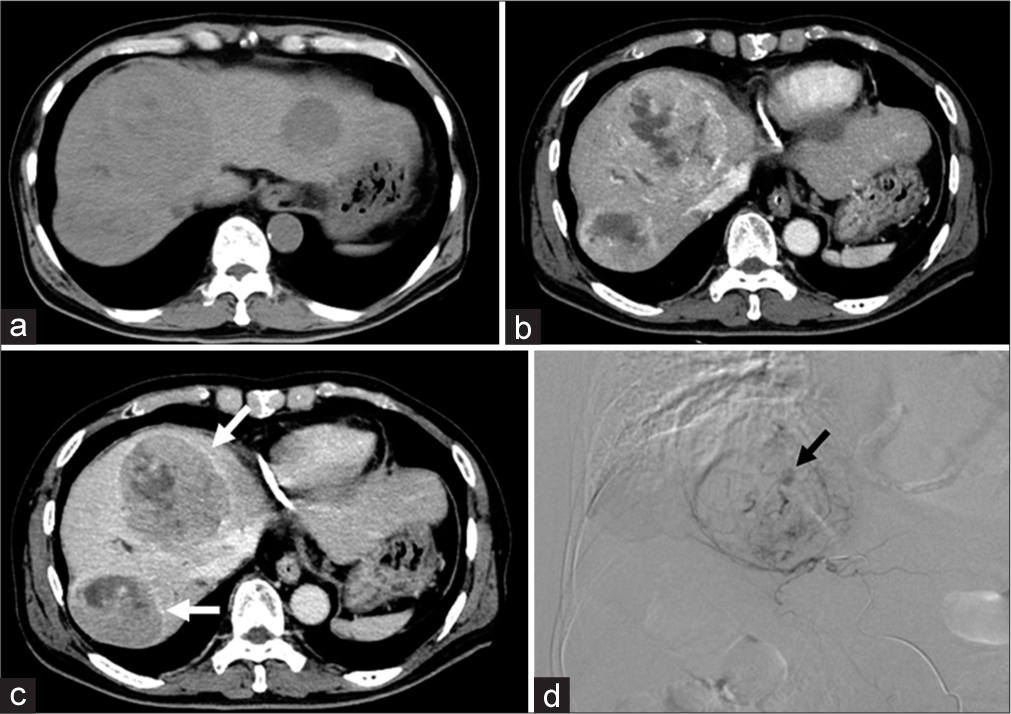
Pre-treatment imaging evaluationDynamic contrast-enhanced (DCE) computed tomography (CT) or DCE magnetic resonance imaging (MRI) performed within 1 month before the DEB-TACE procedure was used to determine whether single- or multinodular HCC was targeted for treatment with DEB-TACE. The tumor diameters of the treated HCCs were measured in the arterial phase of DCE-CT or DCE-MRI. For patient-based analysis, the maximum tumor diameter among them was used. An enhancing capsule is defined as a smooth, uniform, and sharp-bordered rim enhancement found around most or all of the tumor in the portal or delayed phases of DCE-CT or DCE-MRI.[21,22] We evaluated the presence of enhancing capsules in each HCC targeted by the DEB-TACE procedure [Figure 2]. For patient-based analysis, the presence of at least one enhancing capsule was considered positive.
Export to PPT
DEB-TACE proceduresThe right or left femoral artery was punctured using the Seldinger technique to place a 4F arterial sheath. Then, a 4F catheter was selectively inserted into the superior mesenteric and celiac trunks to outline the anatomy of the hepatic artery, identify the tumor’s feeding artery, and assess portal vein patency. A microcatheter was inserted into the tumor’s feeding artery using a super-selective catheterization technique. Subsequently, the IA-call-loaded superabsorbent polymer microsphere (HepaSphere: 50~100 μm) with iodinated contrast agents or epirubicin-loaded superabsorbent polymer microsphere (DC beads: 100~300 μm) with iodinated contrast agents was injected into the tumor’s feeding artery for chemoembolization. The endpoint of embolization was defined as the stagnation of DEBs with iodinated contrast agents observed in the tumors’ feeding arteries. If the endpoint was not achieved after injection of the loading DEBs, additional DEBs were injected until the endpoint was achieved. From the radiological interventional report of the present DEB-TACE procedure, we obtained whether HepaSphere or DC beads were used. In addition, we obtained the number of TAI and cTACE before the present DEB-TACE procedure.
Definition and management for VLPVLP was defined as a localized pooling of contrast agents within the tumor, persisting in the venous phase of angiography and resembling extravasation. VLP was assessed on digital subtraction angiography during and at the endpoint of embolization [Figure 2].
In cases of VLP, additional tumor embolization with gelatin sponge particles was performed until the pooling of the contrast agent disappeared.
Treatment responseThe patients underwent DCE-CT or DCE-MRI at 4~6 weeks after the DEB-TACE procedure in principle. The response of the treated nodules was evaluated by comparing the pre-treatment sum of diameters of target lesions with the post-treatment sum of diameters of target lesions in each patient in patient-based analysis, according to the modified response evaluation criteria in solid tumors guideline.[23] Complete response (CR) was defined as the disappearance of any intratumoral enhancement in all target lesions; partial response (PR) was defined as at least a 30% decrease in the sum of diameters of target lesions; stable disease (SD) was defined as any case that does not qualify for either PR or progressive disease (PD); and PD was defined as an increase of at least 20% in the sum of the diameters of target lesions. The objective response (OR) was defined as the sum of the CR and PR. In nodule-based analysis, the response of the treated nodules was evaluated by comparing the pre-treatment diameters of target lesions with the post-treatment diameters of target lesions to classify them into CR, PR, SD, and PD.
Statistical analysisMann–Whitney U-test was used to compare age, Child-Pugh score, AFP, maximum tumor diameter, number of nodules treated, number of previous TAI, number of previous cTACE, and total number of previous interventional treatments between VLP occurrence and VLP non-occurrence groups, and to compare the tumor diameter between nodules with and without VLP. Fisher’s exact test was used to compare gender, etiology of background chronic liver disease or cirrhosis, BCLC staging, tumor characteristics (single or multinodular), maximum tumor diameter (≥3 cm or <3 cm), the presence of enhancing capsule, two types of DEB size, and two types of loading drugs between VLP occurrence and VLP non-occurrence groups, and to compare tumor location, tumor diameter (≥3 cm or <3 cm), and the presence of enhancing capsule between nodules with and without VLP. For evaluating the treatment response, Fisher’s exact test was used to compare the detailed response (CR, PR, SD, and PD) and OR rate between the VLP occurrence group and VLP non-occurrence group and between the nodules with and without VLP. The factors were selected using a stepwise model, and the multivariate logistic regression analysis was performed to explore the association of factors in predicting VLP occurrence. All statistical analyses were performed using JMP Pro version 17 software (SAS Institute, Cary, NC). A two-sided P < 0.05 was considered statistically significant.
RESULTSA total of 152 nodules were treated by 49 initial DEB-TACE procedures in 49 patients. VLP was observed in 16 patients (32.65%) out of 49 patients in patient-based analysis and 16 nodules (10.52%) out of 152 nodules in nodule-based analysis. The following are the results of the patient-based analysis. There were no significant differences in background factors between VLP occurrence and VLP non-occurrence groups [Table 1]. In pre-treatment imaging characteristics, the maximum tumor diameter in the VLP occurrence group was significantly larger than that in the VLP non-occurrence group (P = 0.0027) [Table 2]. When the maximum diameter was divided into those ≥3 cm and <3 cm, tumors with a maximum diameter ≥3 cm were observed significantly more frequently in the VLP occurrence group (93.75%) than in the VLP non-occurrence group (42.42%) (P = 0.0006). The enhancing capsule was significantly more frequently observed in the VLP occurrence group (56.25%) than in the VLP non-occurrence group (9.09%) (P = 0.0007). There were no significant differences in the items of the present DEB-TACE procedure between VLP occurrence and VLP non-occurrence groups. Regarding the previous interventional treatments, the number of previous cTACE in the VLP occurrence group was significantly lower than that in the VLP non-occurrence group (P = 0.0006). Similarly, the total number of previous treatments in the VLP occurrence group was significantly lower than that in the VLP non-occurrence group (P = 0.0003). Then, the binarized maximum tumor diameter (≥3 cm or <3 cm), enhancing capsule, and the total number of previous treatments were selected using stepwise model for multivariate analysis. The binarized maximum tumor diameter (≥3 cm or <3 cm), the enhancing capsule, and the total number of previous treatments were significantly associated with the occurrence of VLP (P = 0.0048, P = 0.0093, and P = 0.047) [Table 3]. In nodule-based analysis, there were significant differences in tumor diameter, tumor diameter (≥3 cm or <3 cm), and the presence of enhancing capsules between nodules with and without VLP [Table 4].
Table 1: Comparison of background factors between VLP occurrence group and VLP non-occurrence group.
VLP occurrence groupTable 2: Comparison of pre-treatment imaging characteristics and treatments between VLP occurrence group and VLP non-occurrence group.
VLP occurrenceTable 3: Multivariate analysis of imaging characteristics and interventional treatments between VLP occurrence group and VLP non-occurrence group.
VLP occurrence groupTable 4: Comparison of nodule-based imaging characteristics between nodules with VLP and nodules without VLP.
Nodules with VLP (n=16) Nodules without VLP (n=136) P-value Tumor location, n (%) Right lobe/Left lobe 8 (50.00)/8 (50.00) 73 (53.68)/63 (46.32) 0.79 Tumor diameter 59.77±31.27 22.33±18.96 <0.0001* Tumor diameter ≥3 cm/<3 cm, n (%) 14 (87.50)/2 (12.50) 24 (17.65)/112 (82.35) <0.0001* Enhancing capsule (presence/absence), n (%) 9 (56.25)/7 (43.75) 18 (13.24)/118 (86.76) 0.0002*Seven patients were excluded without DCE-CT or DCEMRI images within 1-month-after the DEB-TACE procedure to determine treatment response. There were no significant differences in the detailed treatment responses and OR rate between VLP occurrence and VLP non-occurrence groups [Appendix 1]. Twelve nodules were excluded without DCE-CT or DCE-MRI images within 1-month-after the DEB-TACE procedure to determine treatment response. A significant difference was found in OR rate between nodules with and without VLP (P = 0.032), while there was no significant difference in the detailed treatment responses between the two groups [Appendix 2].
DISCUSSIONIn our present study, the occurrence rate of VLP in the DEBTACE procedure was 32.65% in patient-based analysis and 10.52% in nodule-based analysis. In the previous studies, VLP was observed in 17.1% of the patient-based analysis[15] and in 12.1%,[14] 18.4%,[13] and 16.4%[15] in nodule-based analysis. Overall, including our results, VLP occurs in 17.1–32.65% of patient-based analyses and 10.52–18.4% of nodule-based analyses. The variation in these occurrence rates of VLP may depend on the cases and the characteristics of the nodules treated with DEB-TACE. Since DEB-TACE is often selected in patients with poor liver function and cTACE refractory, and additional embolization with VLP may further degrade liver function after DEB-TACE, the occurrence of VLP should be predicted for each patient before DEB-TACE in daily practice. It should be noted that VLP can occur at an incidence rate of up to 32.65% in patients with HCCs treated with DEB-TACE procedures.
The maximum tumor diameter was larger in the VLP occurrence group than in the VLP non-occurrence group, and the tumor diameter was larger in the nodules with VLP than in the nodules without VLP, which is consistent with previous reports. Li et al. presumed that larger tumors are more prone to VLP because more microspheres, or beads, are used, and the increased pressure in the vascular bed results in the occurrence of VLP.[15] The enhancing capsule was more frequently observed in the VLP occurrence group than in the VLP non-occurrence group and in the nodules with VLP than in the nodules without VLP, which is consistent with the previous reports.[24] The presence of an enhancing capsule is most likely due to tumor growth compressing the surrounding tissue, consisting mainly of fibrous and hepatic tissue, which may inhibit tumor growth to some extent.[15] The presence of an enhancing capsule has been reported to be associated with a favorable treatment response in patients treated with cTACE.[25,26] In giant HCCs, the presence of complete enhancing capsules may have a better long-term outcome than those with incomplete or no enhancing capsules.[27] In our present study, the enhancing capsule may enhance the embolization effect during the DEB-TACE procedure, creating a tumor’s internal pressure gradient that may contribute to the development of VLP. However, this is only a speculation and should be verified by animal studies in the future.
With regard to the bead size, the previous studies reported that smaller beads more easily entered the microvessels, and the accumulation of smaller beads elevated the pressure inside the tumor, leading to the rupture of microvessels of the tumors, resulting in VLP occurrence.[15] However, our study showed no significant difference in the bead size between the VLP occurrence group and VLP non-occurrence group.
It was interesting that the number of previous cTACE and the total number of previous interventional treatments in the VLP occurrence group was significantly lower than that in the VLP non-occurrence group in the univariate analysis of our study. Further, in the multivariate analysis, the total number of previous interventional treatments was significantly associated with the occurrence of VLP (P = 0.047). We speculate on why the previous total number of treatments was related to the occurrence of VLP as follows: in cases with several previous interventional treatments such as cTACE, the embolization effect of previous cTACE on the tumor feeder artery and nearby portal vein might have continued, resulting in fewer beads accumulating in the tumor during the present DEB-TACE procedure. Consequently, this may have led to a lesser pressure gradient within the tumor, thus decreasing the risk of VLP occurrence. On the other hand, the relationship between the total number of previous interventional treatments and the maximum tumor diameter needs to be examined. Indeed, the total number of previous interventional treatments was lower for tumors ≥3 cm than for tumors <3 cm [Appendix 3]. In cases with a high number of previous interventional treatments, the maximum tumor diameter may have been small, and VLP may have been less likely to occur. Nevertheless, we showed that the occurrence of VLP should be carefully considered when the number of previous interventional treatments, including cTACE, is small.
The VLP occurrence group had a higher PR rate and OR rate than the VLP non-occurrence group, which did not reach statistical significance. The nodules with VLP had a significantly higher OR rate than the nodules without VLP. Our results are partially consistent with the previous reports showing that the VLP occurrence group has a significantly better treatment response than the VLP non-occurrence group.[10,15,28] The small sample size due to the exclusion of 7 patients without DCE-CT or DCE-MRI 1-month-after the DEB-TACE procedure may be the reason for the inconsistency of the results.
Although there are few reports on the occurrence of VLP in patients with HCC treated with cTACE, Hu et al. reported the occurrence of VLP in 21.85% in patient-based analysis and 16.81% in nodule-based analysis in cTACE.[29] Furthermore, in this report of cTACE, the VLP occurrence group showed better treatment response than the VLP non-occurrence group as in DEB-TACE.[29] Yamanaka et al. speculated that VLPs may reflect blood spaces secondarily caused by disrupted tumor microvessels and intra-tumor necrosis.[30] A histological study of HCCs that developed VLPs in DEBTACE showed high intra-tumor bead concentrations.[31] Lenvatinib is approved as systemic therapy for patients with unresectable HCC. Despite its rapid inhibition of tumor blood flow, lenvatinib causes tumor-related bleeding and has been shown to resemble VLP.[32] The changes in microvessels within the tumor during systemic therapy may resemble VLP caused by DEB-TACE or cTACE. The obstruction of venous outflow and vascular rupture within the tumor due to high intra-tumor bead concentrations may cause VLP. In both DEB-TACE and cTACE, the occurrence of VLP is not only related to the treatment approaches, such as the need for additional tumor embolization with gelatin sponge particles but also to the treatment response. Further studies are needed to predict the occurrence of VLP using factors including the number of previous interventional treatments.
The locoregional therapies such as cTACE and DEB-TACE are indicated for patients with intermediate stages of the BCLC staging system.[20] Regarding the choice of cTACE versus DEB-TACE, Ikeda et al. suggested that cTACE appeared to have higher CR rates for local tumor control compared to DEB-TACE, although the frequency of post-embolization syndrome was significantly higher in cTACE than in DEB-TACE. This means that DEB-TACE may be indicated for advanced HCC with poor liver function.[9] On the other hand, systemic therapy for advanced HCC achieved remarkable advances, and the combination therapy of atezolizumab (an anti-PDL1 monoclonal antibody) plus bevacizumab (an anti-vascular endothelial growth factor monoclonal antibody) delivered an overall response rate of 29.8% in patients with unresectable HCC.[33] Such advancements in systemic chemotherapy may reduce the reliance on DEB-TACE in the future; however, we believe that understanding the factors associated with VLP can help optimize DEB-TACE procedures, improving patient outcomes and minimizing complications.
Our study had several limitations. First, the number of patients and treated nodules was small. In seven cases, post-operative images were not available to determine the treatment response. Second, in this study, we included patients who underwent initial DEB-TACE after multiple sessions of cTACE. Factors associated with the occurrence of VLP in DEB-TACE after multiple sessions of DEB-TACE need to be studied in the future.
CONCLUSIONThe maximum tumor diameter, enhancing capsule, and the number of previous interventional treatments were significantly related to the occurrence of VLP in patients with HCCs treated with initial DEB-TACE.
留言 (0)