Managing brain metastases (BM) in cancer patients is challenging. The incidence of BM in ovarian cancer (OC) is quite low, being approximately 1.34% (0.49%–6.1%) (1). Despite the variety of existing treatments (surgery, radiotherapy, and chemotherapy), the survival remains very poor with overall survival (OS) around 8–12 months since the diagnosis (2) and there is no established guideline or protocol for their management.
In 15% of cases of high-grade serous ovarian cancer, there is a germline mutation of the BRCA1/2 gene, in 6% of cases a somatic mutation of the BRCA1/2 gene, and in 20% of cases a mutation of other genes involved in homologous recombination, for example, mutations and/or epigenetic silencing of the genes TR, ATM, RAD51/54, CHK1/2, NBS1, PALB2, and PTEN and which equally determine a profile defined as “BRCA-ness” (3, 4). These mutations cause the cell to lose the ability to repair DNA damage caused by external insults, specifically those to the double helix, resulting in a condition defined as homologous recombination deficiency (HRD). This occurrence favors tumor initiation, growth, and evolution. With the acquisition of such molecular, genetic, and biological knowledge, in the last decade, a class of drugs has been identified (4).
Poly-ADP-ribose polymerase inhibitors (PARPi) offer a new option for managing advanced ovarian cancer (OC) in patients with homologous recombination deficiency (HRD) (5–7). However, there is insufficient evidence regarding their effectiveness in ovarian cancer with brain metastases (BM).We present the case of a 54-year-old woman diagnosed with high-grade papillary serous carcinoma of the ovary with a pathogenic germline mutation in BRCA2. During maintenance treatment with olaparib, she developed a single brain lesion consistent with metastasis. After radical surgical excision, she continued olaparib and achieved a radiological complete response (CR) for 15 months. Due to the difficulty of conducting randomized prospective studies in patients with these characteristics, we report our experience and review the current literature based on case reports and retrospective series.
2 Case descriptionA 54-year-old woman with no significant medical or surgical history was diagnosed with ovarian neoplasia after postmenopausal metrorrhagia. She underwent suboptimal cytoreductive surgery for high-grade serous carcinoma involving both ovaries, the uterus, and multiple peritoneal implants. Malignant cells were found in the ascitic fluid, and thoracic tomography showed pleural thickening with associated pleural effusion, with cytology positive for papillary adenocarcinoma.
The patient’s ovarian cancer was initially staged as IVA stage, with a postsurgical CA125 serum level at 646 mg/dl. She received seven cycles of chemotherapy with paclitaxel 175 mg/m2 and carboplatin (AUC 6)–bevacizumab 7.5 mg/kg, achieving a partial response (PR) and normalization of CA125 serum level. Subsequently, she continued bevacizumab for 16 cycles, reaching radiological complete response (CR). Four months after stopping bevacizumab, she experienced peritoneal progression and was switched to cisplatin–paclitaxel, receiving four cycles achieving radiological CR and normalization of tumor markers. A BRCA2 germline mutation (c.2636_2637delCT (p.Ser879Terfs)) was identified, and she started maintenance with olaparib 400 mg/12 h. In August 2021, 37 months from olaparib treatment, she had tonic-clonic seizures, with a 5 cm × 4 cm right frontal brain lesion detected. A multidisciplinary committee recommended complete excision confirming metastasis of high-grade serous papillary carcinoma positive for CK7+, WT1+, and RE+; CK20−. She received adjuvant whole-brain radiotherapy (WBRT) 30 Gy delivered in 10 fractions of 3 Gy each. In January 2022 with no systemic disease, olaparib was restarted at 250 mg/12 h. In April 2023, the patient reported gait instability and headache. A brain local relapse of 13 × 5 mm was detected in the MRI, and she was treated with stereotactic radiosurgery (SRS). After SRS, she experienced slight clinical improvement and continued olaparib. The following brain MRI showed persistence of local recurrence and signs of post-radiotherapy encephalopathy. Despite no changes in size or number of brain lesions, the patient’s neurological condition progressively worsened, leading to inability to walk, loss of sphincters, disorientation, and stupor. The patient died in December 2023.
The treatment and progression periods are summarized in Figure 1.
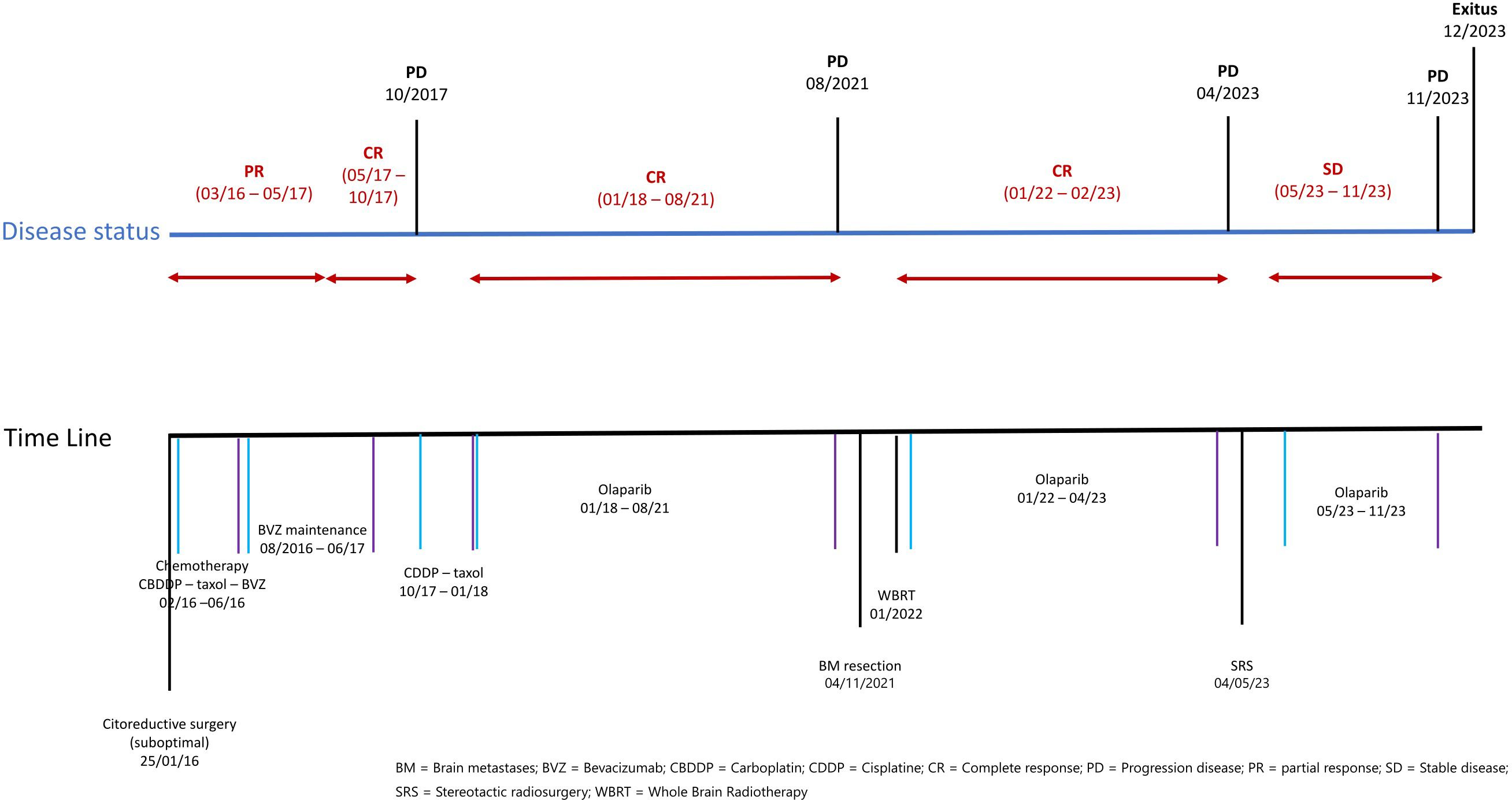
Figure 1 Time line of treatments and disease situation.
3 DiscussionWe present a case in which after a solitary brain metastasis successfully treated by surgery and radiotherapy, maintenance with olaparib was resumed given the absence of extracranial disease and remaining without progression for 15 months.
The incidence of brain metastases in ovarian cancer is approximately 1.34% (0.49%–6.1%) (1, 8–10). In a retrospective study carried out in Israel (with 58.6% of patients of Ashkenazi Jewish ethnicity), the rate of BM in patients with BRCAm was higher than the rate of BM in patients with BRCAwt (5.1% vs. 2.1%, p = 0.013), with an OR of 2.6 (9). In another retrospective study, the hazard ratio (HR) for developing brain metastases in patients carrying BRCA mutations was 3.84 (95% CI: 1.60–9.22, p < 0.001) with no difference in OS between BRCAm and BRCAwt (11, 12). These data support a higher incidence of BM from OC, pointing out a possible tropism for the CNS in BRCAm patients.
In a retrospective study of 174 women with BM from OC, the best survival data are obtained with a trimodal approach (radiation therapy, surgery, and chemotherapy) whereas monotherapy treatment is associated with poorer survival (HR: 2.57, 95% CI: 1.64–3.86) (2). Despite multidisciplinary treatment, the prognosis remains poor with median OS around 1 year since brain progression (1, 12–14). Regarding systemic treatment, platinum derivatives remain the best therapeutic option in ovarian epithelial neoplasms and are capable of crossing the blood–brain barrier (BBB), occasionally achieving adequate control of the disease (2, 12).
PARPi induce the formation of double-stranded DNA breaks by trapping PARP1 and blocking the single-stranded DNA break repair pathway. Tumor cells deficient in HRD pathway eventually die due to the inability to accurately repair DNA double-strand breaks, known as synthetic lethality (4, 15, 16).
Identifying the BRCAness/HRD phenotype is clinically important to optimize the benefit of PARPi (4). The execution of the BRCA test is recommended to identify patients who derive most benefit from PARPi; in the context of the maintenance setting after first-line chemotherapy, it is also recommended to perform the HRD test to establish the extent of the benefit from PARPi in BRCAwt and platinum-sensitive patients (4).
The SOLO2 (olaparib), NOVA (niraparib), and ARIEL3 (rucaparib) clinical trials demonstrated an increase in progression-free survival (PFS) in patients with BRCA mutations compared with placebo: 19.1 vs. 5.5 (HR: 0.30); 21 vs. 5.5 (HR: 0.27); and 16.6 vs. 5.4 (HR: 0.23) when PARPi agents were administered as maintenance therapy in platinum responders (5–7). In addition, niraparib and rucaparib demonstrated increased PFS in the HRD subgroup and in the all-patient subgroup (5, 7).
A meta-analysis carried out in patients with platinum-sensitive recurrent OC confirmed the efficacy of PARPi in the recurrent OC BRCA-mutated population and in the HRD population (17). The current challenge is to investigate the potentiality of combined PARPi with other therapeutic agents in order to enhance efficacy and avoid resistance (4). Despite the presence of a highly immunosuppressive tumor microenvironment that causes poor recognition of tumor cells by the immune system, it is shown that BRCA-mutated and HRD ovarian cancers express higher levels of neoantigens because of defect in DNA repair mechanisms. Moreover, PARP inhibitors are suggested to upregulate PD-L1 expression and stimulate interferon-mediated immune response having therefore a synergic action in immune stimulation (4).
However, these clinical trials and meta-analysis do not make reference to data on patients with brain metastases. The PARPi data published about this population come mainly from clinical cases (8, 10, 15, 18–23) whose main characteristics are presented in Table 1. PFS is defined as time from treatment with PARPi until clinical/radiological progression, even if they continued PARPi beyond progression.

Table 1 Clinical cases of epithelial ovarian cancer with CNS disease treated with iPARPs published in PubMed.
Except for the case presented by Morales (22), all these cases started PARPi in a platinum-sensitive situation. A platinum-sensitive setting has been reported to be a good prognostic factor in patients with BM from OC with an HR of 0.23 in comparation in a platinum-refractory setting (24).
This could be one of the reasons why the duration of the response has been longer in the cases shown in Table 1, compared with the OS data reported around 8–12 months reported in the literature (1, 2, 24). Recently, a study conducted in eight centers in the UK explored the role of PARPi in the management of BM from OC. A total of 29 patients were treated with PARPi, and for the patients whose treatments included PARPi therapy, the median OS was statistically better whether they had additional chemotherapy alone or triplet therapy, achieving the highest OS with chemotherapy + PARPi + other (RT and/or surgery) (14). The authors also pointed out that this better prognosis could be related to a first platinum-sensitive relapse (14).
Another retrospective study of 111 patients with ovarian cancer and brain metastasis analyzed BRCA status, surgical approach, and PARPi treatment. They found that receiving PARPi before brain metastasis delays its diagnosis but negatively affects post-brain metastasis survival in the BRCAm population. However, using PARPi after brain metastasis significantly improves survival expectations. In this cohort, five patients developed brain metastasis during maintenance treatment, and three of them continued PARPi after the diagnosis (25).
Our patient presented BM during maintenance treatment with olaparib. The singularity of the case allowed radical treatment of this lesion while remaining in CR at the extracranial level. In the context of radical treatment of brain metastasis (surgery and/or RT), with no evidence of extracranial disease, a reasonable option could be to maintain treatment with PARPi. In this setting, the role of PARPi has not been described, however, using targeted therapies beyond intracranial progression, which can be seen in other malignancies (10). As discussed in the case presented by Kasherman et al. where olaparib was maintained after cerebral progression, consideration should be given to continuing poly ADP-ribose polymerase inhibitors beyond localized disease control in ovarian high-grade serous carcinoma extracranial oligometastatic progression, given that progression in this context likely occurs within the context of clonal heterogeneity (10). Local treatment such as stereotactic body radiotherapy (SBRT) achieves good local control. In a retrospective study with data of 449 lesions from 261 patients with oligo-recurrent, persistent OC for which salvage surgery or other local therapies were not feasible due to any relative contra-indication to further systemic therapy, SBRT or SRS achieved a rate of 65.2% CR of irradiated lesions (26). After local disease control, several large poly ADP-ribose polymerase inhibitor studies continued treatment beyond progression (10, 27). In our case, after PFS of 15 months, the second intracranial relapse was managed with SRS, continuing olaparib with no evidence of extracranial disease until the patient’s death.
Regarding the differences between the PARPi, niraparib has shown to be able to cross the BBB in animal models (12), being superior to other PARPi. According to a preclinical study in rodents, concentration–time profiles of niraparib in the brain were similar to those in plasma, with mean brain-to-plasma concentration ratios of 0.85–0.99 indicating effective brain penetration in rodents. Niraparib showed significant antitumor activity in both subcutaneous and intracranial xenograft models, with tumor growth inhibition values up to 83%, supporting its potential clinical use against BRCA-mutant tumors metastasized to the brain (28).
Despite the fact that the pharmacodynamic data of olaparib showed little activity at the CNS (29), our case and other similar cases described in the bibliography show a clinical benefit of olaparib in patients with BM from OC, especially in patients with a BRCA 1/2 mutation (14, 19–22); however, the current evidence base in cases and retrospectives studies is not enough to confirm this statement.
4 Conclusion● Brain metastases from OC are rare, representing a clinical challenge whose management requires a multidisciplinary approach. The emergence of PARPi has provided clinicians with new therapeutic options to enhance the prognosis of BRCAm patients.
● The role that PARPi may have in the treatment of brain metastases of ovarian cancer requires more studies. In the context of radical treatment of brain metastasis (surgery and/or RT), with no evidence of extracranial disease, maintaining treatment with PARPi beyond the brain progression should be considered.
● The evidence of PARPi in brain metastases from OC is primarily based on retrospective studies and case reports. Further prospective studies are needed to identify and validate biomarkers to determine which patients will benefit the most.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementEthical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributionsGL: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. TD: Writing – review & editing, Validation, Supervision, Methodology. MI: Writing – review & editing, Validation, Supervision, Methodology, Data curation. LG: Writing – review & editing, Validation, Supervision. EA: Writing – review & editing, Supervision, Validation. AR: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Data curation. AS: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Conceptualization.
FundingThe authors declare financial support was received for the publication of this article. Article processing charges (APC) are funded by IBIMA.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Borella F, Bertero L, Morrone A, Gambella A, Bovetti M, Cosma S, et al. Brain metastases from ovarian cancer: Current evidence in diagnosis, treatment, and prognosis. Cancers (Basel). (2020) 12:1–22. doi: 10.3390/cancers12082156
CrossRef Full Text | Google Scholar
2. Marchetti C, Ferrandina G, Cormio G, Gambino A, Cecere S, Lorusso D, et al. Brain metastases in patients with EOC: Clinico-pathological and prognostic factors. A multicentric retrospective analysis from the MITO group (MITO 19). Gynecol Oncol. (2016) 143:532–8. doi: 10.1016/j.ygyno.2016.09.025
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Pietragalla A, Arcieri M, Marchetti C, Scambia G, Fagotti A. Ovarian cancer predisposition beyond BRCA1 and BRCA2 genes. Int J Gynecol Cancer. (2020) 30:1803–10. doi: 10.1136/ijgc-2020-001556
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Arcieri M, Tius V, Andreetta C, Restaino S, Biasioli A, Poletto E, et al. How BRCA and homologous recombination deficiency change therapeutic strategies in ovarian cancer: a review of literature. Front Oncol. (2024) 14:1335196. doi: 10.3389/fonc.2024.1335196
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 390:1949–61. doi: 10.1016/S0140-6736(17)32440-6
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. (2021) 22:620–31. doi: 10.1016/S1470-2045(21)00073-5
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. New Engl J Med. (2016) 375:2154–64. doi: 10.1056/NEJMoa1611310
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Zhang Z, Xu M, Sakandar A, Du X, He H, He W, et al. Successful treatment of a patient with brain metastasis from ovarian cancer with BRCA wild type using niraparib: A case report and review of the literature. Front Oncol. (2022) 12:873198. doi: 10.3389/fonc.2022.873198
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Limon D, Shachar E, Wolf I, Adar L, Peleg Hasson S, Ferro L, et al. Brain metastases in patients with ovarian cancer. Acta Oncol (Madr). (2022) 61:757–63. doi: 10.1080/0284186X.2022.2066985
CrossRef Full Text | Google Scholar
10. Kasherman L, Madariaga A, Rouzbahman M, Murphy K, Shultz D, Stockley T, et al. Across barriers: poly ADP-ribose polymerase inhibitors beyond progression in high grade serous ovarian cancer with brain metastases. Int J Gynecol Cancer. (2021) 31:139–43. doi: 10.1136/ijgc-2020-001849
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Ratner E, Bala M, Louie-Gao M, Aydin E, Hazard S, Brastianos PK. Increased risk of brain metastases in ovarian cancer patients with BRCA mutations. Gynecol Oncol. (2019) 153:568–73. doi: 10.1016/j.ygyno.2019.03.004
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Stasenko M, Cybulska P, Feit N, Makker V, Konner J, O’Cearbhaill RE, et al. Brain metastasis in epithelial ovarian cancer by BRCA1/2 mutation status. Gynecol Oncol. (2019) 154:144–9. doi: 10.1016/j.ygyno.2019.05.004
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Alizzi Z, Roxburgh P, Cartwright D, McLaren A, Park S, Jones R, et al. Description of a retrospective cohort of epithelial ovarian cancer patients with brain metastases: evaluation of the role of PARP inhibitors in this setting. J Clin Med. (2023) 12:2497. doi: 10.3390/JCM12072497/S1
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Tao M, Cheng J, Wu X. Niraparib as maintenance therapy in germline ATM-mutated and somatic BRCA2-mutated ovarian cancer with brain metastases: A case report and literature review. Onco Targets Ther. (2020) 13:12979–86. doi: 10.2147/OTT.S281302
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Murai J, Huang SYN, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Differential trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. (2012) 72:5588. doi: 10.1158/0008-5472.CAN-12-2753
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Tomao F, Bardhi E, Di Pinto A, Sassu CM, Biagioli E, Petrella MC, et al. Parp inhibitors as maintenance treatment in platinum sensitive recurrent ovarian cancer: An updated meta-analysis of randomized clinical trials according to BRCA mutational status. Cancer Treat Rev. (2019) 80:101909. doi: 10.1016/j.ctrv.2019.101909
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Gray S, Khor XY, Yiannakis D. Niraparib as maintenance therapy in a patient with ovarian cancer and brain metastases. BMJ Case Rep CP. (2019) 12:e230738. doi: 10.1136/bcr-2019-230738
CrossRef Full Text | Google Scholar
19. Gallego A, Garrido D, Yébenes L, Mendiola M, Castelo B, Redondo A. Long-term response to olaparib in BRCA1-related ovarian cancer with brain metastases Case study GYNECOLOGICAL CANCER. Int J Gynecol Cancer. (2021) 31:1292–6. doi: 10.1136/ijgc-2020-002225
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Bangham M, Goldstein R, Walton H, Ledermann JA. Olaparib treatment for BRCA-mutant ovarian cancer with leptomeningeal disease. Gynecol Oncol Rep. (2016) 18:22. doi: 10.1016/j.gore.2016.10.004
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Sakamoto I, Hirotsu Y, Nakagomi H, Ikegami A, Teramoto K, Omata M. Durable response by olaparib for a Japanese patient with primary peritoneal cancer with multiple brain metastases: A case report. J Obstetrics Gynaecol Res. (2019) 45:743–7. doi: 10.1111/jog.13851
CrossRef Full Text | Google Scholar
22. Vásquez FM, Basave HNL, Herrera MDCM, González RRP. Clinically relevant response to treatment with olaparib in a patient with refractory multidrug-resistant ovarian cancer and central nervous system involvement: A case report. Am J Case Rep. (2020) 21:0–0. doi: 10.12659/AJCR.925990
CrossRef Full Text | Google Scholar
23. Cabitza E, Pirola M, Baldessari C, Bernardelli G, Zunarelli E, Pipitone S, et al. Cerebellar metastasis of ovarian cancer: a case report. J Med Case Rep. (2023) 17:1–5. doi: 10.1186/s13256-023-04211-6
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Sehouli J, Pietzner K, Harter P, Münstedt K, Mahner S, Hasenburg A, et al. Prognostic role of platinum sensitivity in patients with brain metastases from ovarian cancer: Results of a German multicenter study. Ann Oncol. (2010) 21:2201–5. doi: 10.1093/annonc/mdq229
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Sassu CM, Marchetti C, Russo G, Minucci A, Boccia SM, Benato A, et al. Epithelial ovarian cancer and brain metastases: might the BRCA status, PARP inhibitor administration, and surgical treatment impact the survival? Int J Gynecol Cancer. (2024) 34:88. doi: 10.1136/ijgc-2023-004980
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Macchia G, Lazzari R, Colombo N, Laliscia C, Capelli G, D’Agostino GR, et al. A large, multicenter, retrospective study on efficacy and safety of stereotactic body radiotherapy (SBRT) in oligometastatic ovarian cancer (MITO RT1 study): A collaboration of MITO, AIRO GYN, and maNGO groups. Oncologist. (2020) 25:e311. doi: 10.1634/theoncologist.2019-0309
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Lazzari R, Ronchi S, Gandini S, Surgo A, Volpe S, Piperno G, et al. Stereotactic body radiation therapy for oligometastatic ovarian cancer: A step toward a drug holiday. Int J Radiat Oncol Biol Phys. (2018) 101:650–60. doi: 10.1016/j.ijrobp.2018.03.058
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Mikule K, Wilcoxen K. Abstract B168: The PARP inhibitor, niraparib, crosses the blood brain barrier in rodents and is efficacious in a BRCA2-mutant intracranial tumor model. Mol Cancer Ther. (2015) 14:B168–8. doi: 10.1158/1535-7163.TARG-15-B168
CrossRef Full Text | Google Scholar
29. Sun K, Mikule K, Wang Z, Poon G, Vaidyanathan A, Smith G, et al. A comparative pharmacokinetic study of PARP inhibitors demonstrates favorable properties for niraparib efficacy in preclinical tumor models. Oncotarget. (2018) 9:37080–96. doi: 10.18632/oncotarget.v9i98
留言 (0)