Dialysis is a treatment that removes wastes and extra fluid from the patient’s blood when the patient’s kidneys are no longer able to work effectively (Hakim and Lazarus, 1995). Patients need dialysis when they develop end-stage kidney failure. Usually, by that time, they lose about 85%–90% of their kidney function and have a glomerular filtration rate that falls below 15 mL/(min · 1.73 m2) (Tattersall et al., 2011). Dialysis has two types: hemodialysis (HD) using a machine/artificial kidney-like apparatus and peritoneal dialysis using a peritoneal membrane as a filter. HD is done for patients with no residual renal function, whereas peritoneal dialysis (PD) is recommended for younger patients due to its flexibility. In chronic or end-stage kidney failure, dialysis is the best method to remove accumulated toxins from the body and improve the quality of life for the rest of life. However, individuals suffering from CRF, who are on dialysis, may have increased cardiovascular and metabolic risk and an increased risk of getting an infection (Vadakedath and Kandi, 2017). Dialysis vintage is associated with an enhanced risk of death, with each additional year of dialysis treatment associated with an increase in the risk of dying by approximately 6% (Chertow et al., 2000). Based on the United States Renal Data System (USRDS) report, the adjusted survival rate for patients receiving HD is 57% 3 years after the onset of ESKD compared with 68% for patients receiving PD. The 5-year survival for patients receiving HD and PD is 42% and 52%, respectively (System, 2018).
Among patients with maintenance dialysis, the mortality rate is high at about 165/1,000 (Saran et al., 2020). Many patients develop malnutrition and a micro-inflammatory state due to tubing during dialysis, reduced food intake and intestinal digestion and absorption, and metabolic acidosis (Kiebalo et al., 2020; Sahathevan et al., 2020). Numerous complications also affect patients’ quality of life and increase mortality. Therefore, improving the complications is extremely important for prolonging the lifespan of patients and improving their quality of life.
Ophiocordyceps sinensis (O. sinensis), also named Chinese caterpillar fungus, is a precious traditional medicine mainly distributed on the Qinghai–Tibetan Plateau (Wei et al., 2021). It has become one of the most valuable biological commodities widely traded in recent years worldwide owing to its medicinal values in terms of anti-fatigue, antitumor, and kidney protection (Liu et al., 2019a; Lee et al., 2021; Long et al., 2021). Modern pharmacological experiments found that the main components of O. sinensis included cordyceps polysaccharide, cordycepin, cordycepic acid, and so forth (He et al., 2020; Su et al., 2020). O. sinensis preparations is overexploited due to the increase in vulnerability and risk for the wild O. sinensis (overexploitation and habitat loss) (Wei et al., 2021) and its surged price (Zhang et al., 2020a), which leads to artificial cultivation to make O. sinensis a more affordable material for commercial trade (Yue et al., 2013a). Synthetic O. sinensis preparation is made from strains extracted from O. sinensis (Liu et al., 2019b). Studies shown that synthetic O. sinensis preparations can benefit patients undergoing dialysis by improving their quality of life, reducing the incidence of cardiovascular events, improving the micro-inflammatory state and malnutrition, and so forth (Liu, 2012; Ashraf et al., 2020; Li et al., 2020). Although a systematic review was published in 2019 to evaluate the efficacy of Cordyceps sinensis as an adjunctive treatment in patients undergoing HD, (Bee and Zoriah, 2019), we aimed to conduct a comprehensive and updated systematic review and meta-analysis to evaluate the efficacy and safety of O. sinensis preparation in both patients undergoing HD and those undergoing PD.
2 ObjectivesThis systematic review aimed to clarify whether O. sinensis preparation in the adjuvant treatment for both patients undergoing HD and those undergoing PD was more effective than the control in anti-infection and reducing cardiovascular events. Our secondary objective was to explore the efficacy of the OS in the two dialysis modalities (HD and PD), various sample size, different treatment course, and follow-up period.
3 Methods and analysis3.1 RegistrationWe drafted the protocol according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocol (PRISMA-P) (Moher et al., 2015). Also, we reported this systematic review in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021). The protocol of the curent review has been registered in the International Prospective Register of Systematic Reviews with the identifier CRD42022324508.
3.2 Eligibility criteriaThe eligibility criteria were formaulated in the Population, Intervention, Comparison, Outcomes, and Study (PICOS) framework as follows.
3.2.1 Type of studyOnly randomized controlled trials (RCTs), with or without blinding, that were published in English or Chinese in peer-reviewed journals were included in this review.
3.2.2 ParticipantsThe study included adult participants aged ≥ 18 years who receive HD or PD, regardless of their primary disease, race, gender, and ethnicity.
3.2.3 InterventionO. sinensis preparations were taken orally combined with dialysis and conventional treatments. No restrictions were imposed on the dosage form, administration, course, or manufacturer. Furthermore, 11 kinds of O. sinensis preparations were identified which are commonly used to treat patients with dialysis, including Bailing Tablets (capsules), Jinshuibao Tablets (capsules), Zhiling Capsules, Cordyceps militaris capsules, Cordyceps militaris powder, cultured C. sinensis powder, powdered Cordyceps mortierella mycelia, Cordyceps cephalosporium mycelia, and fermentative Cordycepis fungal powder. All of them were approved by the National Medical Products Administration in China.
3.2.4 ComparatorThe control group received the same dialysis and conventional treatments as the experimental group. The conventional therapies included low purine, low salt, low fat, low phosphorus quality, a low-protein diet, limited water intake, control of blood pressure, blood lipids, and blood glucose, and the symptomatic treatment for the complications. The study with the control group using other traditional Chinese medicine treatments, including Chinese patent medicine and acupuncture was excluded.
3.2.5 Type of outcomesAfter searching the Core Outcome Measures in Effectiveness Trials (COMET, https://www.cometinitia-tive.org/), we used the outcomes from the Standardized Outcomes in Nephrology-Hemodialysis Dialysis (SONG-HD) and Standardized Outcomes in Nephrology-Peritoneal Dialysis (SONG-PD) core outcome sets (Evangelidis et al., 2017; Manera et al., 2020), which were developed by the Standardized Outcomes in Nephrology-Peritoneal Dialysis (Nephrology-Hemodialysis) initiative. Some of the outcomes were selected for the current reviews, which were divided into primary outcomes (e.g., mortality, CVD, and infection) and secondary outcomes (e.g., vascular access problems, dialysis adequacy, hyperkalemia, and life participation). When the included studies did report the aforementioned outcomes, we used alternative outcomes for meta-analysis.
3.3 Search strategyA search strategy was created with the help of an experienced librarian and adapted for searching the databases, including PubMed, Embase, the Cochrane Library, SinoMed, CNKI, VIP, Wanfang Data and International Clinical Trials Register Search Portal, and ClinicalTrials.gov. Finally, we identified RCTs involving the aforementioned interventions. We conducted the literature search from the inception of all the databases to 31 October 2022, and updated the search on 31 April2024. Studies in accordance with the PICOS were considered. Key search terms (MeSH and free words) used for our searches were “Renal Dialysis” or “O. sinensis” or “RCTs” or “Cordyceps.” The detailed search strategy for all databases is presented in Supplementary Table S1.
3.4 Study selectionAll retrieved records were imported into the Endnote X9.1 software, and the duplicated records were removed. By referring to the eligibility criteria, two researchers (MXL and TYC) independently (1) screened the titles and abstracts of deduplicated studies and removed those that did not meet the eligibility criteria and (2) then rechecked the full texts of the remaining articles and finally included or excluded. A third reviewer (XL) was consulted in the case of disagreement. All excluded studies during the full-text checking were recorded and tabulated with their justification for exclusion (Supplementary Table S1). The selection process followed the PRISMA flow diagram (Moher et al., 2015).
3.5 Data extractionWe extracted information from the included studies, and two researchers (MXL and TYC) filled the extracted data in a pre-designed form designed using an Excel spreadsheet. The data extraction table had information as follows (Tables 2, 3).
1. Study characteristics: published title, author name, journal name, the country where the study was conducted, year of publication, language, sample size, study design, study period, and follow-up period.
2. Participants: male–female ratio, average age, primary disease, disease stage, severity, average duration of disease, and mean history of dialysis.
3. Interventions: hemodialysis or PD; dialysis time, frequency, and duration; comorbidity.
4. Outcomes: Primary outcomes: mortality, CVD, and infection; secondary outcomes: vascular access problems, dialysis adequacy, hyperkalemia, and life participation.
Two researchers (MXL and CJC) independently extracted data from all studies that met the inclusion criteria. All results were cross-examined. When the cross-examination results were inconsistent, the discussion would resolve the disagreement until a consensus was reached or by consulting a third author (TYC and YZC). We contacted the author by phone or email if the critical data of the included study were unavailable or only partly available.
3.6 Assessing risk of biasTwo researchers (MXL and TYC) independently assessed the risk of bias for included studies according to the Cochrane risk-of-bias (ROB) tool for interventions (Higgins et al., 2011). ROB consisted of seven domains on which biases within trials were assessed: (1) sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other biases (baseline imbalance between groups of participants, blocked randomization in trials that were not blinded, and differential diagnostic activity). Each domain was rated as “high,” “unclear,” or “low” risk of bias and reported separately. The assessment was graphed, and Review Manager 5.3 software was used.
3.7 Method for data synthesisQualitative evidence synthesis was performed based on the available results. After describing the baseline characteristics of the studies, the outcome of interest was summarized, that is, the effects of O. sinensis preparations in the adjuvant treatment for patients undergoing HD and those undergoing PD. Furthermore, the effect evaluation for patients undergoing HD and those undergoing PD was performed separately. Statistical significance was set at p < 0.05.
3.7.1 Meta-analysisA meta-analysis was conducted when the number of RCTs corresponded to the same PICOS in two or more. Effect sizes were calculated as either OR (for dichotomous data) and weighted (or standardized) final post-intervention mean differences (for continuous data) with their corresponding 95% confidence intervals. Review Manager 5.3 software (Program, 2014) was used to conduct meta-analyses. The effects models (fixed or random) were used to estimate the effect of O. sinensis preparation by creating forest plots. When heterogeneity was present, the random-effects model was used.
3.7.2 Heterogeneity assessmentWe estimated the between-study heterogeneity in all eligible comparisons, used the χ2-based Q statistic (Cohen et al., 2015), and assessed the extent of heterogeneity with I2, a quantitative measure of inconsistency between studies. When the values were 0% or ≥50%, they represented no heterogeneity or considerable heterogeneity, respectively (Higgins and Thompson, 2002). If the heterogeneity was within the acceptable range, the fixed-effects model was used to affect estimates; otherwise, the random-effects model was used.
3.7.3 Publication biasWe assessed publication bias using funnel plots and Egger tests because more than 10 studies were included in the meta-analysis (Guyatt et al., 2011a). If the funnel plot showed asymmetry, it indicated publication bias. If publication bias existed, trim-and-fill analyses were used to assess the impact of publication bias on the results. Any bias was explained through the analyses and discussions.
3.7.4 Sensitivity analysisThe sensitivity analysis was performed to verify the robustness of the results. We performed this through the leave-one-out strategy (Banach et al., 2015a; Banach et al., 2015b) based on the quality of the included studies to explore the sources of heterogeneity. When one study was excluded, the results and heterogeneity of the remaining studies were reevaluated.
3.7.5 Subgroup analysisThe subgroup analysis was conducted to analyze the causes of heterogeneity. We performed this based on the two dialysis modalities (HD and PD), sample size, treatment course, and follow-up period.
3.8 Quality of the evidenceThe certainty of the evidence was graded for each outcome, from a rating of HIGH to VERY LOW, by following the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (Guyatt et al., 2011b). The GRADE system included five domains that could downgrade the quality of the evidence used in RCTs: limitations, inconsistent results, imprecision, indirectness, and publication bias. The quality of evidence for each outcome was graded as HIGH, MODERATE, LOW, or VERY LOW. A summary of findings (SoF) was created using GRADEPro GDT 2021 (McMaster University, ON, Canada). The SoF presented the following information where appropriate: absolute risks for the treatment and control, estimates of relative risk, and a ranking of the quality of the evidence based on the risk of bias, directness, heterogeneity, precision, and risk of publication bias of the review results. The outcomes reported in the SoF table for this review included CVD, mortality, dialysis adequacy, infection, and so forth.
4 Results4.1 Literature searchA total of 713 studies were retrieved, and 35 studies (Sun et al., 2011; Sun et al., 2012; Wu, 2012; Ye et al., 2012; Liu, 2014; Tian et al., 2014; Wang, 2016; Yao et al., 2016; Chen et al., 2017; Huang et al., 2018; Wang and Sha, 2018; Ying, 2018; Gao and Gao, 2019; Wang, 2019; Wang and Wang, 2019; Xie et al., 2019; Yang et al., 2019; Zhu, 2019; Zhang et al., 2020b; Cui et al., 2020; Zhang et al., 2020c; Zhu and Yu, 2020; He, 2021; Li, 2021; Ling et al., 2021; Ren, 2021; Tian et al., 2021; Gao et al., 2022; Zhang et al., 2022; Zhao et al., 2022; Li et al., 2023; Wang et al., 2023; Zeng, 2023; Lin et al., 2024; Tang and Shao, 2024) with 2,914 patients were included. Further, 295 studies were screened out because of duplication, 367 were excluded after reading titles and abstracts, and 51 were assessed for eligibility by reading full texts. After that, 14 RCTs were excluded for incomplete data and 2 were excluded for unqualified basic characteristics. Ultimately, 35 RCTs were included to conduct meta-analysis (Figure 1).
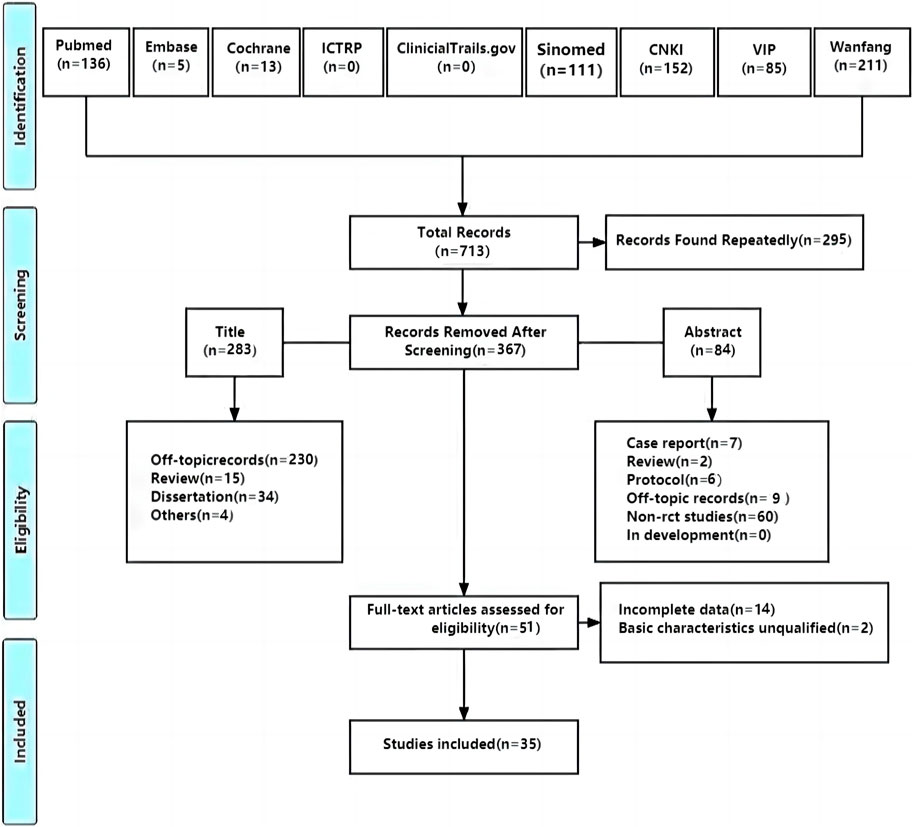
Figure 1. PRISMA flow diagram of the study selecting process.
4.2 Description of the included studiesThe included studies were all from China. The sample size of the included studies ranged from 35 to 150, age from 42.2 ± 15.2 to 74.54 ± 2.06 years, disease duration from 2.21 ± 0.58 to 168.24 ± 21.72 months, and history of dialysis from 10.4 ± 2.0 to 35.16 ± 6.73 months. As for the dialysis modality, 1931 (66%) patients received HD while 983 (34%) patients were treated by PD. In terms of the selection of O. sinensis preparations, 29 of 35 studies used the Bailing capsule and 6 studies used the Jinshuibao capsule. For the doses of O. sinensis preparations, patients in 5 studies took 2 to 3 capsules at a time, patients in 26 studies took 4 to 6 capsules, and patients in 4 studies took more than 6 capsules (Figure 2; Tables 1, 2).
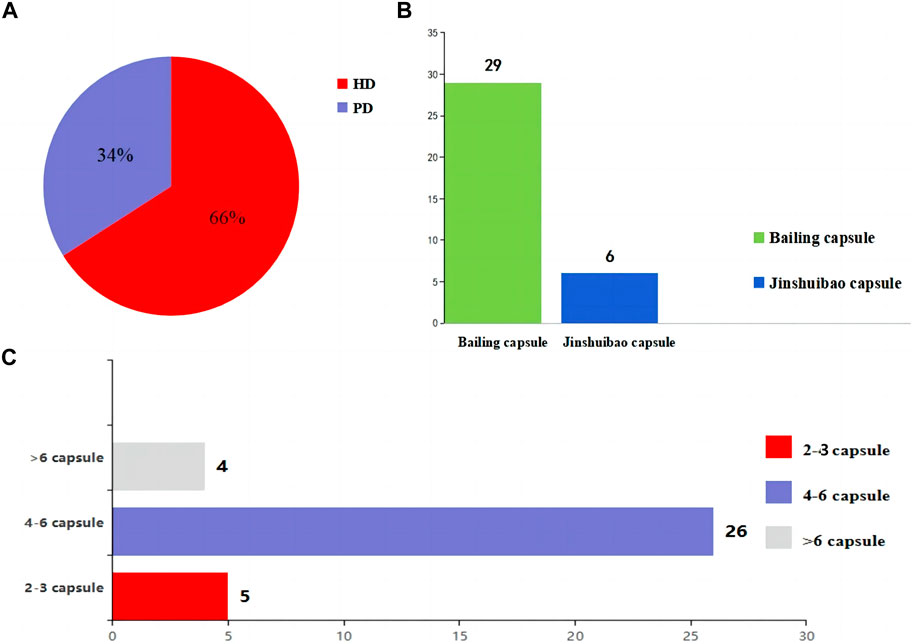
Figure 2. The information about the intervention. (A) Selection of the dialysis method in the patients. (B) O. sinensis preparations. (C) The doses of O. sinensis preparations. HD, Hemodialysis; PD, peritoneal dialysis.
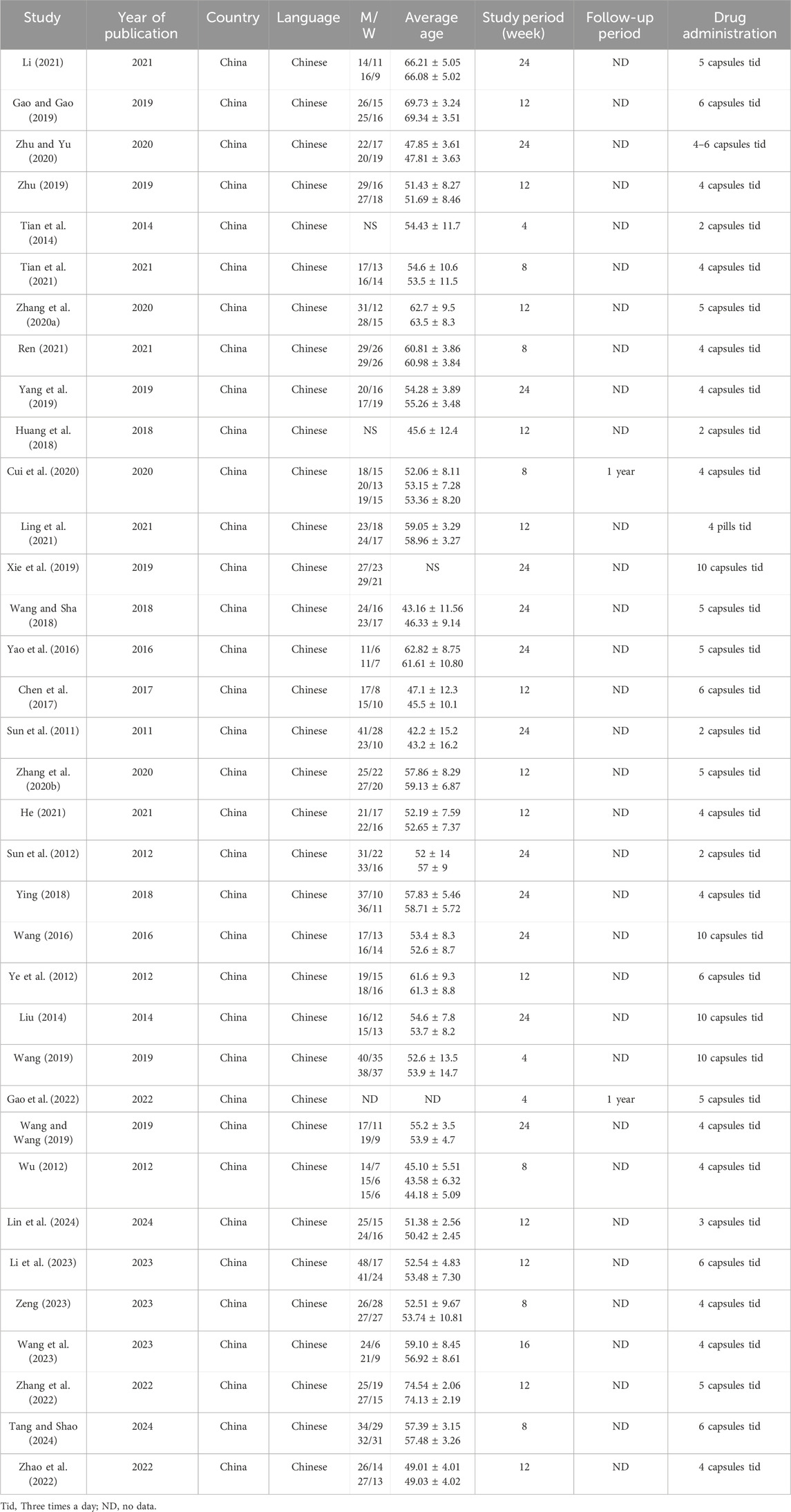
Table 1. Basic characteristics of the included studies (Part Ⅰ).
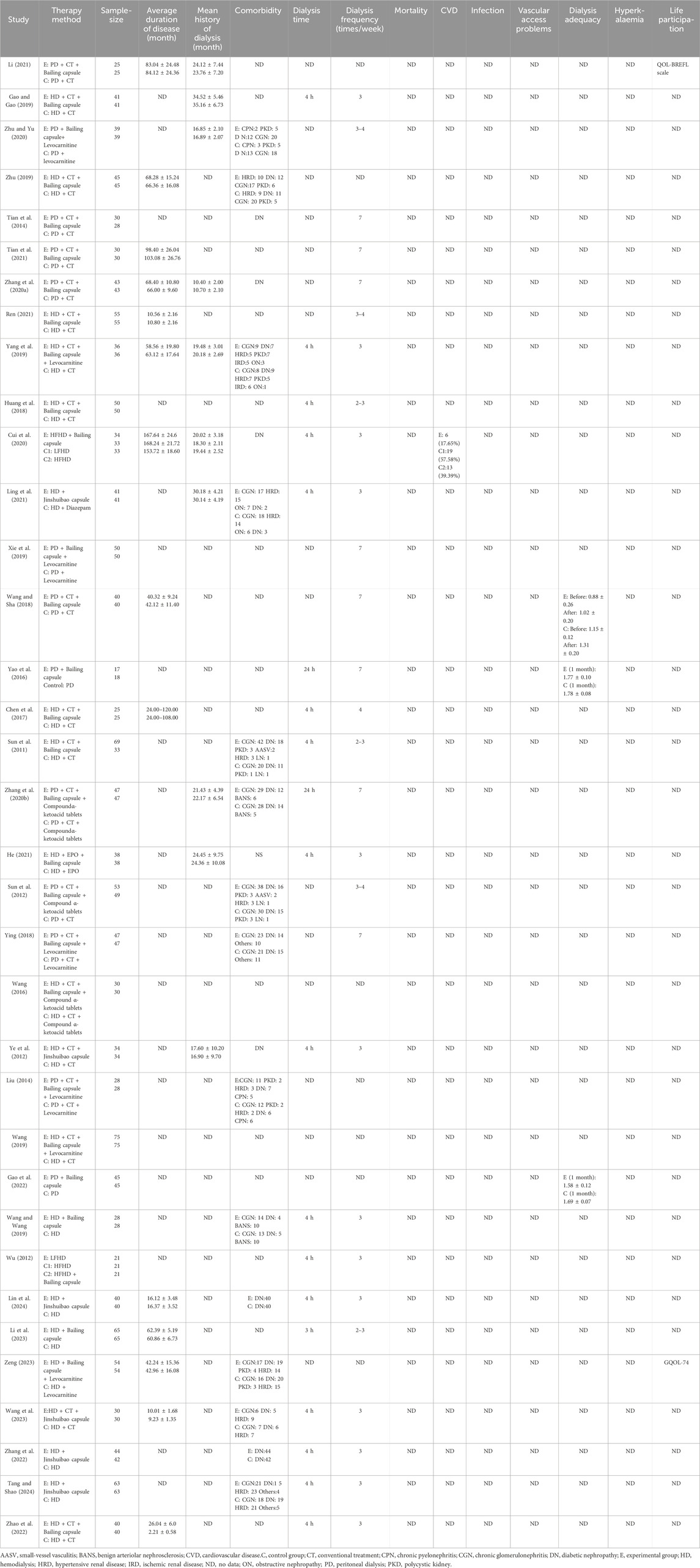
Table 2. Basic clinical characteristics of the included studies (Part Ⅱ).
4.3 Risk of biasWe evaluated 35 RCTs based on the Cochrane ROB tool and found that 30 RCTs used the random sequence generation methods, such as random number table, and 5 studies, due to lack of description, were appraised as” unclear risk” (Wu, 2012; Ren, 2021; Gao et al., 2022; Li et al., 2023; Zeng, 2023). A majority of studies were evaluated as “high risk,” and only nine studies (Sun et al., 2011; Sun et al., 2012; Wu, 2012; Ren, 2021; Gao et al., 2022; Zhang et al., 2022; Li et al., 2023; Wang et al., 2023; Zeng, 2023) were classified as “unclear risk” because of the unclear allocation concealment scheme. Although blinding was not used in any of the studies, the outcome measures were objective and the use of blinding did not affect the evaluation of the results by the system reviewers. Therefore, “Blinding of Participants and Personnel” and “Blinding of Outcome Assessment” were defaulted to “low risk.” All studies had no missing data which were rated as “low risk.” No research proposals were found for any of the studies, and we could not judge whether reporting bias existed due to insufficient information; therefore, it was defined as “unclear.” We were assumed that the “Other bias” were unclear because there was not much time to investigate them for us (Figure 3).
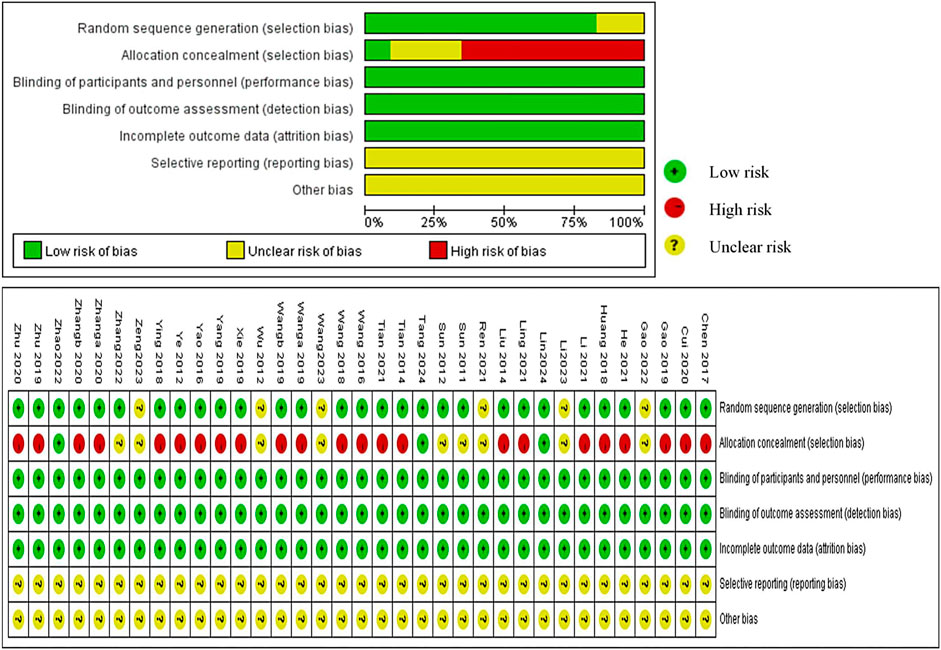
Figure 3. Risk of bias in included studies.
4.4 Outcomes in patients undergoing dialysis4.4.1 CRPA total of 15 RCTs reported CRP levels before and after treatment; 1,191 patients were included. A random-effects model was used to combine the effect sizes (p < 0.00001, I2 = 98%). The CRP levels significantly decreased the intervention group compared with in the control group. [MD = −2.22, 95% CI (−3.24 to −1.20), p < 0.00001] (Figure 4A).
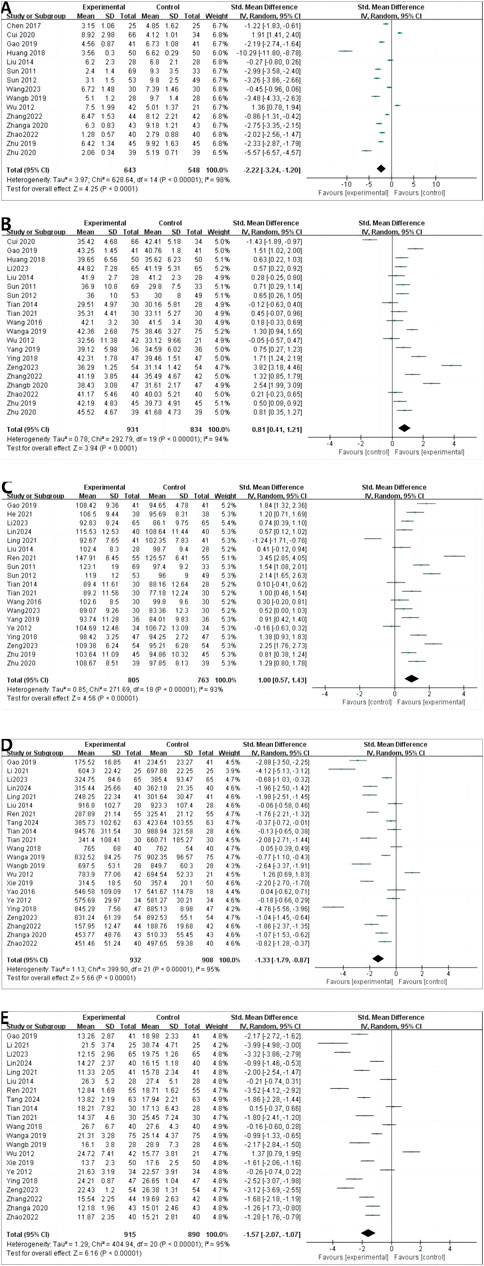
Figure 4. Outcomes in patients undergoing dialysis. (A) CRP; (B) ALB; (C) HGB; (D) CREA; (E) BUN.
4.4.2 ALBA total of 20 RCTs reported ALB levels before and after treatment; 1,765 patients were included. We combined effect sizes using the random-effects model due to large heterogeneity (p < 0.00001, I2 = 94%). The ALB levels significantly decreased in the intervention group compared with the control group [MD =0.81, 95% CI (0.41–1.21), p < 0.0001] (Figure 4B).
4.4.3 HGBHGB levels were described in 19 RCTs; 1,568 patients were involved. The random-effects model was used to combine effect sizes (p < 0.00001, I2 = 93%). The HGB levels significantly increased in the intervention group compared with the control group [MD = 1.00, 95% CI (0.57–1.43), p < 0.00001] (Figure 4C).
4.4.4 CREACREA was reported in 22 RCTs, and 1840 patients were included. The random-effects model was used (p < 0.00001, I2 = 95%). The CREA levels significantly decreased in the intervention group compared with in control group [MD = −1.33, 95% CI (−1.79 to −0.87), p < 0.00001] (Figure 4D).
4.4.5 BUNA random-effects model was used to conduct a meta-analysis of 21 RCTs that reported BUN involving 1805 patients (p < 0.00001, I2 = 95%). The BUN levels significantly decreased in the intervention group compared with in [MD = −1.57, 95% CI (−2.07 to −1.07), p < 0.00001] (Figure 4E).
4.4.6 Adverse drug reactions4.4.6.1 Raw incidence of adverse reactionsA total of 4 studies and 346 patients were enrolled. Data were pooled using a fixed-effects model (p = 0.37, I2 = 5%). The results indicated that the raw incidence of adverse drug reactions was no significant differences in the control group than that in the intervention group [MD = 1.81, 95% CI (0.88–3.74), p = 0.11] (Figure 5).

Figure 5. Forest plot of adverse drug reactions.
4.4.6.2 The details of adverse drug reactionsReports of adverse drug reactions were few in the included studies, with six cases of gastrointestinal reaction, one case of fatigue, and five cases of nausea and one case of infection in the experimental group (Table 3).
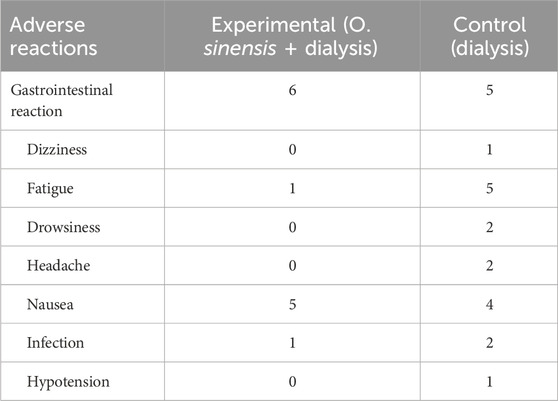
Table 3. The details of reported adverse drug reactions.
4.5 Subgroup analysis4.5.1 CRPThe meta analysis showed that the CRP levels significantly decreased in the intervention group compared with in the control group for patients treated by HD (11 studies, MD: 1.96, 95% CI [−3.17 to −0.75]; I2 = 98%). For patients treated by PD, the CRP levels decreased in the intervention group compared with in the control group (4 studies, MD: −2.93, 95% CI [−4.88 to-0.98]; I2 = 97%) (Supplementary Figure S2A).
In subgroup analyses of different O. sinensis preparations, the CRP levels significantly decreased in Jinshuibao capsule group compared with the control group (6 studies, MD: −0.68, 95% CI [−1.08 to −0.27]; I2 = 30%). The CRP levels significantly decreased in the Bailing capsule group compared with the control group (15 studies, MD: −2.48, 95% CI [−3.70 to −1.26]; I2 = 98%) (Supplementary Figure S3A).
In the subgroup analysis on the impact of intervention duration, the CRP levels significantly decreased in the intervention group compared with the control group (13 studies, MD: −1.95, 95% CI [−3.35 to −0.55]; I2 = 98%) in the duration ≤12 weeks. The CRP levels significantly decreased in the intervention group compared with the control group in the duration>12 weeks (6 studies, MD: −2.64, 95% CI [−4.13 to −1.15]; I2 = 97%) (Supplementary Figure S4A).
In the subgroup analysis of different doses, the CRP levels significantly decreased in the intervention group compared with the control group for patients taking 2–3 capsules (6 studies, MD: −3.32, 95% CI [−6.03 to −0.60; I2 = 99%). The CRP levels significantly decreased in the intervention group compared with the control group for patients taking 4–6 capsules (12 studies, MD: −1.87, 95% CI [−2.93 to −0.82]; I2 = 97%). No significant difference was found between the control and intervention group for patients taking >6 capsules [1 study, MD −0.27, 95% CI (−0.80, 0.26)] (Supplementary Figure S5A).
4.5.2 ALBIn the subgroup analysis of different dialysis methods, the ALB levels significantly increased in the intervention group compared with the control group for patients treated by HD [12 studies, MD: 0.81, 95% CI (0.24 to 1.37); I2 = 95%]. The ALB levels significantly increased in the intervention group compared with the control group for patients treated by PD [8 studies, MD: 0.81, 95% CI (0.24–1.38); I2 = 91%] (Supplementary Figure S2B).
In subgroup analyses of different O. sinensis preparations, the ALB levels significantly increased in the Jinshuibao capsule group compared with the control group [6 studies, MD: 1.32, 95% CI (0.85 to 1.79)]. The ALB levels significantly increased in the Bailing capsule group compared with the control group [19 studies, MD: 0.78, 95% CI (0.36–1.20); I2 = 94%] (Supplementary Figure S3B).
In the subgroup analysis of different intervention duration, the ALB levels significantly increased in the intervention group compared with the control group [14 studies, MD: 0.72, 95% CI (0.13 to 1.30); I2 = 95%] in the duration ≤12 weeks; The ALB levels significantly increased in the intervention group compared with the control group during the study period >12 weeks [8 studies, MD: 0.73, 95% CI (0.38–1.08); I2 = 75%] (Supplementary Figure S4B).
In the subgroup analysis of different doses of O. sinensis preparation, the ALB levels significantly increased in the intervention group compared with the control group for the patients taking 2–3 capsules [10 studies, MD: 0.66, 95% CI (0.34–0.99); I2 = 76%]. The ALB levels significantly increased in the intervention group compared with the control group for the patients taking 4–6 capsules [10 studies, MD: 1.33, 95% CI (0.56–2.09); I2 = 95%]; No significant difference was found in the ALB level between the two groups for the patients taking 4–6 capsules [3 studies, MD: 0.60, 95% CI (–0.17 to 1.38); I2 =91%] (Supplementary Figure S5B).
4.5.3 HGBIn the subgroup analysis of different dialysis methods, for patients treated by HD, the HGB levels significantly increased in the intervention group compared with the control group [12 studies, MD: 1.23, 95% CI (0.75–1.71); I2 = 92%]. The HGB levels significantly increased in the intervention group compared with the control group for patients treated by PD [7 studies, MD: 0.95, 95% CI (0.41–1.49); I2 = 88%] (Supplementary Figure S2C).
In subgroup analysis of different O. sinensis preparations, no significant differences were found in the HGB level between the Jinshuibao capsule group and the control group [6 studies, MD: 0.54, 95% CI (−0.02 to 1.10)]. The HGB levels significantly increased in the Bailing capsule group compared with the control group [16 studies, MD: 1.28, 95% CI (0.88–1.69); I2 = 90%] (Supplementary Figure S3C).
In the subgroup analysis of the different intervention duration, the HGB levels significantly increased in the intervention group compared with the control group in the study period ≤12 weeks [14 studies, MD: 1.18, 95% CI (0.64–1.71), I2 = 93%]. The HGB levels significantly increased in the intervention group compared with the control group during the study period > 12 weeks [8 studies, MD: 1.07, 95% CI (0.63–1.50), I2 = 84%] (Supplementary Figure S4C).
In the subgroup analysis of different doses of O. sinensis preparation, the HGB levels significantly increased in the intervention group compared with the control group for patients taking 2–3 capsules [11 studies, MD: 1.29, 95% CI (0.83–1.76), I2 = 90%]. In HGB levels, the intervention group was significantly increased compared with the control group for patients taking 4–6 capsules [9 studies, MD: 1.08, 95% CI (0.39–1.76), I2 = 92%]. No significant found in the HGB level between the two groups for patients taking >6 capsules [2 studies, MD: 0.36, 95% CI (–0.01 to 0.72), I2 = 0] (Supplementary Figure S5C).
4.5.4 CREAIn the subgroup analysis of different dialysis methods, the CREA levels significantly decreased in the intervention group compared with the control group for patients treated by HD [13 studies, MD: −1.19, 95% CI (−1.70 to −0.68), I2 = 94%]. The CREA levels significantly decreased in the intervention group compared with the control group for patients treated by PD [9 studies, MD –1.56, 95% CI (–2.52 to–0.59), I2 = 96%] (Supplementary Figure S2D).
In the subgroup analysis of different O. sinensis preparations, the CREA levels significantly decreased in Jinshuibao capsule group compared with the control group [6 studies, MD: −1.26, 95% CI (−2.07 to −0.44), I2 = 93%]. The CREA level was decreased in Bailing capsule intervention group compared with the control group [17 studies, MD: −1.35, 95% CI (−1.92 to −0.79), I2 = 95%] (Supplementary Figure S3D).
In the subgroup analysis of different intervention duration, the CREA levels significantly decreased in the intervention group compared with the control group with the study period ≤12 weeks [15 studies, MD: −1.08, 95% CI (−1.52 to −0.64), I2 = 93%]. The CREA levels significantly decreased in the intervention group compared with the control group in the study period > 12 weeks [8 studies, MD: −1.93, 95% CI (−3.27 to −0.59), I2 = 97%] (Supplementary Figure S4D).
In the subgroup analysis of different doses of O. sinensis preparation, the CREA levels significantly decreased in the intervention group compared with the control group for patients taking 2–3 capsules [6 studies, MD: −2.08, 95% CI (−3.07 to −1.10), I2 = 95%]. The CREA levels significantly decreased in the intervention group compared with the control group for patients taking 4–6 capsules [14 studies, MD: −1.06, 95% CI (−1.63 to −0.48), I2 = 94%]. No significant difference in the CREA level was found in the intervention group than in the control group for patients taking >6 capsules [3 studies, MD: −1.01, 95% CI (−2.11 to 0.09), I2 = 94%] (Supplementary Figure S5D).
4.5.5 BUNIn the subgroup analysis of different dialysis methods, the BUN levels significantly decreased in the intervention group compared with the control group for patients treated by HD [13 studies, MD: −1.69, 95% CI (−2.34 to −1.03); I2 = 96%]. The BUN levels significantly decreased in the intervention group compared with the control group for patients treated by PD [8 studies, MD: –1.38, 95% CI (−2.16 to −0.60); I2 = 94%] (Supplementary Figure S2E).
In the subgroup analysis of different O. sinensis preparations, the BUN levels significantly decreased in Jinshuibao capsule group compared with the control group [6 studies, MD −1.36, 95% CI (−1.99 to −0.73), I2 = 89%]. The BUN levels significantly decreased in Bailing capsule group compared with the control group [14 studies, MD: −1.57, 95% CI (–2.27 to −0.86), I2 = 96%] (Supplementary Figure S3E).
In the subgroup analysis of different intervention duration, the BUN levels significantly decreased in the intervention group compared with the control group within the study period ≤12 weeks [15 studies, MD: −1.51, 95% CI (−2.11 to −0.91), I2 = 95%]; The BUN levels significantly decreased in the intervention group compared with the control group in the study period> 12 weeks [7 studies, MD: −1.73, 95% CI (−2.74 to −0.72), I2 = 95%] (Supplementary Figure S4E).
In the subgroup analysis of different doses of O. sinensis preparation, the BUN levels significantly decreased in the intervention group compared with the control group for patients taking 2–3 capsules [6 studies, MD: −1.78, 95% CI (−2.78 to −0.77), I2 = 95%]. The BUN levels significantly decreased in the intervention group compared with the control group for patients taking the 4–6 capsules [13 studies, MD: −1.64, 95% CI (−2.39 to −0.89), I2 = 96%]; The BUN levels significantly decreased in the intervention group compared with the control group for patients taking >6 capsules [3 studies, MD: −0.95, 95% CI (−1.65 to −0.25), I2 = 87%] (Supplementary Figure S5E). Details of the above subgroup analysis are shown in the table below (Table 4).
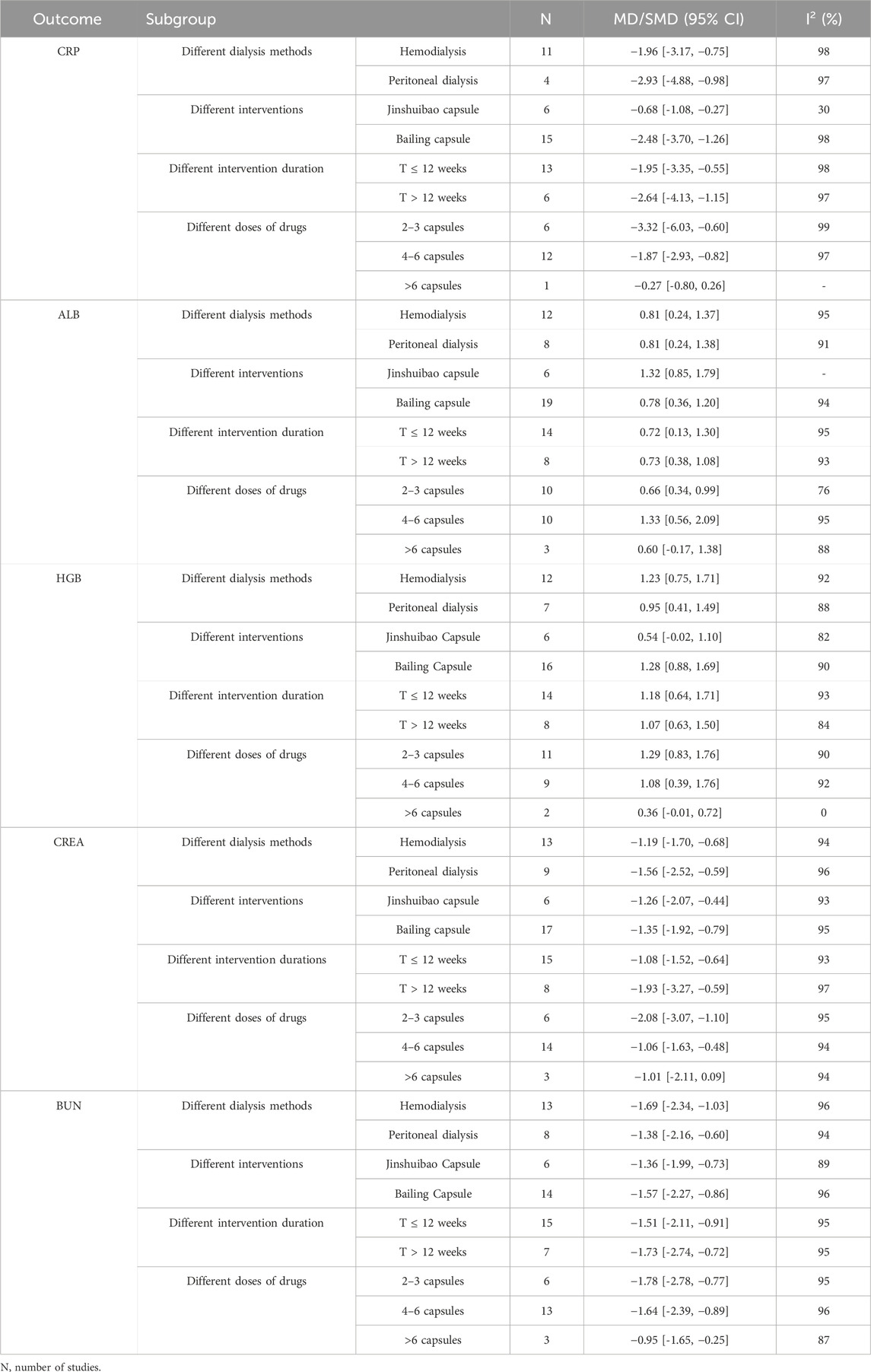
Table 4. Subgroups analysis of outcomes.
4.6 Sensitivity analysisWe performed a sensitivity analysis to assess the robustness of the results and found good robustness after excluding the literature one by one.
4.7 Publication biasWe performed an Egger’s test on five essential outcomes to observe the publication bias, and CRP (p = 0.002 < 0.05), CREA (p = 0.019 < 0.05), BUN(p = 0.025 < 0.05) were showed publication bias, and ALB, HGB p values were >0.05, with no publication bias. After assessing the impact of publication bias on the results with trim-and-fill analyses, the results were found to be reliable (Figure 6).

Figure 6. Funnel plot of publication bias on five essential outcomes. (A) CRP; (B) ALB; (C) HGB; (D) CREA; (E) BUN.
4.8 Quality of the evidenceGRADE was used to assess the quality of outcome evidence for all studies. All outcomes were rated as low or very low according to the GRADE criteria because of serious imprecision and large heterogeneity in findings, and indirectness due to a mix of different interventions and comparators (Table 5).
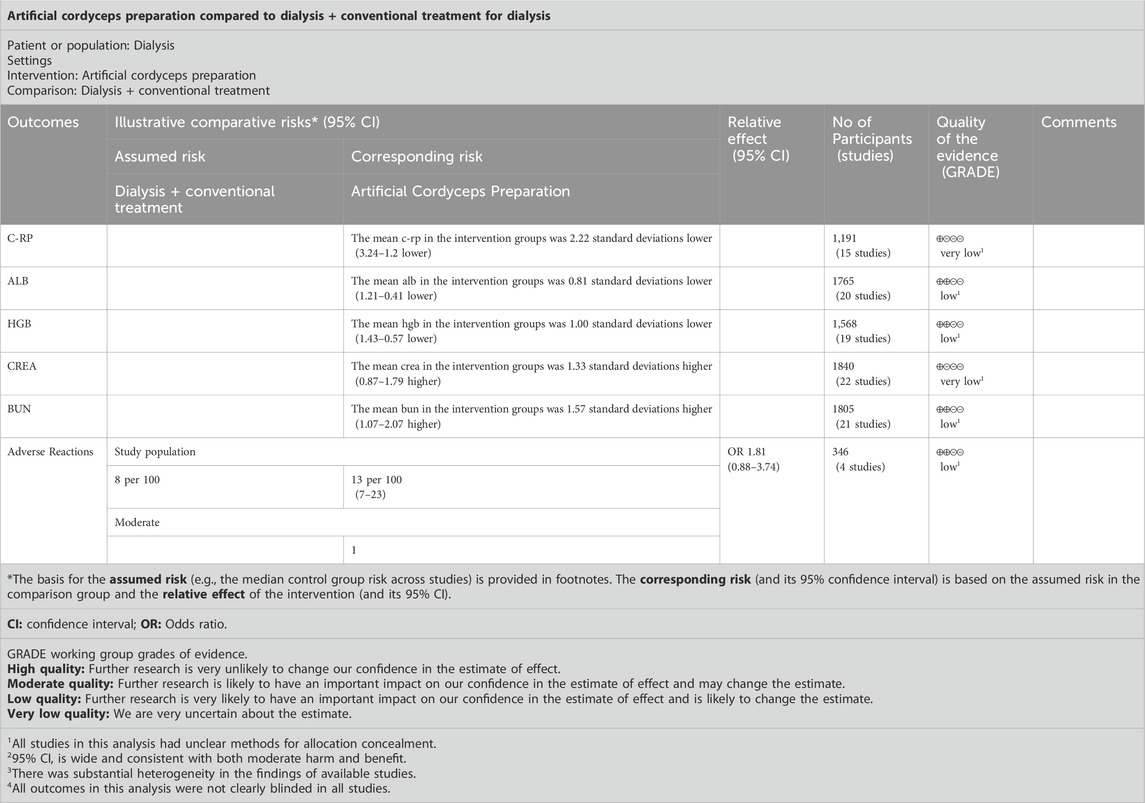
Table 5. Summary of findings table.
5 DiscussionThis review included related RCTs to assess the effects and safety of O. sinensis preparations in adjuvant treatment for patients undergoing dialysis. Alternative outcomes were used due to the lack of reports of the results from the COMET core outcome index set. After meta-analysis, the results showed that O. sinensis preparations could reduce the CREA, BUN, and CRP levels and increase the ALB and HGB levels. Considering the clinical heterogeneity and evidence quality, there are no high-quality evidence to support the use of O. sinensis preparations in adjuvant treatment for patients undergoing dialysis and their harms are under-reported.
From 1990 to 2017, the incidence of dialysis increased by 43.1% with the development of dialysis technology (GBD Chronic Kidney Disease Collaboration, 2020). Approximately 89% of patients undergoing dialysis are treated with HD worldwide, while a minority are treated with PD (Al. and Al). The global dialysis population is proliferating, especially in low- and middle-income countries (Bello et al., 2017); however, many people lack access to kidney replacement therapy, and millions of people die of kidney failure annually worldwide, often without supportive care (Himmelfarb et al., 2020). Thus, new approaches and dialysis modalities that are accessible and offer improved patient outcomes urgently need to be developed.
Chinese caterpillar fungus, or Dong Chong Xia Cao (winter worm summer grass) in Chinese or Tochukaso in Japanese, has been used in China for over 700 years, mainly as a tonic for nourishing the lungs and kidneys (Dong and Yao, 2008). Modern pharmacological studies have shown its therapeutic effect on various diseases and conditions such as the kidneys (Ding et al., 2011; Zhang et al., 2011) as well as on other diseases (Yue et al., 2013b). However, the output of natural O. sinensis cannot fully meet the demands of medical use due to the scarcity of resources and high price, which drives many types of artificial cultivation to make O. sinensis a more affordable material for its use (Qian et al., 2019; Wu et al., 2020; Wang et al., 2022). The highest cordycepin production can be obtained in surface liquid culture using the C. militaris mutant.
The artificial cultivation of C. militaris produces cordycepin. It has a similar pharmacological activity to O. sinensis, which is more accessible; also, multiproduct batch manufacturing has been achieved (Sari et al., 2016; Yue et al., 2013a). This review aimed to assess the role of O. sinensis preparation in the adjuvant treatment for both patients undergoing HD and those undergoing PD. This was the first systematic review to evaluate the efficacy and safety of O. sinensis preparation in adjuvant treatment for two kinds of patients undergoing dialysis (HD and PD). Although a systematic review and meta-analysis of hemodialysis patients was published in 2019, (Bee and Zoriah, 2019), our study, differed greatly from this review. Compared with the previous systematic review, we have some difference in the following aspects. First, our study extended the study population and covered a wider range of subjects, including not only patients undergoing HD but also those undergoing PD. Second, a core outcome set (COS) is the minimum that should be measured and reported in all clinical trials of a specific condition, which also helps streamline the systematic reviewing process (Clarke and Williamson, 2016). Therefore, we searched the COMET database and referred to the dialysis-COS to set the primary and secondary outcomes for our review. Third, we compared the patients with HD to those with PD in response to O. sinensis (Li et al., 2018; Pan, 2019). Altogether, it has been 6 years since the literature search in the last systematic review, and since then, new research evidence has increased. We included latest related studies in recent 6 years, by adding 23 additional studies, and the number of included patients increased to 2,914. Accordingly, new clinical research quesionss with different PICOS have emerged, urging us to carry out a new systematic review. We hope that this systematic review can provide evidence of efficacy and safety for patients undergoing dialysis when using O. sinensis preparation.
5.1 LimitationsThis review was conducted according to a pre-specified protocol and used a highly sensitive search strategy. Two review authors conducted an electronic database search independently and accorded to the evidence certainty for analyzing the results. However, it had several limitations. First, it had restrictions on language, affecting its comprehensiveness. Second, some studies had risk of detection and performance bias due to the lack of blinding. Third, we used COMET outcomes, but only a few studies reported the outcomes. We included other outcomes not directly related to ESRD in this review due to the lack of data, which downgraded the level of evidence. Fourth, the condition of patients in the dialysis period had a certain complexity, and the simultaneous existence of the primary disease and comorbidities led to great clinical heterogeneity and affected our judgment of the results. However, we did not perform a subgroup analysis of patients with different primary diseases or comorbidity due to the lack of study reports, which might account for a risk due to inconsistency.
6 ConclusionIn conclusion, O. sinensis can serve as an adjuvant treatment for patients undergoing dialysis by improving patient renal function, malnutrition, and microinflammation. However, few studies reported clinically relevant outcomes and the methodological quality of the included studies were generally low. Therefore, to using COMET outcomes in trials and providing more reliable evidence through high-quality RCTs are necessary.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributionsML: Writing–review and editing, Writing–original draft, Software, Investigation, Formal Analysis, Data curation, Conceptualization. CC: Writing–review and editing, Writing–original draft, Software, Methodology, Data curation. TC: Writing–review and editing, Writing–original draft, Visualization, Supervision, Software, Data curation, Validation, Methodology, Formal Analysis. QZ: Writing–review and editing, Conceptualization, Visualization. YC: Writing–review and editing, Validation, Resources, Project administration, Methodology, Formal Analysis. SZ: Visualization, Validation, Supervision, Project administration, Funding acquisition, Data curation, Writing–review and editing. XL: Writing–review and editing, Writing–original draft, Visualization, Validation, Supervision, Project administration, Funding acquisition.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by China Academy of Chinese Medical Sciences Innovation Fund (No. CI 2021B003; No. CI 2021A05503), the National Key Research and Development Program (Project number: 2019YFC1709903), Basic TCM evidence-based capacity building Project of the Affiliated Hospital of Changchun University of Chinese Medicine (2019), Chinese Medicine Evidence-based Capacity Enhancing Project (2023).
AcknowledgmentsThe authors thank TC (GCP Department) and YC (GCP Department), from the Affiliated Hospital to Changchun University of Chinese Medicine, Changchun, China, for their assistances in developing the statistical strategy.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be co
留言 (0)