The mitral valve (MV) apparatus modulates blood flow between the left atrium and left ventricle and structurally consists of an annulus, two leaflets, chordae tendineae, and two papillary muscles.[1,2] MV pathologies can involve single or multiple levels of the apparatus, causing MV dysfunction leading to either stenosis or regurgitation or both, diagnosed mostly on echocardiography.[3] These can be caused by rheumatic heart disease, congenital malformations, ischemic heart disease, infections, or collagen-vascular diseases, causing signs and symptoms of MV dysfunction with subsequent need for medical or surgical treatment.[4,5]
In patients with severe or symptomatic MV disease, MV surgery (repair or replacement) is the usual strategy that reduces symptoms and improves survival, other treatment options include percutaneous interventions such as transmitral commissurotomy for mitral stenosis (MS) and transcatheter edge-to-edge repair for mitral regurgitation (MR).[6-9] Surgical treatment includes repair or valve replacement, and the choice of the valve (mechanical or bio-prosthetic) is individualized based on many factors including age, the durability of the valve, need for anticoagulation, patient as well as the surgeon’s preference, and many more.[10,11] The latest guidelines for surgical treatment and prosthetic heart valve selection recommend repair as the preferred treatment. However, in patients with rheumatic or extensive mitral valvular damage/calcification, replacement is usually indicated, with either type of prosthetic valve for patients aged 60–70 years, mechanical prosthetic valve for patients younger than 60 years, and bioprosthetic valve for patients older than 70 years.[6-9] Younger patients face a much higher lifetime risk of reoperation due to a higher rate of bioprosthetic structural valve degeneration, whereas reoperation rates are much lower with a mechanical valve, but there is an increased risk of thromboembolic/hemorrhagic complications and need for lifelong anticoagulation with a mechanical valve.[6,7,12,13] Extensive international literature is available on the outcomes of bioprosthetic MV replacement (MVR), but there are limited local data in South Asian countries.
Recently, it has been observed that surgeons in our population are implanting a larger number of bioprosthetic MV in younger patients, probably due to a greater prevalence of rheumatic heart disease with extensive valvular damage, risk of thrombosis with mechanical valve, and limited heart-valve clinics for decision-making and poor adherence to anticoagulation in less educated population. However, bioprosthetic valve degenerates rapidly in younger people.[10,11] There is no such study that has evaluated the outcomes of patients with bioprosthetic MV in our population.
This study aims to evaluate the clinical characteristics and long-term outcomes of patients with bioprosthetic MVR at a tertiary care hospital in a South Asian country.
This article has been presented as an abstract in the Pakistan Cardiac Society’s Cardiology conference and published in Pakistan Heart Journal on November 16, 2023.
MATERIAL AND METHODS Study designThis was a retrospective observational study.
Study populationAll the patients who underwent bioprosthetic MVR at a tertiary care hospital in Karachi, Pakistan from the year 2006 to 2020, had at least two complete echocardiograms: A pre-surgery echocardiogram and a follow-up echocardiogram which were done within the past 2 years (2021-2022) of our study were included in the study. Patients with incomplete clinical data, mechanical MVR, or MV repair were excluded from the study.
Ethical considerationThe study was started after getting approval from the Ethical Review Board (ERB) committee of our hospital. All the patients who fulfilled the inclusion criteria were included in the study. Data were collected on a predesigned data entry form, after reviewing the electronic medical records of all the patients. The study was conducted in compliance with the protocol and ethical board’s regulatory requirements. Complete privacy was ensured. The patient’s medical record number was the only identifiable information that was collected. Only ERB-approved study personnel had access to the data.
StatisticsClinical and surgical characteristics along with transthoracic echocardiographic findings (pre-surgery and recent most follow-up studies) were noted. The etiology of MV dysfunction was divided into rheumatic, degenerative, and ischemic based on the transthoracic echocardiographic appearance of the MV and relevant past medical history.[5] Bioprosthetic MV dysfunction (BMVD) was defined as a mean gradient of >5 mm Hg with restricted/reduced leaflet motion associated with/without valve degeneration, calcification, thrombus, pannus, vegetation, or patient-prosthesis mismatch, and/or newly developed MR on follow-up transthoracic echocardiography. The severity of bioprosthetic MR was evaluated solely through transthoracic echocardiography, adhering to the latest guidelines.[11] Although transesophageal and 3D echocardiography are considered more reliable in cases where technical limitations exist with transthoracic echocardiography, we did not include patients undergoing these methods. Follow-up data were also collected after reviewing medical records and telephonic interviews of the patients after obtaining informed verbal consent from the patient or their first-degree relative if the patient was not available.
Data were analyzed using statistical software Statistical Package for the Social Sciences version 23. In the descriptive analysis, mean and standard deviation were calculated for continuous variables and percentages for categorical variables. The Chi-square test was applied to all the categorical variables and analysis of variance was applied to the continuous variables on a 95% confidence interval considering P < 0.05 as statistically significant.
RESULTSA total of 502 patients with bioprosthetic MV implantation from 2006 to 2020, who fulfilled the inclusion criteria were included in the study. Out of 502 patients, 322 (64%) were female, the mean age of the patients at the time of surgery was 49.42 ± 14.56 years, and the most prevalent comorbid condition was hypertension in 281 (56%) patients followed by diabetes in 161 (32.1%) patients. The highest number of patients at the time of index surgery was in the age range of 49–58 years [Figure 1].
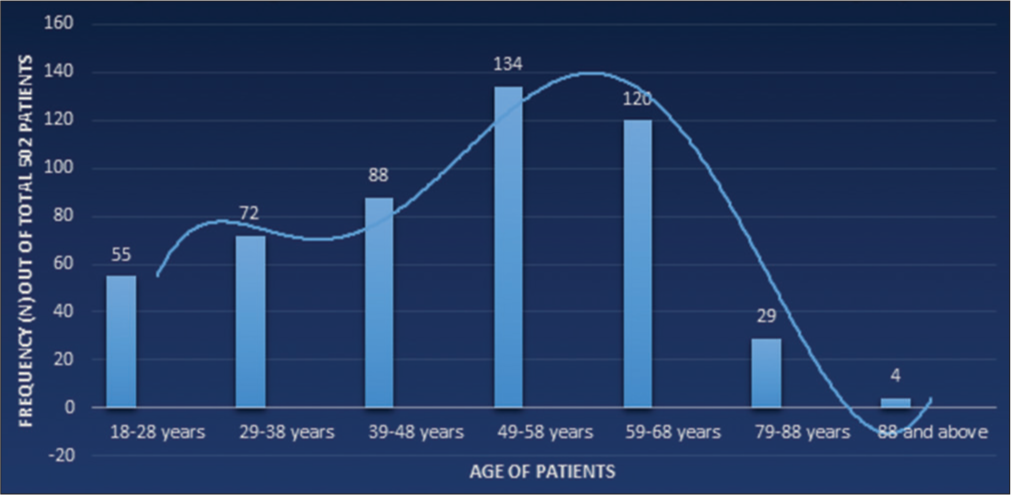
Export to PPT
Baseline characteristics of patients at the time of surgery are shown in Table 1. Most patients (n = 390, 77.7%) showed no significant coronary artery disease (CAD), while 112 (22.3%) patients were diagnosed with moderate–to-severe CAD during pre-surgery assessment. MR was more common, found in 279 (55.6%) patients while MS was the underlying lesion in 188 (37.5%) patients, at the time of index surgery. Combined MS and regurgitation were present in only 35 (7%) patients. Concomitant aortic regurgitation was observed in 243 (48.4%) patients, while aortic stenosis was identified in 97 (19.3%) patients. In addition, tricuspid regurgitation was present in 378 (75.3%) patients. Left ventricular ejection fraction (LVEF) was normal in the majority of patients (n = 399, 79.5%) before surgery.
Table 1: Baseline characteristics.
Clinical characteristics Number (n=502) Percentage Male 180 35.9 Female 322 64.1 Hypertension 281 56 Diabetes Mellitus 161 32.1 Coronary artery disease 112 22.3 Dyslipidemia 95 18.9 Chronic kidney disease 3 0.6 Echocardiographic features MR 279 55.6 MS 188 37.5 Both MS and MR 35 7 Etiology of mitral valve dysfunction Rheumatic 306 61 Degenerative (calcified/myxomatous) 183 36.4 Ischemic 13 2.6 Left ventricular ejection fraction before surgery Normal 399 79.5 Mildly reduced 40 8 Moderately reduced 31 6.2 Severely reduced 32 6.4 Concomitant aortic regurgitation Mild 113 22.5 Moderate 75 14.9 Severe 55 11 Concomitant aortic stenosis Mild 46 9.2 Moderate 15 3 Severe 36 7.2 Concomitant tricuspid regurgitation Mild 146 29.1 Moderate 109 21.7 Severe 123 24.5Surgical details are shown in Table 2. In most of the patients (n = 446, 88.8%) MVR was done as an elective procedure due to NYHA II to IV symptoms at the time of index surgery. Among individuals diagnosed with CAD, 104 (20.7%) patients underwent both coronary artery bypass surgery and MVR. In addition, 5 (1%) patients had undergone percutaneous coronary intervention before surgery, while 3 (0.6%) patients were medically managed due to inadequate surgical targets. Concomitant aortic valve replacement was done in 115 (23%) patients with moderate-to-severe aortic valve disease (isolated or mixed). The mean length of stay during index admission was 8.47 days ± 2.93.
Table 2: Surgical and post-surgical details.
Number (n=502) Percentage Type of surgery Elective 446 88.8 Emergent or urgent 56 11.2 Reason for emergent or urgent surgery Heart failure 41 8.2 STEMI with papillary muscle rupture 3 0.6 Mitral valve abscess 5 1 NSTEMI 7 1.4 Concomitant other valve surgery Aortic valve replacement (mechanical AVR) 115 23 Tricuspid valve repair 140 27.9 Post-surgical complications (During hospital stay) Unstable arrhythmia 55 11 Major Hemorrhage (Thrombolysis in Myocardial Infarction criteria) 6 1.2 Pneumonia 9 1.8 Stable arrhythmia 41 8.2 Respiratory failure 6 1.2 Complete heart block 4 0.8 Stroke 9 1.8 Cardiac tamponade 2 0.4 Septic shock 5 1During the hospital stay, immediate post-surgical complications were observed, with unstable arrhythmia being the most common (11%), followed by stable arrhythmia (8.2%), pneumonia (1.8%), stroke (1.8%), and major bleeding/hemorrhage according to TIMI scoring (1.2%).[14]
In the mean follow-up period of 6.59 ± 2.99 years, BMVD was observed in 183 (36.5%) patients, with valve degeneration as the most common pattern of structural deterioration (n = 139, 27.7%), as shown in Table 3 Nonetheless, redo MV surgery was performed in only 49 (9.8%) patients within our facility.
Table 3: Follow-up details.
Number (n=502)/Mean±SD Percentage Echocardiographic features Left ventricular ejection fraction on follow-up compared to the pre-surgery No change 442 88 Mildly reduced 10 2 Moderately reduced 4 0.8 Severely reduced 46 9.2 Condition of bioprosthetic mitral valve Bioprosthetic mitral valve dysfunction (BMVD) 183 36.5 Degenerative valve 139 Valve calcification 18 Pannus formation 8 Vegetation 8 Patient prosthesis mismatch 4 Malalignment/dislocation 4 Thrombus formation 2 Severity of mitral regurgitation (valvular/paravalvular) Mild mitral regurgitation 18 Moderate mitral regurgitation 94 Severe mitral regurgitation 71 Pressure half time in BMVD 100.137±32.969 Mean gradient in BMVD 13.989±4.202 Peak gradient in BMVD 27.524±5.788 Doppler velocity index (DVI)All the patients were divided into two groups, based on normal functioning bioprosthetic MV or BMVD on follow-up. A comparison between the two groups is shown in Table 4.
Table 4: Comparison between normal functioning bioprosthetic mitral valve and BMVD.
Normal functioning bio-prosthetic mitral valve n=319 (%) BMVD n=183 (%) P-valuea Gender Male 119 (37.3) 61 (33.3) 0.372 Female 200 (62.7) 122 (66.7) Meanb Age (in years) 51.6±14.27 45.639±14.33 0.000 Age categories at the time of MVR (in years) 18–28 25 (7.83) 30 (16.3) 0.000 29–38 38 (12) 34 (18.6) 39–48 60 (19.1) 28 (15.3) 49–58 84 (26.3) 50 (27.3) 59–68 82 (25.7) 38 (20.8) 69–78 26 (8.1) 3 (1.63) 79–88 4 (1.25) 0 Comorbidities Hypertension 195 (61.11) 86 (46.99) 0.002 Diabetes mellitus 114 (35.7) 47 (25.7) 0.020 History of coronary artery disease 80 (25.0) 32 (17.48) 0.049 Type of admission at the time of surgery Elective 278 (87.1) 168 (91.8) 0.111 Emergent/urgent 41 (12.85) 15 (8.2) Presurgery echocardiography Mitral regurgitation 180 (56.4) 99 (54.1) 0.161 Mitral stenosis 122 (38.24) 66 (36.0) Dual mitral valve pathology 17 (5.3) 18 (45.35) Concomitant significant (moderate or severe) aortic regurgitation 79 (24.76) 51 (27.86) 0.784 Concomitant significant (moderate or severe) aortic stenosis 28 (8.8) 23 (12.56) 0.335 Concomitant significant (moderate or severe) tricuspid regurgitation 153 (47.96) 79 (43.16) 0.177 Left ventricular ejection fraction Normal 240 (75.2) 159 (86.9) 0.018 Mildly reduced 30 (9.4) 10 (5.46) Moderately reduced 23 (7. 2) 8 (4.4) Severely reduced 26 (8.15) 6 (3.3) Concomitant aortic valve replacement 72 (22.6) 43 (23.5) 0.812 Concomitant tricuspid valve repair 96 (30.1) 43 (23.5) 0.097 Long-term complications Heart failure 0 16 (8.74) 0.000 Atrial fibrillation 1 (0.31) 11 (6.01) Infective endocarditis 0 8 (4.4) Death after follow-up echo 0 6 (3.28)Females were in the majority in both groups with almost similar percentages, the mean age of patients at the time of index surgery was 51.6 ± 14.27 years in the normal functioning bioprosthetic MV group while it was 45.6 ± 14.33 years in the BMVD group. There were more patients with younger ages in the BMVD group, with a statistically significant difference (P = 0.000).
There was no significant difference in the echocardiographic findings at the time of index surgery among the two groups, except that the LVEF was lower in the normal functioning bioprosthetic MV group.
DISCUSSIONThis study is the first of its kind which has assessed the outcomes of bioprosthetic MVR in a relatively young Pakistani population, as opposed to earlier studies where bioprosthetic MVR was done in middle-aged to elderly patients.
Similar to earlier studies,[4,5,15] this study also showed that MV disease requiring surgical MVR is more common in young females, likely due to rheumatic heart disease involvement at a younger age.
In contemporary clinical practice, there is considerable disagreement about which type of valve to use for patients aged 50–70 who require MVR,[16] and a mutual (doctor-patient) decision-making is considered appropriate to decide about the type of valve, keeping in view the age of the patient, life expectancy, reoperation risk, anticoagulation related adverse events, and the patient preference. On the other hand, the latest guidelines recommend that younger patients (age <50 years) should undergo mechanical valve replacement due to its longer durability as compared to bioprosthetic valves.[6,7]
Similarly, Goldstone et al.[13] showed that mechanical valve replacement was associated with a long-term mortality benefit, as compared to a bioprosthesis, especially in younger patients undergoing surgical MVR. Another retrospective study from Taiwan showed that the all-cause mortality and re-do operation rates in the bioprosthetic valve group were comparatively higher than those in the mechanical valve group, while no group difference was observed in major adverse cardiovascular events.[17] In our study population, bioprosthetic valves are still used in younger patients despite the recommendation against bioprosthetic valves in younger populations. Bioprosthetic valves degenerate rapidly in younger people.[10,11] However, probably due to the risk of thrombosis with mechanical valves, limited medical facilities, and poor adherence to anticoagulation in less educated populations, surgeons still preferring bioprosthetic valves even in younger populations. In the case of younger females, reproductive age might be another factor when considering the choice of prosthetic MV.
One of the studies conducted in South Korea[18] demonstrated that the risk factors associated with BMVD and poor clinical outcomes include young age at operation, chronic kidney disease, and increased pressure gradients across the bioprosthetic MV early after surgery. Studies by Kulik et al. and Ruel et al.[19,20] demonstrated that middle-aged patients with bioprosthetic valves were associated with more major adverse prosthesis-related events as well as a rise in the need for reoperation.
Despite the short follow-up period (6.59 ± 2.99 years) of our study, we also observed that many patients (n = 183, 36.5%) developed BMVD with degeneration and calcification being the most frequent structural deteriorations. This is likely due to using bioprosthetic MV s at a younger age, as previously demonstrated in various studies.[18-21]
In our study, patients who developed BMVD were younger compared to the group with normal functioning bioprosthetic MV, with a statistically significant difference. However, redo surgery was done in only 9.8% of our patients. The small number of redo surgeries may be due to the short follow-up, we have done or due to reluctance to redo surgery by either the patient or the surgeon.
With the increased use of bioprosthetic MVs, the clinical outcomes of patients using bioprostheses require more attention. We observed that patients with BMVD developed significantly more long-term complications compared to those with normal functioning bioprosthetic MVs. Among various complications, heart failure (8.74%) and atrial fibrillation (6.01%) were the most frequent ones, which can be attributed to increased gradients and left atrial dilatation in BMVD group. Six patients died in the BMVD group with multi-organ dysfunction and cardiac arrest as the main causes of death.
However, our study has some limitations as it was a single-center retrospective observational study, so we did not evaluate surgical techniques or the type of bioprosthetic valves, which may have an impact on the results. In addition, multivariate analysis was not done due to a relatively smaller number of patients with bioprosthetic MV abnormalities.
CONCLUSIONTo the best of our knowledge, this is the first study in the existing literature that has been conducted to assess the outcomes of bioprosthetic MVR in a relatively younger South Asian population. We conclude by saying that in our population, younger patients (patients younger than 50 years of age) are undergoing bioprosthetic MVR at a higher rate, resulting in an earlier onset of BMVD and poor long-term outcome.
留言 (0)