Chronic kidney disease (CKD) is defined as evidence of abnormalities in renal structure or function for at least 3 months, with implications for health (Stevens, 2013) including increased bone and cardiovascular morbidity, and significantly reduced life expectancy and quality of life (Ortiz, 2022). Patients in terminal stages (i.e., a 0.13% of the whole population) become dependent on costly replacement therapy in the form of dialysis or renal transplant (Ortiz, 2022). Due to its high prevalence and the absence of an effective treatment, CKD is one of the leading causes of mortality worldwide (Kovesdy, 2022). It is estimated that 10% of the adult population suffers from some degree of CKD, and that by 2100 CKD will be the second leading cause of death from disease (Ortiz, 2022). These data show the need to develop strategies to reduce these numbers, and measures for the prophylactic mitigation of its causes. Several factors have been associated with the risk of developing CKD. Non-modifiable factors include age, sex, race or family history, while others can be averted, treated pharmacologically or reduced, such as obesity, hypertension, diabetes and the use of tobacco or analgesics (Kazancioğlu, 2013). In the last decades, hyperuricemia has been proposed as a potential risk factor for CKD (Feig, 2020).
Hyperuricemia, an excess of uric acid (UA) in the blood, is a common cause of gout (Brook et al., 2010). In 1940, it was observed that a high number of patients with gout also suffered from kidney disorders (Coombs et al., 1940). A later study indicated that 90% of autopsied patients with gout also had kidney damage (glomerulosclerosis, tubulointerstitial fibrosis, and arteriosclerosis) (Talbott and Terplan, 1960). It was suggested that kidney damage was caused by the deposit of urate crystals found in the tubules and the interstitium in these patients. Subsequent studies with animal models confirmed that urate crystals directly cause tubular damage, in part mediated by oxidative stress (Sánchez-Lozada et al., 2002). However, additional pathological factors related to hyperuricemia could exist because urate deposits have also been detected in gouty patients with no evidence of renal damage (Yü and Berger, 1982). Furthermore, a preclinical study determined that mild and transient hyperuricemia, in the absence of urate deposits, also aggravated and accelerated CKD (Mazzali et al., 2001). Other proposed mechanisms of kidney damage caused by hyperuricemia include mitochondrial dysfunction, renin-angiotensin system overactivation, and endothelial dysfunction produced by a reduction of nitric oxide and excessive release of vasoconstrictors (e.g., endothelin and thromboxane) (Mazzali et al., 2001; Mallat et al., 2016; Bonino et al., 2020).
If hyperuricemia plays a significant role in CKD, reducing its levels should have a beneficial effect on renal function (i.e., slowing down or reverting disease progression). In this regard, controversial evidence exists. While numerous clinical studies support a renoprotective effect of hypouricemic therapy [reviewed in (Richette et al., 2018)], others did not find a positive association [reviewed in (Leoncini et al., 2022)]. Meta-analyses that have been performed to date (Bose et al., 2014; 2014; Chen et al., 2020; Sun et al., 2020; Liu et al., 2021; Tsukamoto et al., 2021; Gonçalves et al., 2022) included trials in which the comparator was placebo, usual care, or an alternative medicine, in a mixed manner. Almost invariably, existing meta-analyses only ascribe protection to those treatments that improve renal function and, thus, may underestimate their efficacy. A strict comparison versus placebo/control is necessary to discern whether anti gout treatments improve renal function or merely slow down its progressive decay. Accordingly, with the objective of studying the effect of hyperuricemia-lowering strategy on renal function in CKD, we meta-analyzed only those studies containing a placebo/control arm and carried out a correlation study between hypouricemic efficacy and renal protection.
2 MethodsThe protocol of this systematic review is registered in PROSPERO with the code CRD42022306646 (25/02/2022). The entire procedure described below was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.1 Systematic study miningA bibliographic search of articles published up to September 2023 in MEDLINE and the Web of Science databases was carried out. MeSH terms and keywords were used in order to maximize article mining. In the PUBMED platform, the MeSH terms used were “Chronic Renal Insufficiency” [Mesh]) and “Gout Suppressors” [Pharmacological Action]. In both platforms, the terms were used independently as follows “((“Kidney Failure, Chronic”) OR (“Chronic Kidney Failure”) OR (“Chronic Kidney Injury”) OR (Renal Failure, Chronic) OR (Chronic Renal Injury) OR (chronic renal disease) OR (CKD) OR (CKF)) AND ((“Gout Suppressants”) OR (Allopurinol) OR (Benzbromarone) OR (benziodarone) OR (Colchicine) OR (Febuxostat) OR (halofenate) OR (Probenecid) OR (sulfinpyrazone) OR (tricrynafen) OR (zoxazolamine) OR (pegloticase) OR (rasburicase) OR (losartan))”. In both cases, the human filter was added to select only clinical trials. Subsequently, an additional search was carried out introducing each drug individually along with the terms specified for kidney damage, which further enhanced article identification.
2.2 Inclusion and exclusion criteriaFirst, two members of the research team (A.G.C. and L.V.-V.) independently identified those studies that met at least one of the following exclusion criteria: 1) reviews, protocols, case-reports, congress abstracts, editor letters or comments; 2) pre-clinical studies; 3) only abstract available; 4) unrelated content; or 5) language other than English, Spanish, Italian, French or Portuguese. Among the remaining studies, only those that met all the following inclusion criteria were definitively selected: 1) Randomized studies in which urate-lowering therapy is administered in patients with CKD; 2) Studies that evaluate renal function by estimated Glomerular Filtration Rate (eGFR), serum creatinine (sCr), albuminuria or proteinuria [reporting the mean and a measure of dispersion that allow calculation of the standard deviation (SD)]; 3) Studies that present baseline and follow-up data; and 4) Studies that include a control or placebo group. After comparing the list of articles selected by both researchers, a third member of the team (A.I.M.) was designated to resolve potential discrepancies.
2.3 Data extractionThe following data were extracted from each selected article: study reference (first author and year of publication), design, location, patient recruitment dates, type of population, number of patients in the treated group and in the placebo/control group, evaluated treatment (drug, dose and duration of treatment). Clinical study design quality was calculated according to the Jadad scale (Jadad et al., 1996) (studies with a score of five were considered rigorous, scores between three and five were considered good quality, and scores below three were considered poor quality (and were eliminated). Additionally, the mean SD values of the parameters serum uric acid (sUA), eGFR, sCr, albuminuria and/or proteinuria were registered (or calculated from the standard error of the mean or the confidence interval). When verifying that only two studies evaluated albuminuria, it was decided to dispense with this biomarker. From these numerical data, the mean increase in each biomarker (BMΔ) was calculated in the treated and the control/placebo groups with the formula: BMΔ = BMF− BMB, where BMF is the mean value of the biomarker at the end of the nephroprotective treatment, and BMB is the mean baseline value of the biomarker. The standard deviation resulting from this difference, sΔ, was also calculated as the accumulation of errors: sΔ = sF2+sB2, where sF is the SD value of the biomarker at the end of the nephroprotective treatment, and sB is the SD value of the biomarker at baseline. Since most of the included studies evaluated the drugs allopurinol and febuxostat, these analyzes were performed independently for each of these treatments and for an additional group of drugs called ‘Others'.
2.4 Meta-analysisHeterogeneity between studies was assessed with the Cochran’s Q test under the null hypothesis of homogeneity (p < 0.05 indicated heterogeneity) and the I2 index (I2 > 50% indicated high heterogeneity). After this, the fixed-effects model (for homogeneous studies) or the random-effects model (for heterogeneous studies) was selected to meta-analyze the data. The Hedges’ g value and its 95% confidence interval were calculated for each study and each renal function biomarker with the following formula:
where:
sp=nT−1s∆T2+nC/P−1s∆C/P2nT−1+nC/P−1where BMΔT and BMΔC/P are the biomarker increases in the treatment and in the control/placebo groups, respectively; s∆T2 and s∆C/P2 are the standard deviations of the treatment and the control/placebo groups, respectively; and nT and nC/P correspond to the sizes of the treatment and control/placebo groups, respectively. Forest plots were constructed in which the g parameters of the different included studies were compared.
Finally, funnel plots in which the Hedges’ g of each study was represented versus its standard error were constructed to evaluate potential publication bias. In addition, the asymmetry tests of Begg and Mazumdar (Begg and Mazumdar, 1994) and Egger et al. (Egger et al., 1997) were applied. All the analyses described in this section were carried out with the Meta-Essentials set of workbooks (Suurmond et al., 2017).
2.5 Correlation studyIn order to study the relationship between the ability of the tested treatments to reduce sUA levels and to improve renal function, a Pearson correlation test was performed (for normal data, which was previously verified with the Saphiro-Wilk test). Only the treated groups of those studies that quantified both evolution in sAU and eGFR from the start to the end of treatment were included. p-values lower than 0.05 were considered statistically significant. This analysis was performed with the IBM SPSS Statistics 20.0 software (International Business Machines, Armonk, NY, United States).
3 Results3.1 Description of included studiesA flowchart of the search process followed for the selection of the 29 clinical studies finally included is presented in Figure 1 (Katholi et al., 1998; Siu et al., 2006; Kanbay et al., 2007; Malaguarnera et al., 2009; Nouri-Majalan, 2009; Zhu et al., 2009; Goicoechea et al., 2010; 2015; Momeni et al., 2010; Kao et al., 2011; Shi et al., 2012; Sezer et al., 2014; Bayram et al., 2015; Sircar et al., 2015; Golmohammadi et al., 2017; Krishnamurthy et al., 2017; Ghane Sharbaf and Assadi, 2018; Kimura et al., 2018; Wada et al., 2018; Johnson et al., 2019; Lee and Lee, 2019; Badve et al., 2020; Doria et al., 2020; Perrenoud et al., 2020; Wen et al., 2020; Jeyaruban et al., 2021; Stack et al., 2021; Nata et al., 2023; Yang et al., 2023).
Figure 1. Flowchart of the search process.
The descriptive data extracted from the clinical studies included in this meta-analysis are shown in Table 1. Total patients add up to 4,471 (44.1% receiving an UA-lowering therapy). The drug predominantly used is allopurinol followed by febuxostat.
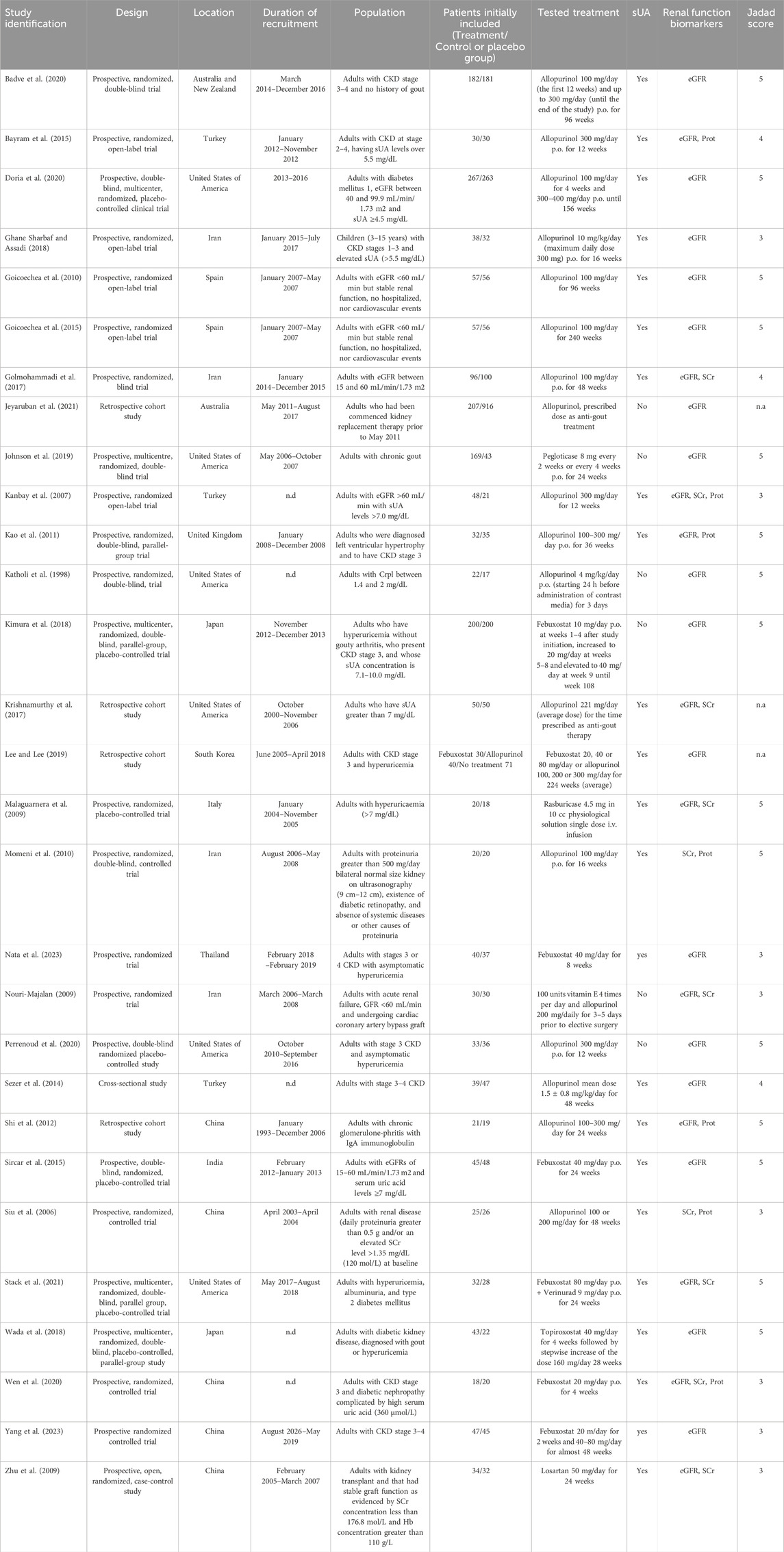
Table 1. Characteristics of the clinical studies included in this meta-analysis. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; i.v., intravenous; n.a., not applicable; n.d., not described; p.o., per os (orally); Prot, proteinuria; SCr, serum creatinine concentration; sUA, serum uric acid concentration.
3.2 Results of the meta-analysis3.2.1 Hypouricemic efficacyThe ability of the treatments to reduce sUA levels in the clinical trials included in this study is summarized in Figure 2. The two most evaluated compounds (allopurinol and febuxostat) significantly reduce sUA levels in 88% and 100% of the trials, respectively. Although higher for febuxostat, the combined meta-analytical result is very favorable for both drugs. In the case of allopurinol, higher effectiveness was observed for doses over 100 mg/day (regardless of the duration of treatment). In contrast, in the case of febuxostat, all tested doses (between 20 and 80 mg) showed similar beneficial effects. On the other hand, among the therapies evaluated to a lesser extent, only topiroxostat showed a highly significant hypouricemic effect.
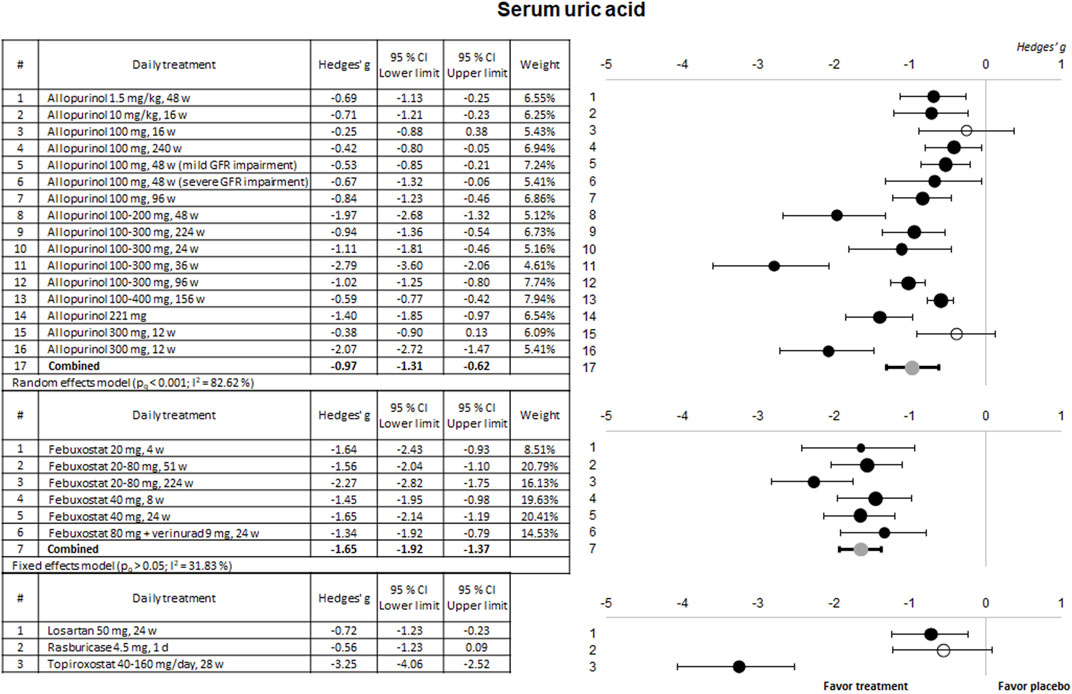
Figure 2. Meta-analytical results of the uric acid-lowering capacity of the therapies evaluated in the included clinical trials. Data are shown as a forest plot representing the difference in the means between the treated group and the control/placebo group for each trial. Effect size is measured as Hedges’ g ± 95% CI. CI: confidence interval; GFR, glomerular filtration rate; w, weeks.
3.2.2 Nephroprotective effectMost of the included trials evaluated the nephroprotective effect through the eGFR. The results of their meta-analysis are presented in Figure 3.
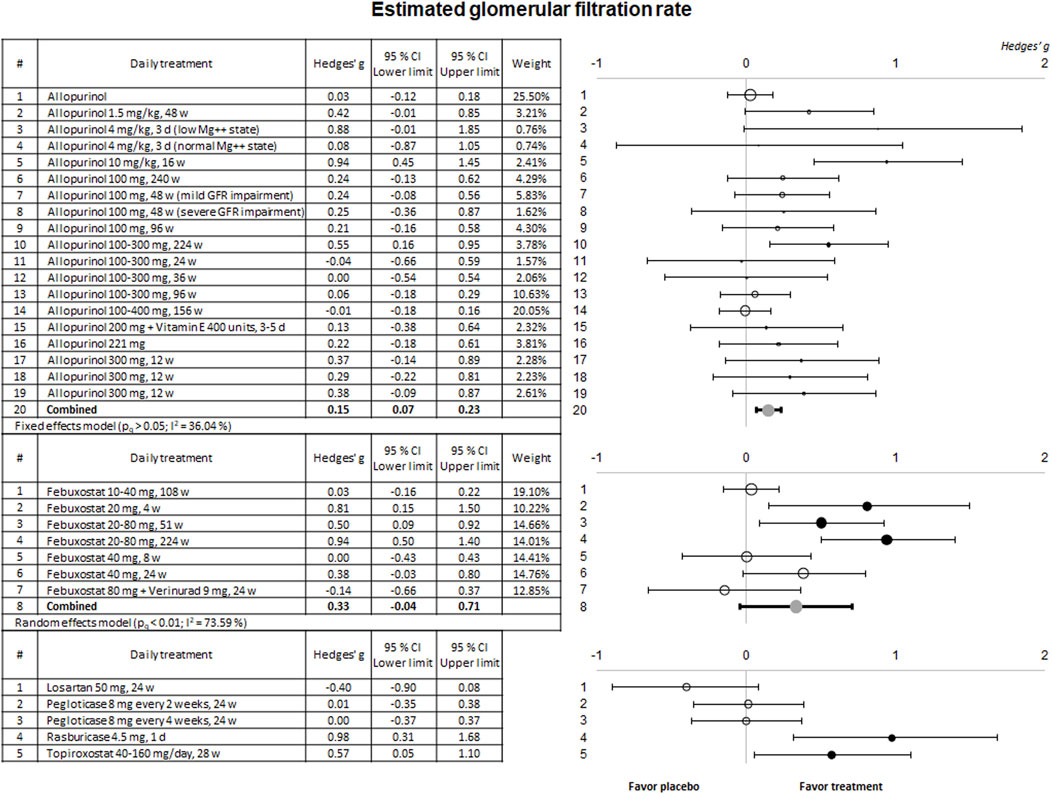
Figure 3. Meta-analytic results of the ability of evaluated therapies to improve or prevent eGFR deterioration. Data are shown as a forest plot representing the difference in the means between the treated and the control/placebo groups for each trial. Effect size is measured as Hedges’ g ± 95% CI. CI: confidence interval; d, days; eGFR, estimated glomerular filtration rate; w, weeks.
The combined result for allopurinol shows a significant nephroprotective effect. Of note, in practically all the studies, the effect on eGFR showed a very high interindividual variability. However, the tendency in almost all the studies using allopurinol shows a beneficial effect on their patients. In no case renal function worsened. In the case of febuxostat, only three studies using the 20 or 20–80 mg/day dosage demonstrated a significant nephroprotective effect. A beneficial effect on renal function was also seen in the only trial using rasburicase or topiroxostat.
Some of the selected clinical trials also evaluated kidney function using sCr and urinary protein excretion. The results of the meta-analysis for these parameters are shown in Figures 4, 5, respectively.
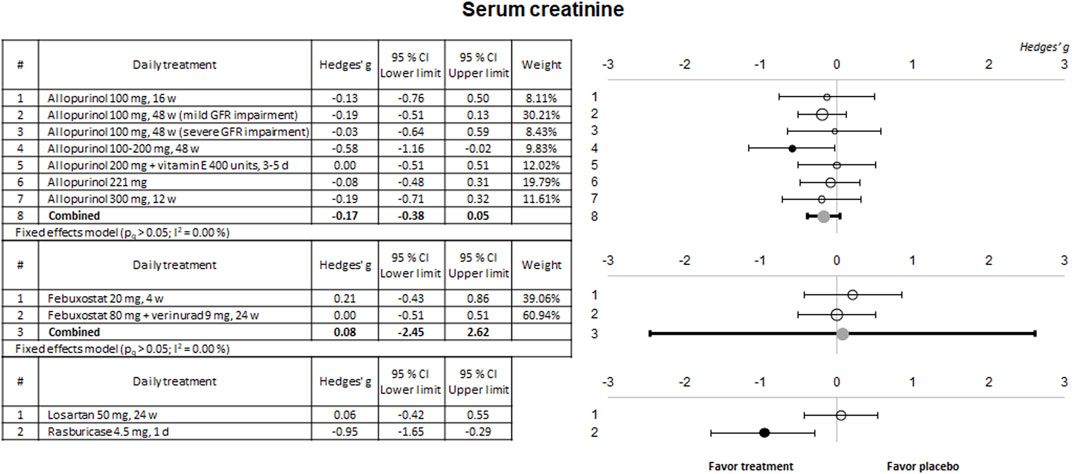
Figure 4. Meta-analytic results of the ability of evaluated therapies to reduce or prevent the in-crease of SCr levels. Data are shown as a forest plot showing the difference in the means between the treated and the control/placebo groups. Effect size is measured as Hedges’ g ± 95% CI. CI: confidence interval; d, days; GFR, glomerular filtration rate; w, weeks.
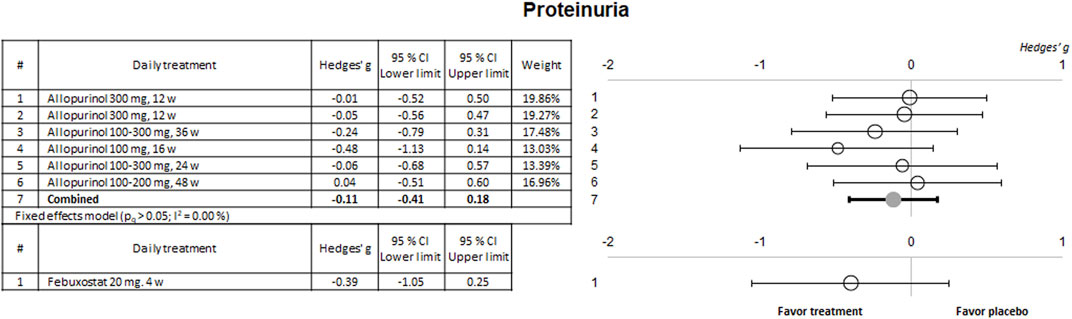
Figure 5. Meta-analytic results of the ability of evaluated therapies to reduce or prevent the in-crease in proteinuria. Data are shown as a forest plot showing the difference in the means between the treated and the control/placebo groups. Effect size is measured as Hedges’ g ± 95% CI. CI: confidence interval; w, weeks.
No significant effects were observed on these two biomarkers for any of the drugs evaluated except for a slight nephroprotective effect observed in a study using 100–200 mg alopurinol, and an evident effect for rasburicase, both on sCr. No study showed a significant effect on proteinuria.
3.2.3 Evaluation of publication biasThe results of the publication bias assessment are shown in Figure 6. Data distribution and asymmetry tests show a notorious publication bias (p < 0.01) for sUA. However, this bias does not affect the object of this meta-analysis, which is focused on whether the anti-gout, hypouricemic therapy exerts beneficial effects on CKD, not on whether the anti-gout therapy reduces uricemia. The asymmetry on sUA is expected, as all the drugs used in the included studies are known to be efficient at reducing hyperuricemia. Moreover, mild publication bias is also found for the eGFR. Specifically, graphical analysis identifies absence of treatments considerably worsening renal function. However, this is not real bias either, because all drugs tested are under clinical use. If deemed nephrotoxic, for ethical reasons they would never be administered to patients with CKD.
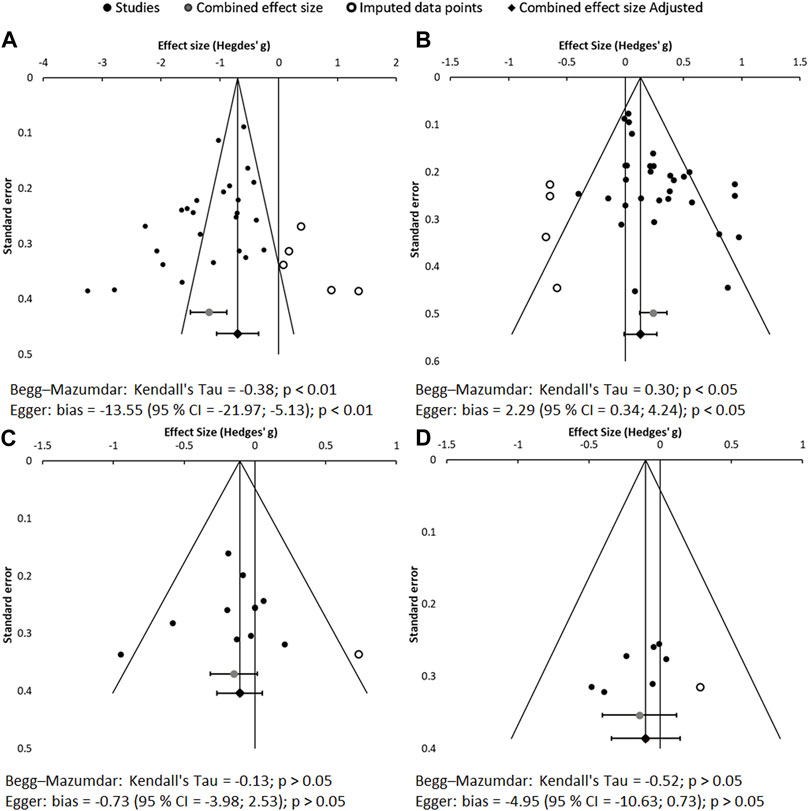
Figure 6. Funnel plots and asymmetry tests corresponding to the meta-analysis of sUA (A), eGFR (B), SCr (C) and proteinuria (D). Effect size is measured as Hedges’ g ± 95% CI. CES: combined effect size; CI: confidence interval.
3.3 Correlation studyThe relationship between sUA reduction and improvement or prevent of eGFR deterioration (a standard renal function biomarker mostly evaluated in the included clinical trials) was studied with the Pearson test. As shown in Figure 7, no correlation was observed between both parameters for any of the drugs evaluated, nor for all of them in general. These results indicate that there is no direct or proportional relationship between them, which suggests that additional mechanisms other than the reduction of sUA contribute to the nephroprotective effect.
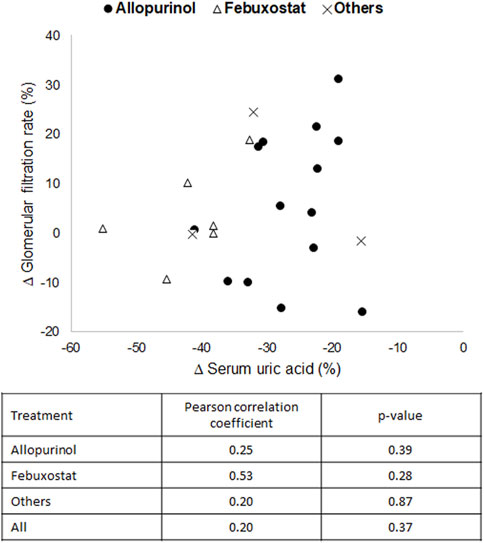
Figure 7. Graphical representation and correlation analysis of the average reductions in serum uric acid versus the average increases in estimated glomerular filtration rate.
4 DiscussionInclusion of a control/placebo group in the evaluation of the nephroprotective effect of hypouricemic therapy in CKD yields only a relevant number of studies for two drugs, allopurinol and febuxostat, from which convincing conclusions can be drawn. Our study demonstrates that both drugs positively affect glomerular filtration, with allopurinol showing a bolder effect. However, this limited casuistry is insufficient to infer whether there is a class effect of hypouricemic therapies on CKD, or the benefits exerted by allopurinol and febuxostat are due to additional mechanisms unrelated to the reduction of UA and specific of these two drugs. To overcome this limitation, a correlation analysis was carried out between the degree of UA reduction and the degree of renal protection for all the drugs included in the study. The results showed that reducing hyperuricemia is beneficial for renal function, but not in a directly proportional manner.
Proportionality might be disrupted by two factors. On the one hand, study population heterogeneity. A large inter-individual variability in the renal effect of antigout therapy is evidenced by the long error bars observed in virtually all the studies. This individual variability may be explained by the enrolment of patients at different stages of CKD and with different risk factors, whose renal damage patterns are heterogeneously caused by hyperuricemia and, thus, respond differentially to hypouricemic therapy. Identifying the phenotype and pathological scenario in which hypouricemic therapy provides nephroprotection to CKD patients poses an immediate research challenge. In this sense, some studies suggest that reducing UA may be more effective in preventing kidney damage in younger people (Feig, 2020) and in the early stages of CKD, which requires confirmation.
On the other hand, additional mechanisms unrelated to UA reduction could uncouple the apparent relationship of the hypouricemic effect on the nephroprotection observed between drugs and between patients. In this regard, allopurinol and febuxostat have strong antioxidant properties as both drugs are xanthine oxidase inhibitors (Becker et al., 2005; Schumacher et al., 2008). Several authors propose this mechanism as the main responsible for their nephroprotection (Okafor et al., 2017; Cicero et al., 2021). However, antioxidants alone are not enough to prevent CKD (Casanova et al., 2021). A marked anti-inflammatory effect on the vascular endothelium has also been reported for both allopurinol (Goicoechea et al., 2010) and febuxostat (Becker et al., 2005; Schumacher et al., 2008). Febuxostat has also been shown to prevent CKD progression in nephrectomized, normouricemic rats, by preserving preglomerular vessel morphology and maintaining glomerular pressure, which unveils an additional protective effect independently of UA levels (Sanchez-Lozada et al., 2018).
Another important observation from this meta-analysis is that, overall, renal protection is not dependent on drug dose or treatment duration. One possible reason is that in some clinical trials the dose was adjusted as treatment progressed, while in others it was not. This could affect the results since possibly not all patients received the most appropriate dose.
Interestingly, protection has been observed even in short-term treatments (i.e., 4–16 weeks) (Kanbay et al., 2007; Momeni et al., 2010; Bayram et al., 2015; Ghane Sharbaf and Assadi, 2018; Perrenoud et al., 2020; Wen et al., 2020). This suggests that, in addition to impinging on slower chronic processes of kidney injury underlying CKD progression, hypouricemic agents may also ameliorate renal function by a relatively swift mechanism. For instance, an improvement in endothelial function bestowed by antioxidant and anti-inflammatory effects and by increased nitric oxide availability (Khosla et al., 2005; Schwartz et al., 2011), may cause renal vasodilation, increase renal blood flow and GFR, and explain the faster response seen in some patients. Distinct acute effects also contribute to the variable effect exerted by different drugs, and for the same drug between patients.
Thus, the overall effect of a drug in a specific study depends on the heterogeneous composition of pathophysiological patterns in the population studied, as determined stochastically or by environmental and social factors. Individual responses depend, in turn, on how the pharmacological mechanisms of the drug used match the patient’s pathophysiological pattern. In perspective, personalized antigout therapy in CKD should be based on the election of the appropriate drug/dose for each scenario.
Of note, only two of the 29 articles included in this meta-analysis considered albuminuria in assessing renal function, despite its importance in the diagnosis of CKD (Hallan et al., 2009). In fact, albuminuria complements the GFR, improves CKD diagnosis, risk stratification, and prognosis of progression (Hallan et al., 2009; Polkinghorne, 2014; Lambers Heerspink and Gansevoort, 2015), and forms part of the Kidney Disease Improving Global Outcomes guidelines (i.e., the international consensus diagnostic criteria) since 2012 (KDIGO, 2013). While GFR only informs on status of the glomerular filtration process, increased albuminuria may reflect a change in glomerular permselectivity and a defect in tubular function, specifically in tubular reabsorption (Levey et al., 2020; Divya et al., 2023). Incomplete renal function diagnosis may cause misinterpretation of the effect of hypouricemic therapies. Curiously, this affects studies performed both before and after public availability of the KDIGO guidelines. We contend that future clinical trials designed to evaluate the nephroprotective effect of UA-lowering therapy should collect both GFR and albuminuria values for a more granular detection of the pathophysiological spectrum underlying CKD.
In conclusion, this work shows that the reduction of hyperuricemia might potentially be an effective strategy in the prevention of CKD in specific pathological scenarios, which needs to be further explored. The phenotype of patients who may benefit from this therapy should be the focus of future studies.
Data availability statementThe raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributionsAC: Writing–original draft, Methodology, Formal Analysis, Data curation. AM: Writing–review and editing, Methodology, Formal Analysis. LV-V: Writing–original draft, Methodology, Formal Analysis, Conceptualization. FL-H: Writing–review and editing, Supervision, Formal Analysis, Conceptualization.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study has been funded by Instituto de Salud Carlos III (ISCIII) through the project “PI20/01,351”and co-funded by the European Union; and RICORS 2040, RD21/0005/0004, co-funded by the European Union–NextGenerationEU, Mecanismo para la Recuperación y la Resiliencia (MRR)
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesBadve, S. V., Pascoe, E. M., Biostat, M., Tiku, A., Boudville, N., Brown, F. G., et al. (2020). Effects of allopurinol on the progression of chronic kidney disease. N. Engl. J. Med. 382, 2504–2513. doi:10.1056/NEJMoa1915833
PubMed Abstract | CrossRef Full Text | Google Scholar
Bayram, D., Tuğrul Sezer, M., İnal, S., Altuntaş, A., Kıdır, V., and Orhan, H. (2015). The effects of allopurinol on metabolic acidosis and endothelial functions in chronic kidney disease patients. Clin. Exp. Nephrol. 19, 443–449. doi:10.1007/s10157-014-1012-z
PubMed Abstract | CrossRef Full Text | Google Scholar
Becker, M. A., Schumacher, H. R., Wortmann, R. L., MacDonald, P. A., Eustace, D., Palo, W. A., et al. (2005). Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N. Engl. J. Med. 353, 2450–2461. doi:10.1056/NEJMoa050373
PubMed Abstract | CrossRef Full Text | Google Scholar
Bonino, B., Leoncini, G., Russo, E., Pontremoli, R., and Viazzi, F. (2020). Uric acid in CKD: has the jury come to the verdict? J. Nephrol. 33, 715–724. doi:10.1007/s40620-020-00702-7
PubMed Abstract | CrossRef Full Text | Google Scholar
Bose, B., Badve, S. V., Hiremath, S. S., Boudville, N., Brown, F. G., Cass, A., et al. (2014). Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol. Dial. Transpl. 29, 406–413. doi:10.1093/ndt/gft378
PubMed Abstract | CrossRef Full Text | Google Scholar
Brook, R. A., Forsythe, A., Smeeding, J. E., and Lawrence Edwards, N. (2010). Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr. Med. Res. Opin. 26, 2813–2821. doi:10.1185/03007995.2010.533647
PubMed Abstract | CrossRef Full Text | Google Scholar
Casanova, A. G., López-Hernández, F. J., Vicente-Vicente, L., and Morales, A. I. (2021). Are antioxidants useful in preventing the progression of chronic kidney disease? Antioxidants 10, 1669. doi:10.3390/antiox10111669
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, Q., Wang, Z., Zhou, J., Chen, Z., Li, Y., Li, S., et al. (2020). Effect of urate-lowering therapy on cardiovascular and kidney outcomes: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 15, 1576–1586. doi:10.2215/CJN.05190420
PubMed Abstract | CrossRef Full Text | Google Scholar
Cicero, A. F. G., Fogacci, F., Kuwabara, M., and Borghi, C. (2021). Therapeutic strategies for the treatment of chronic hyperuricemia: an evidence-based update. Medicina 57, 58. doi:10.3390/medicina57010058
PubMed Abstract | CrossRef Full Text | Google Scholar
Coombs, F. S., Pecora, L. J., Thorogood, E., Consolazio, Wm. V., and Talbott, J. H. (1940). Renal function in patients with gout 1. J. Clin. Invest., 19, 525–535. doi:10.1172/JCI101156
PubMed Abstract | CrossRef Full Text | Google Scholar
Divya, D., Sammi, A., and Chandra, P. (2023). Design and development of opto-electrochemical biosensing devices for diagnosing chronic kidney disease. Biotech Bioeng. bit, 28490. doi:10.1002/bit.28490
CrossRef Full Text | Google Scholar
Doria, A., Galecki, A. T., Spino, C., Pop-Busui, R., Cherney, D. Z., Lingvay, I., et al. (2020). Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N. Engl. J. Med. 382, 2493–2503. doi:10.1056/NEJMoa1916624
PubMed Abstract | CrossRef Full Text | Google Scholar
Ghane Sharbaf, F., and Assadi, F. (2018). Effect of allopurinol on the glomerular filtration rate of children with chronic kidney disease. Pediatr. Nephrol. 33, 1405–1409. doi:10.1007/s00467-018-3943-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Goicoechea, M., de Vinuesa, S. G., Verdalles, U., Ruiz-Caro, C., Ampuero, J., Rincón, A., et al. (2010). Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 5, 1388–1393. doi:10.2215/CJN.01580210
PubMed Abstract | CrossRef Full Text | Google Scholar
Goicoechea, M., Garcia de Vinuesa, S., Verdalles, U., Verde, E., Macias, N., Santos, A., et al. (2015). Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am. J. Kidney Dis. 65, 543–549. doi:10.1053/j.ajkd.2014.11.016
PubMed Abstract | CrossRef Full Text | Google Scholar
Golmohammadi, S., Almasi, A., Manouchehri, M., Omrani, H. R., and Zandkarimi, M. R. (2017). Allopurinol against progression of chronic kidney disease. Iran. J. Kidney Dis. 11, 286–293.
PubMed Abstract | Google Scholar
Gonçalves, D. L. N., Moreira, T. R., and da Silva, L. S. (2022). A systematic review and meta-analysis of the association between uric acid levels and chronic kidney disease. Sci. Rep. 12, 6251. doi:10.1038/s41598-022-10118-x
PubMed Abstract | CrossRef Full Text | Google Scholar
Hallan, S. I., Ritz, E., Lydersen, S., Romundstad, S., Kvenild, K., and Orth, S. R. (2009). Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J. Am. Soc. Nephrol. 20, 1069–1077. doi:10.1681/ASN.2008070730
PubMed Abstract | CrossRef Full Text | Google Scholar
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J. M., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials 17, 1–12. doi:10.1016/0197-2456(95)00134-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Jeyaruban, A., Hoy, W., Cameron, A., Healy, H., Wang, Z., Zhang, J., et al. (2021). Hyperuricaemia, gout and allopurinol in the CKD Queensland registry. J. Nephrol. 34, 753–762. doi:10.1007/s40620-020-00937-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Johnson, R. J., Choi, H. K., Yeo, A. E., and Lipsky, P. E. (2019). Pegloticase treatment significantly decreases blood pressure in patients with chronic gout. Hypertension 74, 95–101. doi:10.1161/HYPERTENSIONAHA.119.12727
PubMed Abstract | CrossRef Full Text | Google Scholar
Kanbay, M., Ozkara, A., Selcoki, Y., Isik, B., Turgut, F., Bavbek, N., et al. (2007). Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int. Urol. Nephrol. 39, 1227–1233. doi:10.1007/s11255-007-9253-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Kao, M. P., Ang, D. S., Gandy, S. J., Nadir, M. A., Houston, J. G., Lang, C. C., et al. (2011). Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J. Am. Soc. Nephrol. 22, 1382–1389. doi:10.1681/ASN.2010111185
PubMed Abstract | CrossRef Full Text | Google Scholar
Katholi, R., Woods, W., Taylor, G., Deitrick, C., Womack, K., Katholi, C., et al. (1998). Oxygen free radicals and contrast nephropathy. Am. J. Kidney Dis. 32, 64–71. doi:10.1053/ajkd.1998.v32.pm9669426
PubMed Abstract | CrossRef Full Text | Google Scholar
Kazancioğlu, R. (2013). Risk factors for chronic kidney disease: an update. Kidney Int. Suppl. 3 (3), 368–371. doi:10.1038/kisup.2013.79
CrossRef Full Text | Google Scholar
Khosla, U. M., Zharikov, S., Finch, J. L., Nakagawa, T., Roncal, C., Mu, W., et al. (2005). Hyperuricemia induces endothelial dysfunction. Kidney Int. 67, 1739–1742. doi:10.1111/j.1523-1755.2005.00273.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Kimura, K., Hosoya, T., Uchida, S., Inaba, M., Makino, H., Maruyama, S., et al. (2018). Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am. J. Kidney Dis. 72, 798–810. doi:10.1053/j.ajkd.2018.06.028
PubMed Abstract | CrossRef Full Text | Google Scholar
Kovesdy, C. P. (2022). Epidemiology of chronic kidney disease: an update 2022. Kidney Int. Suppl. 12, 7–11. doi:10.1016/j.kisu.2021.11.003
CrossRef Full Text | Google Scholar
Krishnamurthy, A., Lazaro, D., Stefanov, D. G., Blumenthal, D., Gerber, D., and Patel, S. (2017). The effect of allopurinol on renal function. JCR J. Clin. Rheumatology 23, 1–5. doi:10.1097/RHU.0000000000000480
PubMed Abstract | CrossRef Full Text | Google Scholar
Lambers Heerspink, H. J., and Gansevoort, R. T. (2015). Albuminuria is an appropriate therapeutic target in patients with CKD: the pro view. Clin. J. Am. Soc. Nephrol. 10, 1079–1088. doi:10.2215/CJN.11511114
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, J.-W., and Lee, K.-H. (2019). Comparison of renoprotective effects of febuxostat and allopurinol in hyperuricemic patients with chronic kidney disease. Int. Urol. Nephrol. 51, 467–473. doi:10.1007/s11255-018-2051-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Leoncini, G., Barnini, C., Manco, L., Nobili, G., Dotta, D., Penso, M., et al. (2022). Uric acid lowering for slowing CKD progression after the CKD-FIX trial: a solved question or still a dilemma? Clin. Kidney J. 15, 1666–1674. doi:10.1093/ckj/sfac075
PubMed Abstract | CrossRef Full Text | Google Scholar
Levey, A. S., Gansevoort, R. T., Coresh, J., Inker, L. A., Heerspink, H. L., Grams, M. E., et al. (2020). Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the national kidney foundation in collaboration with the us food and drug administration and European medicines agency. Am. J. Kidney Dis. 75, 84–104. doi:10.1053/j.ajkd.2019.06.009
PubMed Abstract | CrossRef Full Text | Google Scholar
留言 (0)