Osteosarcoma (OS) is the most common malignant bone tumor, accounting for approximately 56% of bone tumors (Rojas et al., 2021). The incidence of OS is 2–3 cases/million/year in the general population, but is higher in adolescents, with the highest annual incidence of 8–11 cases/million/year in 15–19 year olds (Bessen et al., 2019). The current standard treatment for OS includes extensive surgical resection, neoadjuvant chemotherapy and adjuvant radiotherapy (Thanindratarn et al., 2019). Since the introduction of chemotherapy for OS in the late 1970s, OS patients have received neoadjuvant therapy followed by a combination of postoperative adjuvant chemotherapy, i.e., elevated-dose methotrexate (12 g/m2), etoposide and ifosfamide for children and young adults (<25 years) in the French OS2006/Sarcome-09 study (Gaspar et al., 2018) or other regimens combining doxorubicin (DOX), cisplatin and ifosfamide with or without high-dose methotrexate (Meyers et al., 2005; Bishop et al., 2016) In order to remove the tumor totally, a large amount of tissue needs to be removed during limb-sparing surgery, which is a huge disruption to the body’s structure. At the same time, neoadjuvant radiotherapy and chemotherapy damage normal human tissue while destroying OS tissue, and these methods are not reconstructive. Under these protocols, the 5-year survival rate for children and young adults with limited OS lesions has reached 78% (Heng et al., 2020), but the survival rate for patients with metastases or recurrent OS was still 20% (Gaspar et al., 2018). Furthermore, over the previous 40 years, neither the survival rates for patients without metastases nor the survival rates for those who do have metastases have appreciably improved (Allison et al., 2012). Consequently, OS therapy continues to be a worldwide problem. Thus, the quest for the perfect biomaterial that can simultaneously eradicate tumor cells and encourage bone rebuilding is critical (Ma et al., 2020).
Fortunately, a variety of materials have emerged for OS therapy, with previous research focusing on polymer (He et al., 2022b). These polymers require multiple modifications to be effective in OS therapy, but do not have a significant advantage in promoting bone generation and have weak mechanical properties. 2D nanomaterials are a class of emerging materials that have garnered significant attention from the scientific community. Their unique properties, such as high surface-to-volume ratio, ease of functionalization, and photothermal conversion capabilities, making them highly versatile and suitable for a large number of applications in fields such as optoelectronics and biomedical research. Some examples of these 2D nanomaterials include transition metal dichalcogenides (TMDs), transition metal oxides (TMOs), transition metal carbides, nitrides and carbonitrides (MXenes), BP, layered double hydroxides (LDHs), 2D metal-organic frameworks (MOFs), nanoclay, hexagonal boron nitride (hBN), among others (Murali et al., 2021). When combined with nanomedicine techniques, these new 2D materials hold great potential for cancer therapy, drug delivery, antimicrobial/antimicrobial platforms and tissue engineering (Zeng et al., 2022). The distinct optical or X-ray attenuation properties exhibited by 2D nanomaterials can be utilized for phototherapy (PTT) or radiotherapy in cancer treatment. Furthermore, through integration with other functional nanoparticles or leveraging their intrinsic physical properties, 2D nanomaterials can also serve as effective nanoprobes for multi-modal imaging of tumors. Notably promising examples of 2D nanomaterial-based cancer therapeutics include BiOCl/Bi2O3NSs (Chen et al., 2022a), FeOCl/FeOOH NSs (Kang et al., 2022), As/AsxOy NSs (Kong et al., 2021). In recent years, BP nanoparticles has been used in a wide range of biomedical applications, such as bone tissue engineering (Qiu et al., 2024), wound healing (Bai et al., 2024), cancer therapy (Wu et al., 2024a), epilepsy treatment (Yang et al., 2024), depression treatment (Tan et al., 2024), spinal cord rehabilitation (Liu et al., 2024), antibiotic treatment (Wu et al., 2024b). BP nanoparticles possess excellent biofouling and molecular loading capabilities for use in anticancer therapy. Additionally, their high photothermal conversion efficiency and extinction coefficient make them suitable as photothermal agents (PTAs) in PTT, where localized hyperthermia is generated to eliminate tumors through thermal means. Furthermore, BP nanoparticles can generate singlet oxygen, enabling their application as photosensitizers in photodynamic therapy (PDT) (Qing et al., 2020b). Under physiological conditions, these nanoparticles can be oxidatively degraded into non-toxic phosphonates and phosphates, promoting bone regeneration and eliminating intracellular ROS. In addition, BP nanoparticles have demonstrated the ability to modulate the bone immune microenvironment and promote osteogenesis. Recent studies have shown that BP-based PTT can activate immune responses and alleviate immunosuppression within the tumor microenvironment by detecting T lymphocytes and various immune cytokines. This suggests that BP nanoparticles not only serve as effective PTAs for ablating large solid tumors but also function as immunomodulators for eliminating smaller tumors (Liu et al., 2021a). Therefore, BP nanoparticles offer greater potential for cancer therapy compared to other 2D nanomaterials. In addition, with its features of PTT, PDT, photoacoustic therapy (PAT), drug delivery system (DDS) and biological, BP nanoparticles would become a promising material for OS therapy (Figure 1) (Raucci et al., 2019a; Zhang et al., 2019; Wang et al., 2020b; Hu et al., 2022a; Ma et al., 2022a; Dong et al., 2022; Hou et al., 2022; Li et al., 2023b; Li et al., 2023d).
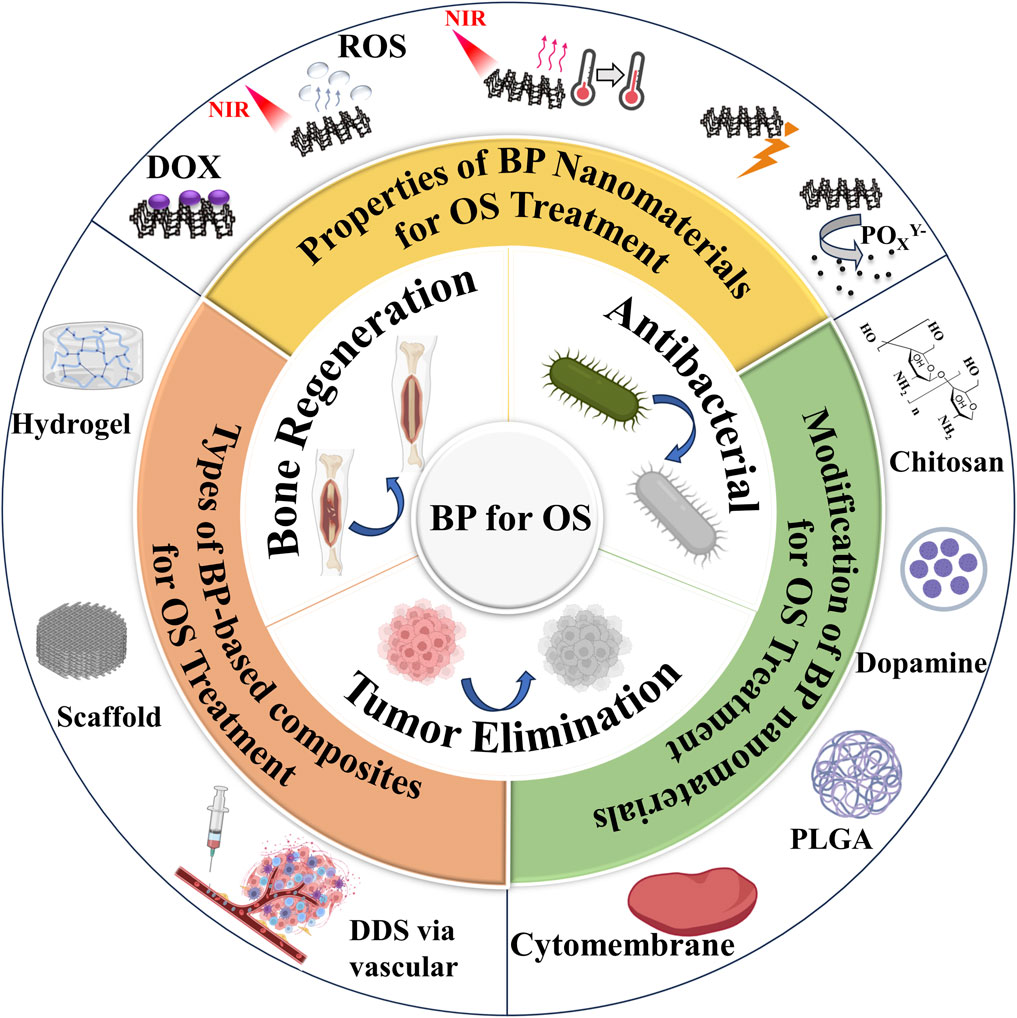
Figure 1. Illustration of BP for OS therapy.
2 Types of BP nanoparticlesPhosphorus has an atomic number of 15 and is in the third period of the periodic table, group VA. Phosphorus is widely distributed in nature, containing 0.12% in the Earth’s crust (Liu et al., 2015), and 0.001–0.1 mg/L in seawater (Wheat et al., 1996) and is the sixth most abundant of all elements in the human body (Calvo and Lamberg-Allardt, 2015). Phosphorus accounts for 1% of the total body mass of humans, with adults containing approximately 660 g of phosphorus, mostly in the form of hydroxyapatite in bones and teeth. In addition, phosphorus plays an essential physiological role as a major component of genetic materials, such as nucleic acids, in sustaining life, transmitting neural stimuli, and catalyzing reactions (Lee et al., 2016). Phosphorus mainly exists three forms of allotropes, including white phosphorus (WP), red phosphorus (RP), and BP (Goodman et al., 1983; Aykol et al., 2017). Among them, BP is the most stable form (Khandelwal et al., 2017). The bulk crystal of BP consists of individual layers. The crystal structure has been illustrated in Figure 2A. The layers of the BP are connected by Van der Waal forces (Figure 2B) (Liu et al., 2015) The interlayer gap varies between 3.21 Å and 3.73 Å (Figure 2C). (Kou et al., 2015) Due to the weak Van der Waal forces, the crystal thickness can be scaled down to the atomic layer scale to obtain phosphorene (or BP nanosheets (BPNSs) and BP nanoribbons (BPNRs) (Watts et al., 2019)) in 2D plane (Liu et al., 2015; Liu et al., 2021b), or even Zero-dimensional (0D) BP quantum dots (BPQDs) (Zhang et al., 2015).
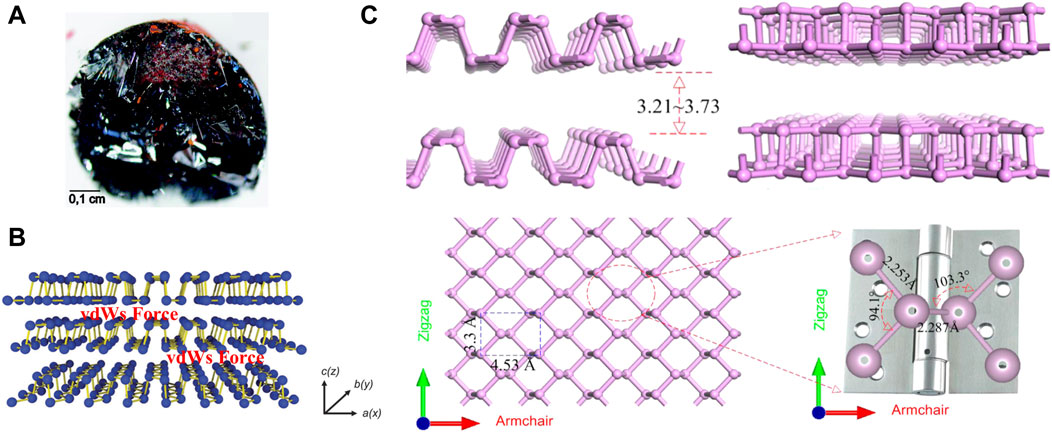
Figure 2. (A) A bulk BP crystal (scale bar = 0.1 cm). (B) Layered structure of BP with Van der Waal forces. (Copyright 2015 (Liu et al., 2015)). (C) Side views, top view of layered BP and the enlarged view of the local atomic structure of the P-P bonded configuration. (Copyright (Kou et al., 2015)).
Phosphorene is single or multi-layered counterpart of BP, which is 2D layered material like graphene (Liu et al., 2014a). Recently, Zhang et al. reviewed the synthesis, properties and applications of phosphenes and provided an outlook on further developments and challenges faced by phosphenes (Zhang et al., 2024). Phosphorene has a pleated structure along the armchair, but a double structure along the zigzag direction. The lattice constants in the two vertical directions are 3.30 Å and 4.53 Å (Figure 2C). (Kou et al., 2015) This structure is similar to a hinge network. The anisotropy of this structure can be distinctly seen in its local bond configuration. Zigzag direction bond angle is 94.1° and the adjacent P-P bond length is 2.253 Å. It is noted that these values are smaller than the corresponding dihedral angle (103.3°) and connected bond length (2.287 Å) along the armchair direction (Figure 2C). (Kou et al., 2015) Since phosphorene was successfully prepared in 2014, there has been an explosion of research on it, revealing that it has considerable potentiality for application in a vast number of fields (Liu et al., 2014a). Apart from graphene, phosphorene is the only stable 2D monolithic material that can be mechanically exfoliated. Unlike graphene, it has inherent, direct, and appreciable band gap (Liu et al., 2014a). Phosphorene includes BPNSs (Thurakkal and Zhang, 2019; Liu et al., 2021b) and BPNRs (Watts et al., 2019). Of all the phosphorene used in OS therapy, BPNSs were the most widely used because of their superior photothermal conversion efficiency and high surface area-to-volume ratio. In early vitro cellular studies have demonstrated that BPNSs can alleviate oxidation-induced apoptosis by clearing reactive oxygen species (ROS) (Liu et al., 2021b). Besides, when combined with near-infrared radiation (NIR), BPNSs trigger a photothermal response that leads to high local temperatures and can effectively ablate tumor cells (Ma et al., 2022a; Li et al., 2023b).
Except BPNSs, BPQDs are also the widely explored nanoplatform for OS therapy. In vivo experiments have proven that BPQDs are non-toxic (Mu et al., 2017). Similar to BPNSs, BPQDs also have good photothermal conversion efficiency and have been used in PTT of tumor (Zhang et al., 2019; Hu et al., 2022a). Unlike the BPNSs, BPQDs are much smaller in diameter, averaging around 10 nm. The smaller size facilitates the distribution of BPQDs in the body and allows them to be protected from phagocytosis by macrophages, extending the half-life of BPQDs (Pandey et al., 2021).
3 General synthesis of BP nanoparticlesIn general, BP nanoparticles are mainly synthesized by top-down methods. The top-down method is the exfoliation of bulk BP into BP nanoparticles by physical or chemical routes. Based on the weak van der Waals forces in BP nanoparticles, it is easy to convert bulk BP into BP nanoparticles. Therefore, we provided a detailed review of synthesis methods for BP nanoparticles in this section.
3.1 Mechanical exfoliation methodMechanical exfoliation was the earliest method to produce BP nanoparticles. In 2014, Xia et al. mechanically exfoliated BPNSs from bulk BP crystals using a 300 nm SiO2 grid (Xia et al., 2014). Meanwhile, Liu et al. also demonstrated that atomic-scale monolayer/multilayer BP nanoparticles can be obtained by mechanical exfoliation (Liu et al., 2014b). However, the mechanical exfoliation method at this time had some drawbacks such as low efficiency, product instability, lack of scalability, adhesive residue and difficulty in controlling the number of layers of BP nanoparticles. To upgrade the traditional mechanical exfoliation method. Metal or plasma-assisted mechanical exfoliation methods emerged and were developed. Gaun et al. prepared a large number of phosphene layers with sizes exceeding 50 μm by combining Au/Ag on a SiO2/Si grid to cut bulk BP (Guan and Xing, 2018). Lu et al. first combined mechanical exfoliation with Ar + plasma to obtain stabilized BPNSs with one to five layers (Lu et al., 2014). The later study by Kuriakose et al. showed that O2 plasma is more suitable than Ar + plasma for mechanical exfoliation of BP nanoparticles (Kuriakose et al., 2018). Recently, Hu et al. invented a modified mechanical stripping method of tape stripping to prepare narrow and edge-flattening black phosphorus nanoribbons (PNRs). (Figure 3A). (Hu et al., 2023) With these improvements, mechanical exfoliation methods have been greatly enhanced for use in the industrial and pharmaceutical industries.
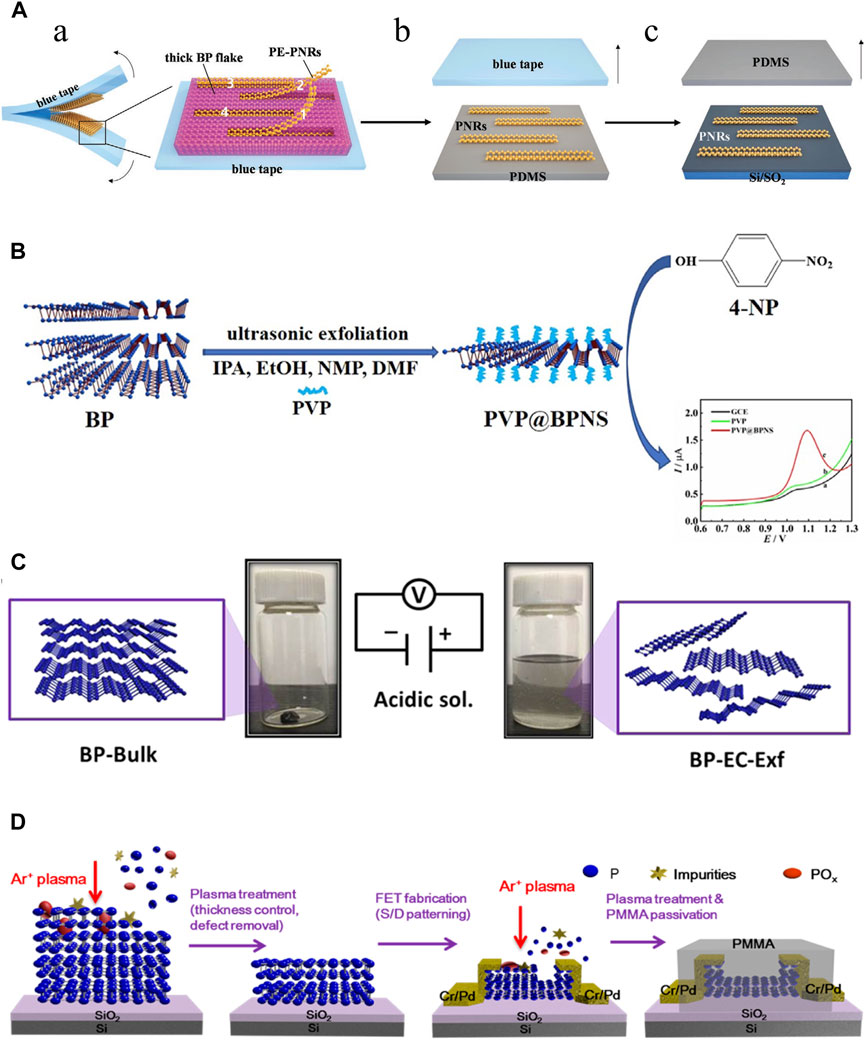
Figure 3. Synthesis of BP nanoparticles. (A) Mechanical exfoliation. (A) Preparation of the partially-exfoliated PNRs (PE-PNRs) connected to a thick BP fake via the blue tape exfoliation (left) and the zoomed-in view of the exfoliated product (right). (B) The PE-PNRs were further peeled off to obtain separated individual PNRs on the polydimethylsiloxane (PDMS) substrate by the PDMS exfoliation. (C) The resulting PNRs in (B) were transferred from the PDMS substrate onto the SiO2/Si substrate by the conventional dry transfer way. (Copyright (Hu et al., 2023)). (B) Liquid exfoliation. BPNSs are simply prepared through ultrasonic exfoliation of bulk BP with the assistance of PVP in different solvents, including IPA, EtOH, NMP and DMF. (Copyright (Shen et al., 2021)). (C) Electrochemical method. The bulk BP crystal is exfoliated in a phytic acid solution by applying a positive voltage. Bulk BP crystals (left) and exfoliated BP-EC-Exf (right) in the solvent. (Copyright (Qiu et al., 2019)). (D) Plasma etching method. Schematic diagram of the effects of the plasma treatment of a BP flake: thickness control, surface defect removal, and device fabrication process. (Copyright (Jia et al., 2015)).
3.2 Liquid exfoliation methodThe liquid exfoliation method is currently the most commonly used technique for large-scale production of BP nanoparticles (He et al., 2024). Immersion of bulk BP crystals in an organic solvent creates ionic exchange between BP layers, which causes bulk BP to exfoliate and form monolayer BPNSs. The forces between the crystal structures of the bulk BP become weak in the solvent, and then the BPNSs are obtained by different methods (ion insertion, ultrasound-assisted, ion-exchange, and shear-exfoliation). The key to the liquid exfoliation method is the choice of solvent. Liquid solvents include N-methyl-2-pyrrolidone (NMP), N-cyclohexyl-2 (or3)-pyrrolidone (NCP), dimethyl sulfoxide and dimethylformamide (DMF), NMP/NaOH mixture and organic/ionic mixture. Unlike mechanical exfoliation, liquid exfoliation can protect the BP nanoparticles from oxidation. Another consideration is to avoid degradation of BP nanoparticles in liquid solvents. In general, solvents with strong surface tension and high dielectric constants can reduce BP degradation in liquids. Shen et al. prepared BPNSs by ultrasonic stripping of bulk BP in different solvents such as iso-Propyl alcohol (IPA), ethanol (EtOH), and DMF using polyvinylpyrrolidone (PVP) as an auxiliary agent, there was found that the stripping efficiencies of IPA and EtOH were much higher than DMF and NMP, and the stripping efficiencies of IPA were better than EtOH. More importantly, PVP reduces the oxidation of BPNSs by forming a protective film, which effectively improves the productivity and environmental protection (Figure 3B). (Shen et al., 2021) In a review, it was detailed that biomolecules such as peptides, proteins, nucleic acids, etc., have been used as exfoliators for the synthesis of high-quality graphene, which opens up possibilities for the production of high-quality BP nanoparticles (Paredes and Villar-Rodil, 2016). Recently, Yang et al. proposed a new synthesis strategy of BPNSs, which used Tween 20 assisted deoxygenation water to efficiently prepared fewer or even single layers of BPNSs (Yang et al., 2020). However, the liquid exfoliation stripping method also has the disadvantages of organic media contamination and time-consuming, and ultrasound-induced damage to the crystal structure can hinder its use in electronics.
3.3 Electrochemical methodIndeed, the electrochemical method belongs to one of the classes of liquid exfoliation methods. The electrochemical method is simple, cost-effective and environmentally friendly methods that has been widely used to prepare 2D nanoparticles such as graphene with satisfactory results. The electrochemical method can be used to synthesize BPNSs on a large scale, which has the advantages of high product yield, high crystalline quality of samples, rapid synthesis process without any additional catalysts, which is commonly used in the fabrication of nano-electronic components. Traditionally, bulk BP is the anode, platinum wire is the cathode, and sulfuric acid solution is used as the electrolyte in the electrochemical methods. With the applied voltage, the BP crystal gradually peels away to form BPNSs. However, when BP crystals are used as the anode, the product is prone to oxidation. Later, Xiao et al. invented an electrochemical cathodic stripping method, in which BP was used as the cathode, a platinum plate as the anode, and tetrabutylammonium hexafluorophosphate as the electrolyte, obtaining BPNSs with a thickness of 2–7 nm, and oxidation could be avoided completely in this process (Xiao et al., 2018). Due to the bare surface nature of this electrochemically stripped BP, it is difficult to disperse and is not compatible with common polymer substrates. To overcome this difficulty, the surface functionalization of graphene has been inspired. Qiu et al. used BP crystal as cathode and phytic acid as electrolyte to generate BPNSs functionalized with cobalt phytate (BP-EC-Exf), and introduced BP-EC-Exf into polyurethane acrylate (PUA) matrix by ultraviolet (UV) curing strategy to prepare superior-performance PUA/BP-EC-Exf (PUA/BP-EC) nanocomposites (Figure 3C) (Qiu et al., 2019).
3.4 Plasma etching methodThe plasma etching method involves placing BP crystals on a silica substrate and exposing them to an oxygen plasma through which the upper layer is oxidized to a PxOy layer, resulting in the separation of the BP crystals and the formation of an arbitrary number of layers of BPNSs. The PxOy layer protects the BPNSs from oxidation during this process. Jia et al. fixed BP crystals on a silicon plate, peeled off the bulk BP crystals with blue Nitto tape, and then treated them with Ar + plasma to obtain BP films with a controlled number of layers, utilized the BP films to fabricate BP field effectiveness transistors (FET), and finally passivated the FET with polymethylmethacrylate (PMMA) (Figure 3D). (Jia et al., 2015) However, this technique has rarely been investigated further in recent years.
4 Properties of BP nanoparticles for OS therapyIn the monolayer BP structure, each phosphorus atom is linked to three neighboring phosphorus atoms by covalent bonds, forming a folded structure. This folded structure endows the BP nanoparticles with high surface area-to-volume ratio, excellent mechanical, optical, thermal and electrical properties (Zhang et al., 2021). Based on the excellent properties, BP nanoparticles have been used in many fields. In this section, we only summarized the properties of BP nanoparticles in the field of OS therapy (Table 1). The wide and adjustable bandgap of BP nanoparticles creates high light absorption efficiency. Moreover, the high photothermal conversion efficiency allows for a good performance of photothermal conversion. Hyperthermia leads to the thermal elimination of tumor cells; thus, BP nanoparticles can be applied as a photothermal agent for PTT. In addition, BP nanoparticles can also be used as a photosensitizer for PDT of OS therapy. Under the NIR, BP nanoparticles can transfer energy to the surrounding oxygen to release ROS, including superoxide anion radicals, hydrogen peroxide, highly reactive hydroxyl radicals, and single-linear oxygen species, which kill tumor cells. ROS can also act as an antibacterial agent to prevent infections in OS therapy (Li et al., 2023d). Besides, the localized warm environment promotes bone regeneration by activating several osteogenic proteins including alkaline phosphatase (ALP) and heat shock protein (HSP).
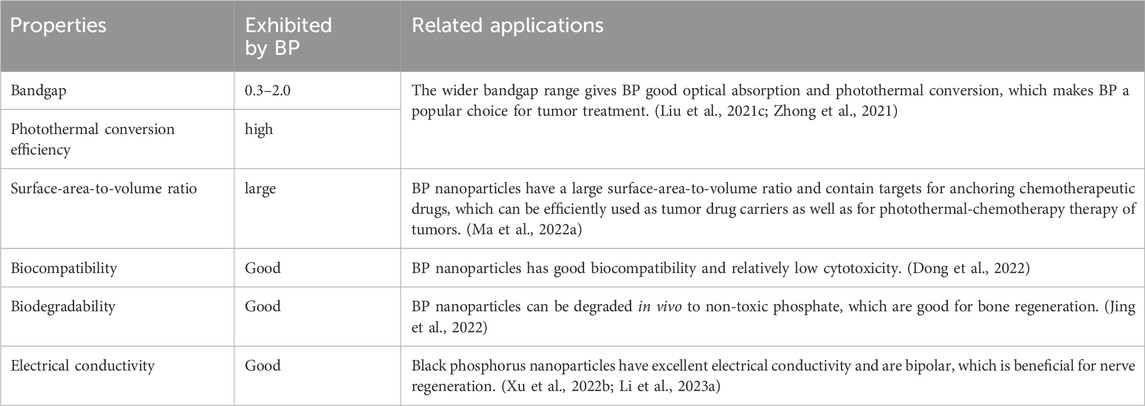
Table 1. Important properties of BP nanoparticles for OS Therapy.
BP nanoparticles have a folded structure that gives them a large surface area-to-volume ratio, allowing them to be efficiently loaded with tumor agents. Chen et al. adsorbed DOX onto BPNSs via electrostatic interactions and showed that the drug loading rate of BPNSs reached 950%, which is higher than any previously reported 2D nanoparticles (Chen et al., 2017). Alternatively, DDS can be combined with PTT to form a photothermal-thermal therapy (Li et al., 2023b; Xu et al., 2023). BP nanoparticles can also form microspheres that encapsulate the drug and reach deeper tumor locations, forming a multimodal therapy of tumors through synergistic PDT and immunotherapy (Li et al., 2019). BP nanoparticles are degradable and their degradation product is phosphate, which is non-toxic to cells. Phosphate is also one of the main components of bone and can promote bone regeneration by trapping calcium to form calcium phosphate (CaP) deposits (Qing et al., 2020a). Moreover, a sudden increase in the concentration of intracellular phosphate can also lead to oxidative stress in tumor cells, which ultimately leads to apoptosis (Zhou et al., 2019). BP nanoparticles also have excellent electrical conductivity and facilitate nerve regeneration during bone regeneration (Qian et al., 2019).
5 Modification of BP nanoparticles for OS therapyApplications for BP nanoparticles in biomedicine are numerous. However, the BP nanoparticle’s instability is currently the issue. It is imperative that BP nanoparticles be modified in order to increase their stability. Recently, An et al. reviewed the progress of polymer-decorated BP nanoparticles for biomedical therapeutic applications (An et al., 2020). Besides, the strategies and recent advances in surface modification of BP nanoparticles for biomedical applications can be accessed through the review of Zeng et al. (Zeng and Chen, 2020) Here we summarize some modification strategies of BP nanoparticles for OS therapy.
5.1 Electrostatic adsorption of cationic polymersBP nanoparticles have negative potentials and can easily adsorb cationic polymers via electrostatic interactions. Currently, cationic polymers used to enhance the stability of BP nanoparticles in OS therapy include chitosan (CS) and dopamine (DA).
5.1.1 ChitosanCS, as a cationic polysaccharide, has good biocompatibility, biodegradability and antibacterial properties (Zhao et al., 2022b). CS promotes osteogenic differentiation by upregulating the expression levels of Osterix and bone sialoprotein (BSP). Electrostatic interactions between positively charged groups on CS and negatively charged groups on the bacterial cell wall can kill bacteria by cutting off their nutrient and oxygen supply. Hydrophobic interactions between the CS and bacteria also have a bactericidal effectiveness. Moreover, the abundant functional groups (hydroxyl and amine) in CS can act as donors and acceptors to promote the formation of intramolecular and intermolecular hydrogen bonds between hydroxyl and amine in DOX, which facilitates the delivery of DOX (Hosseini et al., 2022). Positively charged CS molecules can be adsorbed onto negatively charged BPNSs via electrostatic interactions, which have been shown to enhance the stability of BP nanoparticles in physiological settings. Therefore, CS-modified BP nanoparticles are highly promising for constructing DDS for OS therapy in combination with osteogenesis and antibacterial. There are in vitro and in vivo studies that show: the result of antibacterial and antitumor can be accomplished in one step at a temperature of 49 ± 0.5°C due to the synergistic effectiveness of hydroxypropyl-trimethyl ammonium chloride CS and thermotherapy. At a temperature of 42 ± 0.5°C, NIR can promote osteogenesis (Zhao et al., 2023b). Recently, He et al. designed a multilayered assembled composite coating consisting of negatively charged BPNSs and positively charged CS for 3D printing of polyaryletherether ketone (PEEK) bone scaffolds (He et al., 2022a). The BPNSs in the composite coating inhibited infection via PTT, while the pH-sensitive CS acted as a “smart” DDS. The CS polymer also effectively protects the BP nanoparticles from rapid degradation, allowing for the sustained release of PO43-, which promotes bone regeneration. In this experiment, pristine BPNSs were completely degraded within 1 h, and BP crystals were fully degraded after 440 h. BPNSs encapsulated in CS further inhibited their degradation rate, and they were completely degraded only after 1,351 h (56 d). The results showed that the release of both phosphate and DOX was enhanced at pH 5.5, indicating good pH control of drug release. This may be due to the protonation of CS amino groups in an acidic environment, and polymer swelling can expand the envelope porosity and promote drug release through enhanced diffusion.
5.1.2 DopamineThe high adhesion capacity of mussels is due to Mytilus edulis foot protein (MEFP), which includes DA and its derivatives polydopamine (PDA), is a promising coating in the biological field. PDA coatings can be firmly adhered to the surface of numerous materials and further amine/thiol coupling to secondary biopolymers via Michael additive or Schiff base reactions. It has been demonstrated that PDA has strong NIR absorption and high photothermal conversion efficiency. Therefore, the PDA wrapping BPNSs not only improves the stability of BPNSs by isolating them from the external environment, but also enhances the photothermal conversion efficiency of BPNSs. Ma et al. showed that by encapsulating BP nanoparticles with PDA, the PDA layer enhanced the photothermal properties of BP nanoparticles and that the hydrophilic and biocompatible nature of the PDA layer enhanced cell adhesion and conferred the NIR/PH dual-responsive DOX-release properties of this therapeutic system (Ma et al., 2022b). Recently, researchers found that the irradiation with an 808 nm laser (1W cm−2, 10 min) raised the temperature of the BPNSs solution by 6.3°C when the BPNSs were bound with the PDA films, suggesting the PDA coating effectively enhanced the photothermal activity of BPNSs (Li et al., 2023d). Interestingly, BPNSs protected by PDA (BPNSs@PDA) showed the highest drug release than bare BPNSs, presumably due to the photothermal additive effectiveness of PDA, the higher the temperature, the weaker the electrostatic attraction and the easier the DOX release. In vivo, this integrated system achieves a good photothermal conversion efficiency via BP@PDA, killing bacteria in the initial phase by PTT of BPNSs, and finally killing tumor cells and promoting osteogenesis in the later phase by PTT of BPNSs in synergy with DOX (Li et al., 2023d).
5.2 Polymer vesicle encapsulationPolymer vesicles are micro to nanoscale polymer capsules with bilayer membranes. Poly (lactic-co-glycolic acid) (PLGA) is a linear copolymer of lactic and glycolic acid monomers, one of the most widely used biodegradable polymers. PLGA has excellent biocompatibility, well processability, gradual degradation property and can be loaded with a variety of bioactive factors; therefore, it is often used in drug delivery, bone regeneration, and tumor therapy (Zhao et al., 2021; Feltrin et al., 2022; Lim et al., 2022). BP nanoparticles/PLGA composites were formed when PLGA encapsulated BP nanoparticles, and the BP nanoparticles/PLGA composites had strong hydrophobicity, good biocompatibility and no appreciable toxicity (Shao et al., 2016). Owing to the well hydrophobicity of PLGA, rapid degradation of the BPQDs is prevented, so the BP nanoparticles/PLGA composites possesses good PTT efficiency and tumor targeting ability (Shao et al., 2016).
Wang et al. (Wang et al., 2020a) prepared PLGA-encapsulated BPNSs nanospheres (BPNSs@PLGA) by oil-in-water method for synthetic composite scaffolds for OS therapy and found that the scaffolds could maintain the photothermal conversion capability for more than 3 weeks, which was due to the reduced decomposition rate of BPNSs, as most of the BPNSs were embedded in the polymer matrix and degraded only with the hydrolysis of PLGA, showing a much longer window of time for photothermal therapy operation as compared to other studies in which the BPNSs were deposited only on the surface of the scaffolds (Yang et al., 2018). Recently, Hu et al. (Hu et al., 2022a) encapsulation of BPQDs in PLGA by oil-in-water emulsion solvent evaporation method to form BPQDs/PLGA nanospheres (NSs) and combined with wood and filipin proteins to synthesize hydrogels for the therapy of bone tumors. TEM and FE-SEM results show that BPQDs/PLGA NSs have regular shapes and large radii. Statistical analysis of TEM and AFM data on a sample of 100 BPQDs yielded an average size of 2.39 nm and thickness of 2.21 nm, respectively. The average hydrodynamic size of the BPQDs/PLGA NSs was centered at 139.1 nm, which was larger than the size of the BPQDs, suggesting the PLGAs successfully encapsulated the BPQDs. It was demonstrated by Raman spectroscopy that BPQDs/PLGA NSs were more stable than BPQD in horseradish peroxidase (HRP)/H2O2 solution. Comparing the Raman spectra at 0 and 8 days, no significant difference was recorded in the BPQDs/PLGA NSs, but there was a significant blue shift in the Raman spectra of the BPQDs. In addition, the absorption spectra of both BPQDs and BPQDs/PLGA NSs exhibited a typical broad absorption range across the UV and NIR regions. However, when dispersed in water with HRP/H2O2, the absorption intensity of the BPQDs decreased with dispersion time. In summary, the hydrophobicity of PLGA, which protects BPQDs from rapid oxidative degradation, can allow BPQDs to degrade slowly. At the same time, BPQDs/PLGA NSs exhibit stronger absorption strength and drug carrying capacity than BPQDs due to their larger size.
5.3 Cytomembrane encapsulationRecently, the biomimetic modification strategies of biofilm-encapsulated nanoparticles have gained more and more acceptance. In 2011, Hu et al. first reported the use of erythrocyte membranes to encapsulate PLGA nanoparticles for long-period cargo delivery (Hu et al., 2011). It provides a new idea for the modification of nanoparticles. In later studies, biofilms were widely used in tumor therapy (Zhang et al., 2022b; Choi et al., 2023). It has been recognized that biofilms are non-cytotoxic and highly bioavailable, and that cell membranes carrying cellular immunomodulatory self-labeling proteins can provide the encapsulated nanoparticles with anti-phagocytic activity, prolonged lifespan in vivo, and targeted delivery of the nanoparticles to the lesion, thus enhancing the effectiveness of tumor therapy. Currently, biofilms prepared from various cell membranes (erythrocytes, leukocytes, stem cells, and tumor cells) have been used to encapsulate nanoparticles individually or in a fusion fashion for complex tasks in various physiological environments. Erythrocyte membranes allow the encapsulated nanoparticles to evade immune surveillance, leukocyte membranes are resistant to the action of conditioning agents, stem cell membranes have a naturally high tumor affinity for targeted delivery, and tumor cell membranes have homologous recognition properties. Unfortunately, none of the aforementioned cell membrane encapsulated BP nanoparticles have been reported for OS therapy. The platelet membrane surface contains CD47, which interacts with signal-regulating proteins on immune cells to evade immune clearance and has been used in tumor therapy (Bahmani et al., 2021). Recently, Xu et al. firstly used OS cell membranes (OCM) fused with platelet cell membranes (PM) to construct platelet-OS hybrid membranes (OPMs) to encapsulate BPQDs (BPQDs@OPM), where DOX was loaded on the BPQDs (BPQDs-DOX@OPM) (Xu et al., 2023). Finally, a non-toxic, long-acting, highly effective, targeted chemotherapy in combination with PTT for OS therapy was obtained.
6 Types of BP-based composites for OS therapyIn OS therapy, in order to better exploit the excellent properties of BP nanoparticles, it is necessary to place BP nanoparticles on a suitable platform to form BP-based composites. Currently, BP-based composites for OS therapy include BP-based hydrogels, BP-based bone scaffolds and BP-based DDS via vascular injection.
6.1 BP-based hydrogelHydrogels are one of the most mature materials for biomedical applications and are widely used in many biomedical fields, including tissue engineering, wound healing, drug delivery, and tumor therapy. Whereas the limited properties of conventional hydrogels hamper their potential of applications, and nanocomposite hydrogels combine the properties of nanoparticles to provide superior functionality. BP is commonly incorporated into hydrogels due to its biodegradability, biocompatibility, photothermal properties, electrical conductivity, and high surface area-to-volume ratio, which improves the mechanical strength and stimulus responsiveness of hydrogels. The photothermal conversion efficiency of BP nanoparticles soften the hydrogel, allowing for the release of hydrogel-loaded agents that can be accurately controlled by the intensity of the light, the exposure time, the BP dose, and the hydrogel composition. Hu et al. (Hu et al., 2022a) designed a high-strength WW/RSF hydrogel integrated with PLGA encapsulated BPQDs for efficient mechanical support, bone regeneration, and OS therapy (Figure 4). Better osteogenic differentiation of BMSCs, accelerated bone regeneration was observed in BP/WW/RSF systems, also had good photothermal conversion efficiency and promoted tumor ablation. BPQDs in hydrogel inhibit osteoclast differentiation and have been shown to be photothermal effective against spinal metastases. Moreover, the hydrogel is injectable and can be used for thermal ablation of OS by in situ injection (Li et al., 2023c). Therefore, BP-based hydrogels have frequently been reported for OS therapy in recent years. In addition to being injected in situ into OS lesions for thermal ablation, BP-based hydrogels can also be formed into coatings used to modify hard scaffolds for OS therapy.
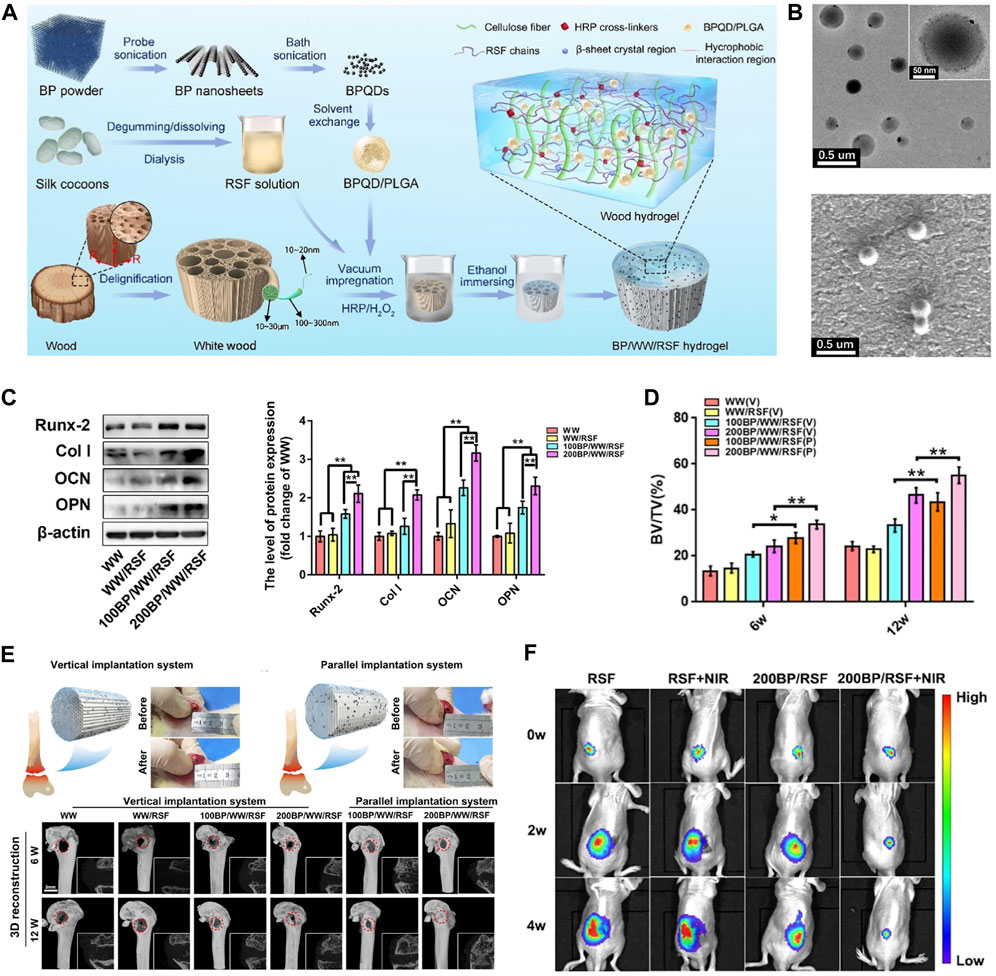
Figure 4. BP-based hydrogel for OS therapy. (A) The fabrication process of BP/WW/RSF hydrogel scaffold. (B) The TEM and SEM images of BPQD/PLGA NSs. (C) Representative Western blot data and quantitative analysis of the expression of Runx-2, Col-1, OCN, and OPN. (D) BV/TV results calculated based on micro-CT. (E) The procedure of implanting hydrogel scaffolds into the critical bone defect in rat femurs and 3D, 2D reconstruction of micro-CT at 6, 12 weeks after implantation. (F) Luminescence images of mice before and after treatments. (Copyright (Hu et al., 2022a)).
6.2 BP-based scaffoldBone scaffolds play a vital role in cell proliferation, differentiation and angiogenesis due to their inherent and excellent osteogenic, bone conduction and bone induction properties, and are often used in the treatment of bone defects in OS therapy. There are many bone scaffolds used in OS therapy, including bio-glass (BG) scaffolds (Yang et al., 2018), PEEK scaffolds (He et al., 2022a), PPENK scaffolds (Li et al., 2023d) and organic polymer composite scaffolds. The surface area of the scaffold provides convenience for the adhesion of BP nanoparticles, and the osteogenic effectiveness of the scaffold is coordinated with the osteogenic effectiveness of BP nanoparticles, and combined with the photothermal and photodynamic effectiveness of BP nanoparticles, which together constitute a multifunctional treatment platform for OS therapy. Recently, Ma et al. (Ma et al., 2022b) designed a multifunctional multiscale therapeutic platform (Fs-BP-DOX@PDA) (Figure 5). First, micro-nanostructures (Fs-NiTi) were fabricated using femtosecond laser direct writing technology. Then, BPNSs were added to Fs-NiTi to construct multilayer structures (Fs-BP). Finally, the DOX-loaded Fs-BP was modified using a PDA. As a result, the Fs-BP had a higher loading efficiency of 93.2% than the Fs-NiTi (35.2%) and P-NiTi (13.3%). Fs-BP-DOX@PDA effectively killed tumor cells (Saos-2 and MDA-MB-231) in vitro, completely eradicated OS cells of mice in vivo and promoted bone regeneration. In summary, BP-based bone scaffolds have promising prospects in OS therapy.
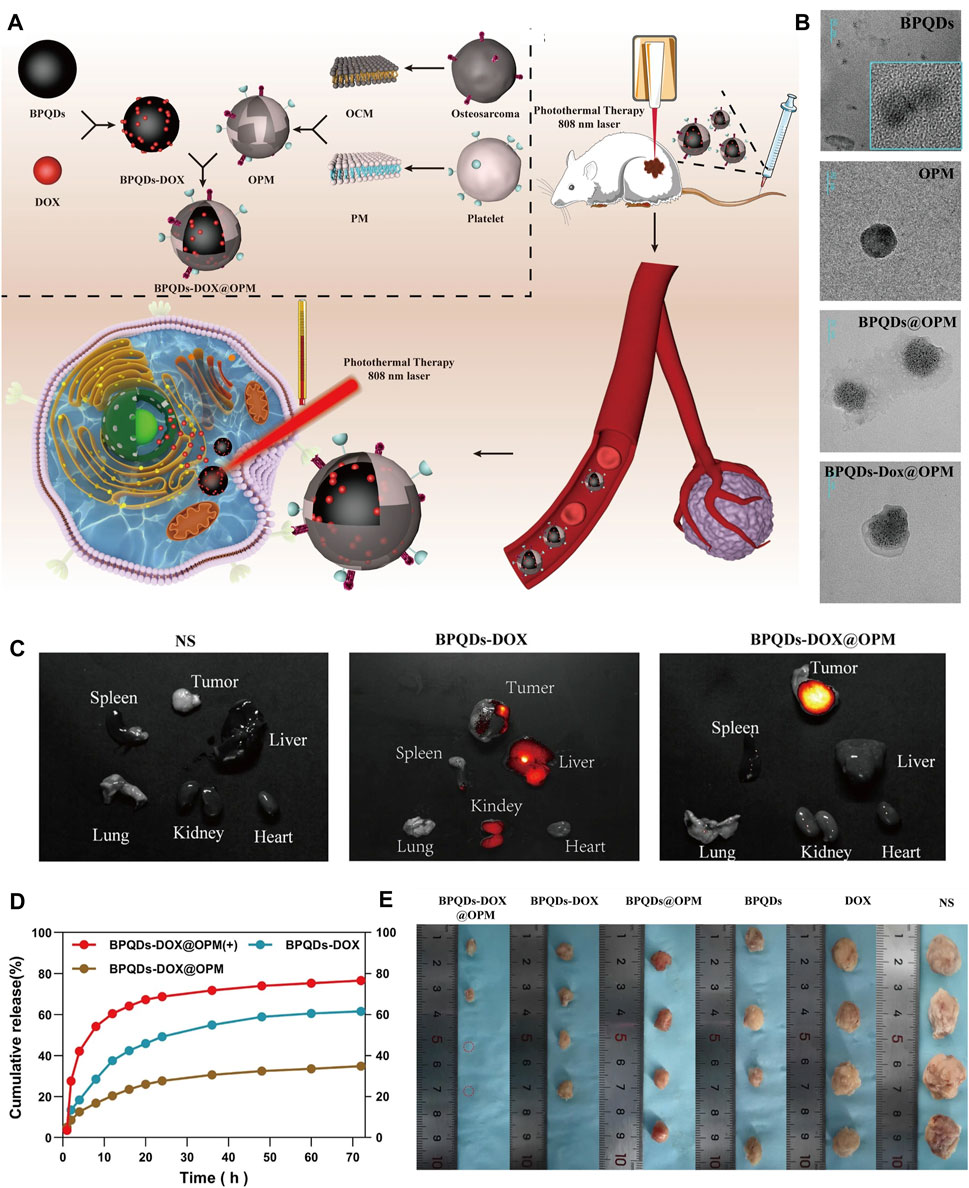
Figure 5. BP-based DDS for OS therapy. (A) Schematic diagram of the role of OPM camouflaged with BPQDs-DOX in combined treatment of OS. (B) Transmission electron micrographs of BPQDs, OPM, BPQDs@OPM, and BPQDs-DOX@OPM (size: 50 nm). (C) BPQDs-DOX@OPM in vivo targeting study. (D) DOX release characteristics of BPQDs-DOX@OPM. (E) Tumor tissues after 18 days of intravenous injection of NS, BPQDs, DOX, BPQDs-@OPM, BPQDs-DOX, and BPQDs-DOX@OPM. (Copyright (Xu et al., 2023)).
6.3 BP-based DDS via vascular injectionThe BP-based DDS via vascular injection has not been studied much, and offers a new idea for targeted treatment of OS. A recent study (Xu et al., 2023) has reported that BPQDs and DOX were encapsulated in OPM to build the BPQDs-DOX@OPM group to achieve a targeted comprehensive treatment strategy of PTT combined with chemotherapy for OS (Figure 6). The OPM encapsulation system also improves the stability of BPQDs by reducing their exposure to oxygen and water, thereby improving their photothermal conversion efficiency. In vitro, Convert the OPM encapsulation system improved stability of BPQDs and increased 72-h release of BPQDs-DOX@OPM and BPQDs-DOX to 34.88% and 61.53%, respectively. This indicated that the OPM hybrid membrane encapsulation system could slow down the release of DOX and prolong the half-life of BPQDs-DOX@OPM in the blood circulatory system. In vivo, after 10 min of irradiation with 808 nm near-infrared light, the temperatures of the BPQDs and BPQDs-DOX@OPM groups increased rapidly, reaching 42.7°C and 48.9°C, respectively. The tumor weight in the BPQDs@OPM group was significantly smaller than that in the BPQDs group. In conclusion, the BPQDs-DOX@OPM group was demonstrated to perform better than the bare BPQDs group. This study proposed a nontoxic, long-circulating, targeted delivery chemotherapy-PTT combined DDS for the OS therapy.
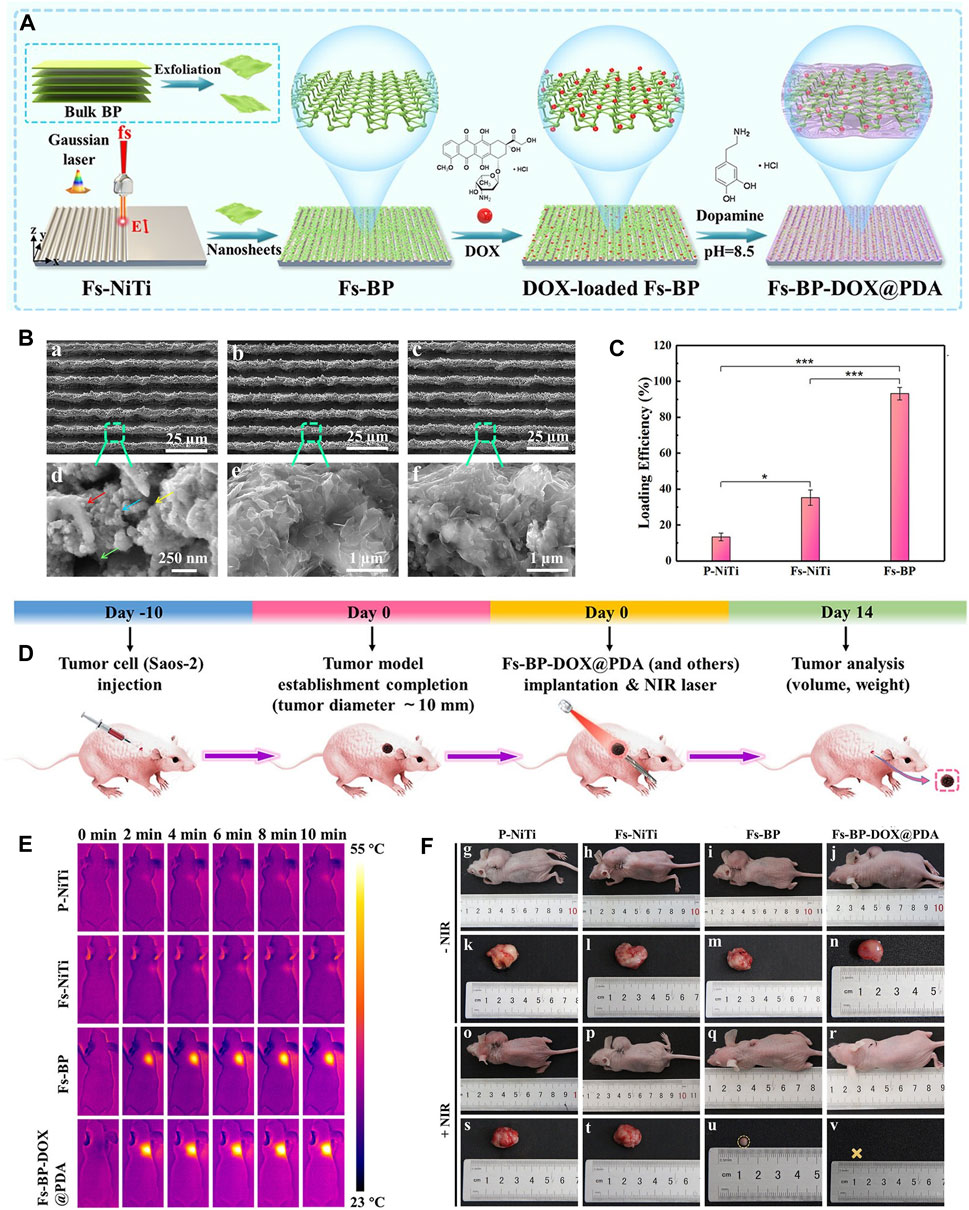
Figure 6. BP-based scaffold for OS therapy. (A) Construction procedure of the Fs-BP-DOX@PDA. (B) SEM images of the (A, D) Fs-NiTi, (B, E) Fs-BP, and (C, F) Fs-BP-DOX@PDA. (C) DOX loading efficiencies. (D) Schematic illustration of ectopic osteosarcoma model establishment and timeline of the therapy schedule. (E) Real-time infrared thermographs of the sample-treated tumor-bearing mice under NIR laser irradiation. (F) Digital images of tumor-bearing mice and the corresponding tumor sites recorded at the end of the treatment period. (Copyright (Ma et al., 2022b)).
7 Applications of BP nanoparticles for OS therapy7.1 BP nanoparticles for tumor eliminationBP is an emerging versatile nanomaterial with good biocompatibility and biodegradability, with promising applications in oncology. BP nanoparticles with high photothermal conversion efficiency can be used as photothermal agents in PTT to generate localized hyperthermia leading to thermal elimination of the tumor. In addition, BP nanoparticles are capable of generating ROS, which makes them useful as photodetectors for PDT. Moreover, BP nanoparticles have a high surface area-to-volume ratio, which improves their efficiency as DDS in tumor therapy. Excitingly, recent studies have shown that BP nanosheets have selective anti-cancer potential against osteosarcoma cells (Bigham et al., 2024). In conclusion, BP nanoparticles can form multiple therapeutic modalities for OS therapy (Table 2).
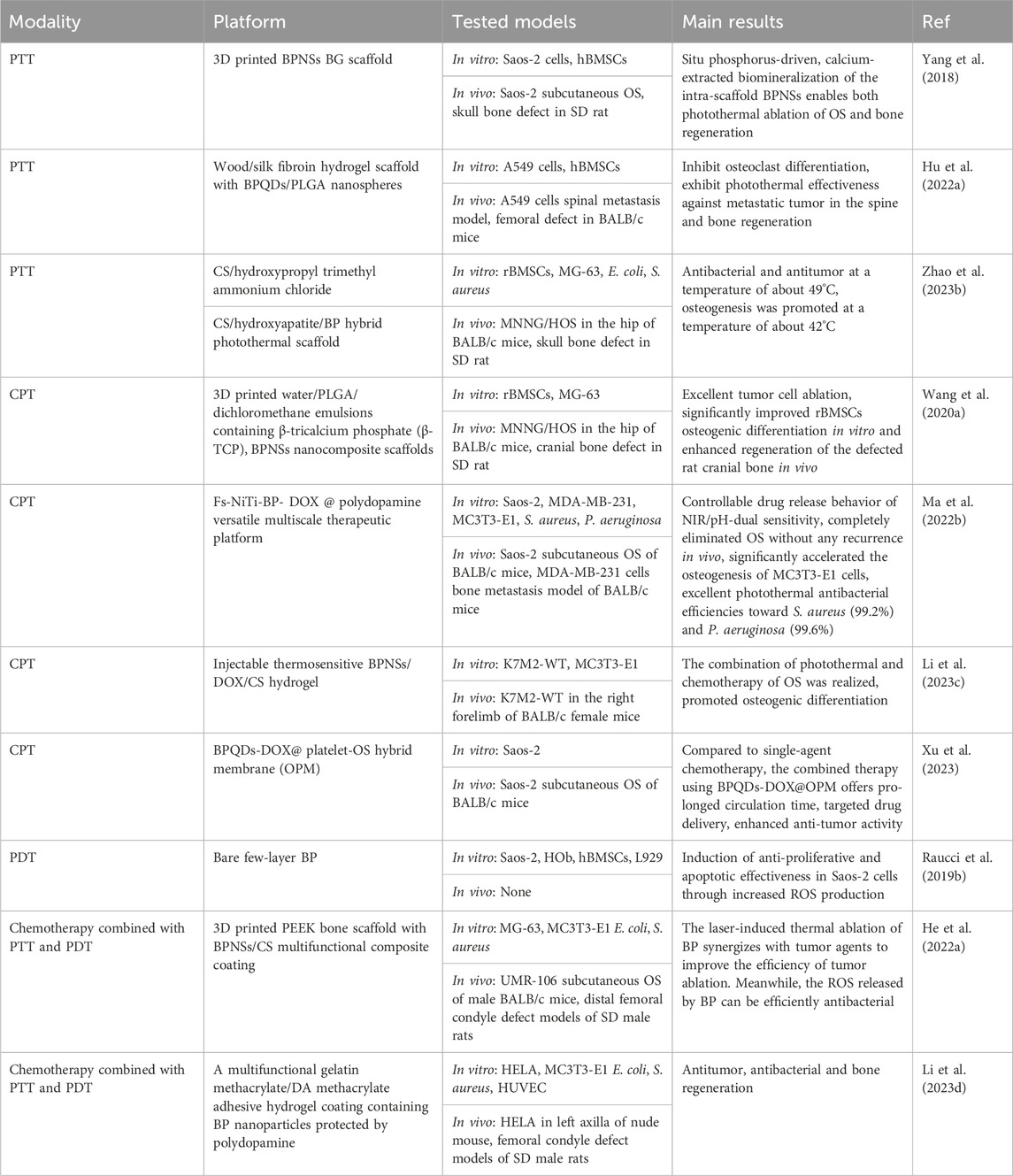
Table 2. Strategies of BP nanoparticles for OS therapy.
7.1.1 PTTPTT, in which light energy is converted into heat energy to ablate the lesion by means of a PTA, has been widely used in tumor treatment (Lv et al., 2021). When the temperature in the localized area of the tumor rises to 41°C–48°C, it results in the death of the tumor cells. In 1995, Chen et al. first found that indocyanine green combined with an 808 nm diode laser produced a photothermal effectiveness that could kill breast tumor cells (Chen et al., 1995). In 2014, Liu et al. first reported that PTT effectively inhibited the growth of OS in vivo. Subsequently, a large number of studies on the in vivo and in vitro applications of PTT in OS had been published since 2014 (Liu et al., 2014c). Two key elements of the PTT, the photothermal agent and the light source. Different types of photothermal agent had been reported, including organic-based materials, carbon-based materials, metal-based materials (Sun et al., 2021). However, due to the limitations of the previous photothermal agent, sometimes heat inevitably leaks out of the target tissue and damages the surrounding normal tissue. Therefore, with the tremendous advances in photothermal agent modification and nanosizing, a number of nanoparticles are continuously being investigated as the most promising photothermal agents for current research.
BP, because of its excellent photothermal conversion efficiency has been used in studies of breast tumor and hepatoma (Chen et al., 2022b; Xie et al., 2023), while there was no review of the photothermal effectiveness of BP about OS. OS, which are often deep and large bone defects that remain after tumor resection, are well suited for NIR-assisted PTT with BP nanoparticles. To achieve the vision of tumor radio-fusion elimination and bone regeneration, Yang et al. first incorporated BPNSs into a 3D printed BG scaffold to treat mice with OS. Under NIR, the temperature of the BG scaffolds increased from 32.4°C to 68.7°C with 808 nm laser irradiation for 5 min (BPNSs concentration = 200 ppm, power density = 1.0 W cm-2), and the photothermal effectiveness did not deteriorate with increasing temperature. In vivo, the temperature of mice OS tissue increased from 30 °C to 55 °C within 1 min and to 58 °C within 5 min. After 14 days, the tumors were completely eliminated without recurrence (Yang et al., 2018). Bacterial infections may also occur following the removal of the OS. Previous studies have shown that long chains of cationic polymers can penetrate the cell membranes of microorganisms, inducing ion leakage and cell death. Negatively charged BP nanoparticles can easily adsorb positively charged macromolecules via electrostatic interactions. Hydroxypropyl-trimethyl ammonium chloride CS (HC) has been shown to improve the stability of BP in physiological environments and to achieve acceptable antibacterial efficacy at 56 °C. This means that BP-based composites can also be antibacterial during the photothermal conversion period. Specifically, Zhao et al. (Zhao et al., 2023b) incorporated BPNSs into a CS/HC matrix mineralized with nano-hydroxyapatite (HA) to prepare CS/HC/HA/BPNSs scaffolds with bone-like hierarchical structure (Figure 7). In vitro and in vivo validation demonstrated that antibacterial and antitumor could be achieved in a one step at 49°C ± 0.5°C due to synergistic effectiveness of HC and thermotherapy; at a temperature of 42°C ± 0.5°C, NIR promotes subsequent osteogenesis. In addition to BPNSs, BPQDs are also used in PTT of tumors. However, the instability of BPQDs is a barrier to their clinical application. After interaction with oxygen, light, and water, BPQDs are susceptible to oxidation and degradation to produce phosphates, leading to a decrease in the efficiency of photothermal conversion and thus in the efficacy of therapy. To overcome this difficulty, Hu et al. (Hu et al., 2022a) encapsulated BPQDs in PLGA nanospheres and integrated them into WW/RSF hydrogel scaffolds (BP/WW/RSF) for tumor abatement and bone regeneration. The results of in vitro photothermal conversion efficiency experiments demonstrated that a power density of 1.0 W cm-2 was sufficient to induce a temperature increase of 27.2°C. In vivo, under 1 W cm-2 irradiation, the temperature of the tumors increased rapidly from 30 °C to 51.9°C in 5 min, and the tumors were effectively controlled over a 4 weeks observation period. Compared to other treatments, such as combining a carrier with a therapeutically toxic chemotherapeutic agent, PTT is able to mitigate unwanted side effects during surgical implantation because the external NIR irradiation after implantation of BP-based composites is much less harmful.
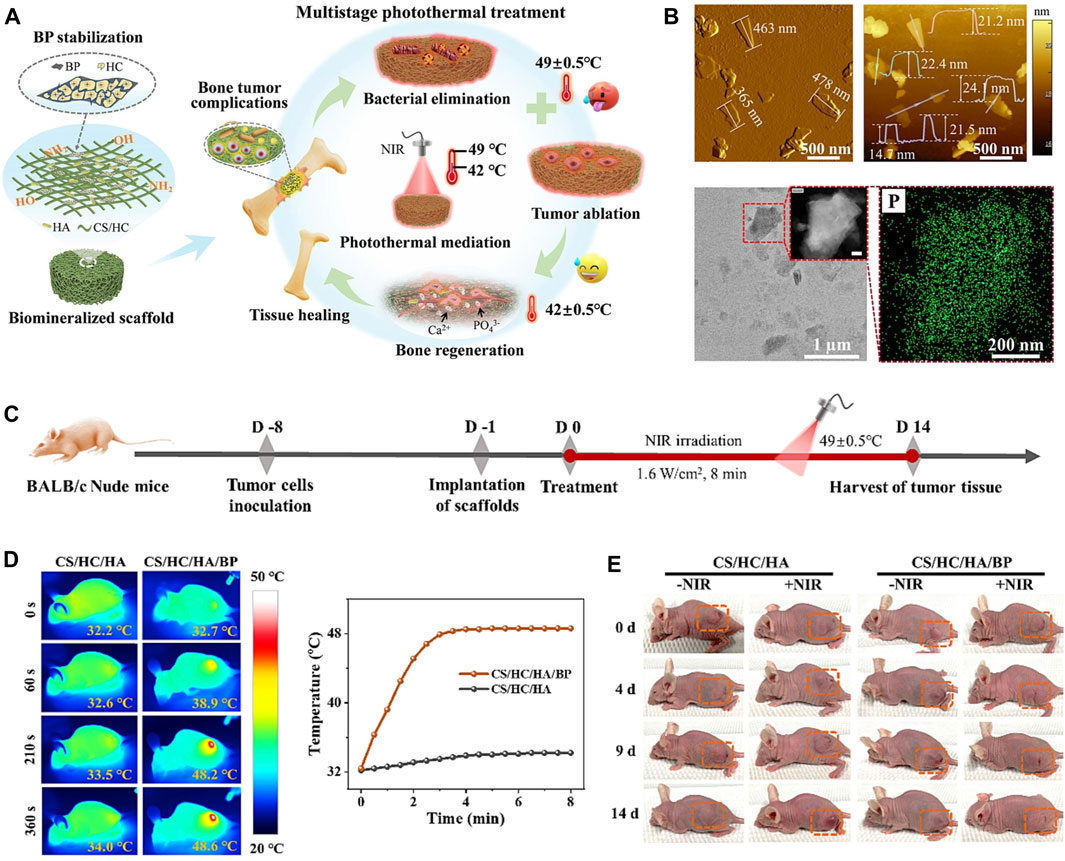
Figure 7. (A) Biomineralized CS/HC/HA/BP scaffold with bone-like hierarchical structure for multistage photothermal treatment of the severe complications associated with bone tumors: bacterial elimination, tumor ablation and subsequent osteogenesis. (B) AFM topographic images, TEM image and STEM-EDX elemental mapping images of BP sheets. (C) Schematic design for the model establishment and photothermal treatment process of the solid tumor in nude mice. (D) The corresponding infrared thermographic maps and time-dependent temperature curve of the tumor-bearing nude mice implanted with CS/HC/HA and CS/HC/HA/BP scaffolds irradiated with NIR laser. (E) Typical photographs of the tumor-bearing mice with or without NIR irradiation. (Copyright (Zhao et al., 2023b)).
7.1.2 Chemical-photothermal therapy (CPT)Treatment of OS usually involves surgical removal of the tumor tissue. However, it is difficult to ensure that the mass is completely removed through surgery, and residual OS cells can cause the tumor to recur. To prevent tumor recurrence, postoperative adjuvant chemotherapy has become the standard treatment strategy for OS (Hu et al., 2022b). However, the side effects and adverse reactions of systemic chemotherapy, such as alopecia, weight loss, and bone marrow suppression, cause severe adverse emotional responses in patients. Although studies have shown that local chemotherapy can do a good job of removing tumor cells and inhibiting recurrence and avoiding systemic medication, subsequent tissue regeneration was hampered by the side effects of locally released high doses of chemotherapeutic agents (Dai et al., 2020). Therefore, finding an efficient treatment option for OS that can reduce the chemotherapy dose is of great clinical application.
BP nanoparticles have a high surface area-to-volume ratio and can be well loaded with chemotherapeutic agents such as DOX and gemcitabine. In addition, the photothermal effectiveness reduces the content of chemotherapeutic agents to minimize the local chemotherapeutic side effects, making PTT in combination with chemotherapy a promising therapeutic strategy. In 2020, the combined chemotherapy with PTT using BPNSs was firstly reported for OS therapy (Wang et al., 2020b). In this report, the release of DOX by BPNSs due to the presence of photothermal effectiveness had an accelerated release behavior at 10 min from the beginning of the release, reaching a 2-fold instantaneous release of 11% ± 1.8% within 24 h. Moreover, tumor recurrence was significant in the PTT group compared to the PTT combined with chemotherapy group. This suggests that PTT and chemotherapy can play a synergistic role in OS therapy. Based on the above theoretical foundation, later, Ma et al. designed a multifunctional photothermal synergistic chemotherapeutic platform for OS therapy (Fs-BP-DOX@PDA) that integrates antibacterial and bone regeneration (Ma et al., 2022a). Shortly, femtosecond laser direct writing was firstly utilized to prepare slot micro-nanostructures (Fs-NiTi), then BPNSs were modified onto Fs-NiTi to c
留言 (0)