Research on incretin hormones has never stopped. The term “incretin” was originally coined by Creutzfeld (Creutzfeldt, 1979) in 1979 to represent the hormone secreted by the gut that stimulates the release of insulin in a glucose-dependent manner and is a critical regulator of energy metabolism. Although several insulin-stimulating hormones are secreted in the gut, there are only two physiological incretins so far, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulin-stimulating peptide (GIP). It was later discovered that these two hormones mediate the gut–bone axis and have a role in regulating bone metabolism. Glucagon-like peptide-2 (GLP-2), which is co-secreted with GLP-1, also regulates bone metabolism and is also part of the gut–bone axis. Several investigations, including human trials (Baggio and Drucker, 2007; Bergmann et al., 2019; Gabe et al., 2022; Gaudin-Audrain et al., 2013; Kreitman et al., 2021; Luo et al., 2016; Ma et al., 2013; Mabilleau et al., 2016; Mieczkowska et al., 2013; Nissen et al., 2019; Skov-Jeppesen et al., 2021; Westberg-Rasmussen et al., 2017), have elucidated that three hormones comprehensively suppress the process of bone resorption, with an added virtue of GIP fervently stimulating bone formation. Another incretin hormone, peptide YY (PYY), which is also secreted along with GLP-1 and GLP-2, has the most prominent role in appetite suppression, but recent studies have also shown that PYY has a strong inhibitory effect on bone formation (Jensen et al., 2021; Leitch et al., 2019; Russell et al., 2009; Scheid et al., 2011; Wong et al., 2012; Wortley et al., 2007). Notably, with increasing research on the gut microbiota (GM) in recent years, it has been found that the GM plays an important role in the regulation of bone metabolism, and the GM regulates bone homeostasis in a variety of ways, including influencing host metabolism, participating in immune regulation, and influencing endocrine bone signaling factors (including influencing the secretion of gut hormones). Herein, we review the relationship, potential mechanisms, clinical application prospects, and challenges of gut hormones, the GM and osteoporosis in the light of emerging literature and based on relevant studies in order to update the knowledge in this research area and to provide some references for future related studies.
1.1 BoneBone, as a highly dynamic organ, is extremely important for the human body. It provides rigidity and shape, supports the body structure, protects the vital organs, and aids locomotion. More importantly, as an endocrine organ, bone also participates in the metabolic processes of the human body. As the storehouse of calcium and phosphorus, bone tissue, along with the intestines and kidneys, is of great significance in maintaining the metabolic balance of phosphate and calcium ions in the body (Hill Gallant and Spiegel, 2017; Jouret et al., 2013).
Bone tissue consists of cellular components in an extracellular matrix. Bone-lining cells, osteoblasts, osteoclasts, and osteocytes are composed of cellular components. Their activity is regulated by mechanical forces, cytokines, hormones (e.g., parathyroid hormone (PTH)), bone cell turnover, and local factors. The differentiation of mesenchymal stem cells (MSCs) depends on the Wnt/β-catenin pathway. Under the regulation of transcription factors such as runt-related transcription factor 2 (RUNX2) and osterix (OSX), MSCs differentiate into osteoblast precursors and, eventually, osteoblasts. Sclerostin encoded by SOST (a soluble molecule that binds to low-density lipoprotein receptor-related protein-5/6 (LRP5/6)) can inhibit the Wnt signaling pathway by competitively inhibiting the coreceptor LRP5/6 in the signal pathway, thereby inhibiting osteoblast differentiation (Krause et al., 2010). Immature osteoblasts activate intracellular protein kinase A (PKA), protein kinase C (PKC), and calcium signal pathways under PTH signaling stimulation, induce osteoclast activation and differentiation, and then establish bone resorption (Rendina-Ruedy and Rossen, 2022). On the other hand, mature osteoblasts are produced and buried in matrix proteins (collagen) and are referred to as osteocytes. Osteocytes account for the majority of cells in mature mineralized bone; they have many linear pseudopods that communicate with osteoblasts, inactive bone-lining cells, and osteoclasts that are recruited on the bone surface to form a network of interconnections. Osteocytes secrete sclerostin in response to mechanical stress, which acts on the Wnt/β-catenin pathway in osteoblasts to inhibit cell proliferation, impair mineralization, enhance apoptosis, and promote osteoclast formation and activity in a RANKL (receptor activator of nuclear factor κB (NF-κB) ligand)-dependent manner (Horwood, 2016). Therefore, sclerostin produced by osteocytes regulates both osteoclasts and osteoblasts. Osteoclasts, the sole bone-resorbing cells, are multinucleated cells tightly attached to the surface of bone, are derived from the macrophage/monocyte lineage, and attach to the bone through a sealing zone where they secrete acids and proteases that break down the mineral and organic phases of bone in a spatially controlled manner. Macrophage colony-stimulating factor (M-CSF) and RANKL are key cytokines required for the survival, expansion, and differentiation of osteoclast precursor cells in vitro (Raggatt and Partridge, 2010). When RANK on the surface of osteoclast precursor cells binds with RANKL released by osteoblasts, the expression of osteoclast-related genes in the nucleus increases through the NF-κB and calmodulin/nuclear factor of activated T-cell (CN/NFATc1) pathways, which ultimately results in the activation of osteoclast-related genes in the osteoclast precursor cells and thus promotes osteoclastic differentiation. Mice lacking functional M-CSF or RANKL genes cannot absorb bone, resulting in ossification (Kim et al., 2020; Salhotra et al., 2020). Osteoprotegerin (OPG) is both a soluble bait receptor of RANKL and a negative physiological regulator of osteoclast formation that can effectively block the binding of RANKL to RANK on osteoclast precursors, delay the activation of osteoclasts, and inhibit bone resorption. Current studies have shown that the proportion of OPG/RANKL expression determines the differentiation and function of osteoclasts (Yang et al., 2020). Downregulation of the OPG/RANKL ratio causes animals to develop osteoporosis due to excessive osteoclastogenesis.
1.2 Bone remodelingBone, a highly dynamic organ, continues to remodel throughout life to help maintain bone strength and function, adapt to the body’s changing mechanical needs, and maintain calcium–phosphorus balance within the body. Healthy bone must be maintained through ongoing skeletal remodeling in order to perform these critical functions throughout life. Bone remodeling is a physiological process that involves the coordinated activity of a group of cells known as the basic multicellular unit (BMU) (Buenzli et al., 2014). The intrinsic communication within the BMU is frequently depicted as an intricate network of regulation among distinct cellular constituents, whereby the osteocyte population regulates the activity of the osteoblast population, and the osteoblast population, in turn, regulates the activity of the osteoclast population. A complete BMU consists of bone-lining cells that regulate the activity of osteoclasts, osteoblasts that are responsible for bone resorption, reversal cells that are responsible for transition, and mature osteoblasts that are responsible for mineralization, which coordinate their activities with each other in time and space to ensure the orderly progression of the entire process of bone reconstruction: activation, resorption, reversal, formation, and termination, a process that will be discussed below and illustrated schematically in Figure 1.
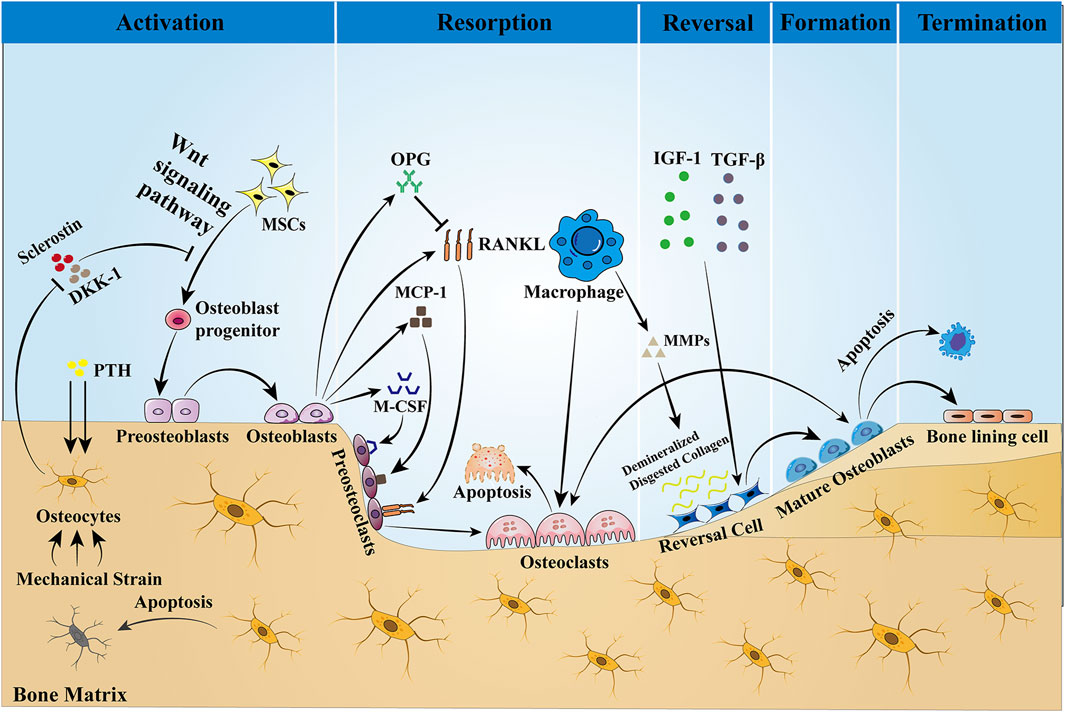
Figure 1. Bone remodeling is a dynamic process that is completed with the participation of BMU. This process is regulated by local intercellular signals and external signaling factors. The cellular components of BMU include osteoblasts, reversal cells, and bone-lining cells. Osteoblasts are derived from MSCs, a process that is dependent on the Wnt pathway and regulated by the transcription factors Runx2 and OSX. Sclerostin is a negative regulator of Wnt, which prevents mesenchymal stem cells from differentiating into osteoblasts. When the bone is stimulated by mechanical traction or PTH, the intracellular PKA and PKC pathways are activated, which initiates bone remodeling by inhibiting sclerostin expression and activating the Wnt signaling pathway. Osteoblasts stimulate osteoclast precursors to differentiate into mature osteoclasts by secreting M-CSF, MCP-1, and RANKL, but they may also inhibit the same cells by secreting OPG that scavenges RANKL, preventing it from binding to the RANKL receptors on the osteoclast precursors. Then, bone resorption begins, producing Howship’s resorption lacunae. After bone matrix resorption, macrophages produce MMPs to degrade and remove the bone matrix, and the release of active TGF-β and IGF-1 triggers osteoblasts to form a new collagen matrix in Howship’s resorption lacunae and fill the absorption space. Finally, the remodeling cycle ends when an equal amount of resorbed bone is replaced. In the bone reconstruction of healthy people, there was no change in bone mass and strength at the end of each reconstruction cycle. However, under some pathological conditions, such as osteoporosis, both bone mass and strength are decreased.
Activation Phase: In the resting state, osteocytes secrete TGF-β, which inhibits osteoclast formation. When osteoblasts are subjected to mechanical loading or when microdamage is detected in old bone (Bonewald, 2007), the signals that inhibit osteoclastogenesis are eliminated and osteoblasts are allowed to be generated (Jann et al., 2020). In addition, PTH signaling activates bone remodeling by inducing osteoblasts to secrete regulatory molecules that recruit osteoclast precursors to specific resorption sites.
Resorption phase: Osteoblasts play a crucial role in establishing bone resorption. As a response to PTH-induced bone remodeling, osteoblasts produce the chemokine monocyte chemotactic protein-1 (MCP-1) in vivo, which is a chemokine for osteoclast precursors and promotes RANKL-induced osteoclast formation in vitro (Li et al., 2007). In addition to the re-recruitment of osteoclast precursors, the major osteoclast factors expressed by osteoblasts, M-CSF, and RANKL are involved in the regulation of bone resorption. M-CSF and RANKL promote the proliferation and survival of osteoclast precursor cells and additionally coordinate the differentiation of osteoclast precursors into multinucleated osteoclasts to promote resorptive activity (Boyce, 2013; Burgess et al., 1999). Matrix metalloproteinases (MMPs) secreted by osteoblasts degrade unmineralized bone-like material arranged on the bone surface, which in turn promotes osteoclast attachment to the RGD-binding site, where the mineralized matrix is dissolved to form the Howship lacuna (Feng and McDonald, 2011). Some of the remaining undigested organic bone matrix is subsequently degraded by collagenases (especially histone K) (Lu et al., 2018).
Reversal phase: Following osteoclast-mediated resorption, the Howship lacunae are covered with a partially digested demineralized collagen matrix (Saftig et al., 1998). To eliminate these collagen remnants and prepare the bone surface for subsequent bone formation orchestrated by osteoblasts, early reversal cells exhibit a pro-resorptive phenotype and secrete matrix metalloproteinases that help osteoclasts to open channels for mineralized bone matrix and degrade the upper layer of non-mineralized collagen. In addition, reversal cells produce RANKL to aid in osteoclast resorption. In addition to reversing cells, macrophages can also produce MMPs, the enzymes required for matrix degradation, and are professional phagocytic cells. Late-differentiated reversed cells with fewer vesicles become mature osteoblasts with a pro-synthesizing phenotype and produce various cytokines such as OPG, which inhibit the proliferation and differentiation of osteoclasts and promote their apoptosis, thus playing an important role in adult bone reconstruction (Abdelgawad et al., 2016). Thus, the ultimate function of reversal cells may lie in their ability to receive or generate coupled signals that allow the transition from bone resorption to bone formation within the BMU.
Formation phase: In the stage of bone formation, many reversal cells close to osteoid proliferate and differentiate into mature osteoblasts, which produce osteoid and are responsible for mineral deposition. Meanwhile, both the insulin-like growth factor-1 (IGF-1) released during bone resorption and TGF-β are key signals for MSC recruitment to the site of bone resorption, and their expression promotes the return of MSCs or early osteoblast precursor cells to the resorption traps, ultimately allowing them to differentiate and secrete the molecules that form the replacement bone (Tang et al., 2009). Finally, to give the bone its full shape, hydroxyapatite is incorporated into this newly deposited bone-like material, ultimately forming high-quality mineralized bone and transitioning bone reconstruction to the termination stage.
Termination phase: The remodeling process concludes once an equal quantity of resorbed bone has been replaced. Sclerostin that inhibits bone formation is re-expressed at the end of remodeling, allowing bone mass to remain stable. Upon completion of mineralization, mature osteoblasts are transformed into bone-lining cells covering the bone surface or embedded in the mineralized matrix and are designated as bone cells. The quiescent bone surface environment is rebuilt and maintained until the next wave of remodeling begins.
Such a dynamic remodeling process is essential for bone self-homeostasis and for maintaining skeletal strength and calcium–phosphorus homeostasis (Siddiqui and Partridge, 2016). In the process of bone reconstruction in healthy people, there was no net change in bone mass and strength after each reconstruction cycle. However, bone mass and strength are reduced under pathological conditions, such as bone remodeling disorders in patients with osteoporosis.
1.3 Bone turnover markersThe International Osteoporosis Foundation and the International Federation of Clinical Chemistry recommend serum type I collagen C-terminal peptide (CTX-I) and type I procollagen N-terminal propeptide (P1NP) as two reference markers (Vasikaran et al., 2011). All research on bone metabolism is supposed to include at least these two markers. CTX-I is a part of the cross-linker decomposed by type I collagen, which is derived only from mature type I collagen, is not degraded or reutilized in vivo, and enters the blood at the time of bone resorption, which directly reflects the degradation of collagen fibers of the bone and decreases during antiresorptive therapy. Therefore, circulating CTX-I levels are used as biomarkers of bone resorption. P1NP originates from type I pre-collagen and is cleaved by protease after translation. Except for a small amount of P1NP deposited in the bone matrix, a large amount of it enters the blood circulation; thus, serum pre-collagen is mainly derived from bone and serves as a marker of bone formation (Schiellerup et al., 2019). Because multiple factors influence the levels of bone formation markers in the body, including hormone levels and body weight (Eastell et al., 2012; Evans et al., 2015), the introduction of a monitoring program is of great importance in determining the validity of bone conversion markers and also contributes to the reduction of fracture risk. However, conducting an extensive and comprehensive long-term study is imperative in order to obtain reliable results. Therefore, researchers may need to accept a degree of uncertainty.
2 Gut–bone axisThe gut and bone are interconnected to form a gut–bone axis, and this interaction is regulated by hormones secreted from the gut. These hormones, which are secreted in response to nutritional intake, lead to decreasing bone resorption (Clowes et al., 2002; Henriksen et al., 2003; Westberg-Rasmussen et al., 2017). Throughout the day, bone is remodeled, absorbed and formed in a coupled process to maintain dynamic balance (Eastell and Szulc, 2017; Zaidi et al., 2018). Eating during the day inhibits bone resorption but increases bone resorption at night, and this day–time suppression is eliminated by fasting, further affirming the role of the gut hormones in the control of bone homeostasis. The gut is one of the most important endocrine organs in the human body, from which many hormones are secreted. Among many hormones, a class of peptides known as incretin hormones has become an important regulator of energy metabolism. “Incretin hormones” are an endocrine signal secreted by the gut after eating that stimulates insulin release in a glucose-dependent manner (Creutzfeldt, 1979). Although the gut secretes a variety of insulin-stimulating hormones, it is currently believed that only GLP-1 and GIP are glucose-dependent insulin secretion stimulators, while other gut hormones, such as GLP-2 and PYY, may indirectly stimulate insulin secretion by increasing blood glucose.
The effects of GIP (secreted by enteroendocrine K-cells) and GLP-1 (secreted by L-cells) on glucose metabolism as mediators of the insulin effect have been extensively studied: enhanced insulin secretion occurs when glucose is ingested orally (Drucker, 2018). As a result, there has been a strong interest in their use in the treatment of type 2 diabetes mellitus (T2DM) and obesity. Many of the drugs widely used in the treatment of T2DM, such as liraglutide and exenatide, are GLP-1 receptor agonists (GLP-1RAs). By promoting postprandial insulin secretion, these hormones have the potential to elicit insulin-mediated enhancements in bone formation. Although short-term insulin injections during normoglycemia have essentially no effect on circulating levels of bone turnover markers (Frost et al., 2018), it is unclear whether long-term increases in insulin levels modulate insulin-mediated increases in bone formation. GLP-2, along with GLP-1, is released from the L-cells of the small intestine, and unlike the hypoglycemic effects of GLP-1 and GIP, GLP-2 is considered more of a pro-intestinal factor, which has led to the development of GLP-2 receptor agonists (GLP-2RAs) as therapeutic agents for short bowel disease (Drucker et al., 1996; Tsai et al., 1997). Unlike GLP-1, the role of GIP in bones is insulin-independent (Christensen et al., 2018). Another hormone, PYY, is similarly secreted along with GLP-1 and GLP-2 in PP cells and may affect bone metabolism by inhibiting bone formation. GLP-1, GLP-2, and GIP are considered to act directly or indirectly on bone cells to prevent bone resorption, while PYY inhibits bone formation. All four incretin hormones are associated with the gut–bone axis and will be summarized in Table 1.
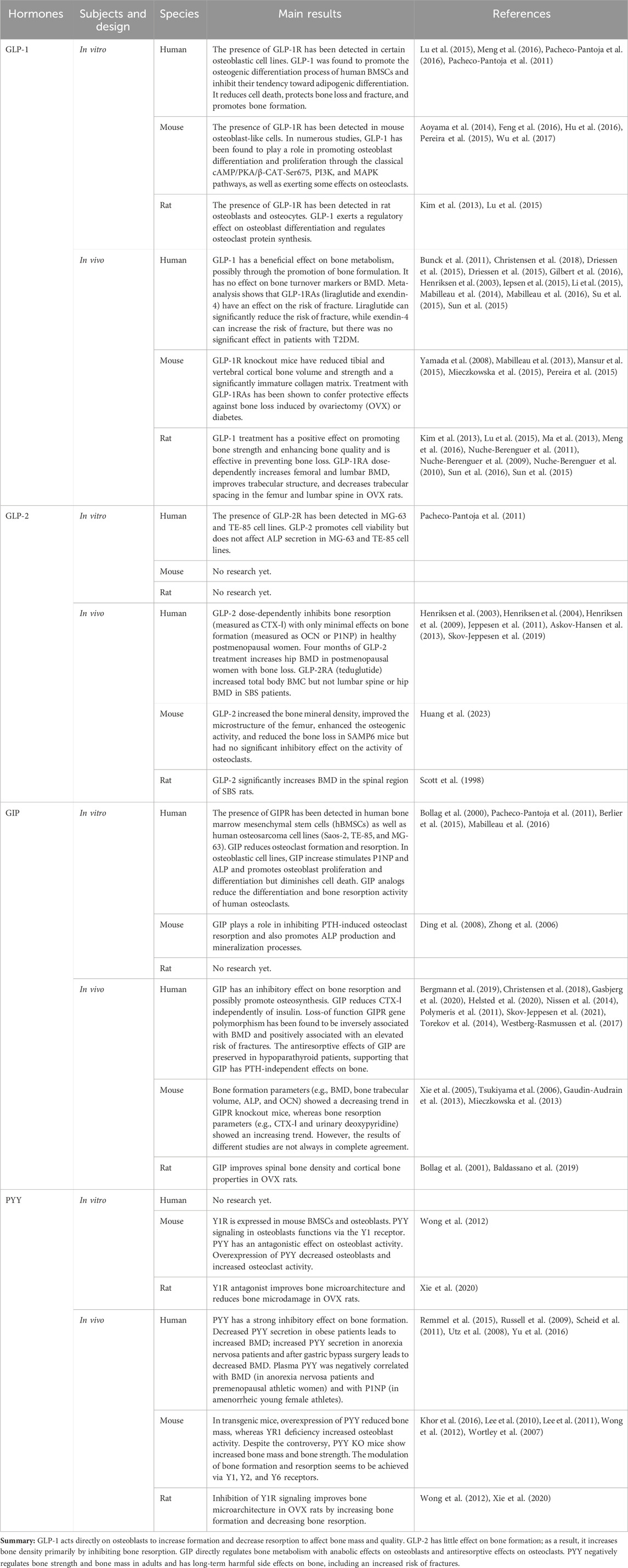
Table 1. Summary of the known effects of gut hormones on bone homeostasis.
3 Gut microbiota (GM)A huge number of microorganisms are attached to the surface and body of the human and play a vital role in the physiological activities and pathological coordination of the body (Cryan et al., 2019). Of the trillions of microorganisms in the human body, the vast majority comprise the more than 1,000 species of GMs found in the gut (Rizzoli, 2019). The gut micro-ecosystem is the largest micro-ecosystem in the human body, with 1,014 micro-organisms known to date and a total number of genes approximately 150 times the number of genes in the human genome (Qin et al., 2010; Zhang et al., 2022). Under normal circumstances, the GM can establish a dynamic ecological balance with the host and the external environment that is conducive to the maintenance of human health (Pröbstel et al., 2020). However, the negative impacts on the GM caused by poor dietary habits, radiation therapy, the misuse of antibiotics in medical treatment, and changes in the living environment may lead to microbial imbalance and metabolic imbalance, which may induce a series of diseases, including various forms of enteritis, diabetes, obesity, rheumatoid arthritis, osteoporosis, and depression (Fava et al., 2019; Jian et al., 2021; Tap et al., 2021; Wang et al., 2021; Zhao et al., 2021). Related studies have shown that regulating the biological abundance of the GM can modulate the onset and progression of diseases, and thus the GM is a potential new target that may offer new therapeutic options for the treatment of metabolic diseases (Tanabe et al., 2019).
Successive studies in recent years have shown that alterations in the GM are strongly associated with the onset and progression of osteoporosis (Chen et al., 2022; Guss et al., 2019; Nilsson et al., 2018). Due to the complexity of the mechanisms involved in the involvement of the GM in osteoporosis, some studies have been conducted in the context of host metabolism, immunity, and the endocrine environment (Behera et al., 2020; Iqbal et al., 2020) (Figure 2).
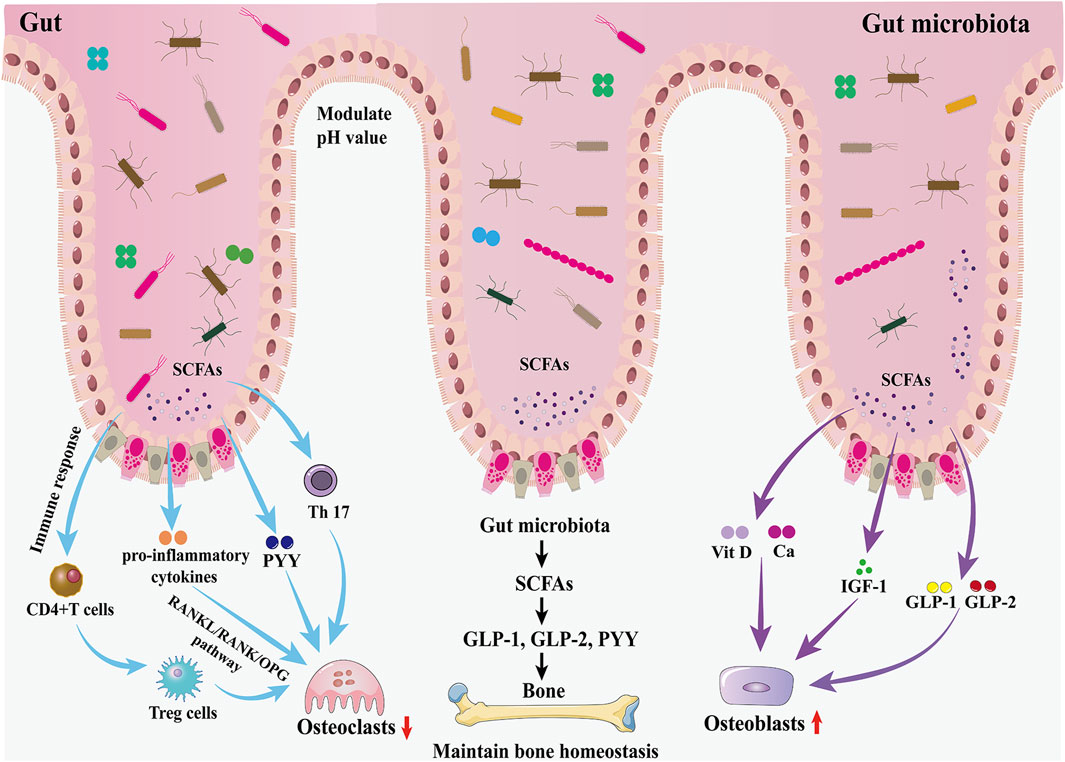
Figure 2. Effects of the GM and gut hormones on bone metabolism. The GM regulates bone homeostasis through multiple pathways, including host metabolism, immunomodulation, and hormonal regulation. On the one hand, the GM promotes the production of GLP-1 and GLP-2 by intestinal cells through SCFAs, thereby promoting bone formation; in addition, the increased secretion of IGF-1 is also favorable to bone formation. On the other hand, the GM inhibits osteoclast activity by inhibiting Th17 cell differentiation and thus inhibiting the release of RANKL.
3.1 GM affects bone homeostasis through host metabolismThe GM may affect bone homeostasis through the metabolism of short-chain fatty acids (SCFAs). In the gut, lactose-based prebiotics are converted by probiotics into SCFAs (butyric, acetic, and propionic acids) and lactic acid (Borka Balas et al., 2023). They promote the synthesis of the gut hormones plasma GLP-1, GLP-2, and PYY by entero-endocrine L cells through the activation of GPCRs (Kasubuchi et al., 2015; Kellow et al., 2014). As described earlier, GLP-1 and GLP-2 play an important role in increasing the activity of osteoblasts and promoting bone formation. Therefore, the GM promotes bone formation through the increase of GLP-1 and GLP-2 secretion mediated by SCFAs. However, SCFA-induced increases in PYY secretion should not be overlooked because PYY has a strong bone resorption-promoting effect. IGF-1 is a hormone that can affect bone growth. It is one of the most abundant growth factors in bone, with receptors in both osteoblasts and osteoclasts, and can regulate the activity of osteoblasts, promote the formation of collagen, inhibit the degradation of collagen, and play a role in the maintenance of bone mass and bone density. The GM may maintain bone mass by increasing serum IGF-1 levels through the production of SCFAs. When SCFAs, a metabolite of GM, were supplemented to antibiotic-treated mice, IGF-1 and bone mass were restored to the levels of normal mice, suggesting that the restoration of the GM in germ-free mice induces circulating levels of IGF-1 through the production of SCFAs, which in turn affects bone metabolism (Yan et al., 2016). In addition, SCFAs can also lower the pH of the gut environment, prevent calcium ions from compounding with phosphorus to increase calcium absorption, and inhibit RANKL signaling pathway-induced osteoclastogenesis by suppressing osteoclast gene expression (Chen et al., 2020). Besides, the butyrate in SCFAs provides energy to the gut, repairs the structure of gut mucosa and villi, increases the absorption area of the gut, and increases the absorption and utilization of calcium in the gut.
3.2 GM affects bone homeostasis through the immune systemThe GM is essential for the function and maturation of the immune system, which stimulates the immune system at the gut mucosal barrier through the release of metabolites and immune cells (including T cells and B cells), the release of pro- or anti-inflammatory mediators as well as cytokines, and the regulation of systemic bone metabolism via the blood circulation (Schluter et al., 2020). Activated immune cells can migrate to skeletal tissue and directly regulate bone metabolism by releasing products that include osteoclast-inducing factors such as RANKL, TNF-α, and interleukin (IL) (Pacifici, 2016). A study of the gut thick-walled bacillus phylum in mice showed that Clostridium difficile promotes the expression of helper T cells (Th cells) in the lamina propria of the colon. Th cells inhibit osteoclast differentiation and impede osteoblast formation, and a reduction in the number of C. difficile strains leads to lower levels of Th cells and an increase in bone turnover (Luo et al., 2011). The GM can also regulate the balance between intestinal Tregs and Th cells by influencing the development of Th cells and the differentiation of Tregs, thereby altering the internal environment of the gut and the body as a whole, regulating the function of osteoclasts and osteoblasts, and influencing the dynamic balance between bone resorption and bone formation (Sun et al., 2020). A GM imbalance may inhibit the differentiation of the Tregs subpopulation, leading to the predominant differentiation of Th17 cells, which belong to the CD4+T-cell osteoblastic cell group and can secrete IL-17a, IL-1, IL-6, tumor necrosis factor, and low levels of interferon-γ. These cytokines induce monocytes and macrophages to form osteoclasts and exacerbate bone loss by promoting the release of RANKL. Kim et al. (2017) transplanted a variety of commensal bacteria isolated from human feces into mice and found that the mouse gut segmented filamentous bacteria and human commensal bacteria promoted the differentiation of Th17 cells in the gastrointestinal tract of mice, which induced pro-inflammatory cytokines (including IL-17, TNF-α, and IL-1β), as well as the production of osteoclasts, and enhanced bone resorption.
3.3 GM affects bone homeostasis through hormonesRecent studies suggest that the GM may be involved in endocrine regulation of the body to alter the relative activity of osteoclasts and osteoblasts, thereby affecting bone metabolism and participating in the regulation of bone mass (Iqbal et al., 2020). Until now, the GM has been found to be strongly associated with the levels of several hormones, including gut hormones, IGF-1, PTH, and estrogen (Behera et al., 2020).
3.3.1 GM affects bone homeostasis through IGF-1The GM may regulate osteogenesis by affecting the level of IGF-1. IGF-1 is an important endocrine hormone that regulates growth and promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) through the Wnt/β-catenin pathway, which is essential for bone accumulation and maturation (Feng and Meng, 2021). When hepatogenic IGF-1 was regularly increased in aseptic mice, they showed bone loss in the short term but increased bone formation and bone mass after 8 months (Yano et al., 2015). When SCFAs, a metabolite of GM, was supplemented to antibiotic-treated mice, IGF-1 and bone mass were restored to the levels of normal mice, suggesting that the restoration of the GM in germ-free mice is mediated by the production of SCFAs, which induces circulating levels of IGF-1 and thus has an effect on bone metabolism (Yan et al., 2016).
3.3.2 GM affects bone homeostasis through PTHPTH is a pro-calcium hormone synthesized by the parathyroid glands. Intermittent PTH treatment (iPTH) plays an important role in the regulation of bone development and maturation by stimulating bone formation and resorption. It has been demonstrated (Li et al., 2020) that this effect is dependent on the GM and its metabolites and that iPTH requires physiological concentrations of butyric acid to regulate Treg and induce bone anabolism. At the same time, iPTH requires microbiota to increase the production of TGF-β and IGF-1 in bone marrow. iPTH failed to induce bone formation in germ-free female mice after microbiota removal with broad-spectrum antibiotics.
3.3.3 GM affects bone homeostasis through estrogenEstrogen plays an important role in bone metabolism, inducing apoptosis in osteoclasts and inhibiting apoptosis in osteoblasts. In an estrogen-deficient environment, the bone conversion cycle is activated more frequently (Ruth et al., 2020). In T-cell-deficient mice, ovariectomy does not lead to bone loss, so TNF plays an important role in bone loss in ovariectomized mice. The key mechanisms by which TNF stimulates bone resorption are activation of RANK and induction of Th17 cells (Yu et al., 2020). IL and TNF inhibition can be used to prevent increased bone resorption due to estrogen deficiency.
In summary, the GM and bone metabolism have a complex and tight association that can affect host metabolism through SCFAs, host immune function through T cells, and host endocrine environment through gut hormones, IGF-1, PTH, and estrogen, thus affecting bone metabolism. As a result, osteoporosis populations have their own specific GM characteristics; that is, diversity and abundance were significantly reduced. Because gut hormones also have a very important influence on bone metabolism, the relationship between the GM, gut hormones, and bone metabolism and their associated mechanisms will be described in detail below.
4 GLP-1GLP-1 is secreted from intestinal L-cells in response to nutrient intake. GLP-1 has two biological activities, namely, GLP-1 (1-36) and GLP-1 (7-36) (Kumar et al., 2016), and GLP-1 (7-36) is predominant in the human body (Ørskov et al., 1994), followed by GLP-1 (7-37) with the same biological activity. Human endogenous GLP-1 cleaves GLP-1 (7-36 amide) and GLP-1 (7-37) on the N-terminal dipeptide very quickly in the presence of dipeptidyl peptidase 4 (DPP-4), generating low-affinity ligands of the GLP-1 receptor, GLP-1 (9-36 amide) or GLP-1 (9-37), which gives it a half-life of only 1.5 min–2 min (Deacon et al., 1995; Kieffer et al., 1995; Mentlein et al., 1993). Subsequently, the kidneys rapidly cleared these intact forms as well as inactivated GLP-1 metabolites. Food composition affects the time and range of GLP-1 secretion. Plasma GLP-1 levels were higher in high-protein diets than in carbohydrate- or fat-rich diets (Lejeune et al., 2006; Raben et al., 2003; Samkani et al., 2018) and increased more slowly after fat than carbohydrate intake (Elliott et al., 1993). Although GLP-1 degradation is not affected by renal function, the elimination of both GLP-1 and its inactive metabolites is prolonged in individuals afflicted by renal insufficiency (Meier et al., 2004).
Evidence has elucidated that GLP-1 orchestrates crucial physiological functions through its interaction with the GLP-1 receptor (GLP-1R), a cAMP-linked G-protein-coupled receptor that is widely distributed throughout the body in various tissues. GLP-1Rs were first identified in pancreatic islet β-cells and the central nervous system (CNS) (Drucker et al., 1987; Shimizu et al., 1987). Subsequently, typical GLP-1R was found to be expressed in the pancreas, kidney, gastrointestinal tract, and blood vessels (Andersen et al., 2018). Recent evidence from Pereira et al. (2015) seems to indicate that the known GLP-1R is also present in bone tissue. GLP-1 acts on pancreatic β to stimulate insulin secretion and improve islet β cell function, on α cells to increase glucose sensitivity and inhibit glucagon secretion, and on δ cells to stimulate growth inhibitory hormone release (Ding et al., 2011; Drucker and Yusta, 2014; Dunning et al., 2005). In addition, activation of GLP-1R in the central nervous system leads to reduced food intake and weight reduction (Holst, 2007), whereas in the stomach, GLP-1 not only inhibits the secretion of gastrointestinal glands but also inhibits gastric motility (especially gastric emptying) and gastric acid secretion (Zhang et al., 2022). In vivo tests on healthy volunteers (Schirra et al., 2002) have shown that GLP-1 increases gastric volume, reduces food consumption, and inhibits gastric emptying. In addition to the effects of GLP-1 in healthy individuals, several studies (Deane et al., 2010; Meier et al., 2003; Näslund et al., 1998) have shown that exogenous GLP-1 inhibits gastric emptying in patients with T2DM, obesity, and critical illness. As a result of these beneficial effects of GLP-1R activation on metabolism, several drugs acting on the GLP-1R have been developed for the treatment of T2DM and obesity, including the GLP-1RAs liraglutide and exendin-4 or its synthetic version exenatide, and additional products are in different stages of development (Nauck et al., 2016; Odawara et al., 2016; Oh and Olefsky, 2016; Sheu et al., 2016). Because of the effectiveness of these drugs in the management of metabolic disorders, interest in them has since gradually shifted to bone metabolism.
4.1 GLP-1’s effect on bone metabolism in vitroSeveral previous studies have shown that GLP-1 affects bone metabolism, although the exact mechanisms involved have not been fully explained. GLP-1R has been identified on immature osteoblastic TE-85 (Pacheco-Pantoja et al., 2011), MG-63 (Pacheco-Pantoja et al., 2011), and MC3T3-E1 (Aoyama et al., 2014) cells, whereas it has not been found in the Saos-2 cell line (Pacheco-Pantoja et al., 2011). It has been found that GLP-1R has glucose-dependent expression during osteogenic differentiation of MC3T3-E1 cells induced by bone morphogenetic protein-2 (BMP-2), and its expression increases with the increase of glucose concentration in the culture medium (Aoyama et al., 2014). GLP-1 affects the differentiation and activity of MC3T3-E1 cells by inducing the hydrolysis of glycosylphosphatidylinositol (GPI) and increasing the activities of PI3K and MAPK signaling pathways in these cells, thus promoting cell proliferation, differentiation, and mineralization (Nuche-Berenguer et al., 2010). Furthermore, liraglutide, a GLP-1RA, was shown to directly promote MC3T3-E1 osteogenesis and osteoblast mineralization through the activation of the cAMP/PKA/β-CAT-Ser675 signaling pathway (Wu et al., 2017). It was also proposed that liraglutide regulated MC3T3-E1 cell differentiation mediated by adenosine monophosphate-activated protein kinase (AMPK), a mammalian target of rapamycin (mTOR) signaling (Hu et al., 2016). Moreover, studies by Pal et al. (2019) showed that liraglutide increased the expression of AdipoR1 in osteoblasts in a time-dependent manner. Because the activation of AdipoR1 in osteoblasts leads to the upregulation of the mitochondrial bioproduction factor, Pgc1α, which in turn contributes to their osteogenic effects, it was hypothesized that liraglutide might stimulate mitochondrial function in osteoblasts by upregulating AdipoR1. In addition, liraglutide promotes mitochondrial DNA synthesis in osteoblasts by mediating the upregulation of TFAM. Notably, liraglutide also promotes anti-apoptotic effects in osteoblasts by upregulating the mitochondrial outer membrane protein VDAC1. Therefore, the enhancement of mitochondrial biosynthesis and mitochondrial function seems to play a key role in the effects of liraglutide-induced bone metabolism. Exendin-4, another GLP-1RA, has been shown to enhance the proliferation and differentiation of osteoblasts partly mediated by MAPK pathways, including extracellular signal-regulated kinase1/2 (ERK1/2), p38, and c-Jun N-terminal kinase (JNK) pathways (Feng et al., 2016). The signaling pathway mediated by GLP-1R prevents apoptosis and promotes cell proliferation (Jiang et al., 2016; Wang et al., 2015). In a study by Pacheco-Pantoja and Ranganath (2011), GLP-1 increased cell survival but decreased secretion of P1NPs (markers of bone formation) in two osteoblast lines, MG-63 and TE-85. In another of their studies, it was also found that GLP-1 induced the expression of c-Fos (a gene critical for osteoblast proliferation and differentiation) in osteoblast TE-85 cells (Pacheco-Pantoja et al., 2016). BMSCs have multidirectional differentiation potential and are capable of osteogenic and lipogenic differentiation. It has been shown that GLP-1R is also expressed in BMSCs, and liraglutide promotes osteogenic differentiation, inhibits lipogenic differentiation and reduces cell death, protects against bone loss and fracture, and promotes bone formation in rat and human BMSCs (Jeon et al., 2014; Lu et al., 2015; Meng et al., 2016; Sanz et al., 2010). The effect of GLP-1 on BMSCs has been shown to be mediated in part by mitogen-activated extracellular signal-regulated kinase (MEK) and protein kinase C (PKC) signaling pathways (Sanz et al., 2010). Another study shows that GLP-1 also promotes BMSCs to differentiate into osteoblasts by acting on the PKA/β-catenin and PKA/PI3K/AKT/GSK3β pathways (Meng et al., 2016). The Wnt pathway may mediate GLP-1 to promote bone formation, as exendin-4 treatment has previously been reported to ameliorate bone loss induced by an impaired Wnt pathway in diabetic rats (Nuche-Berenguer et al., 2010). In addition, the present study also observed that exendin-4 decreased the mRNA and protein levels of sclerostin in osteoblast-like MLO-Y4 cells and also decreased the serum sclerostin levels in T2 DM Otsuka Long-Evans Tokushima obese rats. Therefore, it was further inferred that exendin-4 might first bind to GLP-1R and act on the PKA pathway, which in turn affects the Wnt/β-catenin pathway in the osteoblasts, reduces the expression of sclerostin, and promotes the formation of bone (Kim et al., 2013). To ensure the accuracy of the extrapolation, further research is needed before a definitive explanation of this issue can be provided. Moreover, GLP-1R exists not only in osteoblast lines; Li et al. (2020) found that GLP-1R was expressed in mouse BMMS and RAW 264.7 cells and that knockdown of GLP-1R activity increased the expression of pro-osteoclastogenic biomarkers and promoted osteoclast formation and bone resorption. Exendin-4 and liraglutide increased the number of osteoclast precursors in mice, while the addition of mature osteoclasts reduced the absorption area, suggesting that GLP-1RA promoted osteoclast differentiation but inhibited its absorptive activity. The inhibitory effect of liraglutide on osteoclast formation and bone resorption may be achieved by inhibiting the MAPK and NF-κB pathways, which downregulates the expression of downstream osteoclast marker genes mediated by NFATc1 and NFATc1 by GLP-1R. However, whether GLP-1 or GLP-1RA has a direct effect on osteoclast formation is still controversial.
4.2 GLP-1’s effect on bone metabolism in vivoSeveral in vivo rodent studies have confirmed that GLP-1 is necessary for bone strength (Yamada et al., 2008). Knockouts (KO) are commonly used in modern medical research. Yamada et al. (2008) showed that tibial and vertebral cortical bone volume and strength were reduced in 10-week-old GLP-1R KO mice compared to wild-type control mice. Although there was no significant change in bone mineral quantity or quality in the GLP-1R KO mice, they had a significantly immature collagen matrix, resulting in reduced cortical layer thickness, bone diameter, bone mineral content, and yield strength (Mabilleau et al., 2013). The trabecular density of GLP-1R and GIPR double KO mice was higher than that of the wild-type control group, but the cortical thickness, cortical area, and external diameter of bone decreased (Mieczkowska et al., 2015). In contrast, treatment with GLP-1RAs has been shown to have osteogenic effects. It has been reported that the treatment of diabetic animal models with exendin-4 can improve bone mechanical properties and prevent bone loss by changing the cortical microstructure and bone composition parameters (mineral crystallinity, collagen maturity, acid phosphate content, and carbon–phosphorus ratio) (Mansur et al., 2019). A study on the role of GLP-1RAs in OVX-induced osteoporosis in rats showed that treatment with exendin-4 for 8 weeks improved the trabecular volume, thickness, and number of lumbar vertebrae and femurs; decreased trabecular spacing; and increased bone mineral density (BMD). This was mainly achieved by increasing the expression of Runx2, alkaline phosphatase (ALP), and Col-1 mRNA. In addition, exendin-4 treatment also increased the expression of p38, p42/44, and β-catenin protein; however, GLP-1RAs had little effect on the mechanical resistance of femurs (Pereira et al., 2015). Another study showed that the exendin-4 also inhibited bone resorption by increasing the OPG/RANKL ratio, thereby preventing deterioration of trabecular microarchitecture, reversing the decline in femur and vertebral bone mass, and increasing bone strength (Nuche-Berenguer et al., 2009). Exendin-4 has been shown to act on the Wnt/β-catenin pathway in osteoblasts via the GLP-1R, thereby promoting bone formation and reducing sclerostin levels in T2DM rats (Kim et al., 2013). Calcitonin’s role in bone metabolism is to inhibit osteoclast activity and attenuate the process of osteolysis. It was found that GLP-1R is expressed in thyroid C cells and promotes calcitonin secretion from these cells via a cAMP-mediated pathway (Crespel et al., 1996; Lamari et al., 1996). GLP-1R KO mice showed higher urinary deoxypyridine (bone resorption markers) levels, decreased cortical bone mass, and increased bone fragility, and bone histomorphometrics showed an increased number of osteoclasts enhanced bone resorption activity (Mabilleau et al., 2013). Urinary deoxypyridine levels were effectively suppressed in GLP-1R KO mice after treatment with calcitonin. These findings confirm the important role of endogenous GLP-1R signals in control, which may be through the calcitonin-dependent pathway (Yamada et al., 2008), but this hypothesis needs to be further explored. GLP-1 may also regulate bone metabolism by affecting blood glucose levels. In particular, hyperglycemia has now been found to be negatively correlated with lumbar spine bone density. It has been shown that GLP-1 controls blood glucose levels by stimulating insulin secretion, inhibiting glucagon secretion, and regulating gastric emptying, thus promoting bone formation (Hare et al., 2009; Sasaki et al., 2015). Therefore, it is speculated that GLP-1 can indirectly affect bone metabolism by regulating the level of blood glucose (Terzi et al., 2015).
In preclinical studies in several rodent models, GLP-1 and GLP-1RA have been shown to have significant benefits on bone metabolism, both of which promote bone formation while inhibiting bone resorption and preventing bone degeneration. However, there is a lack of research on GLP-1 on osteoclasts, and increased research in this area, as well as long-term dynamic monitoring, will help determine the effects of GLP-1 on bone and the specific mechanisms.
4.3 GLP-1’s effect on bone metabolism in humansCompared to rodents, results from human studies are inconsistent. Several researchers (Gilbert et al., 2016; Li et al., 2015) examined BMD and bone turnover markers in T2DM patients treated with liraglutide and exenatide but did not find any effect of GLP-1RAs on bone turnover markers or BMD. However, infusion of GLP-1 reduced CTX-Ⅰ in overweight and obese patients (Bergmann et al., 2019), whereas GLP-1RA exenatide also reduced CTX-Ⅰ levels without affecting the levels of P1NP. In contrast, subcutaneous injection of GLP-1 or its major metabolite, GLP-1 (9–36 amide), did not alter CTX-Ⅰ in overweight (Henriksen et al., 2003) or young healthy adults (Nissen et al., 2019), respectively. While inconsistent with previous results, synthesizing these investigations infers that GLP-1 may inhibit bone resorption while leaving bone formation unaffected in humans. However, weight loss caused by GLP-1RA treatment may mean a higher risk of fracture. A meta-analysis (Mabilleau et al., 2016; Sun et al., 2015) showed that the effects of GLP-1RAs (liraglutide and exendin-4) on fracture risk seem to be inconsistent, with liraglutide significantly decreasing fracture risk, whereas exendin-4 increased fracture risk. Driessen et al. (2015) investigated the incidence of fracture between GLP-1RA users and non-users in the United Kingdom and the Netherlands. The findings demonstrated no significant difference between the two groups, suggesting that the role of GLP-1RAs in a T2DM population is neutral. However, inconsistently, meta-analyses from (Cheng et al., 2019) and (Su et al., 2015) have shown that GLP-1RA treatment has a fracture risk reduction effect in patients with T2DM. Considering the limited number of interventional studies on the effects of GLP-1RA on bone and the lack of sufficient preclinical data, it is reasonable to establish in vitro study to investigate the effects of GLP-1 and GLP-1RA on human bone cells, as well as on bone remodeling in individuals with or without diabetes, in order to ensure the accuracy and credibility of the conclusions.
4.4 Relationship between GLP-1, GM, and bone metabolismThe role of GLP-1 in improving bone metabolism and anti-osteoporosis has been discussed above, although the studies on the promotion of GLP-1 secretion by SCFAs are not yet in-depth, and the related mechanisms are not yet clear. GLP-1, as a gut hormone, may be closely related to the GM and bone metabolism to a large extent (Han et al., 2021; Wang et al., 2020). Here, a previous study showed that consumption of the probiotic VSL 3 promoted GLP-1 secretion in mice, accompanied by increased fecal butyric acid levels (Yadav et al., 2013). Butyric acid has also been shown to stimulate the proliferation of intestinal mucosal cells, expanding the surface area of the intestinal epithelium and further enhancing calcium absorption, suggesting an inextricable link (Chen et al., 2019). In contrast, previous studies have shown that some GMs produce serotonin that crosses the intestinal barrier and enters the bloodstream and that serotonin reduces the secretion of GLP-1, which in turn reduces the production of osteoblasts, inhibits bone formation, and leads to osteoporosis (Yadav et al., 2010).
In summary, many studies have shown that GLP-1 affects bone mass and bone quality by affecting bone formation and bone resorption. Although the molecular pathways between GLP-1 and bone metabolism are summarized in this review (Figure 3), the specific processes of GLP-1 or GLP-1RAs affecting bone metabolism and their related molecular mechanisms have not been fully elucidated in different parts of bone tissues from patients with different metabolic states. Therefore, further studies on the effects of GLP-1 or GLP-1RAs on bone metabolism under different metabolic states are necessary. Finally, because GLP-1 is a good metabolic regulator hormone, elucidating the specific mechanism of the effect of GLP-1 on bone metabolism will help guide the development of new drugs for osteoporosis.
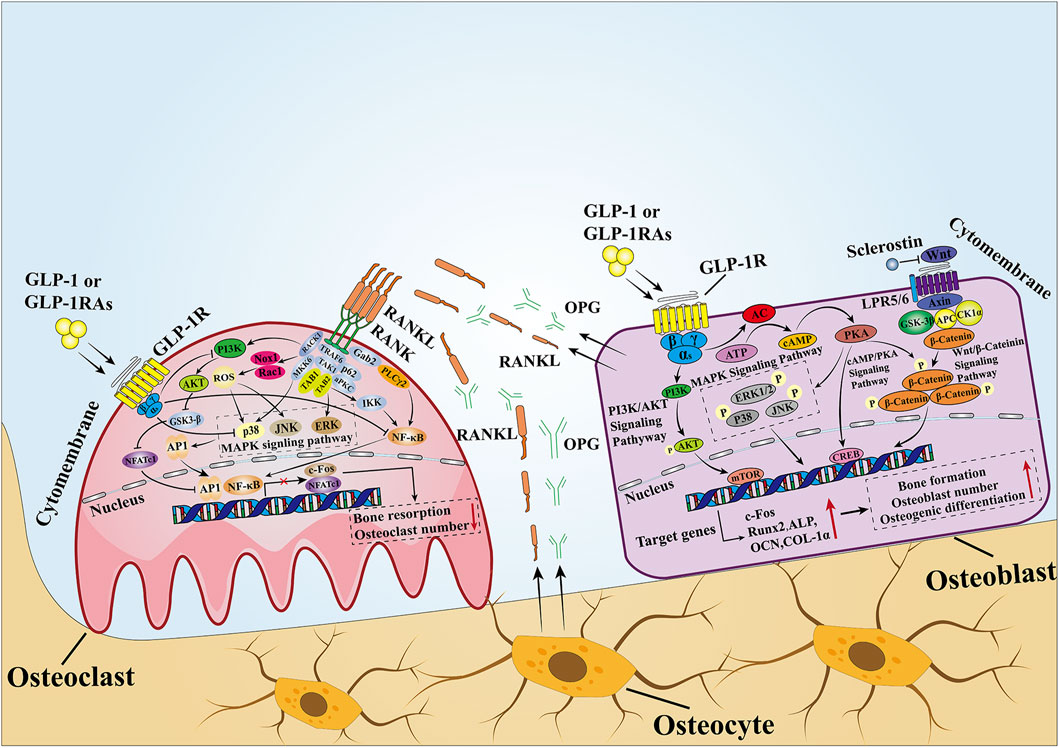
Figure 3. Mechanisms of GLP-1 effects on bone. In MSCs and osteoblasts, GLP-1 activates intracellular signaling pathways by binding to its receptors. After GLP-1R is activated, it stimulates adenylate cyclase to convert ATP into cAMP and then activates downstream PKA signals. Then, PKA continues to increase the phosphorylation of ERK, P38, JNK, and β-catenin, causing them to enter the nucleus to stimulate the expression of osteogenesis-related genes and promote bone formation. In osteoclasts, when GLP-1 binds to GLP-1R, it inhibits the activity of the intracellular MAPK pathway and NF-κB pathway. The former downregulates the transcription of c-Fos to inhibit osteoclast formation, while the latter downregulates the expression of downstream osteoclast marker genes mediated by NFATc1 and NFATc1 to inhibit bone resorption. In addition, GLP-1 directly inhibits the activity of RANKL, thus inhibiting the activity of the NF-κB pathway. OPG and RANKL secreted by osteoblasts and osteoclasts are important signaling pathways that mediate the balance between bone resorption and bone formation, and a proper state of balance is conducive to maintaining bone homeostasis.
5 GLP-2Like GLP-1, GLP-2 is secreted by L-cells in the small and large intestine after nutritional intake, and GLP-2 secretion was not as high after ingestion of a carbohydrate-rich meal as it was after ingestion of protein and fat (Raben et al., 2003). GLP-2 is a short peptide of 33 amino acids derived from the same prepeptide-proglucagon that is decomposed to produce GLP-2 under the action of pre-hormone converting enzyme 1/3 (PC1/3). There are two main forms of GLP-2 in the human body, which are bioactive GLP-2 (1-33) and non-bioactive GLP-2 (3-33) (Hansen et al., 2007). The basal level of plasma GLP-2 is very low during fasting. The concentration of GLP-2 will increase rapidly after eating, and then it will be hydrolyzed by DPP-4 at the first two amino acid residues of GLP-2 (1-33) to non-bioactive GLP-2 (3-33). Although the half-life of GLP-2 (3-33) is about 7 min (Hartmann et al., 2000), it is much longer than that of GLP-1 (1.5 min–2 min). There are two ways to extend the half-life of GLP-2: using DPP-4 inhibitors or substituting 2-position alanine (Hartmann et al., 2000; Jeppesen et al., 2005; Thulesen et al., 2000).
According to the study, most of the physiological effects exerted by GLP-2 are mediated by the GLP-2 receptor (GLP-2R). The GLP-2R belongs to class B1 of the G-protein-coupled receptors (GPCRs) and is highly specific for GLP-2, based on rodent studies. The GLP-2R is widely expressed throughout the intestine but is also found in the central nervous system and possibly to a lesser extent in the lungs (Yusta et al., 2000). In humans, however, the distribution of GLP-2R remains uncertain due to the lack of well-immunolocalized antibodies and the low expression of GLP-2R in cells outside the gastrointestinal tract. Extrapolation from other species may be risky due to interspecies variation (Drucker and Yusta, 2014).
GLP-2 promotes intestinal nutrient absorption. In mice, GLP-2 promoted the growth of the small intestine and large intestine, and inconsistently, the combination of GLP-2 and high-dose GLP-2 (3-33) reduces the growth-promoting effect, speculating that it may be due to competitive antagonism between GLP-2 and GLP-2 (3-33) (Thulesen et al., 2002). GLP-2 acts on intestinal crypts to stimulate cell proliferation and inhibit apoptosis (Dubé et al., 2006). Although less well-established, it possesses the capacity to suppress food intake and bolster the growth of neurons (Askov-Hansen et al., 2013; Bremholm et al., 2010; Henriksen et al., 2009). As a result, a DPP-4-resistant GLP-2 analog (teduglutide) has been used in the treatment of short bowel syndrome (SBS) since 2012 (Jeppesen et al., 2018). GLP-2 promotes the absorption of intestinal nutrients and blood supply. Studies have shown that GLP-2 can increase the blood supply of the superior mesenteric artery through vasoactive intestinal peptide (VIP) and 5-hydroxytryptamine (5-HT) (Guan et al., 2006). GLP-2 inhibits gastric acid secretion. Sham-feeding increased gastric acid secretion, and this increase was reduced by 65% when GLP-2 was given compared to saline, suggesting that GLP-2 can inhibit gastric acid secretion in humans (Meier et al., 2006). Moreover, as GLP-2 regulates glucose metabolism, endogenous GLP-2 can be used as a protective factor against glucose metabolism disorders in mice fed with a high-fat diet, and chronic treatment of GLP-2 (3-33) improves glucose metabolism disorders (Baldassano et al., 2015). The significant role of GLP-2 in participating in the body’s metabolism raises the question: does GLP-2 play a role in bone metabolism?
5.1 GLP-2’s effect on bone metabolism in vitroSimilar to the GLP-1, GLP-2 may have practical value in the treatment of osteoporosis. However, the mechanisms by which GLP-2 acts on bone are not fully understood. Pacheco-Pantoja et al. (2011) discovered the expression of GLP-2R in the immature human osteoblast cell lines TE-85 and MG-63. After incubating GLP-2 with serum-deficient osteoblast cell line TE-85, the level of P1NP decreased, indicating that bone formation may be reduced. However, another parameter associated with bone formation (ALP levels) was unchanged (Pacheco-Pantoja et al., 2011). In contrast, neither P1NP nor ALP changed in the MG-63 osteoblast line, but osteocalcin (OCN) decreased after 5 days of GLP-2 treatment (Pacheco-Pantoja et al., 2011). No significant response was observed in the most mature cell line, Saos-2, which is not surprising because we did not detect the presence of GLP-2R mRNA in the Saos-2 cell line (Pacheco-Pantoja et al., 2011). In addition, recent reports have also indicated that GLP-2R was not detected in human MSC expression (Jeppesen et al., 2018). There seems to be some inconsistency regarding the response triggered by GLP-2, as some bone formation markers were changed while others were unaffected. By contrast, GLP-2R is expressed in human osteoclasts (Skov-Jeppesen et al., 2019), suggesting that GLP-2 may regulate the activity of osteoblasts and possibly that of osteoclasts through coupling factors secreted by osteoclasts. Therefore, it is necessary to investigate the effects of GLP-2 on osteoclasts and osteoblasts and their mechanisms.
5.2 GLP-2’s effect on bone metabolism in vivoIn vivo experiments show a more divergent role and application of GLP-2 in bone mass. The preliminary results of GLP-2 in SBS rats showed that the BMD in the spinal region increased significantly after 5 weeks of GLP-2 treatment (Scott et al., 1998). Huang et al. (2023) showed that GLP-2 treatment for 6 weeks could reduce bone loss in SAMP6 mice, which was characterized by increased bone mineral density, improved microstructure of the femur, and enhanced osteogenic activity. However, the activity of osteoclasts was not significantly
留言 (0)