Inflammatory bowel disease (IBD) including its major clinical forms ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic, recurrent disease characterized by intestinal inflammation whose etiology is still poorly understood. Nevertheless, the pathogenesis of IBD is multifactorial and results from genetic predisposition, immunological status, and environmental factors, including intestinal microbiota disbalance (Graham and Xavier, 2020). Gut microbial dysbiosis was detected both in patients with UC and CD and animal models of IBD (Ni et al., 2017; Facchin et al., 2020) and was associated with a reduced number of short-chain fatty acid (SCFA)-producing bacteria and reduced butyrate concentration (Machiels et al., 2014; Laserna-Mendieta et al., 2018).
SCFAs including butyrate, propionate, and acetate, are produced by the fermentation of nondigestible carbohydrates (dietary fibers) by the gut microbiota and play an important role in maintaining intestinal barrier functions and immune homeostasis. SCFAs, particularly butyrate, have been shown to regulate the proliferation and differentiation of intestinal epithelial cells and their expression of mucins, antimicrobial peptides, and tight junctions proteins; furthermore, butyrate exerts anti-inflammatory effects in immune cells (Gonçalves et al., 2018; Recharla et al., 2023). SCFAs interact with target epithelial or immune cells either via cell-surface G-protein-coupled receptors or by entering cells and controlling gene expression by the direct inhibition of histone deacetylases (Dalile et al., 2019). Previous studies have established that butyrate and other SCFAs can enter enterocytes via passive nonionic diffusion or carrier-mediated transport, facilitating the transport of SCFAs derived from gut microbiota. This transport is mediated by the electroneutral H+-dependent monocarboxylate transporters MCT1, MCT4, and MCT5 and the electrogenic, Na+-dependent monocarboxylate transporter SMCT1, which are encoded by the Slc16a1, Slc16a3, Slc16a4, and Slc5a8 genes, respectively (Gill et al., 2005; Cresci et al., 2010; Al-Mosauwi et al., 2016). Moreover, the efflux of butyrate has been demonstrated through the ABCG2 efflux pump, expressed in the apical membrane of intestinal epithelial cells (Gonçalves et al., 2011). In addition to their interactions with transporters, SCFAs act as ligands capable of activating cell signaling pathways in enterocytes via at least five distinct membrane receptors: GPR109A/HCAR2 (hydrocarboxylic acid receptor 2), FFAR2 and FFAR3 (free fatty acid receptor 2 and 3), OLFR78 (olfactory receptor 78), and OLFR558/OR51E1 (olfactory receptor 558) (Priori et al., 2015; Priyadarshini et al., 2018; Halperin Kuhns et al., 2019; Nishida et al., 2021).
The findings that microbiota-derived butyrate plays an important role in maintaining intestinal barrier integrity, gut homeostasis, and reducing gut inflammation (Gonçalves et al., 2018) led to many studies investigating the therapeutic implications of butyrate treatment for IBD (Recharla et al., 2023). In animal models of colitis (DSS-, TNBS- or IL10−/−-colitis), oral butyrate supplementation attenuated the disease activity index, inflammation and mucosal lesions (Vieira et al., 2012; Ji et al., 2016; Lee et al., 2017; Chen et al., 2018), although some studies failed to show benefits following butyrate treatment (Lee et al., 2022). Moreover, butyrate failed to protect against TNBS-colitis in Hcar2−/− mice (Chen et al., 2018), and Ffar2−/− and Ffar3−/− mice were found to be more susceptible to TNBS-colitis (Kim et al., 2013). Similarly, the beneficial effect of dietary fibers in DSS colitis was diminished in Hcar2−/−, Ffar2−/−, and Slc5a8−/− mice (Gurav et al., 2015; Macia et al., 2015). However, the mechanisms through which oral butyrate operates remain incompletely understood, particularly whether it can directly affect the gut mucosa or if its effects are mediated indirectly through its influence on the gut microbial community (Dou et al., 2020; Facchin et al., 2020; Lee et al., 2022). Therefore, this study aimed to compare the effects of butyrate on SCFA receptors and transporters in the presence and absence of gut microbiota and intestinal inflammation.
Materials and methodsAnimals, treatments, and sample preparationThe experiments were performed on 37 two-month-old specific pathogen-free (SPF) and 34 germ-free (GF) female BALB/c mice (Institute of Microbiology, Nový Hrádek, Czech Republic), which were maintained on a 12 h/12 h light/dark cycle and had free access to autoclaved tap water and an irradiated sterile pellet diet of Altromin 1414 (Altromin, Lage, Germany). The GF mice were kept under sterile conditions in Trexler-type isolators since birth. The sterility was monitored routinely by the aerobic and anaerobic cultivation of mouse feces and swabs from the isolator. Colitis was induced by replacing drinking water with 2.5% dextran sulfate sodium (DSS, M.W. 36–50 kDa; MP Biomedicals, Illkirch, France) in water for 7 days (Hudcovic et al., 2001). Sodium butyrate was administered in drinking water at a concentration of 0.5% (Vieira et al., 2012; Chen et al., 2018).
Both SPF and GF mice were divided into the following six groups (Figure 1): (1) the control group (CTRL), given water without DSS and sodium butyrate; (2) the DSS group, given 2.5% DSS solution in water for 1 week; (3) the butyrate group, given 0.5% sodium butyrate solution in water for 1 week (1BT); (4) the butyrate group, given 0.5% sodium butyrate solution in water for 2 weeks (2BT); (5) the butyrate and DSS group (1BT+1BT/DSS), given sodium butyrate (0.5%) solution 1 week followed by a mixture of sodium butyrate (0.5%) and DSS (2.5%) the following week; and (6) the butyrate and DSS group (2BT + DSS), in which mice received 0.5% sodium butyrate for 2 weeks and 2.5% DSS solution the following week. All mice were anesthetized with isoflurane vapor and decapitated, and the colonic tissue was collected, flash-frozen in liquid nitrogen, and stored at −80°C. The present study builds upon previous research conducted by Jourova et al. (2022) and Satka et al. (2022). Their work demonstrated that administering butyrate to SPF mice for 2 weeks before subjecting them to DSS treatment helped reduce symptoms of intestinal inflammation. These symptoms included clinical indicators, such as colon length shortening, histopathological changes, and decreased colonic epithelial leakiness. Conversely, GF mice showed only a minor increase in clinical score and colon length shortening under similar conditions.
Figure 1. Experimental timeline. DSS, dextran sodium sulfate; BT, sodium butyrate; for more details see the text. The number of animals in each experimental group is given in parentheses (SPF/GF mice).
The experiments were approved by the Committee for the Protection and Use of Experimental Animals of the Institute of Microbiology, v. v. i, Czech Academy of Sciences (approval ID: 21/2018).
Histological assessmentTissues from the distal colon were washed in phosphate-buffered saline, fixed in Carnoy’s fluid, embedded in paraffin, sectioned (7 μm) and stained with hematoxylin and eosin. The samples were observed using an Olympus BX 40 microscope equipped with an Olympus Camedia DP 70 digital camera, and subsequent image analysis was conducted utilizing Olympus DP-Soft software. The colonic crypt length was measured as described previously (Hausmann et al., 2011). Colonic crypt length was assessed solely in well-oriented crypts where the entire invagination from the colonic surface was distinctly visible. Twenty individual crypt measurements per animal were analyzed from six mice in each group.
Sample preparation and gene expression analysisTotal RNA was isolated from 30–50 mg of the frozen distal colon using RNeasy Plus universal Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Due to DSS contamination of samples and inhibition of the subsequent reactions, RNA samples were purified using the GenElute Mammalian Total RNA miniprep kit (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and DNase treatment using the On-Column DNase I Digestion set (Sigma-Aldrich). Isolated total RNA was transcribed to cDNA using random hexamers and the HighCapacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, Ca, USA). Quantitative polymerase chain reactions were performed in the LightCycler 480 PCR System (Roche Diagnostic GmbH, Mannheim, Germany) using 5x Hot FIREpol Probe qPCR Mix Plus (ROX) (Solis BioDyne, Tartu, Estonia) and predesigned TaqMan Assays (Life Technologies) for monocarboxylate transporter 1 (Slc16a1, Mm01306379_m1), monocarboxylate transporter 4 (Slc16a3, Mm00446102_m1), monocarboxylate transporter 5 (Slc16a4, Mm00525195_m1), sodium-coupled monocarboxylate transporter 1 (Slc5a8, Mm00520629_m1), breast cancer resistance protein (Abcg2, Mm00496364_m1), hydrocarboxylic acid receptor 2 (Hcar2, Mm01199527_m1), free fatty acid receptor 2 (Ffar2, Mm02620654_s1), free fatty acid receptor 3 (Ffar3, Mm07294891_g1), olfactory receptor 78 (Olfr 78, Mm00628116_m1), olfactory receptor 558 (Olfr558, Mm01279850_m1), mucin 2 (Muc2, Mm01276696_m1), gut hormone peptide YY (Pyy, Mm00520715_m1), tumor necrosis factor α (Tnfα, Mm00443258_m1), and interleukin 1β (IL-1β, Mm00434228_m1). The quantity of the transcripts was determined using the standard curve method with serial 3-fold dilutions of the mixed cDNA sample, and the quantity of the transcripts of the genes of interest was calculated relative to the geometric mean of the reference genes succinate dehydrogenase subunit A (Sdha, Mm01352366_m1) and hypoxanthine-guanine phosphoribosyl transferase 1 (Hprt, Mm01545399_m1).
Statistical analysisAll quantitative data were analyzed using GraphPad Prism 8 software (GraphPad, La Jolla, CA, USA) and are presented as the mean ± SEM. Data were assessed for normality (Shapiro-Wilk test) and for variance equality (Brown-Forsythe test). When necessary, data were transformed to fit assumptions of normality and homogeneity of variance before analysis (data in graphs are nontransformed). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was performed to examine statistical significance in datasets of multiple groups. Two-way ANOVA followed by Tukey’s post hoc test was used to analyze the interaction between the effects of butyrate treatment and gut microbial status on the expression of butyrate transporters and receptors in the murine colon. Student’s t-test was performed to examine the statistical significance between two groups. p values less than 0.05 were considered statistically significant.
ResultsThe regulation of SCFA transporters and receptors in GF and SPF mice given oral butyrateDiets containing dietary fibers, the main substrates for bacterial fermentation and the production of SCFAs, stimulate colonic SCFA transport. To test how oral butyrate affects the mRNA abundance of colonic SCFA transporters and receptors, mice received sodium butyrate in drinking water for 1 or 2 weeks (experimental design, Figure 1). Comparison of the groups CTRL, 1BT, and 2BT showed that the responses of GF and SPF mice were different (Figure 2). In SPF mice, butyrate significantly decreased the expression of the solute carriers Slc16a1, Slc16a3, Slc16a4, and Slc5a8 and increased the expression of the efflux pump Abcg2 after 2 weeks. The receptors Hcar2 and Olfr78 were also significantly decreased, whereas Olfr558 expression was upregulated and Ffar2 and Ffar3 expression was not changed. In contrast, butyrate in GF mice altered Slc16a3 and Olfr78 expression, but in the opposite direction compared to that of SPF animals.
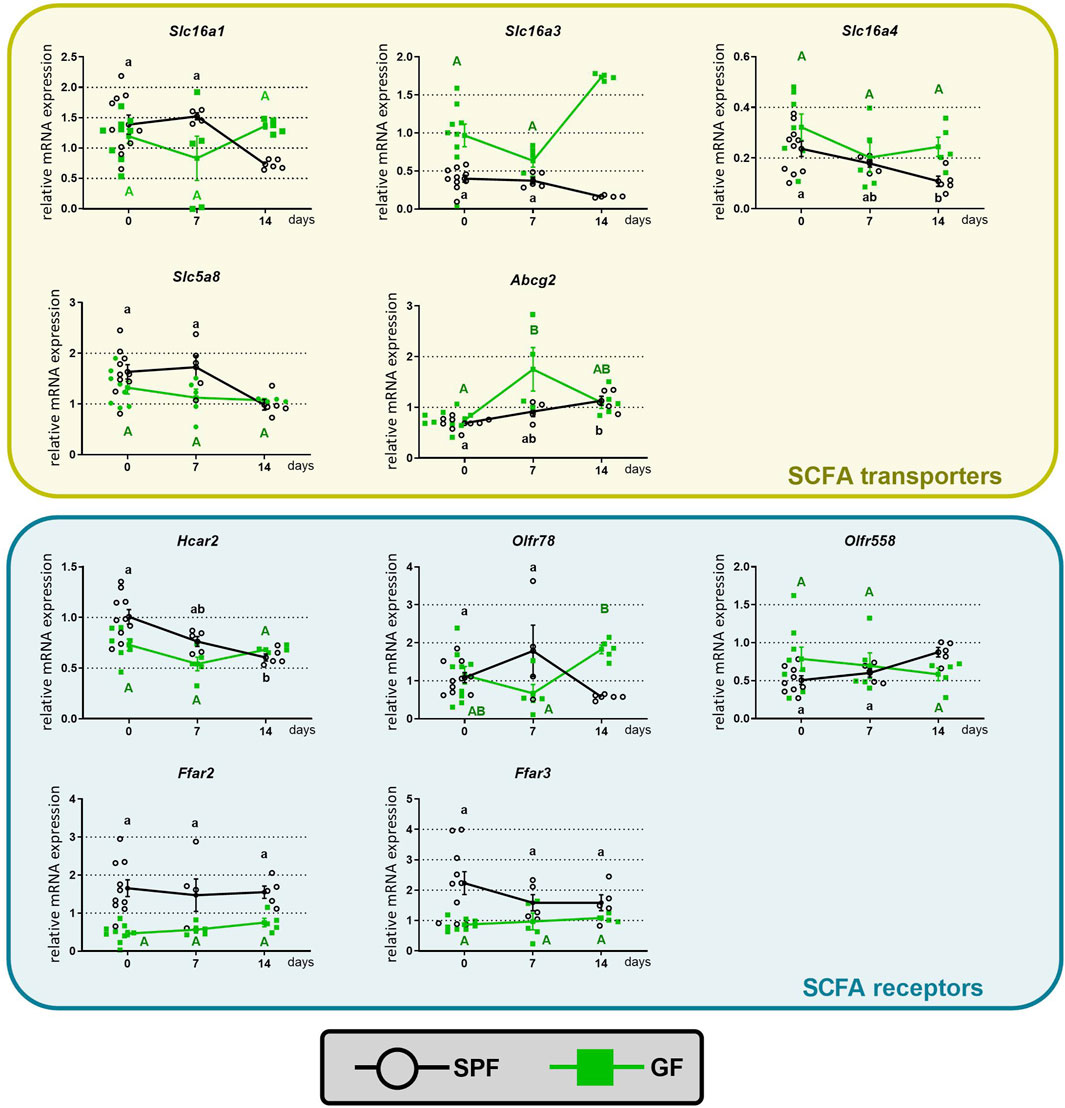
Figure 2. Time course of the effect of butyrate on the expression of colonic SCFA transporters and receptors in specific pathogen-free (SPF) and germ-free (GF) mice. The mice drank either water (CTRL group) or 0.5% sodium butyrate solution for 7 (1BT group) or 14 days (2BT group). The results are presented as the mean ± SEM (n = 4–10 per group). The data for SPF (lowercase letters) and GF (uppercase letters) were analyzed separately by one-way ANOVA followed by Tukey’s post hoc test. Values with same letters are not statistically different.
To investigate the influence of the microbiota on the effect of butyrate, we further analyzed the interaction between the gut microbiota and the 2-weeks butyrate treatment on the expression of SCFA transporters and receptors in the distal colon of mice (Figure 3). Two-way ANOVA revealed that microbial status had a significant effect on the expression of the Slc16a3 and Slc16a4 transporters and the Olfr78, Ffar2, and Ffar3 receptors, and butyrate had a significant effect on the treatment on Slc16a4, Slc5a8 and Abcg2 transporters and the Hcar2 receptor. The interaction between butyrate treatment and microbial status significantly impacted the transporters Slc16a1 and Slc16a3 and some receptors (Hcar2, Olfr78, and Olfr558).
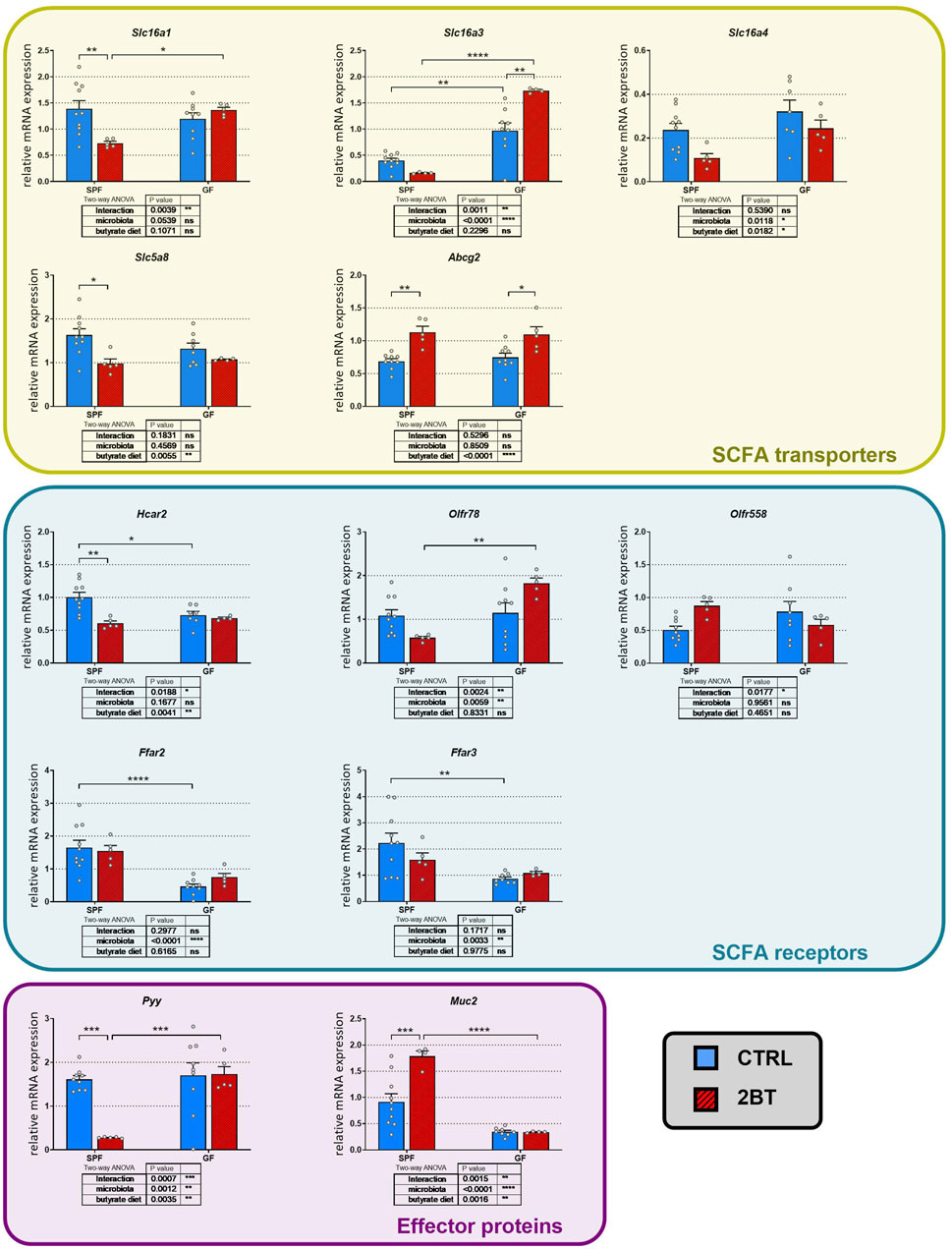
Figure 3. Effects of the microbiota and butyrate on the expression of genes encoding colonic SCFA transporters and receptors, gut hormone PYY, and mucin 2 in specific pathogen-free (SPF) and germ-free (GF) mice. CTRL, mice receiving standard diet and water ad libitum; 2BT, mice receiving standard diet and sodium butyrate solution (0.5%) replacing drinking water in the last 14 days before sacrifice. The results are presented as the mean ± SEM (n = 4–10 per group) and were analyzed by a two-way ANOVA followed by Tukey’s post hoc test. The results of two-way ANOVA are given in tables and the results of post hoc tests in the graphs: *p < 0.05; **p < 0.01; ***p < 0.001, ****p < 0.0001; ns, not significant.
Interestingly, post hoc comparison revealed that butyrate downregulated the expression of Slc16a1 (p < 0.01), Slc5a8 (p < 0.05), and Hcar2 (p < 0.01) in SPF but not GF mice, whereas in the case of Slc16a4, the decrease was not significant in SPF mice. In contrast, butyrate treatment upregulated Slc16a3 expression in GF mice (p < 0.01) and Abcg2 expression in both SPF and GF mice (p < 0.05 and 0.01, respectively). The effect of microbiota on the expression of SCFA transporters and receptors differed from that of butyrate. Microbiota downregulated the expression of Slc16a3 (p < 0.01) but had an opposite effect on the expression of Hcar2 (p < 0.05), Ffar2 (p < 0.0001), and Ffar3 (p < 0.01). In addition, the absence of microbiota prevented the decrease in Slc16a1, Slc5a8 and Hcar2 expression induced by increased butyrate intake.
To investigate whether the interplay between microbial status and oral butyrate can extend its influence on other local processes in the gut, we conducted additional studies to examine the effects of butyrate and microbiota on the expression of Pyy and Muc2 genes. These genes encode the gut hormone PYY and mucin 2, respectively, both of which are known to be modulated by butyrate. Additionally, the secretion of PYY is linked to the activation of FFAR2 receptors, as reviewed recently (Dalile et al., 2019). For both Pyy and Muc2, two-way ANOVA showed that butyrate treatment, microbial status and the butyrate treatment x microbial status interaction had a significant effect (Figure 3). Butyrate administration significantly downregulated Pyy expression (p < 0.001) and upregulated Muc2 expression (p < 0.001) in SPF but not GF mice and the GF conditions suppressed expression of Muc2 just at the level of significance (p = 0.0504).
These data suggest that butyrate administration for 2 weeks has an impact on the colonic expression of SCFA transporters and receptors, mucin, and the gastrointestinal hormone PYY and that their expression is modulated by the presence/absence of gut microbiota.
Effect of butyrate on acute DSS-induced colitisAs SCFAs exert anti-inflammatory effects in the intestinal mucosa, we investigated the expression of SCFA transporters and receptors during inflammation in mice with acute DSS-induced colitis. First, we studied the manifestation of the disease at the level of colonic crypt length shortening and inflammation (Figure 4). The histological examination (Figure 4A) of SPF mice revealed significant inflammatory cell infiltration into the lamina propria, thickening of the submucosa, loss of the epithelial layer, and disappearance of mucosal crypts in the colonic wall of DSS-treated controls (group DSS; grade 3.3 ± 0.3). These changes were also observed to a lesser extent in mice pretreated with butyrate for 1 week and cotreated with butyrate and DSS the following week (group 1BT+ 1BT/DSS; grade 2.2 ± 0.2). In contrast, mice pretreated with butyrate for 2 weeks displayed a notable inhibitory effect on DSS-induced histological changes (group 2BT + DSS; grade 1.9 ± 0.1) compared to the DSS controls. These mice exhibited reduced inflammatory cell infiltration and fewer pathological changes in the mucosa or epithelial layer. Conversely, histological alterations in the colonic mucosa of GF mice were mild. Control mice treated with DSS displayed increased infiltration of inflammatory cells into the lamina propria and partial disappearance of mucosal crypts in the colonic wall (group DSS; grade 1.7 ± 0.3). Mice pretreated with butyrate for 1 week and cotreated with butyrate and DSS the following week (1BT + BT/DSS group; grade 1.1 ± 0.3) exhibited subtle signs of inflammation. However, mice pretreated with SB for 2 weeks showed a reduced impact on DSS-induced histological changes (2BT + DSS group; grade 1.4 ± 0.2) compared to the DSS controls.
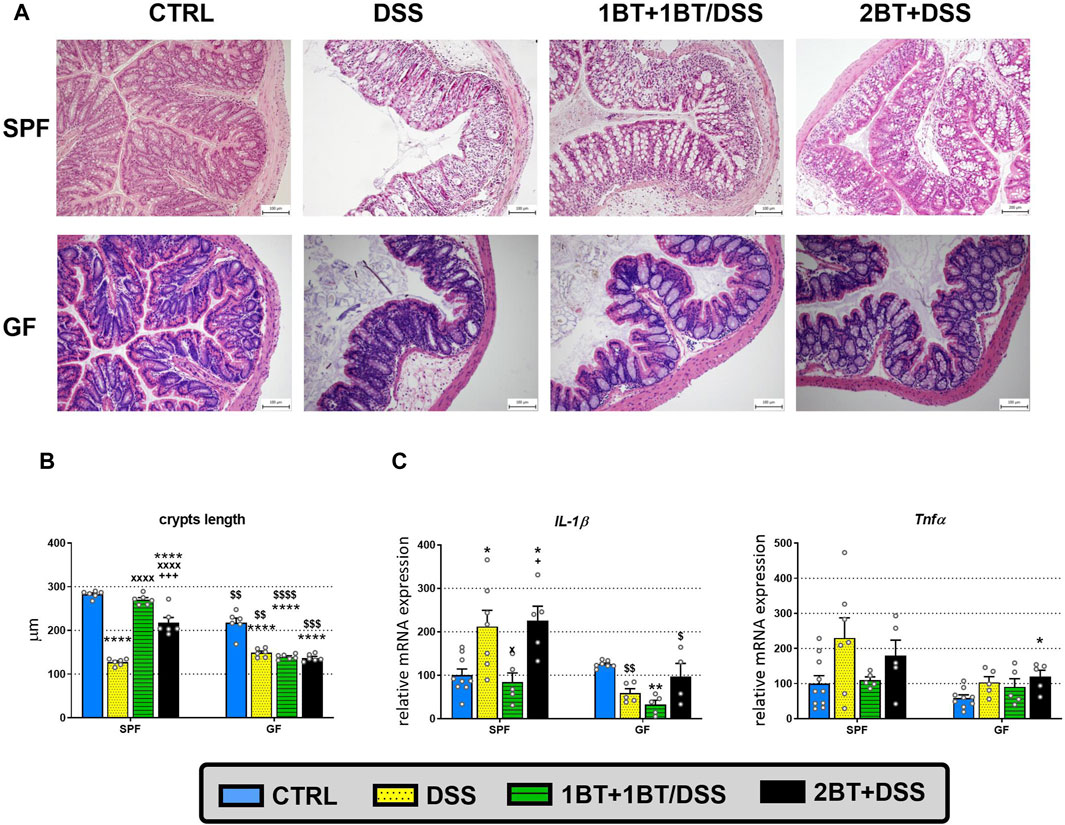
Figure 4. Dextran sodium sulfate (DSS)-induced colitis and the effect of oral butyrate (BT) on crypt length and expression of proinflammatory cytokines. (A) Hematoxylin and eosin staining of colon tissue from specific pathogen-free (SPF) and germ-free (GF) mice treated with DSS. The histopathological changes in the colonic mucosa following DSS treatment were assessed through histological scoring at the end of the experiment. These changes are illustrated using representative histological sections. (B) The length of colonic crypts and (C) mRNA expression of interleukin 1β (IL-1β) and tumor necrosis factor α (Tnfα) in SPF and GF mice treated with DSS and BT. CTRL, control, untreated mice; DSS, mice that drank 2.5% DSS for 7 days; 1BT+1BT/DSS, mice that drank 0.5% BT solution for 1 week and then the mixture of 0.5% BT and 2.5% DSS for the second week; 2BT + DSS, mice pretreated with 0.5% BT for 2 weeks before drinking 2.5% DSS the following week. The results are presented as the mean ± SEM (n = 6 per group for the measurement of crypt lengths; n = 4–10 per group for the analysis of proinflammatory cytokine expression) and were analyzed by one-way ANOVA separately for SPF and GF mice. Different from the CTRL group: *p < 0.05, **p < 0.01, ****p < 0.0001; different from the DSS group: xP < 0.05, xxxxP < 0.0001; different from the 1BT+1BT/DSS group: +p < 0.05, +++p < 0.001. Differences between SPF and GF mice in identical experimental groups were compared using Student’s t-test: $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001.
Compared with control animals (CTRL group), we found a significant reduction in crypt length in the DSS group, independent of microbial status, with the length being 2.2 times shorter in SPF mice and 1.5 times shorter in the GF group (p < 0.0001; Figure 4B); there was also detected more than 2 times upregulation of IL-1β expression in DSS-treated SPF mice compared to control animals (p < 0.05; Figure 4C). In SPF mice, the co-administration of butyrate and DSS (1BT+1BT/DSS group) prevented crypt shortening (p < 0.0001) and the upregulation of IL-1β expression (p < 0.05). On the other hand, pretreatment of SPF mice with butyrate before induction of DSS colitis (2BT + DSS group) did not mitigate the impact of DSS on the expression of IL-1β, although we observed significant reduction in the effect of DSS on crypt length, compared to mice of DSS group. In GF mice, the administration of butyrate failed to alleviate the DSS-induced reduction in crypt length in both 1BT+1BT/DSS group and 2BT + DSS group, and the expression of IL-1β was decreased following the co-administration of butyrate and DSS (1BT+1BT/DSS group) compared to that of untreated, control GF mice (CTRL group) (Figures 4B, C). The patterns of butyrate and DSS effects on Tnfα expression were similar but did not reach statistical significance. These data indicated that a serious inflammatory response occurred in the mouse colon and that tissue damage could occur simultaneously. A detailed description of the clinical score and the histopathological changes in SPF mice was already published in our previous publication (Jourova et al., 2022), as well as the clinical score and the length of the colon in GF mice (Satka et al., 2022).
Challenge with DSS did not change the mRNA expression of SCFA transporters in the colon regardless of the presence or absence of microbiota (Figure 5). Co-administration of DSS and butyrate (1BT+1BT/DSS group) significantly altered the expression of Slc16a1 (p < 0.01), Slc16a4 (p < 0.05), Slc5a8 (p < 0.001) and Abcg2 (p < 0.0001) in SPF but not GF mice treated with DSS (DSS group). Except for Abcg2, DSS had a similar effect on the expression of SCFA transporters if it was applied alone or after a preceding 2-weeks administration of butyrate. The expression of SCFA receptors was only partially affected by DSS. DSS significantly downregulated the expression of Ffar2 (p < 0.001) in SPF mice and upregulated the expression in GF animals (p < 0.01); this effect showed a tendency to persist in the case of co-administration or pretreatment with butyrate. In contrast, DSS upregulated Olfr78 expression in both SPF (p < 0.001) and GF animals (p < 0.01) and co-administration of DSS and butyrate (1BT+1BT/DSS group) prevented this upregulation in SPF but not GF mice. Like Olfr78, the expression of Pyy was also upregulated in the presence of DSS in both SPF and GF mice and the co-administration of DSS and butyrate successfully prevented this upregulation in SPF mice but not GF mice.
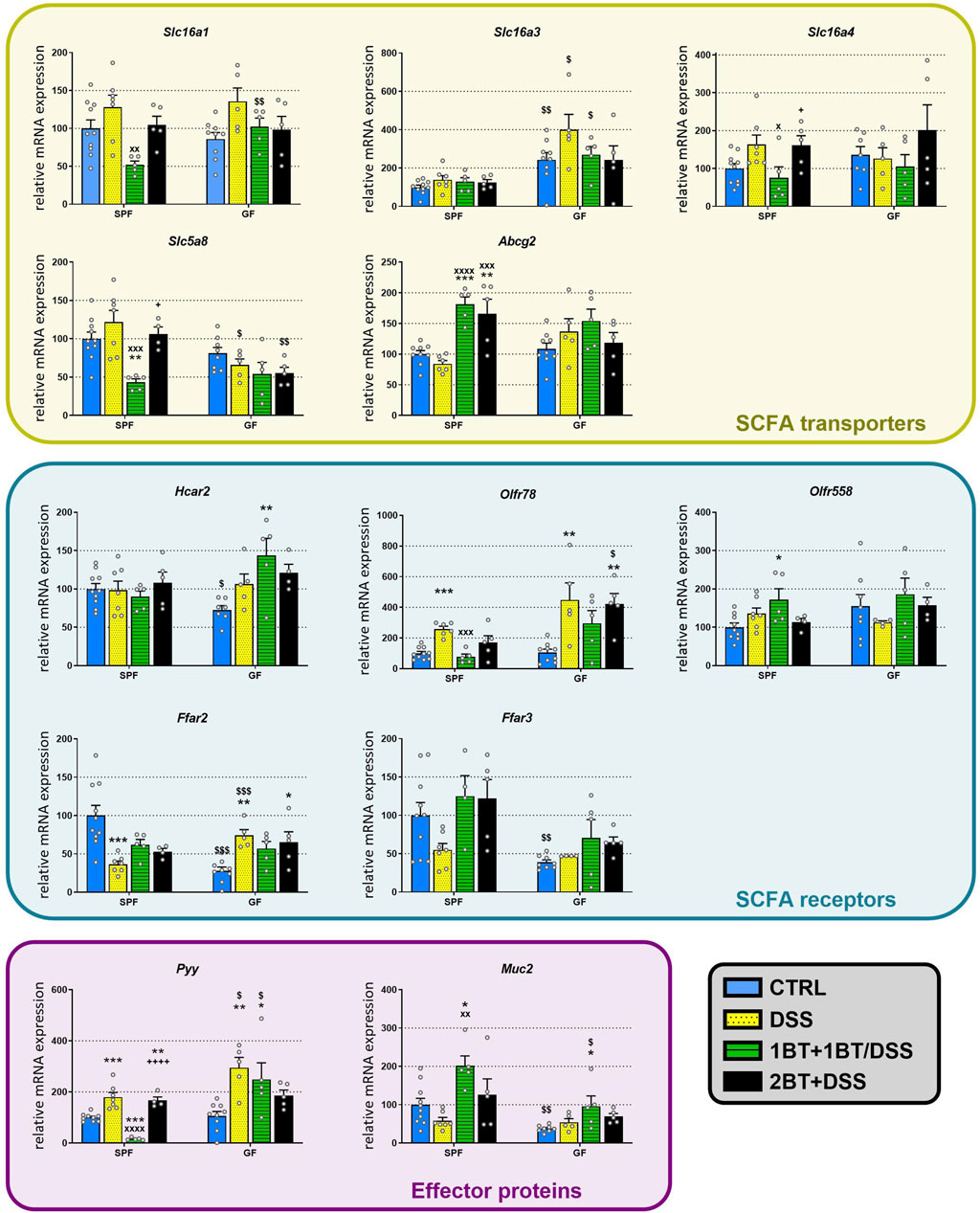
Figure 5. Effect of oral butyrate administration on colonic SCFA transporters and receptors, gut hormone PYY, and mucin 2 in specific pathogen-free (SPF) and germ-free (GF) mice treated with DSS. CTRL, control, untreated mice; DSS, mice that drank 2.5% DSS for 7 days; 1BT+1BT/DSS, mice that drank 0.5% BT solution for 1 week and then the mixture of 0.5% BT and 2.5% DSS for the second week; 2BT + DSS, mice pretreated with 0.5% BT for 2 weeks before drinking 2.5% DSS the following week. The results are presented as the mean ± SEM (n = 4–10 per group) and were analyzed by one-way ANOVA separately for SPF and GF mice. Different from the CTRL group: *p < 0.05, **p < 0.01, ***p < 0.001; different from the DSS group: xP < 0.05, xxP < 0.05, xxxP < 0.001, xxxxP < 0.0001; different from the 1BT+1BT/DSS group: +p < 0.05, ++++p < 0.0001. Differences between SPF and GF mice in identical experimental groups were compared using Student’s t-test: $p < 0.05, $$p < 0.01, $$$p < 0.001.
DiscussionThe mechanism by which SCFAs, particularly butyrate, promote immunity and improve IBD treatment efficacy is not completely understood, even though it is known that SCFAs act at the cell surface as endogenous ligands for some G protein-coupled receptors and intracellularly as inhibitors of histone deacetylases (Gonçalves et al., 2018; Dalile et al., 2019). Previous studies have shown that the administration of sodium butyrate in drinking water ameliorates inflammation and epithelial barrier dysfunction (Vieira et al., 2012; Ji et al., 2016; Lee et al., 2017; Chen et al., 2018; Jourova et al., 2022); nevertheless, orally administered butyrate is thought to be absorbed and utilized before reaching the colon (Daniel et al., 1989; Wang et al., 2023). Here, we demonstrate that a relatively low concentration of sodium butyrate in a drinking solution modulated the expression of the SCFA carriers Slc16a1, Slc16a3, Slc16a4, and Slc5a8 and the efflux pump Abcg2. The observed effect of butyrate on the expression of SCFA transporters does not seem to reflect direct substrate-induced regulation via butyrate. First, the effect of oral butyrate differed between SPF and GF mice. Second, we found downregulated expression of SCFA solute carriers in SPF mice treated with butyrate, but butyrate stimulated the expression and function of Slc16a1 (MCT1) and Slc16a3 (MCT4) in colonic epithelial cell lines (Borthakur et al., 2008; Ziegler et al., 2016)) or in the intestinal epithelium (Dengler et al., 2015). Third, oral administration of butyrate significantly upregulated Slc16a3 expression but only in GF and not SPF mice.
In this report, we also showed that not only the expression of SCFA transporters but also that of SCFA receptors is modulated by the gut microbiota and by oral intake of butyrate. We found that the expression of Ffar2, which is expressed both in enteroendocrine cells (EEC) and colonocytes, and Ffar3, which is expressed in EEC and enteric neurons but not colonocytes (Priyadarshini et al., 2018), strongly depends on gut microbiota but not on oral butyrate treatment. A similar effect of the microbiota on the expression of Ffar2 was observed in the mouse ileum (Yajima et al., 2016). In contrast to Ffar2 and Ffar3, the expression of Hcar2, a colonic SCFA receptor (Priyadarshini et al., 2018), depended not only on gut microbiota, as was already shown earlier by Cresci et al. (Cresci et al., 2010), but also on oral butyrate. Similar to the colonic Slc16a1 and Slc5a8 transporters, oral butyrate dampened Hcar2 expression in SPF but not GF mice. These data indicate a relationship between the regulation of Hcar2, the principal butyrate receptor in the colon, and the colonic butyrate transporters Slc16a1 and Slc5a8. A similar link between the expression of Hcar2 and Slc5a8 was shown by Cresci et al. (Cresci et al., 2010). The importance of the microbiota for the effect of oral butyrate on the gut is also supported by the results of Pyy and Muc2 expression. In both cases, the effect of butyrate was evident only in SPF but not GF animals, with downregulation in the case of Pyy expression and upregulation in the case of Muc2 expression; this effect is similar to the effect of rectal butyrate enemas on Muc2 expression in conventional mice (Gaudier et al., 2009). The lack of the inhibitory effect of butyrate on Pyy expression observed in our in vivo experiments with GF mice aligns with findings from experiments conducted using sterile mouse intestinal epithelial cell culture (Larraufie et al., 2018).
Indirect transcriptional regulation of colonic SCFA transporters and receptors may depend on the interaction of oral butyrate with the gut microbiota or with upstream parts of the gastrointestinal tract. In the case of the effect of oral butyrate in SPF mice, we cannot exclude the possibility that oral butyrate modulates the gut microbiota and microbiota-secreted soluble factors, which might control the expression of SCFA transporters and receptors. There is strong evidence that oral sodium butyrate remarkably alters the gut microbiota (Dou et al., 2020; Lee et al., 2022) and that Lactobacillus plantarum-derived soluble factors or Lactobacillus delbrueckii consumption upregulate Slc5a8 and Slc16a1 expression (Hou et al., 2022; Kim et al., 2022). Furthermore, the SMCT1, MCT1, and MCT4 transporters and the FFAR2 and FFAR3 receptors have been found in the duodenum (Akiba et al., 2015; Kaji et al., 2015), and butyrate has been shown to activate vagal afferents and release gut hormones that might modulate intestinal transport (Dalile et al., 2019).
Since butyrate has ameliorative effects in the treatment of colitis (Recharla et al., 2023), and we have shown that butyrate together with the microbiome affects the expression of transporters and receptors in the colon, we further investigated the interaction between oral treatment of butyrate and the presence/absence of microbiome in a model of acute colitis. Even though downregulation of Slc16a1 and MCT1 protein expression and upregulation of Slc16a3 and MCT4 expression were detected in biopsies of patients with IBD and in the colon of rats or mice with DSS-induced colitis (Thibault et al., 2007; Erdmann et al., 2019; Zhang et al., 2019), our results did not show any effect of DSS on SCFA transporters in SPF mice. This discrepancy may result from differences in the colitis models used including strain, time of colitis, and degree of inflammation. In this regard, the expression levels of SCFA transporters depend on the inflammatory state of the colonic mucosa (Thibault et al., 2007; Zhang et al., 2019). Moreover, DSS treatment significantly alters the structure of the intestinal microbiota (Lin et al., 2023), whose diversity and composition might differ among various locations/research groups.
In addition, we analyzed the expression of SCFA receptors in mice with colitis and demonstrated that DSS treatment leads to upregulation of Olfr78 expression regardless of the presence or absence of gut microbiota and significant downregulation of Ffar2 expression in SPF mice and upregulation in GF mice. In addition, Ffar3 expression in SPF mice was also decreased, but the decrease was not significant. In contrast, Hcar2 and Olfr558 expression was not changed in either SPF or GF mice. In this regard, acute DSS colitis in conventional C57BL/6 mice decreased the expression of Ffar2, Ffar3, and Hcar2 (Lin et al., 2023; Nan et al., 2023), although this result was not confirmed by others (Kotlo et al., 2020; Han et al., 2021). These findings indicate that Olfr78, Ffar2, and Ffar3 may play a more important role in our model of colitis/inflammation than other SCFA receptors and that the response of Ffar receptors to inflammation depends on the gut microbiota.
In conclusion, our study demonstrates that the intestinal microbiota actively modulates the response of genes encoding colonic SCFA transporters and receptors, mucin, and the gut hormone PYY to oral butyrate administration. It is noteworthy that the responses to butyrate did not differ significantly between the colons of healthy subjects and those with experimentally induced murine colitis. Our observations indicate alterations in the expression of certain SCFA receptors and the hormone PYY in colitis, while the expression of SCFA transporters remained unchanged. It is important to note that these changes occurred regardless of the presence or absence of gut microbiota. Overall, our results suggest a promising avenue for further research in this area. These findings highlight the potential indirect influence of butyrate supplementation on its anti-colitic effects.
Data availability statementThe raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statementThe animal study was approved by the Ethics Committee, Ministry of Education of the Czech Republic. Experiments were approved by the Committee for the Protection and Use of Experimental Animals of the Institute of Microbiology, v. v. i, Czech Academy of Sciences of the Czech Republic (Approval ID: 21/2018). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributionsKV: Methodology, Visualization, Writing–original draft, Data curation. TH: Funding acquisition, Methodology, Project administration, Writing–review and editing, Funding acquisition. MV: Conceptualization, Funding acquisition, Methodology, Writing–review and editing, Project administration. PE: Methodology, Writing–review and editing. PK: Methodology, Writing–review and editing. PPH: Methodology, Writing–review and editing. DS: Methodology, Writing–review and editing. JP: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing–original draft.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Czech Science Foundation (Grants Nos 19-08294S and 21-10845S), and the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104)–funded by the European Union–Next-Generation EU. MV was supported by the Czech Academy of Science (PPLZ project L200112201).
AcknowledgmentsThe authors want to thank Ivana Muricová and Kateřina Balounová from the Institute of Physiology, CAS, Prague, for her support in daily activities associated with sample preparation and Barbora Draboňová, Jarmila Jarkovská, Marta Stojková and Kamila Michaličková from the Institute of Microbiology, CAS, Nový Hrádek for their excellent technical assistance and animal care.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAkiba, Y., Inoue, T., Kaji, I., Higashiyama, M., Narimatsu, K., Iwamoto, K., et al. (2015). Short-chain fatty acid sensing in rat duodenum. J. Physiol. 593, 585–599. doi:10.1113/jphysiol.2014.280792
PubMed Abstract | CrossRef Full Text | Google Scholar
Al-Mosauwi, H., Ryan, E., McGrane, A., Riveros-Beltran, S., Walpole, C., Dempsey, E., et al. (2016). Differential protein abundance of a basolateral MCT1 transporter in the human gastrointestinal tract. Cell Biol. Int. 40, 1303–1312. doi:10.1002/cbin.10684
PubMed Abstract | CrossRef Full Text | Google Scholar
Borthakur, A., Saksena, S., Gill, R. K., Alrefai, W. A., Ramaswamy, K., and Dudeja, P. K. (2008). Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-κB pathway. J. Cell. Biochem. 103, 1452–1463. doi:10.1002/jcb.21532
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, G., Ran, X., Li, B., Li, Y., He, D., Huang, B., et al. (2018). Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine 30, 317–325. doi:10.1016/j.ebiom.2018.03.030
PubMed Abstract | CrossRef Full Text | Google Scholar
Cresci, G. A., Thangaraju, M., Mellinger, J. D., Liu, K., and Ganapathy, V. (2010). Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 14, 449–461. doi:10.1007/s11605-009-1045-x
PubMed Abstract | CrossRef Full Text | Google Scholar
Dalile, B., Van Oudenhove, L., Vervliet, B., and Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478. doi:10.1038/s41575-019-0157-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Daniel, P., Brazier, M., Cerutti, I., Pieri, F., Tardivel, I., Desmet, G., et al. (1989). Pharmacokinetic study of butyric acid administered in vivo as sodium and arginine butyrate salts. Clin. Chim. Acta. 181, 255–263. doi:10.1016/0009-8981(89)90231-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Dengler, F., Rackwitz, R., Benesch, F., Pfannkuche, H., and Gäbel, G. (2015). Both butyrate incubation and hypoxia upregulate genes involved in the ruminal transport of SCFA and their metabolites. J. Anim. Physiol. Anim. Nutr. Berl. 99, 379–390. doi:10.1111/jpn.12201
PubMed Abstract | CrossRef Full Text | Google Scholar
Dou, X., Gao, N., Yan, D., and Shan, A. (2020). Sodium butyrate alleviates mouse colitis by regulating gut microbiota dysbiosis. Anim. (Basel) 10, 1154. doi:10.3390/ani10071154
CrossRef Full Text | Google Scholar
Erdmann, P., Bruckmueller, H., Martin, P., Busch, D., Haenisch, S., Müller, J., et al. (2019). Dysregulation of mucosal membrane transporters and drug-metabolizing enzymes in ulcerative colitis. J. Pharm. Sci. 108, 1035–1046. doi:10.1016/j.xphs.2018.09.024
PubMed Abstract | CrossRef Full Text | Google Scholar
Facchin, S., Vitulo, N., Calgaro, M., Buda, A., Romualdi, C., Pohl, D., et al. (2020). Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 32, e13914. doi:10.1111/nmo.13914
PubMed Abstract | CrossRef Full Text | Google Scholar
Gaudier, E., Rival, M., Buisine, M.-P., Robineau, I., and Hoebler, C. (2009). Butyrate enemas upregulate Muc genes expression but decrease adherent mucus thickness in mice colon. Physiol. Res. 58, 111–119. doi:10.33549/physiolres.931271
PubMed Abstract | CrossRef Full Text | Google Scholar
Gill, R. K., Saksena, S., Alrefai, W. A., Sarwar, Z., Goldstein, J. L., Carroll, R. E., et al. (2005). Expression and membrane localization of MCT isoforms along the length of the human intestine. Am. J. Physiol. Cell Physiol. 289, C846–C852. doi:10.1152/ajpcell.00112.2005
PubMed Abstract | CrossRef Full Text | Google Scholar
Gonçalves, P., Araújo, J. R., and Di Santo, J. P. (2018). A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm. Bowel Dis. 24, 558–572. doi:10.1093/ibd/izx029
PubMed Abstract | CrossRef Full Text | Google Scholar
Gonçalves, P., Gregório, I., and Martel, F. (2011). The short-chain fatty acid butyrate is a substrate of breast cancer resistance protein. Am. J. Physiol. Cell Physiol. 301, C984–C994. doi:10.1152/ajpcell.00146.2011
PubMed Abstract | CrossRef Full Text | Google Scholar
Gurav, A., Sivaprakasam, S., Bhutia, Y. D., Boettger, T., Singh, N., and Ganapathy, V. (2015). Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem. J. 469, 267–278. doi:10.1042/BJ20150242
PubMed Abstract | CrossRef Full Text | Google Scholar
Halperin Kuhns, V. L., Sanchez, J., Sarver, D. C., Khalil, Z., Rajkumar, P., Marr, K. A., et al. (2019). Characterizing novel olfactory receptors expressed in the murine renal cortex. Am. J. Physiol. Ren. Physiol. 317, F172–F186. doi:10.1152/ajprenal.00624.2018
CrossRef Full Text | Google Scholar
Han, R., Ma, Y., Xiao, J., You, L., Pedisić, S., and Liao, L. (2021). The possible mechanism of the protective effect of a sulfated polysaccharide from Gracilaria lemaneiformis against colitis induced by dextran sulfate sodium in mice. Food Chem. Toxicol. 149, 112001. doi:10.1016/j.fct.2021.112001
PubMed Abstract | CrossRef Full Text | Google Scholar
Hausmann, M., Leucht, K., Ploner, C., Kiessling, S., Villunger, A., Becker, H., et al. (2011). BCL-2 modifying factor (BMF) is a central regulator of anoikis in human intestinal epithelial cells. J. Biol. Chem. 286, 26533–26540. doi:10.1074/jbc.M111.265322
PubMed Abstract | CrossRef Full Text | Google Scholar
Hou, G., Yin, J., Wei, L., Li, R., Peng, W., Yuan, Y., et al. (2022). Lactobacillus delbrueckii might lower serum triglyceride levels via colonic microbiota modulation and SCFA-mediated fat metabolism in parenteral tissues of growing-finishing pigs. Front. Vet. Sci. 9, 982349. doi:10.3389/fvets.2022.982349
PubMed Abstract | CrossRef Full Text | Google Scholar
Hudcovic, T., Štĕpánková, R., Cebra, J., and Tlaskalová-Hogenová, H. (2001). The role of microflora in the development of intestinal inflammation: acute and chronic colitis induced by dextran sulfate in germ-free and conventionally reared immunocompetent and immunodeficient mice. Folia Microbiol. (Praha) 46, 565–572. doi:10.1007/BF02818004
PubMed Abstract | CrossRef Full Text | Google Scholar
Ji, J., Shu, D., Zheng, M., Wang, J., Luo, C., Wang, Y., et al. (2016). Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 6, 24838. doi:10.1038/srep24838
PubMed Abstract | CrossRef Full Text | Google Scholar
Jourova, L., Satka, S., Frybortova, V., Zapletalova, I., Anzenbacher, P., Anzenbacherova, E., et al. (2022). Butyrate treatment of DSS-induced ulcerative colitis affects the hepatic drug metabolism in mice. Front. Pharmacol. 13, 936013. doi:10.3389/fphar.2022.936013
PubMed Abstract | CrossRef Full Text | Google Scholar
Kaji, I., Iwanaga, T., Watanabe, M., Guth, P. H., Engel, E., Kaunitz, J. D., et al. (2015). SCFA transport in rat duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G188–G197. doi:10.1152/ajpgi.00298.2014
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, H.-J., An, J., and Ha, E.-M. (2022). Lactobacillus plantarum-derived metabolites sensitize the tumor-suppressive effects of butyrate by regulating the functional expression of SMCT1 in 5-FU-resistant colorectal cancer cells. J. Microbiol. 60, 100–117. doi:10.1007/s12275-022-1533-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, M. H., Kang, S. G., Park, J. H., Yanagisawa, M., and Kim, C. H. (2013). Short-chain fatty acids activate GPR4
留言 (0)