Respiratory distress syndrome (RDS) is a clinical syndrome characterized by respiratory distress and progressive aggravation in affected neonates soon after birth and is one of the most common clinical conditions dealt with in the neonatal intensive care unit (NICU) (1). It is estimated to affect up to 7% of neonates and is among the major causes of neonatal mortality (2–4).
Previous studies have suggested that the pathogenesis of neonatal RDS is more complex than commonly recognized and inflammation may be involved (5). Nupponen et al. demonstrated a systemic inflammatory reaction in preterm infants with RDS (6). In recent studies, infection and inflammation have been identified as risk factors for RDS, which can cause organ dysfunction (5, 7, 8).
The neonatal sequential organ failure assessment (nSOFA) scoring system is an operational definition of organ dysfunction, which is widely used to identify the presence of life-threatening organ dysfunction among preterm infants with inflammation-related diseases such as infection and sepsis (9, 10).
This study was developed to clarify whether nSOFA score could be used to predict mortality in patients with neonatal RDS. In order to broaden the clinical application of the nSOFA score and to find a new method to assist clinicians to quickly and accurately identify high-risk neonates that require more aggressive intervention.
Methods Study designThis is a retrospective cohort study with data from the Marketplace for Medical Information in Intensive Care III (MIMIC-III) database, a longitudinal single-center database that contains information related to patients admitted to the intensive care unit at Beth Israel Deaconess Medical Center (Boston, MA, United States) between 2001 and 2012 (11). The database is maintained by the Massachusetts Institute of Technology (MIT) Computational Physiology Laboratory. The project was approved by the institutional review boards of MIT and the Beth Israel Deaconess Medical Center (BIDMC) and was granted a waiver of informed consent. After successfully completing the National Institutes of Health (NIH) web-based training course and the Protecting Human Research Participants examination (no. 41897755), permission was given to extract data from MIMIC-III.
Selection of participantsThe MIMIC-III database contains a total of 7,870 patients admitted to the NICU and patients who didn’t develop RDS were excluded. A total of 1,281 patients with RDS were included in the final study cohort, grouped according to nSOFA score level and survival status, respectively. The flow chart of patient screening is shown in Supplementary Figure 1. The diagnosis of RDS was carried out according to the Canadian neonatal network (CNN) (4) and defined as babies requiring respiratory support exceeding 24 h, intubation, surfactant administration (but not for meconium aspiration, pneumonia, or pulmonary hemorrhage), fraction of inspired oxygen (FiO2) exceeding 25% for a minimum of 24 h or according to the international statistical classification of diseases and related health problem ninth revision (ICD-9) codes including code 769. For patients who were admitted to the NICU more than once, only the first NICU stay was included for analysis.
Variable extractionClinical data were extracted from the MIMIC-III database for the first 24 hours of the patient’s NICU admission, including demographics, vital signs, laboratory tests, diagnostic codes, medications and survival data. Clinical data required to calculate the nSOFA score were also collected and included the receipt of intubation and mechanical ventilation, the FiO2 to achieve the peripheral oxygen saturation (SpO2), the platelet count and any requirement for glucocorticoid, inotropic, or vasoactive drugs. The SpO2/FiO2 were converted using the partial pressure of arterial oxygen (PaO2)/FiO2 ratio conversion where SpO2/FiO2 = 64 + 0.84 × (PaO2/FiO2) (12). Missing values in the nSOFA score were assumed to be in the normal range as in previous studies (13, 14) and if a variable was recorded more than once in the first 24 h, the most serious record was used. Comparison of baseline characteristics of patients with missing and non-missing SpO2/FiO2 is shown in Supplementary Table 1. Comorbidities identified based on documenting the ICD-9 codes included hemolytic disease of the newborn (HDN), congenital heart disease (CHD), acidosis, anemia, bleed, pneumonia, jaundice, and septicemia. Acute kidney injury (AKI) was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines as an increase in serum creatinine (Scr) of 0.3 mg/dL or more from baseline within 48 h (15).
The primary outcome of this study was classified as all-cause mortality. Inpatient mortality information was obtained from the Hospital Information System, and mortality information for discharged patients was obtained from the US Social Security Death Index.
Application of the neonatal sequential organ failure assessment scoreThe nSOFA score uses categorical scores with a total score range from 0 as best to 15 as worst to objectively describe (1) receipt of mechanical ventilation and oxygen to maintain a physiologic peripheral saturation which was scored 0 to 8; (2) inotropic or vasoactive drug support, including the use of corticosteroids for presumed adrenal insufficiency or catecholamine-resistant shock scored 0 to 4; and (3) the presence and severity of thrombocytopenia based on the most recent platelet measure scored 0 to 3 (9) (Table 1).
TABLE 1
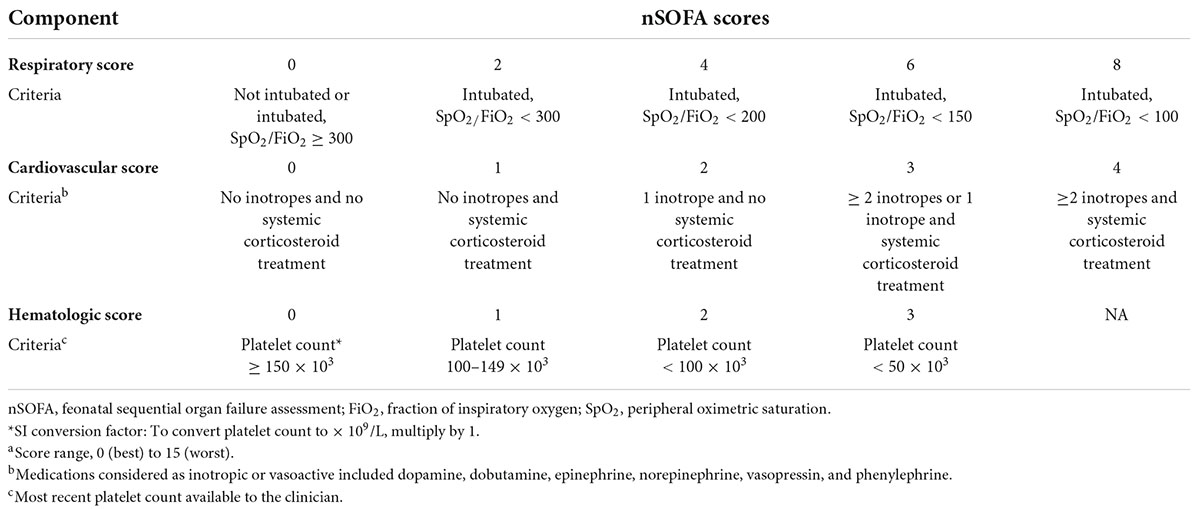
Table 1. Neonatal sequential organ failure assessment (nSOFA) components and scoring.a
Statistical analysisValues are presented as the means ± standard deviations or medians as interquartile ranges (IQRs) for continuous variables and categorical variables are presented as total numbers and percentages. Comparisons between groups were made using the Student’s t-test or the Mann-Whitney U test for continuous variables and χ2-test or Fisher’s exact test for categorical variables.
The area under the receiver operating characteristic curve (AUROC) was calculated to assess the accuracy of the nSOFA score and its subscales in predicting the primary outcome in patients with RDS. The cut-off values of the receiver operating characteristic (ROC) curve were identified by calculating the Youden index, which divided the high and low nSOFA groups. The Kaplan-Meier survival analysis were performed to evaluate the incidence rate of primary outcome events among groups according to various levels of the nSOFA score and discrepancies among groups were evaluated by log-rank test. Cox proportional hazards models were used to estimate the relationships between nSOFA score (per 1 score)/nSOFA groups (high nSOFA groups and low nSOFA groups) and primary outcomes. Characteristic variables with significant baseline differences or clinical significance were used as candidate predictors in the multivariate Cox regression model. Variables with more than 20% missing data were excluded from the analysis. The details of missing data are summarized in Supplementary Table 3.
The propensity score matching (PSM) was used to adjust for ethnicity, sex, and gestational age to ensure the comparability across of high and low nSOFA groups and between survival and non-survival groups in the analysis of baseline characteristics. Baseline characteristics of the original and matched cohorts were presented separately. Multivariate Cox regression analysis was also performed on the matching cohort. Subgroup analyses was used to investigate whether the risk was modified by sex and septicemia. All analyses were first unadjusted as model one, then adjusted for sex, ethnicity, gestational age, weight, and heart rate to get model two and finally for anemia, bleed, septicemia, and pulmonary surfactant to get model three.
All data analyses were performed using R software (version 4.0.4; R Foundation for Statistical Computing, Vienna, Austria) and SPSS statistical software (SPSS Statistics 24.0). Bilateral p-values < 0.05 for all analyses were considered statistically significant.
Results Baseline characteristicsThere were 1,281 patients with RDS who were analyzed between 2001 and 2012 of whom 733 (57.2%) were male and 1,127 (93.1%) were less than 37 weeks of gestational age. There were 40 (3.1%) patient deaths in this cohort. The optimal cut-off value for nSOFA score associated with death was defined as 2.5 and this was used to divide the cohort into low and high nSOFA groups.
There was a total of 347 (27.1%) patients with a high nSOFA score, of whom 37 (10.7%) died, compared with 3 (0.3%) of the 934 (72.9%) patients with a low nSOFA score. Patients in the high nSOFA group had more gestational age less than 28 weeks, smaller height, weight and head circumference, lower SpO2/FiO2, less urine output, additional complications such as acute kidney injury, bleeding and infection and had higher rates of ventilator and vasoactive drug use on the first day of admission (all p < 0.05). Baseline information for the original cohort and matched cohort of RDS patients with high and low nSOFA scores is shown in Table 2. Baseline characteristics of grouping according to the occurrence of follow-up deaths is shown in Supplementary Table 4.
TABLE 2
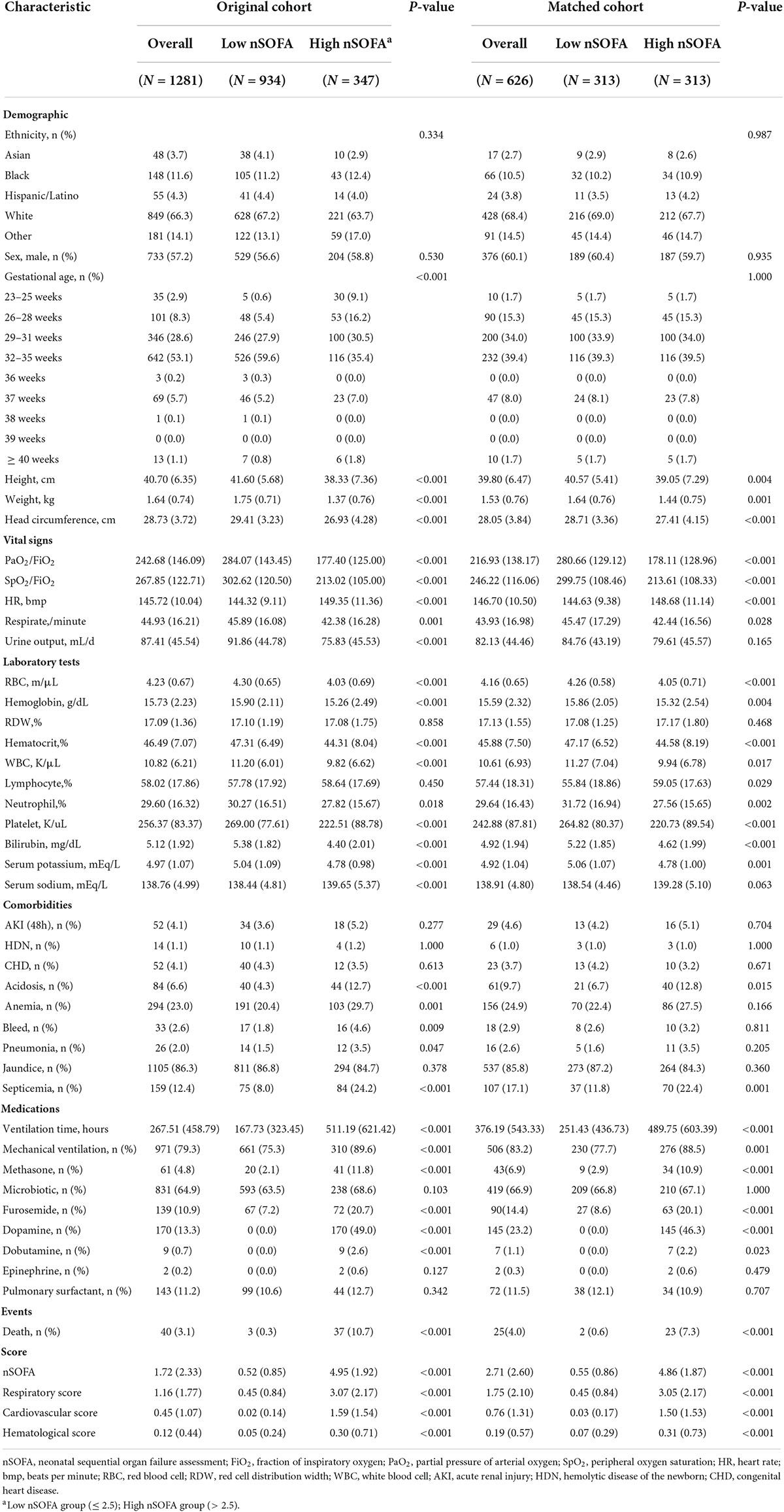
Table 2. Demographic data and comparisons between the low and high nSOFA groups.
Comparisons of neonatal sequential organ failure assessment scoreCompared with survivors, non-survivors had higher nSOFA scores (6.20 vs. 1.58, p < 0.001) and subscales (respiratory score: 3.45 vs. 1.09; cardiovascular score: 2.45 vs. 0.38; hematologic score: 0.30 vs. 0.11). The mortality rate of RDS patients increased with increasing nSOFA score (0–2: 0.4%; 2–4: 1.6%; 4–6: 7.0%; 6–8: 12.2%; 8–10: 29.0%; 10–15: 42.1%). The area under curve (AUC) for the nSOFA score predicting death in patients with RDS was 0.89 (95% CI, 0.84–0.95, p < 0.001), which was higher than the predictive efficacy of the subscale [respiratory score: 0.80 (95% CI, 0.72–0.86, p < 0.001); cardiovascular score: 0.85 (95% CI, 0.79–0.91, p < 0.001); hematologic score: 0.55 (95% CI, 0.49–0.61, p = 0.690)] (Figure 1). The optimal cut-off value of the nSOFA score for predicting death in patients with RDS was 2.5, a sensitivity of 92.5% and a specificity of 75.0% (Supplementary Figure 2). We added gestational age to the original nSOFA score for scoring and analysis. The results showed that the AUC of the modified nSOFA score was 0.91 (95% CI, 0.86–0.95, p < 0.001), which was higher than that of the original nSOFA score and gestational age alone [0.73 (95% CI, 0.62–0.84, p < 0.001)].
FIGURE 1
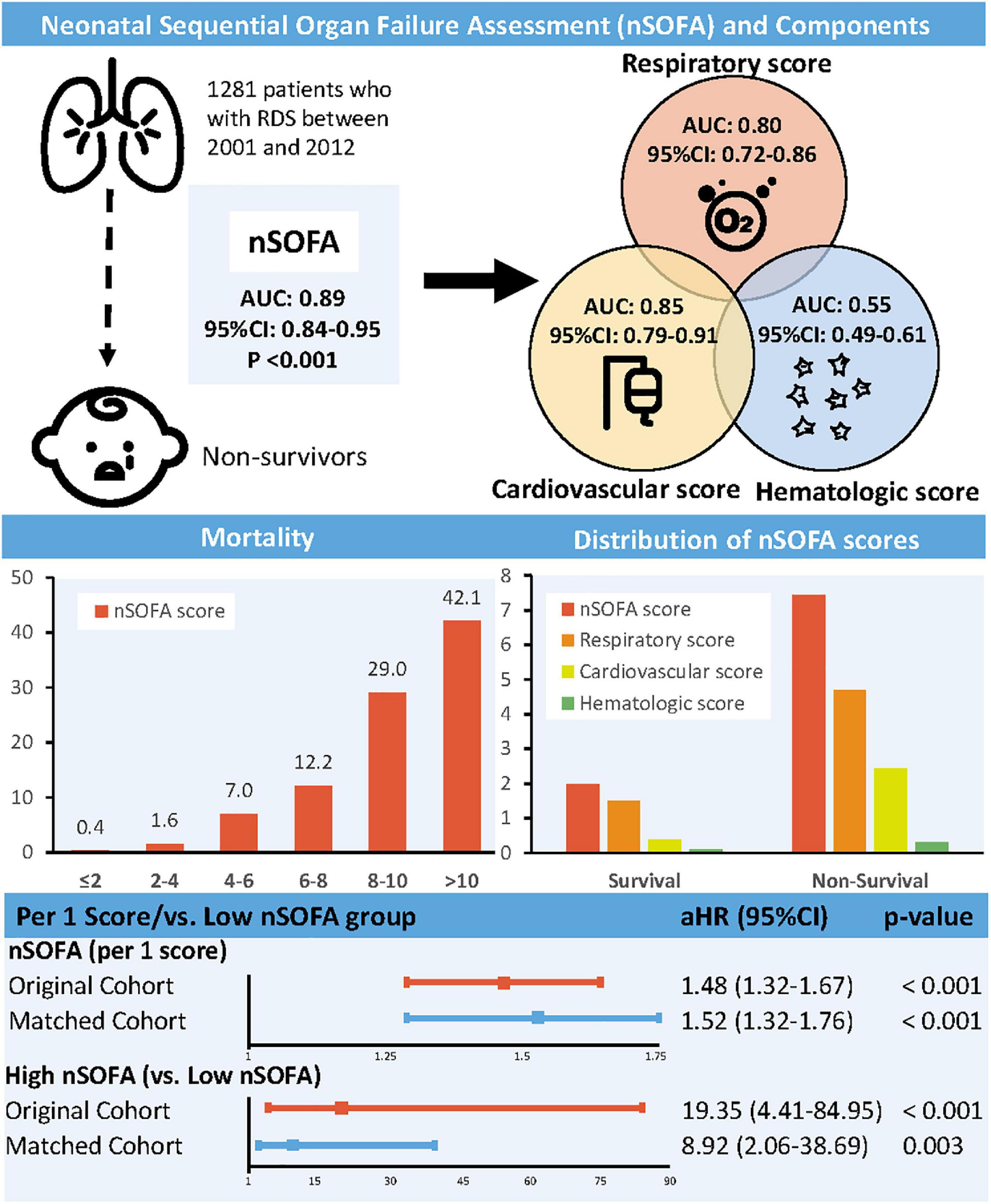
Figure 1. Association between neonatal sequential organ failure assessment and mortality. nSOFA, neonatal sequential organ failure assessment; RDS, respiratory distress syndrome; AUC, area under curve; CI, confidence interval; HR, hazard ratio.
Association between neonatal sequential organ failure assessment scores and mortalityThe prognostic impact of nSOFA scores on patients who with RDS was studied. The Kaplan-Meier survival analysis curves for assessing the mortality between groups based on low and high nSOFA groups are shown in Figure 2. There was a statistically significant difference in mortality between low nSOFA and high nSOFA groups (log-rank p < 0.001). Univariate Cox proportional risk analysis showed that each unit increase in nSOFA scores was significantly associated with mortality (HR: 1.59, 95% CI: 1.46–1.73; p < 0.001). Even after adjusting for sex, ethnicity, gestational age, weight, heart rate, anemia, bleed, septicemia, and pulmonary surfactant, each unit score increase in nSOFA score remained significantly associated with mortality in patients with RDS (aHR: 1.48, 95% CI: 1.32–1.67; p < 0.001). Similarly, the high nSOFA group was significantly associated with higher mortality in RDS patients compared with the low nSOFA group (aHR: 19.35, 95% CI: 4.41–84.95; p < 0.001; Table 3).
FIGURE 2
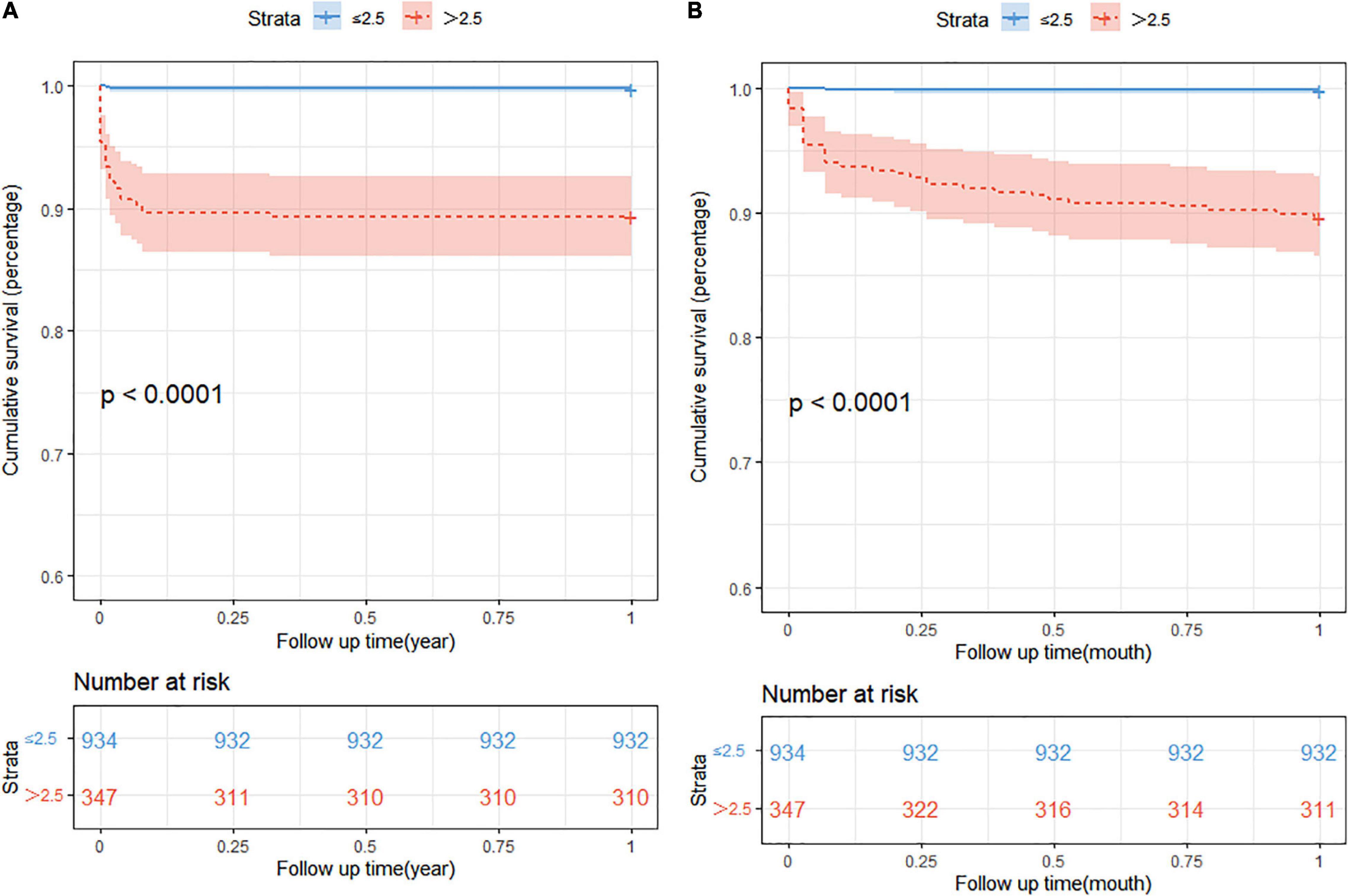
Figure 2. The Kaplan-Meier survival analysis curves. (A) The 1-year all-cause mortality; (B) 1-month all-cause mortality. nSOFA, neonatal sequential organ failure assessment.
TABLE 3
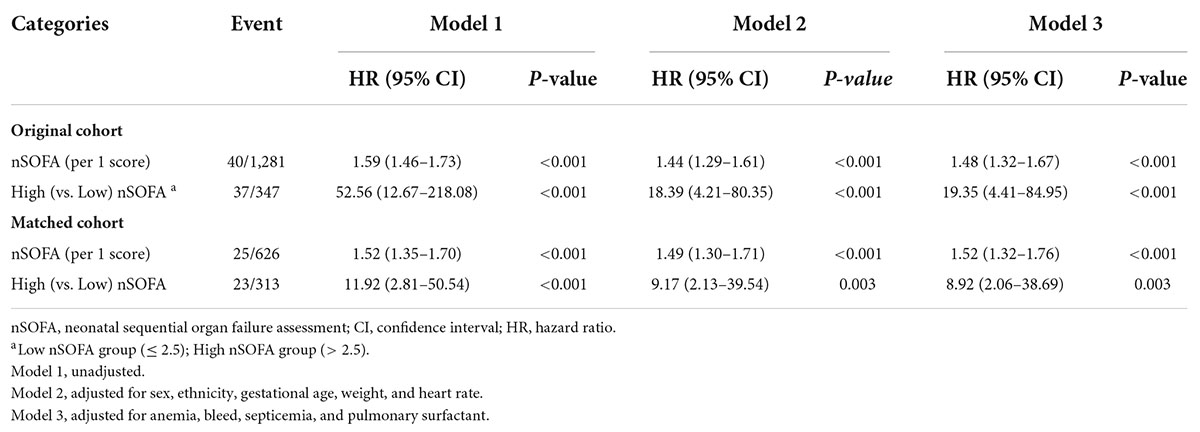
Table 3. Association between nSOFA scores and mortality.
Sensitivity analysisStratified and interaction analyses were used to evaluate whether the association differed by sex and septicemia. The results showed that the association between nSOFA score and mortality in RDS patients did not vary with sex and septicemia status (Supplementary Table 5). PSM did not change any of the conclusions (Table 3).
DiscussionThis retrospective study investigated the relationship between nSOFA score and the risk of death in neonates with RDS and found that the nSOFA score was independently associated with the risk of death in neonates with RDS. Each unit increase in nSOFA score correlated with a 48% increase in the risk of death in patients with RDS. The risk of death was higher in neonates in the high nSOFA group compared to the low nSOFA group and the relationship persisted after multivariable adjustment for traditional neonatal mortality risk factors.
The clinical condition RDS is one of the most common to be managed in the NICU. The incidence of RDS is inversely proportional to the gestational age of the neonate, with smaller and earlier neonates having more severe disease and it is the leading cause of morbidity and mortality in preterm neonates (16). Despite rapid advances in modern medicine, neonates who have developed RDS still have a high mortality rate (17–19) and there is an urgent need for a method to alert clinicians to intervene in such high risk neonates. However, there is still a lack of validated tools to predict the prognosis of neonates with RDS. Recent studies have shown that not just premature birth, multiple pregnancies, and low birth weight will increase the risk of neonatal RDS (5, 6). Infection and inflammation has been identified as a risk factor for RDS (5) and Nupponen et al. showed that neutrophils were activated in neonates with RDS (6). The nSOFA score is an operational definition of organ dysfunction that can identify those preterm neonates with infection and sepsis and an increased risk of mortality (9, 10), as sepsis is associated with a systemic inflammatory response which is implicated in the occurrence and development of RDS (6, 20). This study showed that the nSOFA score was strongly predictive of the mortality in neonates with RDS. As the first study to link nSOFA score with neonatal RDS, these results showed that the nSOFA score had a good predictive power for mortality risk in neonates with RDS and should be used for the assessment of prognostic risk in neonates with RDS.
In adults, the SOFA score includes aspects of central nervous system (CNS), liver dysfunction, and renal dysfunction, but these systems are more difficult to measure in neonates (10). The nSOFA score includes only three subscales defined as the respiratory, cardiovascular and hematological systems. The SpO2/FiO2 in the respiratory score is based on the Berlin definition to assess the severity of RDS, while mechanical ventilation maintains adequate ventilation and oxygenation to support organ function, both of which accurately reflect respiratory function. Corticosteroids have a wide range of potential effects in terms of anti-inflammatory, antioxidant, pulmonary vasodilatory and anti-edema actions (21), whereas vasoactive drugs optimize the hemodynamic status and improve blood perfusion to the organs (22). In the cardiovascular system, the use of vasoactive drugs and corticosteroids can be effective in assessing the severity of the disease. This study also observed that the cardiovascular subscale of the nSOFA score was the strongest predictor of neonatal death in RDS. According to a related article, coagulation dysfunction and progression of organ dysfunction in patients with thrombocytopenia have a significant correlation with each other (23), so the coagulation score based on platelet count can effectively reflect the progression of organ dysfunction. This study found that the hematological subscale of the nSOFA score appeared to perform poorly in predicting the prognosis of RDS neonates, whereas the nSOFA score demonstrated good predictive efficacy in terms of prognosis of RDS neonates. In addition, gestational age is known to be an independent predictor of incidence of RDS in neonates and is strongly associated with poor prognosis. Therefore, we added gestational age to the original nSOFA score for scoring and analysis. The results showed that the AUC of the modified nSOFA score was higher than that of the original score. This result provides a theoretical basis for the application of the nSOFA score in patients with RDS.
This study filled a gap in this field and broadens the clinical application of the nSOFA score, by determining the predictive power of the nSOFA score for mortality in RDS patients and its use to identify RDS patients at increased risk of adverse outcomes. As an objective measure of clinical course, nSOFA score has a potential utility both as a proxy variable for disease severity and as a risk assessment indicator for short- and long-term clinical endpoints (9). In this study, the nSOFA score showed good correlation in most cases and its findings will be useful for clinicians and researchers who need tools to objectively measure the risk of death, as well as for healthcare leaders and policy makers who develop resource allocation protocols for critically ill patients (24).
This study had several limitations. The data were only extracted from MIMIC-III data, which was a single-center retrospective study and it was difficult to control for a variety of confounders and bias in the analyses. In addition, individual patient-level data on treatment variables such as fluids, time to pulmonary surfactant and time to vasopressors was not available. Maternal medication data during pregnancy and delivery were not available. The SpO2/FiO2 was converted and replaced by using PaO2/FiO2. All the above factors may have influenced us to draw more definitive conclusions. Finally, this study did not examine the non-ICU population, so its applicability to neonates with suspected RDS in the rest of the hospital on the wards or in the Emergency Department could not be determined by this study. In the future, more large-scale, multicenter studies on neonatal mortality should be conducted to further verify these results.
ConclusionThis study indicated that nSOFA score was associated with the risk of mortality in the neonatal RDS of NICU. The active use of the nSOFA score may help clinicians to quickly and accurately identify high risk neonates and implement more aggressive interventions.
Data Availability StatementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statementThe studies involving human participants were reviewed and approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributionsYL conceptualized the research aims, planned the analyses, and guided the literature review. SS extracted the data from the MIMIC-III database. SS and JG participated in processing the data and performing the statistical analysis. LL, JT, JX, and WC wrote the first draft of the manuscript. SS, JG, MF, QL, KC, and YL provided comments and approved the final manuscript. All authors contributed to the article and approved the submitted version.
FundingThis work was supported by the Longyan City Science and Technology Plan Project (2021LYF17039); Longyan City Science and Technology Plan Project (2021LYF17025). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Conflict of InterestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s NoteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AcknowledgmentsWe thank all the participants and staff of the Marketplace for Medical Information in Intensive Care III for their valuable contributions. We also thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Supplementary MaterialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.911444/full#supplementary-material
References1. Wu X, Feng Z, Kong J, Lai Y, Jia C, Xu Z, et al. Efficacy and safety of surfactant administration via thin catheter in preterm infants with neonatal respiratory distress syndrome: a systematic review and meta-analysis. Pediatr Pulmonol. (2021) 56:3013–25. doi: 10.1002/ppul.25545
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Cresi F, Maggiora E, Borgione SM, Spada E, Coscia A, Bertino E, et al. Enteral Nutrition Tolerance And REspiratory Support (ENTARES) Study in preterm infants: study protocol for a randomized controlled trial. Trials. (2019) 20:67. doi: 10.1186/s13063-018-3119-0
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Nupponen I, Pesonen E, Andersson S, Makela A, Turunen R, Kautiainen H, et al. Neutrophil activation in preterm infants who have respiratory distress syndrome. Pediatrics. (2002) 110(1 Pt 1):36–41. doi: 10.1542/peds.110.1.36
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Stylianou-Riga P, Boutsikou T, Kouis P, Kinni P, Krokou M, Ioannou A, et al. Maternal and neonatal risk factors for neonatal respiratory distress syndrome in term neonates in Cyprus: a prospective case-control study. Ital J Pediatr. (2021) 47:129. doi: 10.1186/s13052-021-01086-5
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Meert KL, Banks R, Holubkov R, Pollack MM, Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Morbidity and mortality in critically Ill children. II. A qualitative patient-level analysis of pathophysiologies and potential therapeutic solutions. Crit Care Med. (2020) 48:799–807. doi: 10.1097/CCM.0000000000004332
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Fleiss N, Coggins SA, Lewis AN, Zeigler A, Cooksey KE, Walker LA, et al. Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Netw Open. (2021) 4:e2036518. doi: 10.1001/jamanetworkopen.2020.36518
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Wynn JL, Polin RA. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr Res. (2020) 88:85–90. doi: 10.1038/s41390-019-0517-2
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. (2007) 132:410–7. doi: 10.1378/chest.07-0617
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Sanchez-Pinto LN, Parker WF, Mayampurath A, Derrington S, Michelson KN. Evaluation of organ dysfunction scores for allocation of scarce resources in critically ill children and adults during a healthcare crisis. Crit Care Med. (2021) 49:271–81. doi: 10.1097/CCM.0000000000004774
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Wynn JL, Mayampurath A, Carey K, Slattery S, Andrews B, Sanchez-Pinto LN. Multicenter validation of the neonatal sequential organ failure assessment score for prognosis in the neonatal intensive care unit. J Pediatr. (2021) 236:297–300.e1. doi: 10.1016/j.jpeds.2021.05.037
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Yadav S, Lee B, Kamity R. Neonatal Respiratory Distress Syndrome. Treasure Island, FL: StatPearls Publishing LLC (2022).
17. Abdelrahman SM, Hamed SM, Nasr A. Neonatal respiratory distress in Omdurman Maternity Hospital, Sudan. Sudan J Paediatr. (2014) 14:65–70.
PubMed Abstract | Google Scholar
18. Bhutta ZA, Yusuf K. Profile and outcome of the respiratory distress syndrome among newborns in Karachi: risk factors for mortality. J Trop Pediatr. (1997) 43:143–8. doi: 10.1093/tropej/43.3.143
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Matsuo S, Sharma A, Wang P, Yang WL. PYR-41, a ubiquitin-activating enzyme E1 inhibitor, attenuates lung injury in sepsis. Shock. (2018) 49:442–50. doi: 10.1097/SHK.0000000000000931
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Brennan CA, Osei-Bonsu P, McClenaghan RE, Nassar A, Forget P, Kaye C, et al. Vasoactive agents in acute mesenteric ischaemia in critical care. A systematic review. F1000Res. (2021) 10:453. doi: 10.12688/f1000research.52782.1
CrossRef Full Text | Google Scholar
23. Ogura H, Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, et al. SIRS-associated coagulopathy and organ dysfunction in critically ill patients with thrombocytopenia. Shock. (2007) 28:411–7. doi: 10.1097/shk.0b013e31804f7844
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Ehmann MR, Zink EK, Levin AB, Suarez JI, Belcher HME, Daugherty Biddison EL, et al. Operational recommendations for scarce resource allocation in a public health crisis. Chest. (2021) 159:1076–83. doi: 10.1016/j.chest.2020.09.246
留言 (0)