The gut microbiota with trillions of microbial cells and thousands of species profoundly influences human physiology, including various diseases and disorders. The human gut microbiota starts colonizing during the pregnancy period (Collado et al., 2016). Collectively the genome of all these microorganisms represents the microbiome. The process of development and colonization of gut microbes co-occurs with brain development during pregnancy in a coordinated way until the first few years after birth (Jena et al., 2020; Acuña et al., 2021). An imbalance in the gut microbiome during the critical developmental phase can influence the overall developmental process, primarily neuronal plus glial development and maturation (Borre et al., 2014; Marín, 2016). The microbiota composition exhibits the highest intra- and inter-individual variability during the first 12 months of post-natal development until it reaches a stable adult-like organization at the age of ∼ 3 years and can influence the process associated with brain development and in shaping the immune profile of the individual. (Koenig et al., 2011; Bäckhed et al., 2015; Gensollen et al., 2016). Early-life colonization of the host’s mucosal surfaces is crucial for the development and maturation of the host’s immune system in a healthy individual (Gensollen et al., 2016).
However, exposure to factors such as maternal immune activation (MIA), poor diet, disease/infections and antibiotic overdose can lead to early life gut dysbiosis (Vangay et al., 2015; Neuman et al., 2018; Li et al., 2021). The altered gut microbiome can cause dysregulated immune activation, igniting systemic inflammation resulting in atypical brain development leading to symptoms associated with neurodevelopmental psychiatric disorders (NPD) (Figure 1) (Garay and McAllister, 2010; Han et al., 2021; J.; Lu and Claud, 2019; Munawar et al., 2021). Hence, the presence of a balanced microbiome is needed for the proper functioning of the immune system, which in turn regulates the neurodevelopmental trajectories (Garay and McAllister, 2010; Gensollen et al., 2016; Sotgiu et al., 2020).

FIGURE 1. Significant risk factors affecting events with in human developmental window could bring on neurodevelopmental psychiatric disorders. The critical developmental window spans from the fetal stage until childhood, which consists of early colonization and development of the microbiome, development of the brain and nervous system, and development and maturation of the immune system. Within this period, the composition and diversity of the gut microbiome, genetics, maternal, and other reverent factors can alter the overall developmental homeostasis by causing disturbances in the immune system. Gut dysbiosis, immune alteration, and other factors induce microglial activation via inflammatory response, which leads to systemic and neuroinflammation. This negatively affects the brain development process and leads to abnormal brain development and functionality, including anxiety, depression, intellectual disability, and behavioral abnormality that can be seen in neurodevelopmental and psychotic disorders.
NPDs are a spectrum of disorders arising from atypical brain development resulting in cognitive, emotional, and motor deficits (Kapoor, 2002; Villagomez et al., 2019; Munawar et al., 2021). These include attention deficit hyperactivity disorders (ADHD), autism spectrum disorders (ASD), and schizophrenia (SCZ) which affect children in their daily lives through the early adolescence and later periods of life. These NPDs are also associated with psychotic symptoms and behavioral abnormalities characterized by positive symptoms such as psychotic hallucinations, the eccentric overflow of thoughts, and negative symptoms like insensibility, emotional and interactive withdrawal, lethargy, etc. (Cowen et al., 2012). These behavioral abnormalities often change or maturate and become more severe as a child grows older; some disabilities and behavioral abnormalities remain permanent (Klimkeit et al., 2016).
An increasing number of preclinical and clinical studies have been focusing on how the interaction between the complex gut-microbial ecosystem and central nervous system can regulate brain development and its association with NPDs (Figure 2) (Yap and Martin, 2015; Ernesto Martínez-González and Andreo-Martínez, 2019; Bull-Larsen and Hasan, 2019; Yuan et al., 2019; Checa-Ros et al., 2021; Kelly et al., 2021). However, most of these studies have explored the relationship on core pathology associated with one clinical subtype of NPDs. It is evident that there is a need for a greater understanding of the complex coordinated underlying pathways that’s span major NPDs. In this timely review, we discuss the recent evidence establishing the direct role of commensal gut microbes and associated metabolites on human brain development and how alterations in gut microbiome brain axis mediated communications play a role in the pathology of ASD, ADHD, and SCZ, which stems from aberrant brain development. Secondly, we propose a hypothetical framework illustrating an association between gut microbiota and inflammation affecting brain development and preventive measures in association with diet and probiotic therapy at crucial time points to mitigate the initiation and proper management of existing neurodevelopmental psychiatric disorders.

FIGURE 2. Role of the gut microbiome in healthy brain development. The gut microbiome plays an essential role in various processes of brain development such as neurogenesis, myelination, microglial maturation, development and maintenance of blood-brain barrier integrity, development of HPA-axis, and HPA-axis stress response. Any alterations in this developmental process can significantly increase the risk for neurodevelopmental disorders.
2 Development of Gut MicrobiotaFollowing birth, the colonization of microbes initiates and is very much influenced by the mode of delivery (normal delivery or C-section) (Biasucci et al., 2010; Wampach et al., 2017). Some studies have shown that microbial colonization begins in-utero with the discrete microbial community present in the umbilical cord blood, placenta, and amniotic fluid and may carry out their role in the foetal developmental process (Aagaard et al., 2014; Collado et al., 2016). Although some studies have defined the microbial composition of the fetal meconium has been defined in several studies (Moles et al., 2013; Stinson et al., 2019), others have linked premature birth with the decreased microbial diversity within the meconium microbiome (Ardissone et al., 2014). Interestingly some recent studies suggest a similarity within the microbial profile in between placenta, fetal meconium, and amniotic fluid (He et al., 2020; Senn et al., 2020). Younge et al. have confirmed maternal-fetalin utero-translocation of the gut microbiota using a mouse model (Younge et al., 2019). The preterm neonate meconium has been shown to have abundant microbes belonging to Lactobacillus, Staphylococcus, and Enterobacteriales (Madan et al., 2012). With low diversity and high inter-individual variability, the meconium microbiome is more abundant with genera Bacillus, Escherichia/Shigella, and Enterococcus, whereas Bacteroides and Bifidobacterium are less abundant concerning the fecal microbiome of infants (Moles et al., 2013; Bäckhed et al., 2015). The placenta is most abundantly colonized by phyla Proteobacteria and Bacteroidetes and various other phyla like Firmicutes, Fusobacteria, Tenericutes, and genera Mycoplasma and Ureaplasma (Brotman, 2011; Aagaard et al., 2014). At the time of birth, the babies born through standard delivery through the vagina are colonized mainly by the maternal vaginal microbial population, predominantly Lactobacillus and Prevotella (Dominguez-Bello et al., 2010). In contrast, cesarean section delivered babies are more exposed and colonized by Staphylococcus and Corynebacterium closer to the skin microbiome (Dominguez-Bello et al., 2010). The typical microbiome composition of a newborn is mainly dominated by Proteobacteria and Actinobacteria, where the former dominates immediately after birth and later towards the 4 months of age (Bäckhed et al., 2015; Dogra et al., 2015). During the 1st year of life, the gut of the new-born infant is predominantly inhabited by Bifidobacterium, Enterococcus, Escherichia/Shigella, Streptococcus, Bacteroides, and Rothia gut-microbial population closer to maternal microbiota in addition to Clostridium, Ruminococcus, Veilonella, Roseburia, Akkermansia, Alistipes, Eubacterium, Faecalibacterium, and Prevotella, species. Moreover, variation in the maternal microbiota has also been suggested as modulating factor of microbiome composition in offspring (Dotterud et al., 2015). The 1st 3 years of the lifetime are more crucial for establishing a healthy and stable structured microbiome. It has been reported that the process of the microbiome and neuronal development coincides in an intense and coordinated way within this critical time frame and is most vulnerable to disruption (Sharon et al., 2016; Jena et al., 2020; Acuña et al., 2021). A gut dysbiosis at such period has been reported to bring about many NDD/NPD like ADHD, ASD, SCZ, intellectual as well as learning disability, and behavioral problems (Borre et al., 2014; Chrobak et al., 2016; Bojović et al., 2020). Moreover, after this period, microbiota reconstitution does not normalize the behavioral phenotype or neurochemical disturbances during the critical developmental period (Sudo et al., 2004; Heijtz et al., 2011; Click or tap here to enter text; Clarke et al., 2013). Hence, maintaining a healthy and well-structured microbiota is essential during prenatal and postnatal periods up to a particular developmental phase.
3 Role of Gut Microbes in Early Brain DevelopmentEmerging studies provide evidence about the active role of microbiota during central nervous system development. It is now clear that gut microbes play an active role in the neurodevelopmental processes (Figure 2), including the establishment of the blood-brain barrier (BBB) (Parker et al., 2020), neurogenesis (Cerdó et al., 2020), maturation of microglia (Erny et al., 2015), and myelination (Hoban et al., 2016; Duncan and Watters, 2019). These processes are critical in shaping animal behavior and cognition. Various dietary components released from the gut are needed for the developing brain for neuronal cell maturity and proper functions (Prado and Dewey, 2014; Ratsika et al., 2021). Also, recent evidence describes that gut microbes can directly facilitate the development processes in the brain, which have long-lasting consequences in health (J. Lu and Claud, 2019).
3.1 Blood-Brain BarrierThe BBB establishes early in-utero, which constitutes capillary endothelial cells sealed by tight junction proteins, pericytes, and astrocytes, forming the restrictive barrier between the brain and systemic circulation. It also facilitates the exchange of molecules and nutrients for proper maintenance and functioning of the brain (Obermeier et al., 2013; Braniste et al., 2014). The presence of balanced gut microbiota and microbial-derived metabolites such as SCFAs are essential in regulating the formation and maintenance of intact BBB (Obermeier et al., 2013; Braniste et al., 2014; Michel and Prat, 2016). The permeability of BBB in developing sterile fetuses decreases towards adulthood (Mollgaard and Saunders, 1986). In germ-free (GF) mice, the permeability of BBB increases for macro-molecules due to reduced expression of basic junctional proteins occludin and claudin-5 in the brain endothelial layer (Braniste et al., 2014). Microbial colonization of the gut or administration of butyrate, a short-chain fatty acid (SCFA) produced by gut-microbial fermentation, has been shown to reduce the blood-brain permeability in GF mice (Braniste et al., 2014).
3.2 NeurogenesisNeurogenesis refers to the development of new functional neurons through differentiation of the neural stem/progenitors’ cells (Gage, 2019; Cosacak et al., 2020). The functionality of neurogenesis and neuronal plasticity is critical for learning, memory, cognition, and stress response, especially in the hippocampus, as the cognitive center (Kempermann, 2019). A balanced gut-microbiota is involved directly or indirectly in maintaining the microenvironment to support the process of neuronal development (Sarubbo et al., 2022). A recent comparative study between GF and SPF mice outlines a range of gut microbial metabolites that can cross through the placenta into the foetal compartment, with the ability to induce and regulate the prenatal developmental process (Pessa-Morikawa et al., 2022). In addition, PG, a bacterial cell wall component, gets crossed through the placenta to reach the foetal brain, there it activates Toll-like receptor 2 (TLR2), triggering an increase in FOXG1 expression, a crucial transcription factor in regulating the development and neurogenesis, thereby inducing neuronal proliferation in the forebrain area (Kaul et al., 2012; Humann et al., 2016). A recent study provides direct evidence linking modulation of microbial function and associated cytokine by microbiota to influence the process of neurogenesis (Salvo et al., 2020).
Further, the gut-microbes may indirectly affect neuronal-plasticity by regulating neuronal migration and maturation in CNS may be via regulation of ephrin B and reelin pathway, where ephrin B plays a pivotal role in the maintenance of the gut epithelial barrier integrity and reelin, a membrane glycoprotein responsible for neuronal migration (Allam-Ndoul et al., 2020; Grandi et al., 2019; D’Arcangelo, 2014; White and Getsios, 2014; Sentürk et al., 2011; Hafner et al., 2005).
Accumulating evidence suggests that gut microbes can impact the neural stem cells’ fate by coordinating with intricate pathways of differentiation and survival through neurotrophins and neurotransmitters in different areas of the brain (Sharon et al., 2016). The process of synapse development and maturation is associated with neuronal maturation and plasticity. Experimental administration of neonatal prebiotic (BGOs) in comparison to other prebiotic in 22 days old rats has been reported to elevate hippocampal expression of synaptophysin and brain-derived neurotrophic factor (BDNF). Synaptophysin, a synaptic vesicle protein, which controls the synaptic vesicle endocytosis kinetics, and BDNF, a nerve growth factor secreted by neurons, which also act as a signaling molecule for neuronal survival, growth, maturation maintenance of various brain cell populations, as well as the establishment of neuronal circuitry through the formation of the synapse (S. Williams et al., 2016).
Serotonin, a neurotransmitter and signaling molecule, can also be synthesized and released by gut microbes into the gut lumen, known to promote adult neurogenesis (Alenina and Klempin 2015). In addition, gut microbes have been reported to have an essential involvement in serotonergic signaling pathways in the gut and multiple regions of the brain (O’Mahony et al., 2015). Several studies have also reported the role of gut microbiota in regulating adult neurogenesis. By labeling proliferating cells with bromo-deoxyuridine GF mice brain, an increase in the adult dorsal hippocampal neurogenesis was reported compared to conventionally grown mice, and even upon microbial colonization, the phenotypical condition couldn’t be reversed. This indicates that the absence of microbes induces a dysregulated increase in adult dorsal hippocampal neurogenesis, and the microbial signals during crucial early life developmental window act as a controlling force in regulating neurogenesis in the hippocampus (Ogbonnaya et al., 2015).
Furthermore, the usage of antibiotics, which has a negative impact on gut microbiota, was associated with decreased neurogenesis (Möhle et al., 2016). The molecular mechanism that regulates the process of adult neurogenesis via microbiota and its associated metabolites is not very clear. It is suggested that neuroinflammatory mechanisms mediate this process along with humoral and metabolic pathways (Liu et al., 2022). A number of recent studies provide evidence to support the role of neuroinflammatory mechanisms. Intestinal bacteria maintain the enteric nervous system in adult mice through Toll-like receptor 2-induced neurogenesis (Yarandi et al., 2020).
Additionally, a decrease in BDNF mRNA level in the whole hippocampus was reported, particularly with a significant decline in neuronal proliferation and survivability in vagotomized mice (O’Leary et al., 2018). Furthermore, microbiome also indirectly influences hippocampal neurogenesis via regulating the neuronal immune system. Induction of acute colonic inflammation via administrating dextran sodium sulfate in mice reported a dysbiotic gut microbial composition, the increased hippocampal expression level of pattern recognition receptor and T-helper 17 cell-associated cytokines and ionized calcium-binding adapter molecule 1, a microglial activation marker, concomitant to depreciated adult hippocampal neurogenesis with consequent behavioral deficits (Salvo et al., 2020).
Early life stress, like a lack of social interaction, can also alter the stability of the gut microbiome (Cong et al., 2015), along with reduced neurogenesis and IL-6 and IL-10 level in the hippocampus of socially isolated mice as compared to grouped controls (Dunphy-Doherty et al., 2018). A reduction in hippocampal neurogenesis is strongly associated with impaired learning, anxiety, depressive-like behaviors, neuroinflammation, which again have an explicit association with structural alterations gut microbiome (Liu et al., 2022).
3.3 MyelinationA healthy/intact gut microbiome has been reported to modulate myelination (Hoban et al., 2016; Jena et al., 2020). Humans are born primarily with unmyelinated axons in the central nervous system (CNS) at the time of birth. Rapid myelination of maturing axons occurs within just a few years after childbirth by oligodendrocytes through the process of engagement and ensheathment (Lu et al., 2002; Williamson and Lyons, 2018), with a variable rate of myelination and myelin content over time (Benes, 1989), until early adulthood. (Lebel et al., 2012; Williamson and Lyons, 2018). Any aberration in this process may lead to long-lasting defects. Myelination has the most crucial role in cognitive function, and the scale of myelination has been linked with neuronal plasticity and function (Almeida and Lyons, 2017; Kaller et al., 2017). The gut microbiota regulates the critical process of myelination by regulating myelination-related gene expression in oligodendrocytes. Myelin deformities can have a detrimental impact on brain function and behavior (Gacias et al., 2016; Hoban et al., 2016; Ntranos and Casaccia, 2018). Most notably, the prefrontal cortex (PFC) area of the brain exhibits myelination at a later period, during the initial phase of an infantile life, which makes it more vulnerable to external influencing factors, like intestinal dysbiosis (Hoban et al., 2016). As in the case of GF mice, irregulated myelin formation in the PFC region has a harmful effect on social behavior (Gacias et al., 2016; Hoban et al., 2016). Furthermore, bacterial metabolites such as SCFAs have been demonstrated to have a beneficial impact over stress-induced behavioral difficulties, intestinal barrier dysfunctionalities, and in the regulation of the myelination process (Gacias et al., 2016; van de Wouw et al., 2018). SCFA butyrate, when orally administered, caused recovery of myelination impairments, intestinal physiology, and behavioral deficit in antibiotic-treated mice, indicating a pivotal role of gut microbiota in developing the microbiome-gut-brain (MGB) axis through regulation of myelination process in the PFC region (Keogh et al., 2021). Thus, the microbiota is crucial for myelination and maintenance of the plasticity of the myelin sheath.
3.4 Hypothalamus-Pituitary-Adrenal AxisThe endocrine-neurocrine interaction between the hypothalamus, pituitary gland, and adrenal gland in response to stress is known as the hypothalamus-pituitary-adrenal axis, where the corticotropin-releasing factor (CRF) plays the central role in the stress response via initiating a course of events that leads to the release of glucocorticoids from the adrenal cortex by regulating the HPA-axis (S. M. Smith and Vale, 2006). In developing the HPA-axis, commensal microbiota also plays a significant role (Sudo, 2012). In GF mice, dopamine deficiency, particularly in the frontal cortex hippocampus and striatum, is one of the major causes of improper stress regulation and anxiety-like behavior (Zarrindast and Khakpai, 2015). The administration of probiotic formulation consisting of probiotic strains Lactobacillus helveticus and Bifidobacterium longum, the level of anxiety decreased substantially (Partrick et al., 2021). Also, the comparison between GF and specific-pathogen-free (SPF) mice revealed increased CRF mRNA level in the hypothalamus of the GF mice, indicating an enhanced stress response related to HPA-axis (Sudo et al., 2004).
3.5 Microglial Development and PhysiologyMicroglia are resident immune cells (macrophages) that belong to the glial system. They account for 10–15% of the whole total glial cell count in the CNS (Hugh Perry, 1998), thoroughly distributed across the brain and the spinal cord (Lawson et al., 1990). Unlike the neuronal cells, microglial cells that constitute the innate immune system of CNS are derived from a subset of primitive macrophages that originates from the progenitor cell of the yolk sac (Ginhoux et al., 2013). The functionalities of microglia include immune defense and maintenance of the CNS. The microglial cells consistently survey their local microenvironment to detect pathogenic invasion or tissue damage throughout the CNS, guarded by the BBB (Daneman, 2012). Microglia also regulate neuronal proliferation, differentiation, and formation of synaptic connections (Graeber, 2010; Hughes, 2012), that helps in the refurbishing of post-natal neural circuits via controlling synaptic truncation during postnatal development of the mice (Tremblay et al., 2010; Paolicelli et al., 2011). Microglia contributes to both innate and adaptive immune system defense in the CNS. Abnormal activation of microglia aberrantly induces inflammation, which has been observed in most brain-related pathologies. Emerging evidence also suggests microglia directly affect neuronal pathology and contributes to disease progression (Perry et al., 2010; Helmut et al., 2011; Kingwell, 2012). Recent research data have demonstrated that microbiota has a vital role in the development and maturation of microglia (Erny et al., 2015; Abdel-Haq et al., 2019). However, upon damage or depletion of microglial macrophages and the yolk progenitor cells the bone marrow-derived macrophages can replenish them by developing and differentiation with the help of microbial signals at an early point (Erny et al., 2015; J.; Han et al., 2019). Moreover, in GF mice, the microglial cells show a significantly altered developmental state, with morphological characteristics and gene expression profile of a developmental and maturation arrest condition. These GF mice-derived microglia usually displayed a limited response towards viral infections and microbe-associated molecular patterns (MAMP), which interestingly rescued by SCFA administration (Hanamsagar et al., 2017).
4 Altered Gut Microbiota, Inflammation, and Neurodevelopmental Disorders4.1 Altered Microbiome and InflammationIn response to eliminating invading microorganisms such as bacteria, viruses, and other pathogens, the innate immune system triggers an intracellular signaling pathway via activation of toll-like receptors (TLRs) for gene expression of interferons, cytokines, and other immunological mediators (El-Zayat et al., 2019). The molecules during pathogenic elimination can also contribute to the development of inflammation response and so-called inflammatory mediators. Inflammation can also be triggered in response to endogenous factors such as predisposition to genetic abnormalities, behavioral anomalies of immune cells (autoimmunity/allergy), as well as external factors such as trauma, heat and cold stress, environmental toxicants, pathogens, diseases, etc. (Chen et al., 2018). Among pro-inflammatory mediator IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-12, TNF-α, INF-γ and among anti-inflammatory mediator IL-4, IL-5, IL-10, TGF-β, C-reactive protein (CRP), and serum amyloid-A are considered as inflammation biomarkers (Brenner et al., 2014; Sands, 2015; Kany et al., 2019; Morris et al., 2020).
The gut microbes also have an essential role in the induction of inflammation. More specifically, the pathogenic/opportunistic pathogenic strains such as E. coli and B. fragilis from the Enterobacteriace family contribute to pathogenesis when outnumbered and eventually to inflammation (Hiippala et al., 2020). Lipopolysaccharides (LPS), a specific cell membrane component present mostly in Gram-negative bacteria like E. coli and B fragilis is endotoxic and, upon interaction with macrophages, cause the release of pro-inflammatory cytokine TNF-α, IL-6, IL-1, which lead to endocytic septic shock and often have fatal outcomes (Agarwal et al., 1995). The LPS from Bacteroides is also a potential stimulator of the innate immune system (Alexander and Rietschel, 2001). In systemic inflammation, there is a reported elevation in the expression of pro-inflammatory cytokines IL-6 and IL-8, which have been correlated with microbial taxa belonging to phylum Proteobacteria within total fecal microbiota (Biagi et al., 2010).
Moreover, the combination of different taxa of microbes can enhance the pathogenic effects that lead to inflammation. For example, co-infection of E. coli and B. fragilis can induce an increase in expression of TNF-α, keratinocytes-derived chemokine mRNA (KC mRNA), and proteins in peritoneal tissue and cause early peritonitis, abscess development, and death in the experimental mice model, which is not observed when infected singularly (J. M. Kim et al., 2000). Colonization of Chloristidium difficle has been reported to have an association with frequent occurrence of eczema, sensitization to allergy, and ectopic dermatitis (Lee et al., 2017).
In contrast to inflammation, inducing microbes, a fraction of gut microbiota can counteract the inflammation. These microbes can act either by directly inhibiting inflammation-inducing microbes, strengthening the gut epithelial/mucosal barrier or directly interacting with inflammation-inducing components of the immune system or all three ways simultaneously (Hakansson and Molin et al., 2011). Faecalibacterium prausnitzii from the family Ruminococcaceae has been reported to secrete anti-inflammatory metabolites that block the activation and secretion of NF-κB and IL-8 in Caco-2 cells (Sokol et al., 2008). Also, F. prausnitzii can induce an increase in the anti-inflammatory ratio of IL-10 and IL-12 (Alameddine et al., 2019). An endoscopic recurrence of Chron’s disease has been reported when there is a lower proportion of F. prausnitzii within the composition of the patient’s group (Sokol et al., 2008).
Moreover, oral administration of F. prausnitzii can reduce the disease severity induced by 2, 4, 6-trinitrobenzene sulfonic acid in the colitis mouse model (Y. Zhou et al., 2021). Lactobacillus and Bifidobacterium are well-known probiotics and are the most studied taxa having anti-inflammatory properties and are the most crucial part of the gut microbiota (Cristofori et al., 2021). Microbes belonging to taxa Lactobacillus and Bifidobacterium have been reported to suppress an undesired activation of the immune response as in case of autoimmunity and allergy, while capable of inducing an increase in nonspecific IgA antibody secretion in the intestine (Sjögren et al., 2009; Kandasamy et al., 2015). Gut dysbiosis can be the consequence of an increase in inflammation-inducing microbes in the gut. Both dysbiosis and inflammation have a negative effect on brain development and can result in NDDs (Figures 3, 4).

FIGURE 3. Gut-brain homeostasis and inflammatory mechanisms in neurodevelopmental psychiatric disorders due to gut brain dysbiosis. In infancy, the dysbiotic gut is characterized by less diverse microbiota with an abundance of pathogenic microbes, less beneficial microbes, and disrupted gut epithelial barrier with consequent GI tract-related disorders. When severe, the pathogenic microbes may cross through and enter the blood, which holds a massive immune response and releases inflammatory cytokines that lead to inflammation. Cytokine imbalance can induce microglial activation in the brain, which again causes neuroinflammation. This is associated with a disruption in brain development, and delay in neurodevelopmental subsequently may lead to NDD and NPDs.
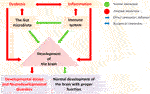
FIGURE 4. The gut microbes regulate the development and maturation of the immune system and play a crucial role in brain development (Green: normal interaction; Red: aberrant interaction). Gut dysbiosis and the immune system in brain development led to neurodevelopmental disorders. A gut dysbiosis alters the immune system homeostasis and leads to the activation of inflammatory response. Such aberration during brain development can disrupt the developmental pathways, leading to developmental delays and neurodevelopmental disorders.
4.2 The Gut Microbiota and Neurodevelopmental DisordersAny alterations in the above neurodevelopmental process lead to a class of disorders affecting brain development and function that are collectively referred to as neurodevelopmental disorders, affecting 15% of the worldwide estimated population (Boyle et al., 2011). The gut microbiota has a significant role in neuronal development through a complex bidirectional communication, also known as the gut-brain axis. Alteration in the colonization of gut microbiota has a major impact on mammalian brain development that can influence the aberration in adult behavior by altering the expression pattern of several genetic events during the process (Heijtz et al., 2011). Also, the neurocrine disturbances (Clarke et al., 2013) and consequent behavioral phenotypes resulting upon disruption of the gut microbiota colonization during the developmental period cannot be reversed via reconstitution of microbiota at a later period (Sudo et al., 2004; Heijtz et al., 2011; Desbonnet et al., 2014). Inflammation-induced by dysregulation of the maternal immune system via maternal infection, maternal gut dysbiosis, or metabolic disorders during pregnancy has also been considered as modulating risk factors for abnormal brain development prenatally as well as neonatally, subsequently leading to a range of neurodevelopmental and neuropsychiatric disorders in the later period (Boulanger-Bertolus et al., 2018; Guma et al., 2021). Fetal exposure to maternal gut microbial products (propionic acid, LPS, or peptidoglycan), immune complexes, antibodies, and inflammation mediators can alter the normal neurodevelopmental process. Some of these specific factors are known to cross the placenta and the immature prenatal BBB and modulate several processes of brain development directly or by inducing neuroinflammation (Madore et al., 2016). Dysregulation of immune homeostasis and systemic inflammation share a direct connection with management complications of the gastrointestinal (GI) system (Chen et al., 2018), which eventually resulted in increased intestinal permeability (leaky gut) accompanied by various GI problems, and interestingly has a reciprocal correlation with gut microbial dysbiosis. Such GI complications have a reported association with various NDDs (Maguire and Maguire, 2019). After immediate birth among the first microbial colonizers, Bifidobacteria are the most important and dominant bacterial genera in the infant as well as adults’ gut commensals (Milani et al., 2017). Also, Bifidobacteria are considered among most potential probiotics and known to have anti-inflammatory, antimicrobial properties can utilize indigestible polysaccharides and can produce various B-group vitamins (O’Callaghan and van Sinderen, 2016). In addition, Bifidobacteria can reduce stress levels and help with depression by improving HPA axis stress response (Sudo et al., 2004), elevating the serotonergic precursor-tryptophan (Desbonnet et al., 2014), and having an anxiolytic effect (Bercik et al., 2011). Deficiency of intestinal Bifidobacteria has been linked with indigestion, vitamin B12 deficiency, dysregulated immune system, gut inflammation, depression, and anxiety-like behavior in individuals suffering from NDDs (Jolanta Wasilewska and Klukowski, 2015; Y.; Zhang et al., 2016). Moreover, in NDD patients, there is a significant decrease or complete absence of Bifidobacteria, while no such case was observed in any of the control subjects (Bojović et al., 2020). An association between the total SCFAs level and abundance level of Faecalibacterium, Ruminococcus, and Bifidobacterium was found (de Angelis et al., 2013), whereby R. champanellensis and F. prausnitzii are present in lower abundance in the patients, with several other strains from the genus Bifidobacterium. Desulfotomaculum guttoideum, Romboutsia ilealis, and Intestinibacter bartlettii are the microbial sp closely related to Clostridium clusters (Gerritsen et al., 2014) were commonly found in the NDD patients (Bojović et al., 2020). Moreover, microbes classified under genera Bifidobacterium and Ruminococcus cannot self-produce SCFA butyrate but can induce the production of SCFAs because of their ability to digest undigestible carbohydrates. They can induce butyrate production by providing substrates for butyrate-producing microbes in the colon area (Belenguer et al., 2006). SCFAs are important molecular intermediaries of the gut-brain axis, suggesting their involvement in developing autism-like symptoms (Rogers et al., 2016). Lower numbers of SCFA-producing bacteria, particularly the butyrate-producing bacteria in the affected individual’s gut, have been reported to exert a substantial impact on the etiopathology of NDDs (Bojović et al., 2020). One of the microbial species, namely Dialister invisus, is isolated from the oral cavity usually present in the gut microbiota of healthy humans (Downes et al., 2003), and a decrease in its abundance has been noticed in the NDD patient group (Bojović et al., 2020). This depicts that the variance in general commensal microbes in the NDD patients is significantly lower, with an abundance of potentially harmful bacteria than the healthy control group. Besides, people suffering from NDD commonly suffer from gut microbiota dysbiosis. However, there is a difference between the type of microbial richness and diversity within the gut microbiota and its association with different NDDs (Lacorte et al., 2019). Tables 1–3 provide a detailed summary of gut microbial abundance and altered cytokine levels in the case of NDDs.

TABLE 1. Gut microbes and Level of cytokines in autism spectrum disorder (ASD).

TABLE 2. Gut microbes and level of cytokines in attention-deficit/hyperactivity disorder (ADHD).

TABLE 3. Gut microbes and Level of cytokines in Schizophrenia (SCZ).
4.3 Role of Inflammation in Brain Development and Neurodevelopmental DisordersEvidence suggests that dysregulated inflammation may be at the root of deficits that occur in numerous neurodevelopmental disorders, including schizophrenia and autism spectrum disorder (Ransohoff et al., 2015). Correlation between inflammation and NPDs in children has been gaining interest increasingly due to the harmful effects of inflammation on neuronal plasticity, neurogenesis, and overall neuronal development (Garay and McAllister 2010; Nona M.; Jiang, 2016). Inflammatory molecules affect brain development via interacting with MHC class-I molecule, glial cells, monoamine metabolism pathway, and HPA axis (Nona M. Jiang, 2016). In addition, immune cells, including B-cell, T cells, and macrophages, play a crucial role in the processes of brain development and are responsible for the onset of NPD (Shogo and Toshihide, 2018). Besides B-1a cell, one of the B-cell subtypes prevalently localizes to the neonatal brain in response to choroid plexus, reportedly involved in oligodendrocyte maturation in the developing brain (Shogo and Toshihide, 2018). However, in normal physiological conditions, B-1a cells preferentially localize in the intra-peritoneal cavity and participate in the neuro-immune defense system against any pathogenicity through the innate immune capability to secrete IgM antibodies, along with suppressing neuroinflammation, hence maintaining the neuroimmune homeostasis of the developing brain (Baumgarth, 2011; Kurnellas et al., 2015). Likewise, T-cells producing interferon-γ (IFN-γ) and IL-4 have been reported to regulate neuronal connectivity, development of cognitive, social behavior, and learning-related memory, respectively (Derecki et al., 2010; Filiano et al., 2016). The complete absence of T-cells in nude mice shows significant impaired neurogenesis and brain dysfunctionality (Ziv et al., 2006). Additionally, M2 macrophages subtypes are known to secrete neurotrophic factors such as IL-4, IGF-1, and upon induction BDNF, which promotes hippocampal neurogenesis, neuronal survival, and enhancement of spatial memory (Rőszer, 2015; Qi et al., 2017). IGF-1 and IL-4 are also produced by microglia and T cells, respectively (Derecki et al., 2010; Ueno et al., 2013). Also, perivascular macrophages support BBB development by providing pericytes progenitor cells (Daneman et al., 2010; Yamamoto et al., 2017).
Throughout the pregnancy, the maternal immune system and a fetal-placental immune response protect both mother and fetus in coordination with each other via systematic expression of various immune receptors that recognize diverse extra/intracellular pathogen and related products. And with pathogenic encounters, effectively produce a moderated inflammatory response, downstream signaling pathways related to pathogen clearance along with the secretion of antimicrobial peptides, and Indoleamine 2,3-dioxygenase (IDO) that are directly involved with pathogen clearance at the uterine-placental interface (Hoo et al., 2020). Thus, the immune system is likely to be present in an activated state rather than in the modulated state. And any case of immune alteration during this period can result in an increased susceptibility towards microbial and parasitic infections (Mor and Cardenas, 2010). This may trigger the fetal inflammatory response syndrome (FIRS) and results in an increased cytokine level of IL-1, IL-6, IL-8, and TNF-α, which has reported an increased risk of fetal developmental abnormalities (Gotsch et al., 2007). Moreover, human studies have also demonstrated a significant correlation between MIA, FIRs and the development of neurodevelopmental psychiatric disorders such as ASD, schizophrenia, neurosensorial deficits, ADHD, and psychosis in early as well as in the later period of adulthood (Knuesel et al., 2014; Brown and Conway, 2019; Choudhury and Lennox, 2021).
Further, research in the experimental mouse model has characterized high pro-inflammatory cytokine levels in the blood and amniotic fluid upon immune stimulation during pregnancy (Mor and Cardenas, 2010). It may likely contribute to the immunopathology of NDDs in their offspring. Certain inflammatory and immune-related conditions specific to particular NDD conditions, associated with inflammation induced by MIA, FIRS induction, nvolved in the development of NDD in offspring (Gotsch et al., 2007; Knuesel et al., 2014; Jung et al., 2020). Tables 1–3 provides a detailed summary of elevation and downfall in cytokine level during the inflammatory conditions in NDDs. The association of well-known neurodevelopmental-psychiatric disorders with inflammation and gut microbiome are discussed below.
4.4 Autism Spectrum Disorder4.4.1 The Gut Microbiome in ASDChildren with ASDs show a range of communication problems like lack of interest in social involvement, trouble expressing their feelings, and mostly avoiding physical contact, with trouble in speaking tendency (NIH, 2016). In addition, abnormality restrictive and repetitive behaviors, like aligning toys, flapping hands, swinging their body, or revolving in circles, have been the hallmarks of ASD (S. H. Kim and Lord, 2010). Around 80% of the children with ASD suffer from GI symptoms that include dyspepsia, abdominal pain, and constipation. The main reason for these severe GI problems could be the disruption of the gut microflora (Jolanta Wasilewska and Klukowski, 2015). In a recent study, restrictive food preference-based diet has also been linked with dysbiosis and decreased gut microbial diversity in children with ASD (Yap et al., 2021). Whilst the gut microbiome can regulate dietary preference, food choices as well as eating-related behavior, and a healthy gut microbiome is associated with healthy food choices (Alcock et al., 2014; de Wouters d’Oplinter et al., 2021; Koponen et al., 2021). Moreover, during pregnancy, dysbiosis of the maternal gut effectively alters gut-microbial diversity and immunity in the offspring, bringing an early onset of NDD (Nyangahu et al., 2018; Di Gesù et al., 2021). Additionally, altered immunity and inflammation also cause dysbiosis (Zeng et al., 2017).
Increasing evidence supports an association between gut microbiota dysbiosis and ASD (Xu et al., 2019). Through culture methods, aggressive forms of Candida species have been identified in the stool samples of 57% of children suffering from ASD compared to healthy controls (Iovene et al., 2017). Investigation of correlation between microbiome and ASD revealed that Clostridium histolyticum, a pathogenic anaerobic bacterium (Clostridium clusters I and II), was highly abundant in the fecal microbiota of ASD individuals. Several research groups have also detected an overgrowth of Clostridium sp. in the microbiota of children with autism (Finegold et al., 2002; Parracho et al., 2005; de Angelis et al., 2013). Erysipelatoclostridium ramosum was isolated from the fecal specimens of autistic children (Finegold et al., 2002). Clostridium tetani produce a neurotoxin p-cresol and other toxic metabolites (phenols and indole derivatives) that cause anxiety-like behavior (Bolte, 1998; Hsiao et al., 2013). Clostridium bartlettii is known to synthesize trans-3-indole acrylic acid (IAA), and by glycine conjugation, it can get converted into Indolyl-3-acryloyl glycine (IAG), which is a speculative ASD urinary diagnostic marker (Shattock and Whiteley, 2002; Bull et al., 2003). Overgrowth of these kinds of microbes has been associated with ASD pathology. Abnormality in sulfur metabolisms, such as the decline of serum sulfur in blood with a higher urinary excretion, decreased trans-sulfuration, and reduction in methylation capacity, in addition to chronic oxidative stress (Finegold et al., 2012b), have been detected in children with autism. This may be due to an increase in another sulfate-reducing organism, D. guttoideum, similar to Desulfovibrio (Finegold et al., 2012a; Tomova et al., 2015). In addition to Chloristidium cluster bacteria, Sutterella, associated with gut-immune homeostasis, and GI symptoms, has been found closely related with the intestinal epithelial layer among autistic children while absent in controls (B. L. Williams et al., 2012). Moreover, a particular relation between microbes Actinomycetaceae, Allisonella, Barnesiella, Coprobacter, Fusobacterium, Olsenella in ASD individuals and constipation have been observed. In contrast, there was an increase in Holdemanella in the case of ASD without associated constipation (Lacorte et al., 2019). The microbiome of ASD individuals represents a phylum level ratiometric increase in Firmicutes/Bacteroidetes due to a significant increase in Firmicutes and a reduction in the abundance of Bacteroidetes (Finegold et al., 2010; Kang et al., 2013). A lower abundance of Eubacteriaceae, Erysipelotrichaceae, Faecalibacterium, Peptostreptococcaceae, Ruminococcaceae, and Streptococcaeceae, has been reported at a taxa level (de Angelis et al., 2013). At the family level, a considerably higher abundance of Bifidobacteriaceae, Lactobacillaceae, Enterobacteriaceae, and Veillonellaceae have been observed (Bezawada et al., 2020). At the genus level, there was a significant increase in Bifidobacterium, Bacteroides, Bacillus, Biophila, Faecalibacterium, Lactobacillus, Lachnospira, Lactococcus, Lachnobacterium, Megamonas, Megasphera, Mitsuokella Oscillospira, Parabacteroides, Sutterella (Lacorte et al., 2019), Collinsella, Corynebacterium, and Dorea (Strati et al., 2017). A lower abundance of genera Streptococcus, Veillonella, Escherichia (Zhang et al., 2018), Alistipes, Dialister, Parabacteroides (Strati et al., 2017) were observed in autistic children. Additionally, Yap et al. reported a specific decline in Romboutsia timonensis abundance, especially in the gut microbiota of ASD children (Yap et al., 2021). Furthermore, SCFAs are crucial for maintaining BBB integrity and essential during the process of brain development (Silva et al., 2020), with proper maintenance of the functional intestinal epithelial barrier (Peng et al., 2009). SCFAs like butyrate, propionate and acetate solely or in combination with others can stimulate the tight junctions (TJ) formation (Ohata et al., 2005; Peng et al., 2007, 2009). Especially butyrate regulates the formation of BBB (Nankova et al., 2014). Further, butyrate and propionate exert a strong epigenetic effect over-regulation of the gene expression profile of neurotransmitter systems, neuronal cell adhesion molecules, inflammation, mitochondrial function, oxidative stress, and lipid metabolism. All these have been reportedly associated with the development of ASD (Nankova et al., 2014). Lower SCFAs levels may disrupt epithelial barrier function in the intestine, and BBB thus increases the permeability as reported in ASD patients (Fiorentino et al., 2016). Decreased SCFAs levels in the gut can be related to the absence of potential SCFA-producing bacteria in ASD patient microbiota (Bojović et al., 2020). Assisting all these reports, lower Bifidobacterium sp. levels were found in children with autism (Wang et al., 2011).
4.4.2 Inflammation in ASDCytokines regulate immune response and are interestingly involved in neuronal development synaptic functions, differentiation, migration, proliferation, and behavioral impairments (Deverman and Patterson, 2009; Mousa and Bakhiet, 2013). Neuropoietic cytokines such as IL-6, IL-1β, and TNF-α have been reported to exert direct influence on the cortical neuron, dendrite development, neural activity, long-term potentiation, neurite outgrowth, regulation of synaptic plasticity in the hippocampus, overall neurodevelopment as well as behavior (Deverman and Patterson, 2009; Mousa and Bakhiet, 2013). Dysregulation in the cytokine levels creates disturbances in immune homeostasis, which potentially contribute to communication and behavioral impairment at the neuronal level in early childhood and, upon becoming severe, can give rise to NDDs like ASD (Goines and Ashwood, 2013; H. K.; Hughes et al., 2018). In children with ASD increased number of monocytes and plasma concentrations of IL-8, TNF-α, IL1β are observed, which indicate an abnormal inflammation condition (Rodríguez et al., 2017). Increased plasma concentration of IL-8 has been reported as a result of increased IL-17 released by activated Th17 cells in response to epithelial and endothelial infections (Suzuki et al., 2011). Immune dysfunction in ASD patients like abnormal T helper cell profile (Ashwood and Wakefield, 2006), increased concentration of complement factors (Corbett et al., 2007), pro-inflammatory interleukins (IL-1β, IL-6, IL-12p40) (Ashwood et al., 2011), TNF-α, and a decreased level of Transforming growth factor β (TGF-β) have been reported to cause acute inflammation analogous to ASD condition. Abnormal cytokine levels have been associated with poor health and communication, impaired social interaction, poor cognition/memory, and behavioral and neuronal dysfunction in ASD (Ashwood et al., 2011; Tye et al., 2019). Further, more studies conducted in children with ASD have revealed an elevated plasma level of IL-1β, IL-6, IL-17, IL-12p40, and IL-12p70, including both Th1 and Th2 cytokines, with down-regulation of IL-2 (Molloy et al., 2006; Jácome et al., 2016; Eftekharian et al., 2018). There also has been a report suggesting a sex-specific correlation of cytokine expression profile. According to which the mRNA expression level of TNF-α has been linked with expression of TGF-β, INF-γ, IL-17, and IL-6 in the case of males, whereas it's not the case with the females (Eftekharian et al., 2018). Besides, reports also show a difference in cytokine expression profile as per the disease severity in children with ASD. At a mildly severe condition, there is an elevation in plasma level of IL-12p40. In contrast, patients with moderate disease severity have higher plasma levels of TNF-α, which explains the correlation of disease severity with increased plasma concentration of TNF-α (Jácome et al., 2016; Xie et al., 2017).
In addition to all these inflammatory factors that contribute to ASD pathology, dysregulation in the maternal immune system during pregnancy has also been implicated in the development of ASD (Bilbo et al., 2018). Fetal brain reactive antibody, autoimmunity, or IgG antibody from mother can cross the placental barrier, thus entering into the fetal compartment where they can interfere with the developmental process by recognizing self-proteins. Also, in the fetus brain, the BBB is not fully formed or functional (Croen et al., 2008; Braunschweig and van de Water, 2012; Fiorentino et al., 2016). Therefore, the probability of immune-complexes and inflammatory mediators in action can reach the brain stem by crossing the BBB, contributing to microglial activation and neuronal inflammation eventually (Morgan et al., 2010; Fiorentino et al., 2016). Neuroinflammation within the CNS is also associated with severe disease conditions in ASD (Dipasquale et al., 2017; Eissa et al., 2020). Animal and human studies have suggested an elevated IL-6 expression in the autistic brain, causing anatomical aberration. Such overproduction of IL-6 can alter synapse formation and neurotransmission along with distorted patterns and distribution of dendritic spines (Wei et al., 2011, 2012, 2013). Moreover, the ASD animal model studies showed systemic inflammation with up-regulated IL-β, IL-6, IL-18, IL-33, IL-17, and TNF-α along with microglia activation and neuroinflammation principally contributing to the pathological mechanisms of ASD (Prata et al., 2017).
4.5 Attention-Deficit/Hyperactivity Disorder4.5.1 The Gut Microbiome in ADHDAttention-deficit/hyperactivity disorder is commonly characterized by persistent inattention and hyperactivity-impulsivity onset during childhood. Genetic factors (Faraone et al., 2005), streptococcal infection (Leslie et al., 2008), and environmental factors (Mulligan et al., 2013; Guney et al., 2015) have a close association with ADHD. However, dopamine (DA) deficiency and an altered level of norepinephrine (NE), serotonin, GABA has also been proposed for ADHD (Blum et al., 2008; Wilens and Spencer, 2010), including a decline in cortisol level, indicating disruption of the HPA-axis as well (Fortier et al., 2013). The microbiota has an important role in the development of the HPA-axis, and individuals with ADHD experience an imbalance in their microbiota and a reduced microbial diversity and composition, which resulted in lower levels of cortisol to cause improper management of the HPA-axis stress response (Huo et al., 2017; Checa-Ros et al., 2021). Children with ADHD unusually have a higher load of the family Bacteroidaceae, Neisseriaceae differing from a higher level of Prevotellaceae, Catabacteriaceae, and Porphiromonadaceae in the control group. An increased microbial load of family Neisseriaceae and Bacteroidaceae has been reported causing a significant decline in the gut microbial diversity in ADHD (Lacorte et al., 2019). At a family level, the ADHD-specific fecal microbiome represents a substantial rise in the richness of Moraxellaceae, Peptostreptococcaceae, Peptococcaceae, Xanthomonadaceae, and a firm decline in Alcaligenaceae (Jiang et al., 2018). While, at a genus level, Dialister, Faecalibacterium, Lachnoclostridium, and Sutterella were the major representatives that brought the differences among children with and without ADHD, respectively (Prehn-Kristensen et al., 2018). Besides, a certain group of gut microbiota can synthesize neuroactive monoamine molecules (dopamine, noradrenaline, serotonin, GABA) and its precursors (phenylalanine, tyrosine, tryptophan) that are involved in ADHD pathomechanism. These microbes also induce intestinal epithelial cells to synthesize neuroactive compounds and indirectly control the neurotransmission system to have a crucial impact on brain functioning and behavior (Strandwitz, 2018; Y.; Chen et al., 2021; Huang and Wu, 2021). In a study including 28 participants, functional magnetic resonance imaging (fMRI) revealed a nominal increase in the genus Bifidobacterium in ADHD (Hiergeist et al., 2020). This increase has a significant association with enhanced bacterial gene functionality that encodes the enzyme cyclohexadienyl dehydratase (Aarts et al., 2017), an enzyme involved in the phenylalanine (a dopamine precursor) synthesis pathway (Lou, 1994). Phenylalanine can cross the BBB and regulate dopamine synthesis positively or negatively by inhibiting tyrosine hydroxylase during dopamine synthesis (Lou, 1994). Dopamine deficiency can cause decreased neural responses to reward anticipation, which is one of the hallmarks of ADHD (Aarts et al., 2017). On the other hand, diminished Bifidobacterium population during early childhood has been correlated with a greater risk of dev
留言 (0)