Esophageal cancer (EC) is a malignant neoplasm of the gastrointestinal tract characterized by high morbidity and mortality rates. The etiology of EC is multifaceted and potentially linked to various factors, including poor dietary habits and genetic predisposition. EC exhibits an insidious onset, with minimal symptomatic manifestation in early stages, and is predominantly diagnosed in advanced stages, resulting in an overall poor prognosis with a 5-year survival rate of 15-25% (1, 2). Currently, surgical intervention remains the primary treatment modality for early-stage EC. The majority of patients eligible for direct surgery undergo comprehensive treatment, principally centered on surgical intervention (3). However, patients with locally advanced EC exhibit low R0 resection rates, frequently experience high recurrence and metastasis rates following surgical treatment alone, and do not achieve high 5-year survival rates (4). With advancements in surgical techniques and the incorporation of neoadjuvant therapies, the prognosis for patients with locally advanced EC has significantly improved. Relevant clinical studies have substantiated the safety and efficacy of neoadjuvant radiotherapy and neoadjuvant chemotherapy (5).
For locally advanced EC, neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy are common treatment options that can effectively enhance prognosis, efficacy, and confer survival benefits to patients (6). Furthermore, advancements in targeted and immunological drugs have provided new perspectives on neoadjuvant therapy selection (7). Current neoadjuvant treatment strategies encompass chemotherapy, radiotherapy, molecular-targeted therapy, immunotherapy, and other integrated approaches. However, the optimal selection among these options remains a subject of debate and necessitates further investigation.
Recent immunotherapy studies have demonstrated that the combination of chemotherapy and immunotherapy is more effective than chemotherapy alone in the first-line treatment of advanced EC (8). However, the efficacy and safety of immunotherapy combined with chemotherapy for neoadjuvant treatment of locally advanced EC remain controversial. While research has shown that immunotherapy significantly improves the 5-year survival rate in patients with advanced EC, there is limited data on neoadjuvant therapy for resectable locally advanced EC. This study aims to investigate the impact of neoadjuvant therapy, with or without immunotherapy, on the prognosis of patients with Stage II or III EC (T2-T4, N0-N+), providing a reference for clinical treatment decisions.
2 Materials and methods2.1 Subjects of the studyPatients with EC undergoing neoadjuvant therapy at Shandong Cancer Hospital between January 2021 and December 2023 were enrolled in this study. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) EC patients with American Joint Committee on Cancer 8th edition staging of Stage II or III (T2-T4, N0-N+) confirmed by histopathologic and imaging tests; (3) Eastern Cooperative Oncology Group performance status score of 0-2; (4) no contraindication to chemotherapy and receipt of at least one cycle of platinum-based agent with paclitaxel chemotherapy (21 days apart between each period); and (5) if neoadjuvant radiotherapy was received, the radiotherapy regimen was required to be Dt: 41.4 Gy, 1.8 Gy * 23f, 1.8 Gy/f, 5f/w. The exclusion criteria were (1) complicated severe organ diseases; (2) incomplete clinical data; and (3) unacceptable treatment toxicities or treatment delays. This study was approved by the ethics committee of Shandong Cancer Hospital (approval number: SDTHEC202412018). Informed consent was obtained from all patients.
2.2 General informationA retrospective study methodology was employed to gather patients’ demographic and clinical data, including age, sex, BMI, smoking and drinking status, primary tumor location, pathological type, chemotherapy cycles, treatment modality, initial clinical staging, and postoperative pathological staging. Follow-up assessments were conducted quarterly for the first two years post-treatment, then biannually thereafter, to determine survival status and time to fatal event, with follow-up extending to September 1, 2024. Overall survival (OS) was defined as the duration from diagnosis to death from any cause or the date of the last follow-up visit. Clinical benefits were categorized according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, encompassing complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
2.3 Statistical analysisFor data analysis, R 4.2.2 software was utilized. Count data were presented as frequency and percentage (n, %). Differences in the general clinicopathological data among different treatment benefit groups were evaluated using the chi-square test or Fisher’s exact test. Kaplan-Meier analyses were employed for survival assessments. To analyze independent risk factors affecting postoperative survival of patients with EC treated with various modalities, univariate and multivariate Cox regression analyses were conducted. Statistical significance was set at P < 0.05.
3 Results3.1 Baseline characteristicsA cohort of 175 patients with EC who had undergone previous neoadjuvant therapy was analyzed. The majority of patients were under 65 years old (100, 57.1%) and male (150, 85.7%). Treatment modalities included chemotherapy (36, 20.6%), immunotherapy combined with chemotherapy (89, 50.9%), chemoradiotherapy (34, 19.4%), and immunotherapy combined with chemoradiotherapy (16, 9.1%). Following neoadjuvant therapy, clinical benefit was assessed: 64 (36.6%) patients achieved CR, 70 (40%) PR, 33 (18.9%) SD, and 8 (4.6%) PD. These outcomes were determined based on pathologic analyses of tumor specimens post-surgery. CR was defined as the complete disappearance of all target lesions with no new lesions for at least 3 months. PR was characterized by a ≥ 30% decrease in the sum of target lesion diameters. PD was identified by the emergence of new lesions or at least a 20% increase in the sum of target lesion diameters. SD was classified as a reduction in the sum of the maximum diameter of target lesions that did not meet PR criteria or an increase that did not meet PD criteria. Table 1 presents the baseline characteristics of the patients.
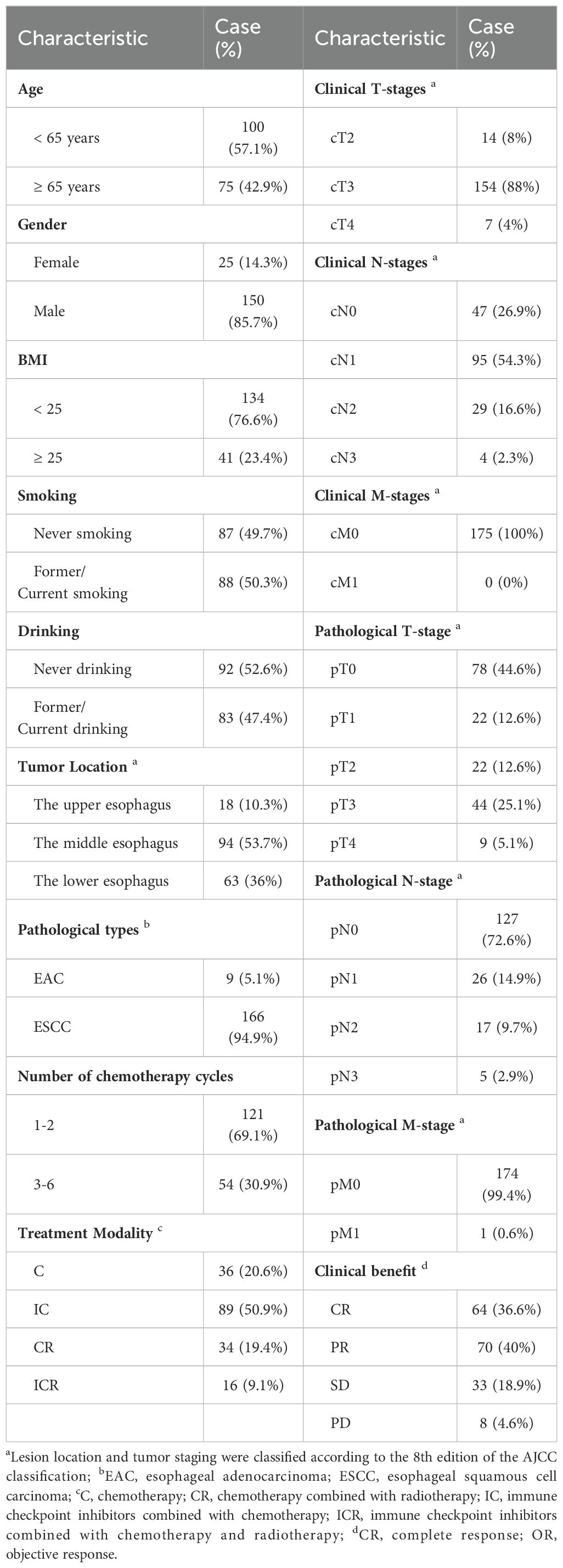
Table 1. Demographic, clinical and pathologic features of patients.
3.2 Analysis of differences in different clinical benefitsIn this study, OR was defined as CR plus PR. As presented in Table 2, among the 175 patients included in this study, 64 (36.6%) achieved CR and 134 (76.6%) achieved OR. Notably, CR was attained more frequently in patients younger than 65 years (P = 0.028) and in those diagnosed with esophageal squamous cell carcinoma (ESCC) (P = 0.027). The clinical N-stage demonstrated a statistically significant influence on OR achievement, with a higher proportion of N1 patients achieving OR (P < 0.001).
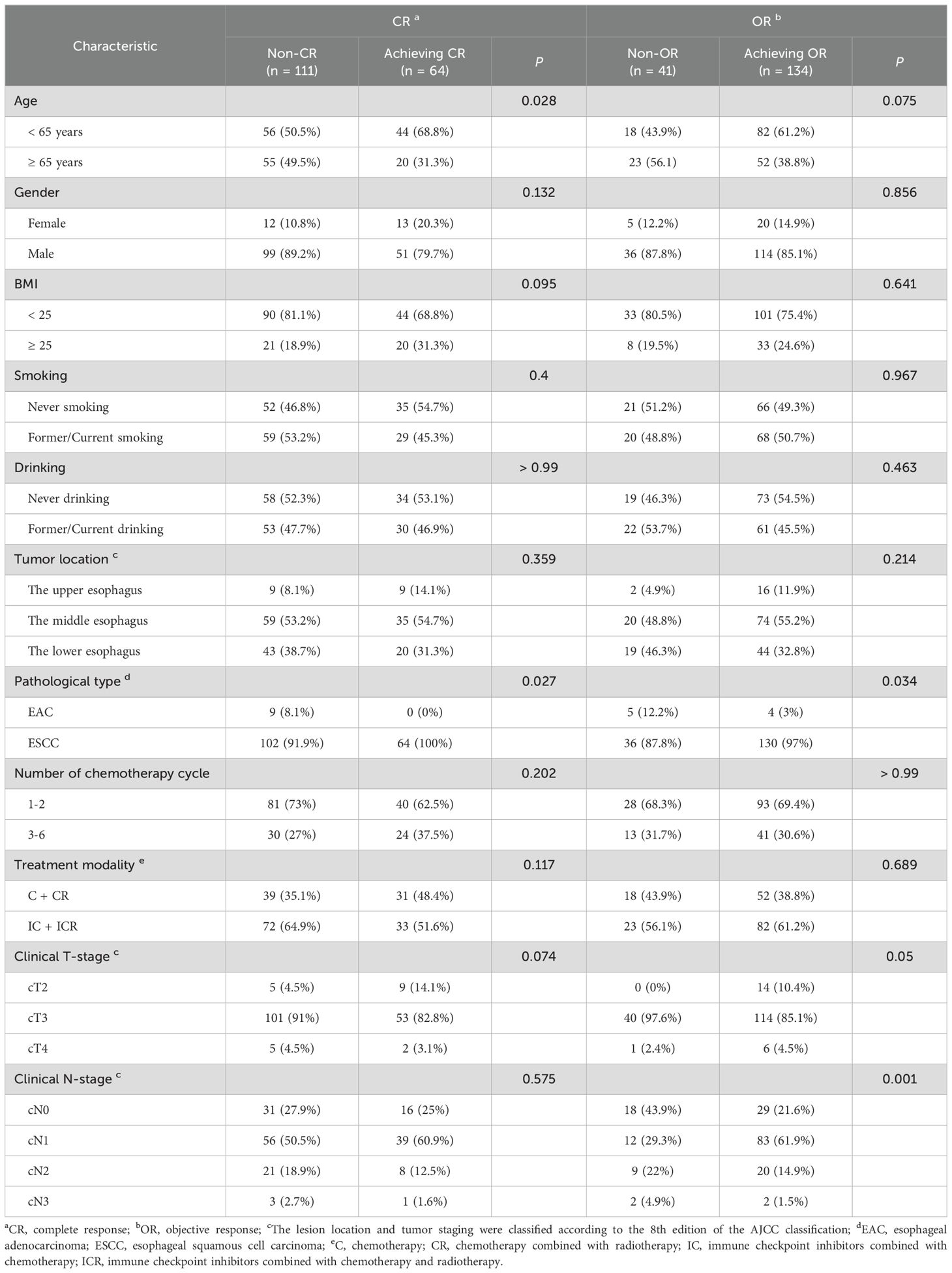
Table 2. The differences in clinical benefit among patients with diverse clinical characteristics.
Regarding therapeutic factors, 40 (62.5%) of the 64 patients who achieved CR received 1-2 cycles of neoadjuvant therapy. Additionally, 93 (69.4%) of the 134 patients who achieved OR received 1-2 cycles of neoadjuvant therapy. Among the 121 patients who received 1-2 cycles of treatment, 40 (33.1%) achieved CR and 93 (76.9%) achieved OR. Of the 54 patients who received 3-6 cycles of treatment, 24 (44.4%) achieved CR and 41 (75.9%) achieved OR. The number of neoadjuvant cycles did not demonstrate statistically significant differences in the achievement of CR or OR (P >0.05). Furthermore, 31 (48.4%) of the 64 patients who achieved CR did not receive immunotherapy during their neoadjuvant therapy regimen. Of the 134 patients who achieved OR, 52 (38.8%) did not receive immunotherapy during their neoadjuvant therapy regimen. In the group without combination immunotherapy (70 patients), 31 (44.3%) achieved CR and 52 (74.3%) achieved OR. Among the 105 patients in the combination immunotherapy group, 33 (31.4%) achieved CR and 82 (78.1%) achieved OR. The study found no statistically significant differences in the effects of combination immunotherapy on achieving CR and OR (P > 0.05).
3.3 Survival analysisSurvival analysis was conducted using the Kaplan-Meier method. As illustrated in Figure 1E, patients who abstained from alcohol consumption demonstrated improved OS compared to those with a history of or current alcohol use (P = 0.008). Interestingly, patients who did not undergo immunotherapy as part of their treatment regimen exhibited better survival rates than those who did (P = 0.002) (Figure 1I). Figure 1K reveals that regarding pretreatment N-staging, N3 patients had significantly poorer survival outcomes compared to N0 (P = 0.004), N1 (P = 0.003), and N2 (P = 0.003) patients. No statistically significant differences in OS were observed among patients with other varying general clinicopathological factors (P > 0.05).
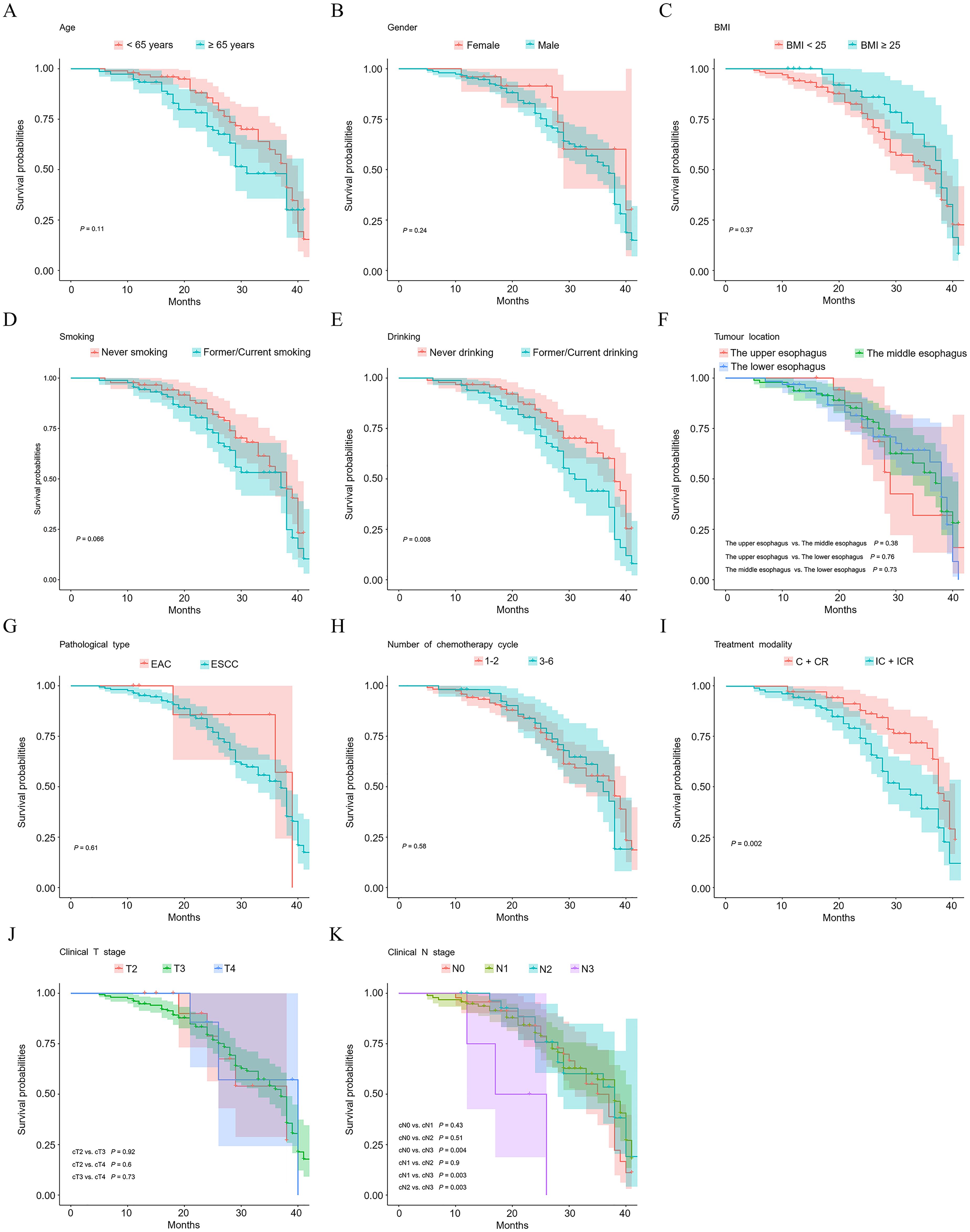
Figure 1. Kaplan–Meier plots of overall survival probability of esophageal cancer patients. Kaplan-Meier survival curves illustrate survivals of patients with different age (A), gender (B), BMI (C), smoking status (D), drinking status (E), tumour location (F), pathological type (G), number of chemotherapy cycle (H), treatment modality (I), clinical T stage (J), and clinical N stage (K).
To elucidate the influence of CR on overall survival in EC patients who underwent neoadjuvant therapy, statistical analyses were conducted. The findings indicated that CR following neoadjuvant therapy did not significantly affect the overall survival of the 175 patients studied (P = 0.92), as illustrated in Figure 2A. Furthermore, no substantial survival differences were observed in either subgroup of patients: those who did not received immunotherapy therapy as part of their neoadjuvant regimen (Figure 2B, P = 0.63) and those who did (Figure 2C, P = 0.24).
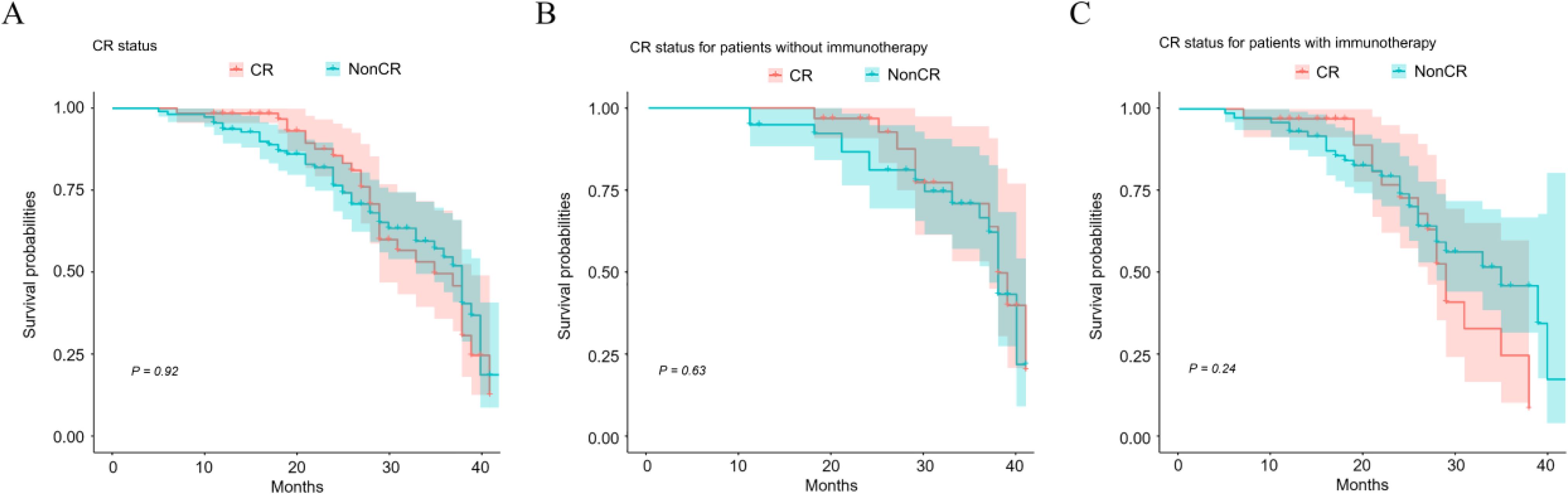
Figure 2. Impact of CR status on overall patient survival after neoadjuvant therapy. Kaplan-Meier survival curves illustrate survivals of patients with different CR status (A), CR status for patients without (B) or with immunotherapy (C).
3.4 Univariate and multivariate Cox analysesIn this study, univariate and multivariate Cox analyses were conducted to identify independent prognostic factors in patients receiving different treatment modalities. Table 3 presents the results of the univariate Cox analysis. Factors with P < 0.15 were included in the multivariate Cox analysis, and the results were visualized as forest plots. Figure 3A illustrates that among patients with EC after neoadjuvant therapy who did not receive immunotherapy, those with primary tumors located in the middle (HR, 0.181; 95%CI = 0.044-0.739; P = 0.017) and lower esophagus (HR, 0.163; 95%CI = 0.032-0.821; P = 0.028) had a better prognosis compared to patients with tumors in the upper esophagus. Importantly, among EC patients who received neoadjuvant therapy without combination immunotherapy, those who underwent 3-6 cycles of therapy demonstrated a worse prognosis than those who received 1-2 cycles of neoadjuvant therapy (HR, 2.731; 95%CI = 1.187-6.284; P = 0.018). This finding suggests that for patients receiving neoadjuvant chemotherapy or chemoradiotherapy, increasing the number of treatment cycles did not confer a survival benefit. Instead, it led to a worse prognosis. Furthermore, in EC patients who received neoadjuvant therapy containing immunotherapy, no significant effect of alcohol consumption and T-staging on patient prognosis was observed (Figure 3B).
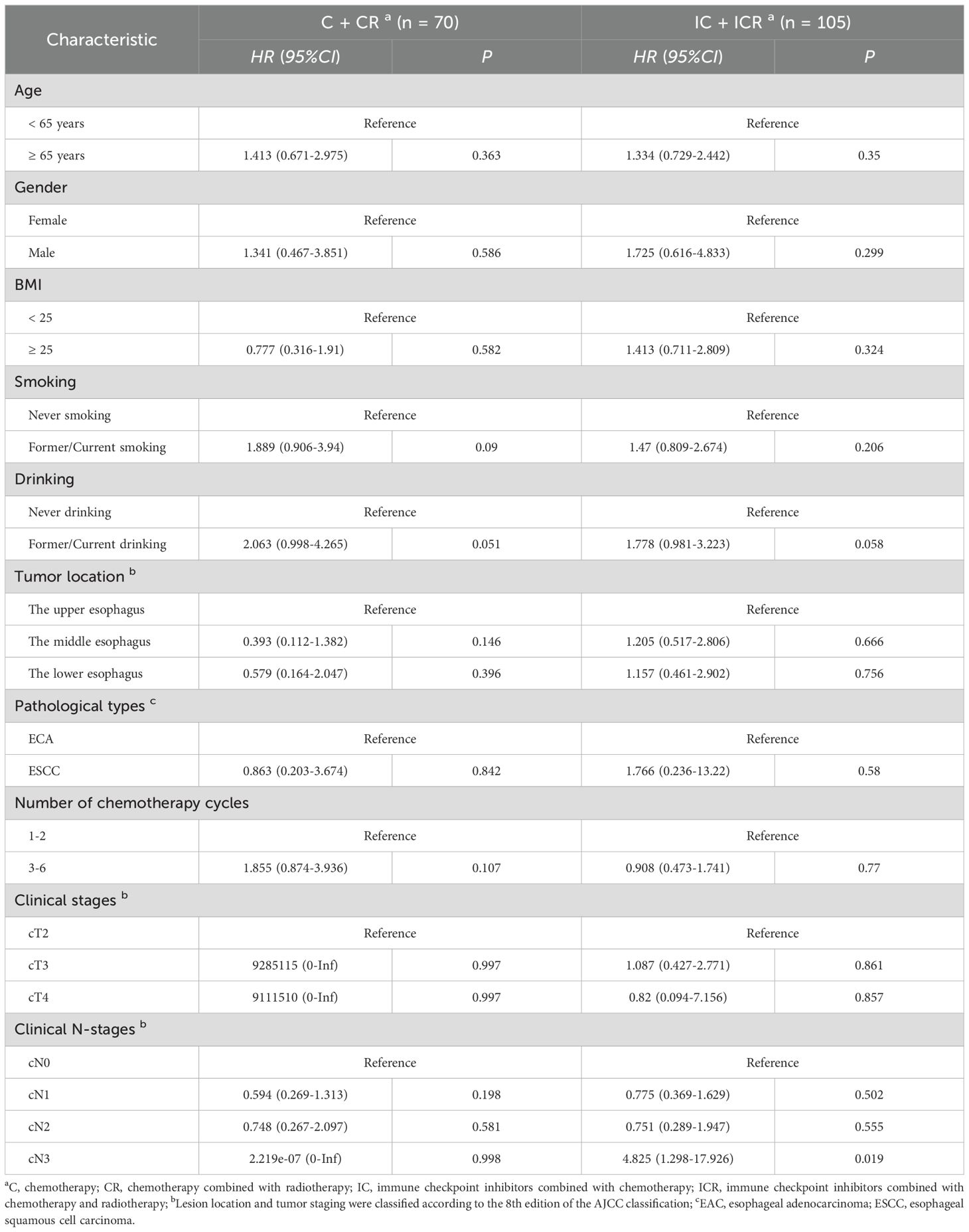
Table 3. Univariate Cox regression analysis of factors influencing patient prognosis across different treatment groups.

Figure 3. Multivariate cox regression analysis of the factors associated with overall survival of esophageal cancer patients. Forest plots from multivariate Cox regression analysis of neoadjuvant regimens in EC without (A) or with (B) combined immunotherapy.
4 DiscussionPatients with locally advanced EC often present with large tumors that are closely associated with surrounding tissues. In some cases, these tumors invade adjacent structures such as the aorta and thoracic duct, and may exhibit local lymph node metastasis. The complexity of these cases precludes direct surgical intervention, resulting in low R0 resection rates and poor long-term postoperative prognoses (9). In recent years, the widespread adoption of neoadjuvant therapy has significantly altered the treatment landscape for patients with locally advanced EC (10, 11). This study aims to further investigate the independent prognostic factors in EC patients following neoadjuvant therapy through retrospective analysis. The objective is to provide a reference basis for informed decision-making regarding EC neoadjuvant therapy regimens.
Multiple studies have confirmed that neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy are more efficacious than surgery alone, without increasing the incidence of perioperative complications (12). As immunotherapy has gained widespread clinical application for advanced EC, researchers have begun exploring its combination with chemotherapy in neoadjuvant treatment to enhance EC prognosis (13). However, consensus on optimal EC neoadjuvant therapy remains elusive. Research indicates that preoperative immunotherapy can activate the immune system, enhance tumor-specific T cell activity through tumor antigens, and elicit therapeutic responses in primary tumors and metastatic lesions (14). Nonetheless, this study did not demonstrate a significant difference in clinical benefit or OS between neoadjuvant therapy regimens with or without immunotherapy.
Yang et al. conducted a study involving 16 patients with locally advanced ESCC who underwent neoadjuvant therapy. The treatment consisted of two cycles of carilizumab combined with a TC regimen of chemotherapy (paclitaxel plus carboplatin), followed by surgery 4 weeks after completion. Their findings indicated an objective remission rate of 81.3% and a CR rate of 31.3% following neoadjuvant therapy (15). Similarly, in the present study, 36.6% of patients achieved CR and 76.6% achieved OR after receiving neoadjuvant therapy. Research has shown that immunotherapy can suppress tumor angiogenesis and enhance the body’s anti-tumor response, while chemotherapy can amplify this effect (16). Furthermore, immunotherapy may augment the cytotoxic impact of chemotherapy on tumor cells by increasing chemosensitivity in patients with locally advanced EC (17). In this study, 44.3% of patients in the non-combination immunotherapy group and 31.4% in the combination immunotherapy group achieved CR. Although the CR rate was higher in the non-combination immunotherapy group, no statistically significant difference was observed in the effect of the combination immunotherapy regimen on achieving CR within the treatment modality. This outcome may be attributed to the fact that 28.6% of patients in this study received synchronized radiotherapy. Radiotherapy plays a crucial role in EC treatment and has consistently held a significant position in neoadjuvant therapy for EC (18, 19). Neoadjuvant chemotherapy and radiotherapy work synergistically, not only controlling local tumors but also addressing other hidden foci, thereby reducing the risk of disease recurrence and ultimately improving patient survival rates.
Neoadjuvant regimens for EC are widely utilized in clinical practice, with variations in treatment protocols and cycle numbers, including weekly regimens and 21-day cycles (20, 21). The number of neoadjuvant therapy cycles a patient receives depends on both the lesion’s response to treatment and the clinician’s decision-making process. In this study, 121 (69.1%) patients received 1-2 cycles of neoadjuvant chemotherapy, while 54 (30.9%) received 3-6 cycles. Multivariate Cox analysis results indicated that among EC patients who underwent neoadjuvant therapy without immunotherapy, those receiving 3-6 cycles had a poorer prognosis compared to those receiving 1-2 cycles. This suggests that for patients undergoing neoadjuvant chemotherapy or chemoradiotherapy, an increase in treatment cycles may negatively impact prognosis. However, this study did not find that the number of neoadjuvant therapy cycles affected the prognosis of EC patients receiving neoadjuvant therapy that included immunotherapy.
Recent years have witnessed continuous advancements in EC neoadjuvant therapy. Research has demonstrated that this approach not only effectively eliminates subclinical metastatic foci and reduces tumor stage, but also enhances surgical resection rates while minimizing the risk of tumor implantation and metastasis, thereby conferring survival benefits to EC patients (22, 23). Our findings indicate that following neoadjuvant therapy, a higher proportion of patients under 65 years of age or with ESCC pathology achieved CR. Notably, we observed that patients who did not receive immunotherapy in their treatment regimen exhibited better survival outcomes compared to those who did. Furthermore, among post-neoadjuvant EC patients not receiving immunotherapy, those undergoing 1-2 cycles of neoadjuvant therapy demonstrated a more favorable prognosis than those receiving 3-6 cycles. However, the present study has some limitations because it is a single-center retrospective analysis. Future prospective multicenter studies are required to corroborate our findings to further clarify the prognosis of different neoadjuvant treatment regimens for EC and the differences in survival among patients with different clinicopathological factors. Such investigations will facilitate more standardized and personalized treatment approaches, ultimately enhancing patients’ quality of survival.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsJD: Formal Analysis, Software, Validation, Writing – original draft, Writing – review & editing. CL: Data curation, Methodology, Writing – original draft, Writing – review & editing. BW: Investigation, Validation, Writing – original draft, Writing – review & editing. YL: Data curation, Software, Writing – original draft, Writing – review & editing. SW: Supervision, Validation, Writing – original draft, Writing – review & editing. HC: Data curation, Validation, Writing – original draft, Writing – review & editing. MG: Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by National Natural Science Foundation of China (62273329), Medical Science and Technology Project from Department of Health of Shandong Province (202209030779), and Wu Jieping Medical Foundation (320.6750.2022-09-54, 320.6750.2022-18-41 and 320.6750.2024-17-23).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Liu M, Yang W, Guo C, Liu Z, Li F, Liu A, et al. Effectiveness of endoscopic screening on esophageal cancer incidence and mortality: A 9-year report of the endoscopic screening for esophageal cancer in China (ESECC) randomized trial. J Clin Oncol. (2024) 42:1655–64. doi: 10.1200/JCO.23.01284
PubMed Abstract | Crossref Full Text | Google Scholar
2. Su X, Fu C, Liu F, Bian R, Jing P. T-cell exhaustion prediction algorithm in tumor microenvironment for evaluating prognostic stratification and immunotherapy effect of esophageal cancer. Environ Toxicol. (2024) 39:592–611. doi: 10.1002/tox.23887
PubMed Abstract | Crossref Full Text | Google Scholar
3. Heinrich K, Heinemann V, Stintzing S, Müller L, Ettrich TJ, Büchner-Steudel P, et al. Adjuvant treatment with S-1 in patients after R0-resection of adenocarcinoma of the stomach and esophagogastric junction: A multicenter phase I/II feasibility study (GMBH-STO-0114). Oncol Res Treat. (2024) 47:251–61. doi: 10.1159/000538143
PubMed Abstract | Crossref Full Text | Google Scholar
4. Deboever N, Jones CM, Yamashita K, Ajani JA, Hofstetter WL. Advances in diagnosis and management of cancer of the esophagus. BMJ. (2024) 385:e074962. doi: 10.1136/bmj-2023-074962
PubMed Abstract | Crossref Full Text | Google Scholar
5. Tang H, Wang H, Fang Y, Zhu JY, Yin J, Shen YX, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol. (2023) 34:163–72. doi: 10.1016/j.annonc.2022.10.508
PubMed Abstract | Crossref Full Text | Google Scholar
6. Yang Z, Guan F, Bronk L, Zhao L. Multi-omics approaches for biomarker discovery in predicting the response of esophageal cancer to neoadjuvant therapy: A multidimensional perspective. Pharmacol Ther. (2024) 254:108591. doi: 10.1016/j.pharmthera.2024.108591
PubMed Abstract | Crossref Full Text | Google Scholar
7. Yan Y, Feng X, Li C, Lerut T, Li H. Treatments for resectable esophageal cancer: from traditional systemic therapy to immunotherapy. Chin(Engl). (2022) 135:2143–56. doi: 10.1097/CM9.0000000000002371
PubMed Abstract | Crossref Full Text | Google Scholar
8. Chen W, Cao K, Zhang L, Zhao X, Chen B, Li W, et al. Efficacy and safety evaluation of frontline immunotherapy combinations in advanced esophageal squamous cell carcinoma: a network meta-analysis highlighting the value of PD-L1 expression positivity scores. Front Immunol. (2024) 15:1414753. doi: 10.3389/fimmu.2024.1414753
PubMed Abstract | Crossref Full Text | Google Scholar
9. Stahl M, Maderer A, Lordick F, Mihaljevic AL, Kanzler S, Hoehler T, et al. Perioperative chemotherapy with or without epidermal growth factor receptor blockade in unselected patients with locally advanced oesophagogastric adenocarcinoma: Randomized phase II study with advanced biomarker program of the German Cancer Society (AIO/CAO STO-0801). Eur J Cancer. (2018) 93:119–26. doi: 10.1016/j.ejca.2018.01.079
Crossref Full Text | Google Scholar
10. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. (2021) 156:721–9. doi: 10.1001/jamasurg.2021.2373
PubMed Abstract | Crossref Full Text | Google Scholar
11. Shang X, Xie Y, Yu J, Zhang C, Zhao G, Liang F, et al. A prospective study of neoadjuvant pembrolizumab plus chemotherapy for resectable esophageal squamous cell carcinoma: The Keystone-001 trial. Cancer Cell. (2024) 42:1747–1763.e7. doi: 10.1016/j.ccell.2024.09.008
PubMed Abstract | Crossref Full Text | Google Scholar
15. Yang P, Zhou X, Yang X, Wang Y, Sun T, Feng S, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol. (2021) 19:333. doi: 10.1186/s12957-021-02446-5
PubMed Abstract | Crossref Full Text | Google Scholar
16. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. (2014) 21:15–25. doi: 10.1038/cdd.2013.67
PubMed Abstract | Crossref Full Text | Google Scholar
17. Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med. (2018) 283:110–20. doi: 10.1111/joim.12708
PubMed Abstract | Crossref Full Text | Google Scholar
18. Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: A randomized clinical trial. JAMA Surg. (2021) 156:444–51. doi: 10.1001/jamasurg.2021.0133
PubMed Abstract | Crossref Full Text | Google Scholar
19. Zhang C, Zhang G, Sun N, Zhang Z, Xue L, Zhang Z, et al. An individualized immune signature of pretreatment biopsies predicts pathological complete response to neoadjuvant chemoradiotherapy and outcomes in patients with esophageal squamous cell carcinoma. Signal Transduct Target Ther. (2020) 5:182. doi: 10.1038/s41392-020-00221-8
PubMed Abstract | Crossref Full Text | Google Scholar
20. Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. (2021) 39:1995–2004. doi: 10.1200/JCO.20.03614
PubMed Abstract | Crossref Full Text | Google Scholar
21. Lagarde SM, Phillips AW, Navidi M, Disep B, Immanuel A, Griffin SM. The presence of lymphovascular and perineural infiltration after neoadjuvant therapy and oesophagectomy identifies patients at high risk for recurrence. Br J Cancer. (2015) 113:1427–33. doi: 10.1038/bjc.2015.354
PubMed Abstract | Crossref Full Text | Google Scholar
22. van der Wilk BJ, Eyck BM, Hofstetter WL, Ajani JA, Piessen G, Castoro C, et al. Chemoradiotherapy followed by active surveillance versus standard esophagectomy for esophageal cancer: A systematic review and individual patient data meta-analysis. Ann Surg. (2022) 275:467–76. doi: 10.1097/SLA.0000000000004930
PubMed Abstract | Crossref Full Text | Google Scholar
23. Mantziari S, Gronnier C, Renaud F, Duhamel A, Théreaux J, Brigand C, et al. Survival benefit of neoadjuvant treatment in clinical T3N0M0 esophageal cancer: results from a retrospective multicenter european study. Ann Surg. (2017) 266:805–13. doi: 10.1097/SLA.0000000000002402
留言 (0)