Transsphenoidal resection is the primary treatment approach for pituitary adenomas (PA) and other tumors near the sella turcica. However, the success rate of surgical procedures varies significantly across studies, as do the associated complication rates. Twenty-five years ago (1), a higher success rate for the surgical treatment of GH-secreting adenomas was reported in patients operated by a single experienced neurosurgeon. Furthermore, these outcomes improved with increasing neurosurgical experience (2). Subsequent studies have corroborated these findings in the context of acromegaly (3, 4) and also in Cushing’s disease (CD) and prolactinomas, with a significantly higher remission rate in centers performing more than ten operations per year for CD (5).
The rate of tumor recurrence at five years after TSS is largely reduced from 44% to 4% in patients with non-secreting pituitary adenomas (NSPA) who have undergone gross total resection (GTR) of the tumor (6). It is increasingly recognized that GTR in NSPA is more frequently achieved through the endoscopic approach compared to the traditional microscopic TSS (7). A study conducted in 2016 demonstrated that even a surgeon with limited experience can achieve comparable outcomes to those of highly experienced surgeon using a microscopic technique, when utilizing endoscopic methods in a cohort of patients with NSPA (8). Moreover, the rate of GTR in NSPA was found to improve with the surgeon’s experience (9).
The complication rate of TSS depends on the annual volume of operations performed by a neurosurgeon and his completion of a learning curve (10). In the last century, some reports suggested a minimum learning curve of 200 operations for microscopic TSS (11) which has been reduced to 40-50 surgeries with the use of endoscopy (12, 13).
The proposal to establish Pituitary Tumors Centers of Excellence (PTCOE) as the ideal model for managing pituitary pathology (14) has received widespread support. Recently, core criteria for the PTCOE accreditation process have been published (15). These criteria include an annual volume of 100 pituitary surgeries per center, mainly TSS. More recently, a large study of 1149 patients in nine PTCOE showed optimal rates for complications of TSS in pituitary adenoma (16).
Despite the growing body of evidence supporting the establishment of PTCOE and the impact of surgical experience on TSS outcomes, there is a lack of comprehensive data on TSS outcomes for PA in Spain. While several countries have published their results in line with PTCOE criteria (17), the situation in Spain remains largely underreported. This absence of data hinders the evaluation of surgical outcomes, the understanding of complication rates, and the potential benefits of centralizing care in specialized centers.
Thus, our aims were to evaluate the rate of successful TSS and the incidence of surgical complications in a large multicenter series in Spain. Considering the influence of surgical experience, case volume and surgical specialization on these outcomes. This will provide a clearer picture of how TSS outcomes align with international benchmarks. Additionally, these findings may serve as a foundation for policy recommendations to optimize pituitary tumor management nationwide.
2 Materials and methods2.1 Study design and participantsWe evaluated the results of TESSPAIN (TranssphEnoidal Surgery in SPAIN), a retrospective, multicenter, nationwide project that included all the TSS that were performed in the 29 participating centers from January 1, 2018, to December 31, 2022. The study was endorsed by the Spanish Society of Endocrinology and Nutrition (SEEN) and distributed to all members of the SEEN Neuroendocrinology Task Force, which includes most of the endocrinologists who take care of these patients in Spain. The study was reviewed and approved on May 24, 2023, by the Regional Ethics Committee of the Basque Country (CEIm-E) (PI2023077). The study was conducted in accordance with the requirements of the Declaration of Helsinki and good clinical practice. Due to the retrospective nature of the study, patient consent was waived.
For each patient, the coded identity of the neurosurgeon, tumor type, year of surgery, therapeutic goal and outcome, and postoperative complications were recorded.
Six types of lesions were identified: Clinically non-secreting pituitary adenoma (NSPA); Growth hormone-secreting pituitary adenoma, including those cosecreting prolactin or other hormones (ACRO); Cushing’s disease (CD); prolactin-secreting adenoma (PRLoma); thyroid-stimulating hormone-secreting adenoma (TSHoma); and other non-adenomatous tumors, mainly craniopharingiomas, meningiomas, chordomas, and Rathke’s cleft cysts (OTHER). ACRO, CD, PRLoma, and TSHoma were evaluated globally as Secreting Pituitary Adenoma (SPA).
Centers that were accredited in Spain during the study period (referred to as CSUR centers in Spain) and those performing more than 25 surgeries per year were classified as high-volume (HV) centers. These HV centers were compared with those not meeting these criteria, referred to as non-high-volume (non-HV) centers.
In Spain, CSUR centers meet several specific requirements (see footnote), including performing more than 20 pituitary surgeries annually over the past three years, having a multidisciplinary team, managing more than 250 patients with pituitary diseases regularly attended, and conducting teaching and research activity, among others.
HV centers with a dedicated neurosurgeon who performed more than 75% of all TSS or with a stable team of up to 3 dedicated neurosurgeons were compared with HV centers without a dedicated neurosurgeon or without an established team.
2.2 Definitions and objectivesSurgical success for SPA was defined according to the published remission criteria for each disease: age normalized serum IGF-1 value and a random GH <1.0 µg/L for ACRO (18); postoperative basal cortisol less than 5 µg/dl and adrenal insufficiency requiring steroid replacement for more than 3 months for CD (19); serum prolactin less than 10 ng/ml for PRLoma, recently reported as associated with low recurrence (20), and resolution of hyperthyroidism for TSHoma. For NSPA, surgical success was considered when GTR was confirmed on the MRI three to six months after TSS. At each participating center, the assessment of GTR for NSPA was performed locally. MRIs were reviewed by neuroradiology teams as part of routine follow-up at three to six months postoperatively. These teams included experienced neuroradiologists with expertise in pituitary imaging. While MRI review was not centralized, all centers adhered to established criteria for reporting residual tumor to ensure consistency of assessment (21).
The following surgical complications were evaluated: postoperative cerebrospinal fluid (CSF) leak, reoperation for bleeding or CSF leak, infection, permanent anterior pituitary hormone or arginine vasopressin deficiency (AVPD) persisting beyond 6 months of surgery, venous thromboembolism, cerebrovascular accident, cranial oculomotor nerve or optic nerve injury, death, and other (including pneumocephalus and vasospasm). New anterior pituitary deficiencies were based on the prescription of new hormonal replacement therapy after surgery, without specifying either the number of axes or which ones.
Both outcomes, surgical success and surgical complications, were evaluated based on whether the hospital met or not the HV criteria. For HV centers, the impact of having a dedicated neurosurgical team (DNT) on these outcomes was also evaluated.
In addition, adenomas retrospectively deemed resectable (R-PA) were evaluated for surgical success and complications. To qualify as resectable, those PA with preoperative advanced cavernous sinus invasion (Knosp 3b or 4) documented in the radiologic report or after the revision of the preoperative MRI (if not previously specified), were excluded.
2.3 Statistical analysesCategorical variables were expressed as absolute values (n) and percentages (%), while quantitative data were presented as mean ± standard deviation or median and interquartile range for non-normally distributed parameters. Success rate and complication rate were the main outcomes. Outcomes were calculated as percentages with total numbers and reported with 95% confidence intervals calculated using data weighted by the number of cases operated in each center. Outcomes were compared using the chi-squared test or the Fisher´s exact test to evaluate the influence of being treated in an HV center versus not being treated in an HV center on both success and complication rates, and to assess the effect of having a dedicated neurosurgical team between HV centers with and without a dedicated neurosurgical team. Spearman’s coefficient (Rho) was calculated to describe the correlation between total TSS and number of TSS for PA at each center with success rate. The correlation between surgical volume and complication rates at each center and the correlation between success rates and complication rates at each center were also evaluated using this coefficient. Statistical analyses were performed using IBM SPSS Statistics version 27.0.
3 Results3.1 Population studyA total of 2815 TSS procedures were performed at the 29 study centers. The most frequent PA type was NSPA (n=1421; 50.5%), followed by SPA (n=911; 32.4%): 436 patients with ACRO, 323 patients with CD, 127 patients with PRLoma, and 25 operated for TSHoma. The remaining 483 TSS (17.1%) were performed for non-adenomatous pituitary tumors, usually with extrasellar extension, mainly for craniopharyngioma, Rathke´s cleft cyst, meningioma, chordoma, and others (Figure 1). More than 80% of SPAs were considered R-PA by the referring endocrinologists, with the exception of PRLomas where only 56.7% were considered resectable.
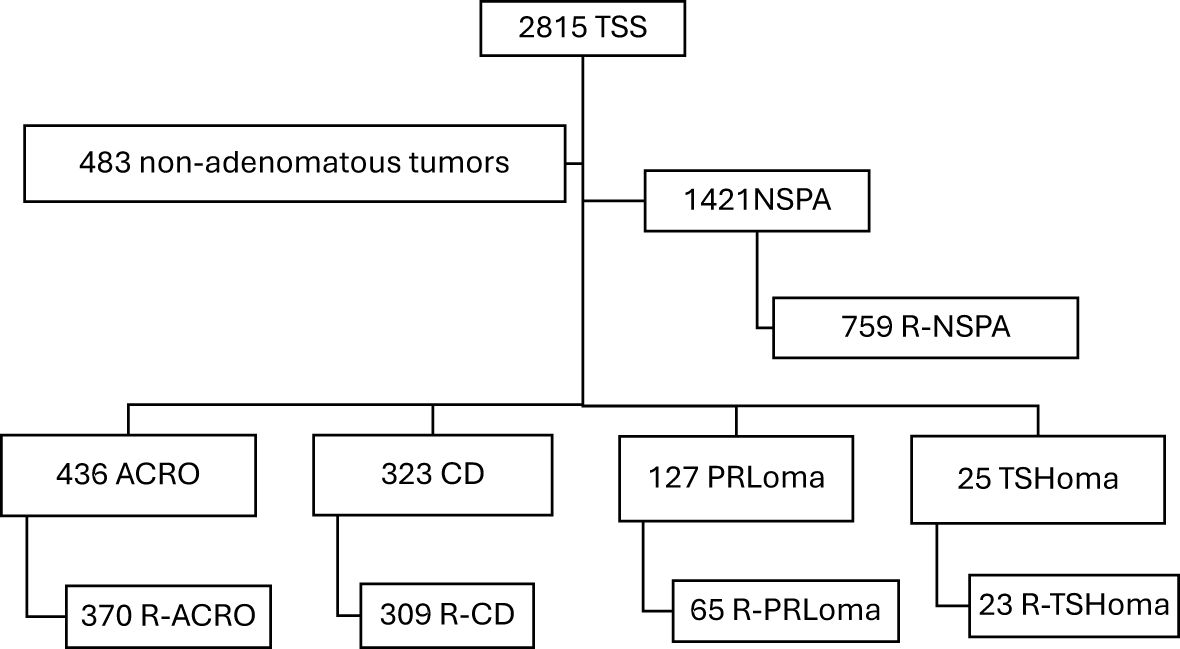
Figure 1. Flowchart of the patients included in the TESSPAIN Registry.
Nine centers had the recognition as CSUR, and these, along with two others performing more than 25 TSS/year formed the HV group. This group had performed a higher percentage of TSS on non-adenomatous tumors and R-NSPA than the non-HV group, and HV group also had a lower percentage of R-ACRO than non-HV group. Table 1 shows the details of the included cases and their comparison in both groups.
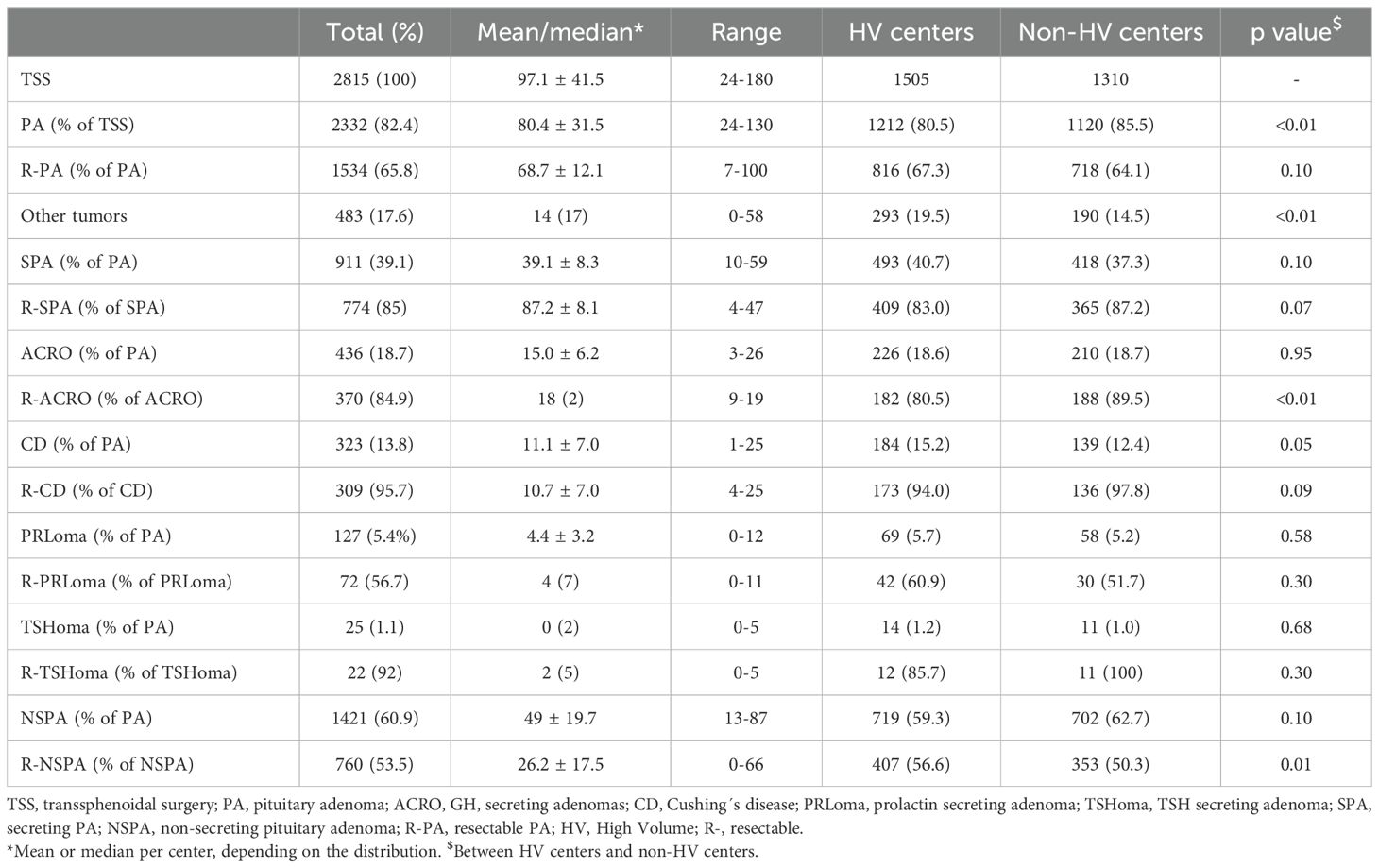
Table 1. Number of cases and comparison divided by the expertise of the operating center.
Six of the eleven HV centers had a dedicated neurosurgical team, while five of them had more than three neurosurgeons, and none of them performed more than 75% of the TSS.
3.2 Success rate of TSSThe overall success rate of TSS for PA was 50.5%, increasing to 76.8% for R-PA. TSS success for each PA subtype increased when considered for R-PA separately, particularly for PRLoma and NSPA. TSH-secreting adenomas had the highest success rate, 88% (CI95: 75.7-100%), increasing to 95.6% for R-TSHoma. These results are shown in Table 2.
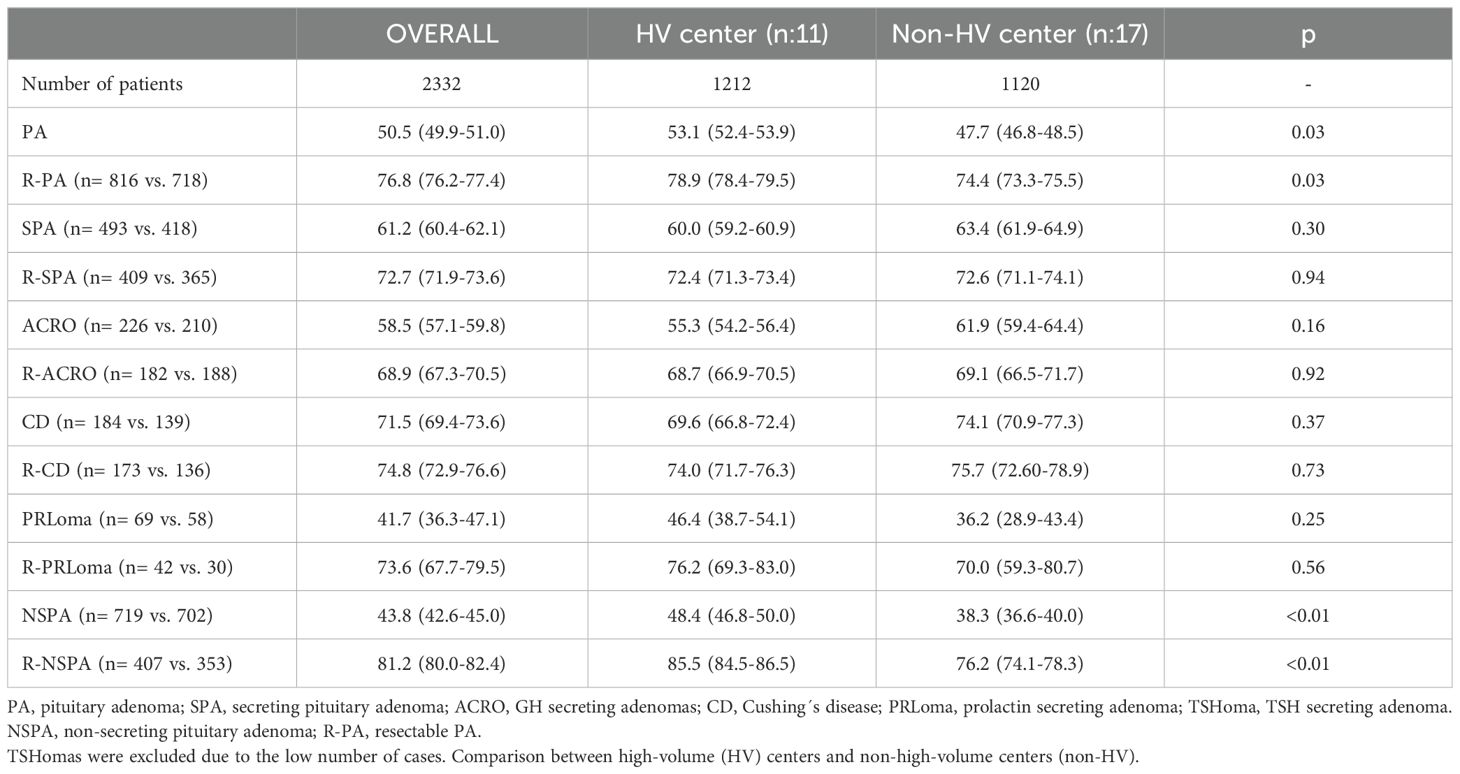
Table 2. Success rates (95% CI) of transphenoidal surgery for PA.
The overall success rate of TSS for PA was significantly higher in HV centers than in non-HV centers (53.1 vs 47.7%; p=0.03). Better outcomes with TSS for NSPA accounted for this difference. This difference persisted when including only R-PA, despite a higher percentage of NSPA considered amenable to GTR in HV centers (Table 1). The difference in SPA did not reach statistical significance for any subtype, either overall or for R-PA (Table 2).
Linear correlation analysis showed a positive correlation between the global success rate in PA and the number of TSS procedures at each center (Rho: 0.49; p <0.01; Figure 2), mainly driven by this correlation in R-NSPA (Rho: 0.57; p: <0.01). The success rate in SPA showed only a weak positive correlation with the number of TSS for PRLoma (Rho: 0.38; p: 0.04).
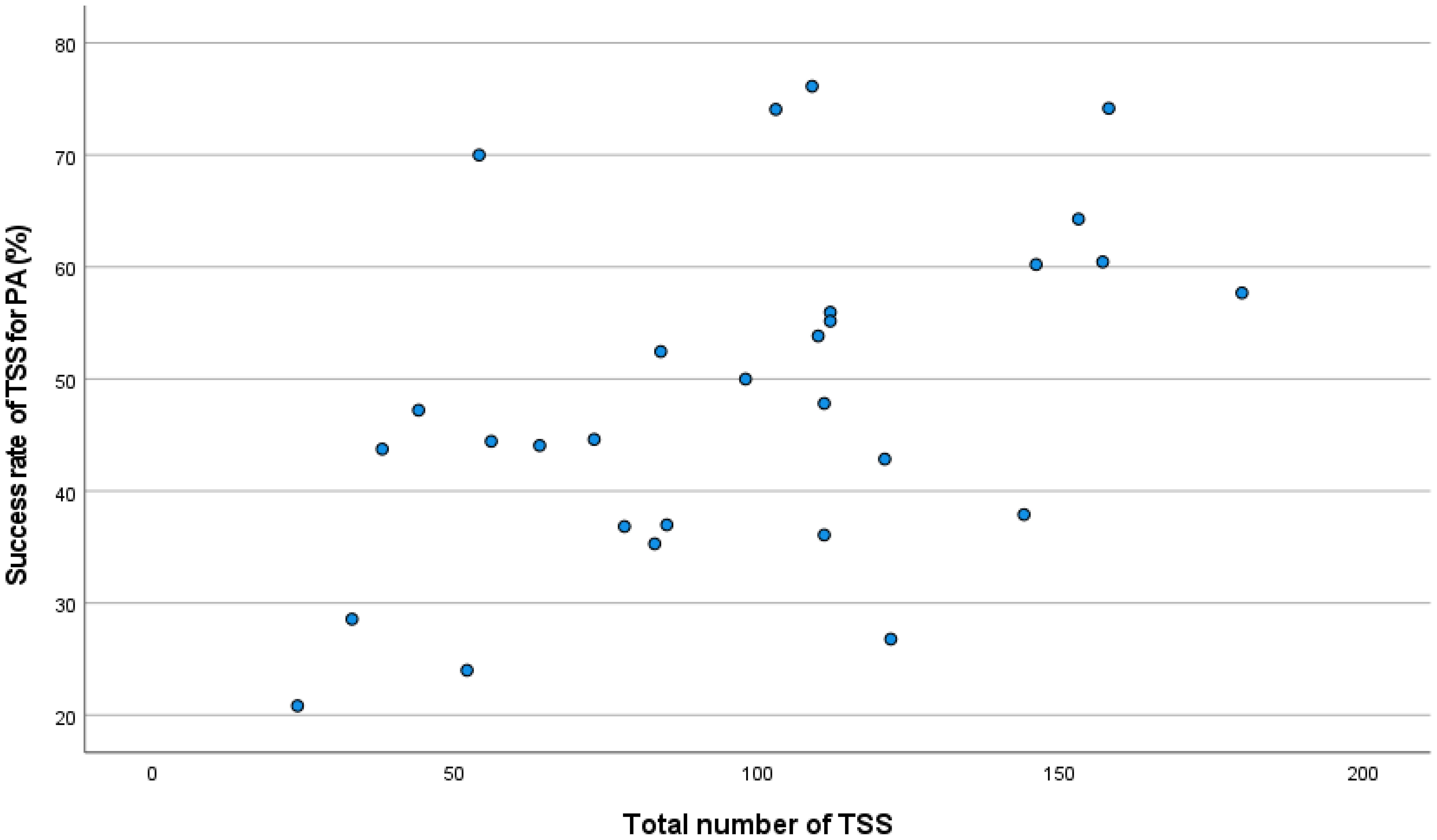
Figure 2. Correlation between total TSS and success rate in PA.
3.3 Complications of TSSThe overall surgical complication rate of TSS for all PA was 22.1%, which was higher for NSPA compared to SPA (25.0% vs. 17.7%; p<0.001). The complication rate ranged from 0 to 42.9% in R-SPA. Only one of the five centers with no complications performed more than 10 TSS procedures, corresponding to a HV center with a DNT. The complication rate in R-NSPA was 0% in only one center, which performed 12 cases during the study period. Persistent AVPD occurred in 5.4%, CSF leak in 3.1% and reoperation for complications in 2.8% of all TSS for PA. For SPA, the mean complication rate for R-SPA was not significantly different from the mean complication rate for non-resectable adenomas (18.5% vs. 13.1%; p: 0.13), while this rate was lower for TSS for ACRO than for TSS for CD (13.1% vs. 24.1%; p: 0.01), even when only R-PA were considered (13.5% vs. 24.3%; p<0.001). Complications were more common in TSS for NSPA in non-resectable adenomas compared to R-NSPA (28.1% vs. 22.2% p: 0.01). The mean rate of damage to anterior pituitary function was 14.7% but showed a wide range between centers from 0 to 37.1%. There was a positive correlation between the rate of postoperative anterior pituitary deficiencies for NSPA and the rate of NSPA deemed resectable (Rho: 0.386; p<0.001).
The overall complication rate of TSS for PA was significantly higher in non-HV centers than in HV centers (24.0% vs 20.4%; p<0.01). The difference did not reach statistical significance for any subtype of PA, neither overall nor for R-PA. Ten out of the 2332 patients who underwent TSS for PA died in the postoperative period (surgical mortality for adenomas: 0.4%). The mortality rate was significantly higher in non-HV centers than in HV centers, but with a small number of cases (2 deaths vs. 8 deaths). These results are presented in Table 3.
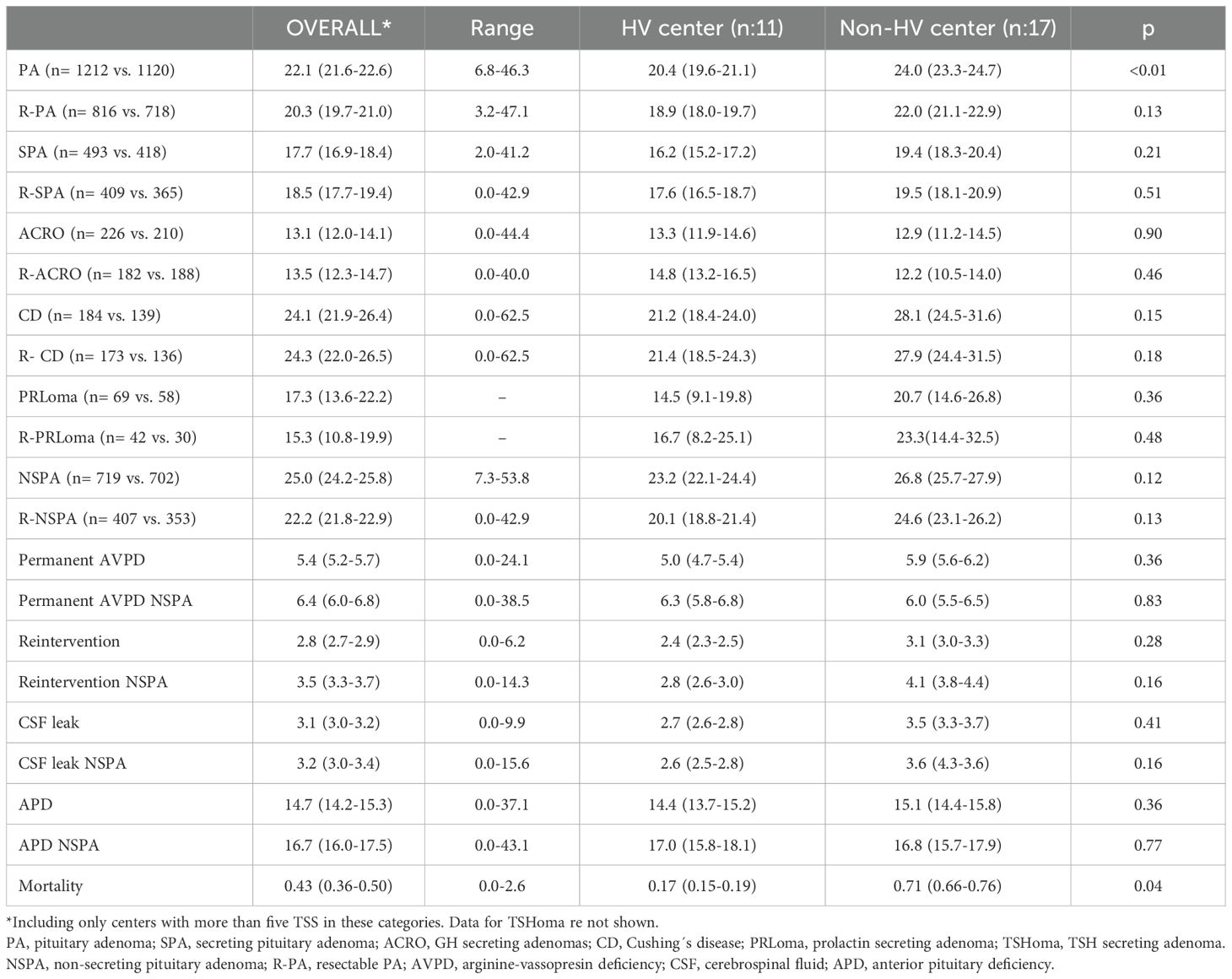
Table 3. Global complication rates (95% CI) of transsphenoidal surgery for PA and comparison between high-volume (HV) centers and non-high-volume centers (non-HV).
Linear correlation analysis showed no positive or negative correlation between the overall complication or AVPD rate in PA and the number of TSS procedures in each center. Neither the total number of TSS nor the number of TSS for PA in each center had any correlation with the complication rate in all groups (divided by tumor type or complication type). However, a correlation was found between the global success rate in PA and the complication rate in R-PA (Rho: 0.499; p: 0.006). This positive correlation with global success rate remained significant when R-SPA (Rho: 0.454; p: 0.006) and R-NSPA (Rho: 0.488; p: 0.008) were evaluated separately.
3.4 Influence of a dedicated neurosurgical team in HV centersThe six HV centers with a DNT operated on 712 pituitary adenomas, of which 60.9% were NSPA (n: 434) and 48.4% (n: 210) were considered to be R-NSPA. The five HV centers without a DNT operated 500 pituitary adenomas, of which 57% were NSPA (n: 285) and 69.1% (n: 197) R-NSPA, significantly higher rate than in centers with a DNT (p<0.001). The success rate for PA was significantly higher in centers without a DNT due to a higher rate of GTR in NSPA. However, this difference disappeared when only R-NPSA were included. Success rate was higher without statistical significance for all SPA types except PRLoma, with greater difference for R-SPA. These data are shown in Table 4.
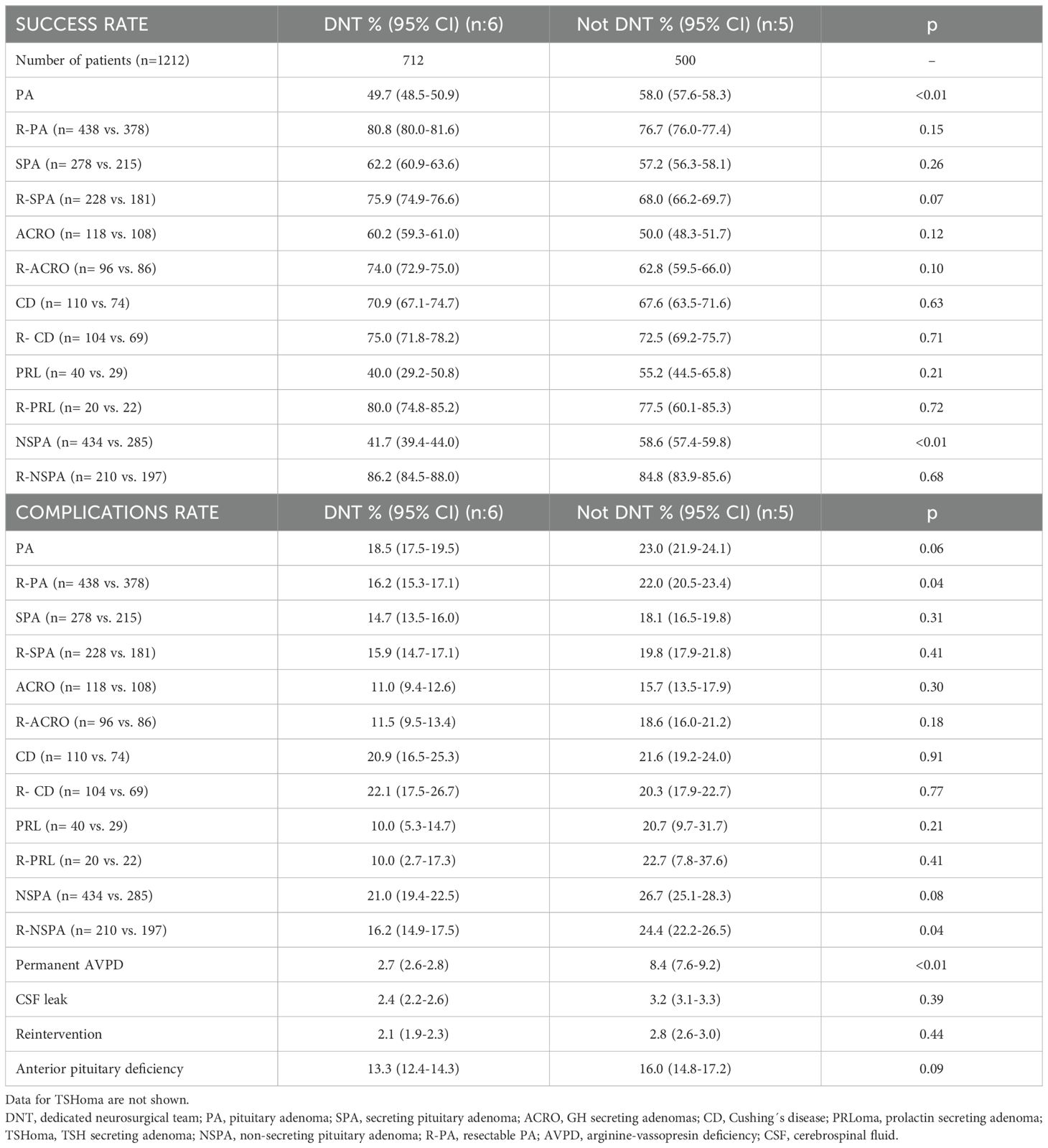
Table 4. Comparison of success rates and complications rates between patients operated in a HV center with and without a dedicated neurosurgical team.
Complication rates were lower in R-PA operated at HV centers with a DNT (16.2 vs. 22.0; p=0.04) due to less surgical complications in R-NSPA, with no differences in secreting adenomas. The rate of permanent AVPD (2.7 vs. 8.4%) was the most significant difference. These data are also shown in Table 4.
4 DiscussionThe outcomes of transsphenoidal surgery (TSS) are mostly influenced by tumor characteristics, -particularly its size and invasiveness-, as well as the expertise and experience of the medical team performing the surgery. Higher success rates with increasing neurosurgical experience have been reported for the surgical treatment of secreting adenomas, particularly in patients operated on by a single experienced neurosurgeon (2–5). The same has been demonstrated for TSS for NSPA, with improved outcomes and a higher rate of GTR after completing a learning curve (9). Previously reported series refer to high expertise centers performing high volume TSS (12), but there is a lack of data including daily practice in a nationwide setting like TESSPAIN.
The TESSPAIN study provides a comprehensive evaluation of TSS outcomes for pituitary PA across 29 centers in Spain. This study’s findings offer valuable insights into the effects of surgical experience, case volume, and specialized neurosurgical teams on surgical success and complication rates in treating PA, contributing essential data to the ongoing discussions about PTCOE. The overall success rate of TSS was higher in centers with HV with lower complications. The annual number of TSS recommended to reduce the risk of complications from TSS was set at 25 transsphenoidal operations for PA per year in the review by Honegger et al. (5), and this number was selected to classify a center as HV, in addition to nationally recognized centers of excellence for pituitary surgery.
4.1 Comparison of high- and low-volume centersOur results show that compared to non-HV centers, HV centers with greater experience achieved a higher overall success rate for PA surgery. This association between surgical volume and outcomes aligns with international literature (5, 9), which supports the importance of high case volumes in improving surgical success. Specifically, higher success rates were particularly notable in NSPA in HV centers, where the ability to achieve GTR plays a critical role. Moreover, a significant positive correlation was observed between the global success rate and the number of TSS procedures per center, reinforcing the impact of experience and surgical practice volume on clinical outcomes.
HV centers demonstrated a lower overall complication rate, highlighting the safety advantages associated with increased surgical volume. However, this trend did not consistently reach statistical significance across all PA subtypes, possibly due to variations in adenoma characteristics and surgical approaches across different centers. These findings support the need for PTCOE designation, where volume thresholds can help standardize care quality.
The lack of a significant difference in the overall success rate for each type of secreting adenoma between centers with and without a dedicated neurosurgical team may be explained by the limited number of operations performed in each center included in the study.
4.2 Impact of a dedicated neurosurgical teamIn HV centers, having a DNT was associated with notable benefits, especially regarding postoperative outcomes for NSPA. Centers with a DNT showed lower complication rates, particularly in persistent AVPD, with a rate similar to the 2% (95% CI, 0.02-0.03) recently reported in a systematic review of the literature (22). The presence of a stable, specialized team may contribute to refined procedural skills, enabling safer resections and quicker management of complications.
Interestingly, while HV centers with a DNT showed significantly fewer complications in R-NSPA, the success rate for all NSPA was higher in HV centers without a DNT. This difference disappeared when only R-NSPA were considered. This fact may reflect case selection dynamics, as HV centers without a DNT handled a lower proportion of complex cases attempting to achieve GTR (48.4% vs. 69.1%) and performed more TSS without curative intention. Regardless, the lower complication rates observed in centers with a DNT highlight the potential value of such teams in optimizing surgical safety.
4.3 Surgical Complications and Influencing FactorsThe overall complication rate in this study was 22.1%, with new anterior pituitary deficiencies being the most common postoperative morbidity, followed by persistent AVPD. Although there was no significant difference in AVPD rates between HV and non-HV centers, the incidence was notably lower when surgeries were performed by a dedicated DNT. This finding suggests that although AVPD remains a risk, its incidence may be mitigated by increased surgical experience and procedural improvements. A positive correlation was noted between the rate of postoperative anterior pituitary deficiency and the percentage of NSPA deemed resectable. In addition, the lower rate of NSPA amenable to GTR in non-HV centers suggests that these centers usually operated on more invasive NSPA with worse preoperative anterior pituitary function that could not be impaired by TSS, often performed without curative intention. Dedicated neurosurgeons in HV centers achieved the same success rate in R-NSPA with significantly fewer complications than non-dedicated neurosurgeons in HV centers, as reported many years ago (11).
The mortality rate for TSS was low at 0.4%, in line with international standards (23). However, the mortality rate was higher in non-HV centers, which may be due to the differences in experience in the management of complex cases. This finding highlights the importance of specialized and experienced surgical teams in minimizing risks associated with PA surgeries.
4.4 Outcomes by tumor typeLongitudinal studies have shown that the success rate of TSS in acromegaly continues to improve over decades, especially with increasing experience (24, 25) and a dedicated neurosurgeon (3, 26). With data collected from nine PTCOE, the remission rate for TSS in ACRO with macroadenomas was 49% without taking into account the invasiveness of the tumor (15). The remission rate in noninvasive adenomas was 62.9% in another study that showed an improvement rate with increasing experience, 50 to 73.6% in two periods (24). Our success rate was similar, 68.9% for R-ACRO. This rate improved with DNT in HV centers (74%), but did not reach statistical significance, probably because of the small number of cases in each center. However, the success rate for R-ACRO was similar, with no difference between HV and non-HV centers.
Two recent studies reported a global remission rate of 72.5% and 88.1% in TSS for CD (27, 28), slightly higher than ours (71.5%). We found a non-significant higher success rate in HV centers with DNT for CD and R-CD, and there was no significant difference in success rate when comparing HV centers with non-HV centers. This finding may be partially explained by the lower rate of R-CD in HV centers.
Invasion of the cavernous sinus by PRLoma is a poor prognostic factor (29) for successful TSS. Almost half of our PRLomas (43.3%) were not resectable. In a review published in 2020 (30), long-term disease control after surgery was 67% (95% CI, 60-74), 83% for microprolactinomas and 60% for macroprolactinomas. HV centers with a dedicated neurosurgical team achieved a success rate of 80% for R-PRLomas, but the small number of patients precluded evaluation of these results.
An earlier diagnosis of TSHomas allows more microadenomas to be identified, as in our case with a resectable rate of 92% for TSHomas and a success rate of 88%, which is much higher than that published in older series (31).
The ideal outcome of TSS for NSPA should be GTR, as regrowth of residual tumor can occur in up to 50% of cases (6). Invasiveness is the main determinant of the likelihood of GTR. In their review of 28 previous publications, Honegger et al. found no significant correlation between GTR and the annual number of TSS cases (5). HV centers had higher GTR than non-HV centers in our series. Similarly, there was a positive correlation between the total number of TSS and GTR for both NSPA and R-NSPA. This correlation of GTR rate has less significance with the number of TSS for PA at each center, suggesting that performing TSS for other tumors may predict better outcomes in NSPA. In addition, endocrinologists from HV centers were more likely to consider NSPA as amenable to GTR, suggesting a more demanding attitude towards GTR for NSPA in centers with a higher number of TSS. A DNT did not significantly increase the rate of GTR for R-NSPA in HV centers (86.2 vs. 84.8%), although they had fewer surgical complications for R-NSPA. The 75th percentile cut-off for residual tumor in the benchmark outcomes study for transsphenoidal surgery of pituitary adenomas for R-NSPA was less than 23.2% (16). In our study, the GTR rate for R-NSPA was 81.2%, which is better. However, this value may be influenced by the criteria used by each endocrinologist to consider an NSPA as resectable.
4.5 Study implications and future directionsThe TESSPAIN study bring out the importance of establishing PTCOE standards in Spain, as this could enhance consistency in TSS outcomes across centers. By concentrating resources and expertise in designated centers, Spain could improve surgical success rates and minimize complications associated with PA surgery. This model could particularly benefit patients in non-HV centers, where access to specialized teams and high case volumes remains limited.
Additionally, the correlation between surgical success and volume supports the necessity of experience for optimal outcomes, reinforcing the value of implementing volume thresholds in accreditation criteria.
4.6 LimitationsThe retrospective design of this study and the reliance on self-reported data from multiple centers may introduce reporting biases or inconsistencies in data collection methods. This is particularly true for the assessment of resectability, which was based on radiologic report or review of images at each center, and the detection of new postoperative hormonal deficiencies, which was based on the prescription of new hormonal replacement therapy after surgery without detailed data. Furthermore, while surgical success rates and complications were correlated with volume and specialization, causal inferences are limited. Prospective studies with standardized data collection protocols are warranted to validate these findings.
The main strengths of this study are the large number of operations and the wide range of centers reflecting the real-world experience in Spain.
4.7 ConclusionThe TESSPAIN study provides essential data on TSS outcomes for PA in Spain, revealing that surgical experience, high case volumes, and specialized neurosurgical teams are instrumental in achieving optimal surgical success and minimizing complications. These findings support the establishment of PTCOE in Spain to ensure that patients with PA receive the highest quality of care. With standardized benchmarks and focused expertise, PTCOE could reduce variability in outcomes, aligning Spain’s care standards with international best practices for managing pituitary adenomas.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe study was reviewed and approved on May 24, 2023, by the Regional Ethics Committee of the Basque Country (CEIm-E) (PI2023077). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin due to the retrospective nature of the study and non-interventional design. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article due to the retrospective nature of the study.
Author contributionsMP: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. ASo: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. FH: Data curation, Writing – original draft, Writing – review & editing. FG-P: Data curation, Writing – original draft, Writing – review & editing. RC: Data curation, Writing – original draft, Writing – review & editing. DM: Data curation, Writing – original draft, Writing – review & editing. AG: Data curation, Writing – original draft, Writing – review & editing. ASi: Data curation, Writing – original draft, Writing – review & editing. RV: Data curation, Writing – original draft, Writing – review & editing. MC: Data curation, Writing – original draft, Writing – review & editing. AV: Data curation, Writing – original draft, Writing – review & editing. JR-C: Data curation, Writing – original draft, Writing – review & editing. GS: Data curation, Writing – original draft, Writing – review & editing. PR-M: Data curation, Writing – original draft, Writing – review & editing. PP-R: Data curation, Writing – original draft, Writing – review & editing. MA-C: Data curation, Writing – original draft, Writing – review & editing. SL: Data curation, Writing – original draft, Writing – review & editing. AI: Data curation, Writing – original draft, Writing – review & editing. DO: Data curation, Writing – original draft, Writing – review & editing. SA: Data curation, Writing – original draft, Writing – review & editing. FM: Writing – review & editing, Data curation, Writing – original draft. ÁA: Data curation, Writing – original draft, Writing – review & editing. LG-F: Data curation, Writing – original draft, Writing – review & editing. RG-C: Data curation, Writing – original draft, Writing – review & editing. NE: Data curation, Validation, Writing – original draft, Writing – review & editing. TG-V: Data curation, Writing – original draft, Writing – review & editing. EM: Data curation, Writing – original draft, Writing – review & editing. AD: Data curation, Writing – original draft, Writing – review & editing. JA: Data curation, Writing – original draft, Writing – review & editing. JS: Data curation, Writing – original draft, Writing – review & editing. AP: Data curation, Methodology, Writing – original draft, Writing – review & editing. CN: Data curation, Writing – original draft, Writing – review & editing. IO: Data curation, Writing – original draft, Writing – review & editing. CT: Data curation, Writing – original draft, Writing – review & editing. RL: Data curation, Writing – original draft, Writing – review & editing. PP-V: Data curation, Writing – original draft, Writing – review & editing. CC: Data curation, Writing – original draft, Writing – review & editing. DP: Data curation, Writing – original draft, Writing – review & editing. GD-S: Writing – original draft, Writing – review & editing, Data curation. MP-D: Data curation, Writing – original draft, Writing – review & editing. BB: Conceptualization, Data curation, Supervision, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. Publication fees were supported by the Spanish Society of Endocrinology and Nutrition.
AcknowledgmentsWe are particularly grateful to all the patients included in the study and to the Spanish Society of Endocrinology for its support.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes Ministerio de Sanidad.https://www.sanidad.gob.es/profesionales/CentrosDeReferencia/CentrosCSUR.htm [Accessed September 10, 2024]References1. Gittoes NJ, Sheppard MC, Johnson AP, Stewart PM. Outcome of surgery for acromegaly–the experience of a dedicated pituitary surgeon. QJM. (1999) 92:741–45. doi: 10.1093/qjmed/92.12.741
PubMed Abstract | Crossref Full Text | Google Scholar
2. Ahmed S, Elsheikh M, Stratton IM, Page RC, Adams CB, Wass JA. Outcome of transphenoidal surgery for acromegaly and its relationship to surgical experience. Clin Endocrinol (Oxf). (1999) 50:561–7. doi: 10.1046/j.1365-2265.1999.00760.x
PubMed Abstract | Crossref Full Text | Google Scholar
3. Sala E, Ferrante E, Locatelli M, Rampini P, Mantovani G, Giavoli C, et al. Diagnostic features and outcome of surgical therapy of acromegalic patients: experience of the last three decades. Hormones (Athens). (2014) 13:95–103. doi: 10.1007/BF03401325
PubMed Abstract | Crossref Full Text | Google Scholar
4. Hazer DB, Işık S, Berker D, Güler S, Gürlek A, Yücel T, et al. Treatment of acromegaly by endoscopic transsphenoidal surgery: surgical experience in 214 cases and cure rates according to current consensus criteria. J Neurosurg. (2013) 119:1467–77. doi: 10.3171/2013.8.JNS13224
PubMed Abstract | Crossref Full Text | Google Scholar
6. Chen Y, Wang CD, Su ZP, Chen YX, Cai L, Zhuge QC, et al. Natural history of postoperative nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Neuroendocrinology. (2012) 96:333–42. doi: 10.1159/000339823
PubMed Abstract | Crossref Full Text | Google Scholar
7. Almutairi RD, Muskens IS, Cote DJ, Dijkman MD, Kavouridis VK, Crocker E, et al. Gross total resection of pituitary adenomas after endoscopic vs. microscopic transsphenoidal surgery: a meta-analysis. Acta Neurochir (Wien). (2018) 160:1005–21. doi: 10.1007/s00701-017-3438-z
PubMed Abstract | Crossref Full Text | Google Scholar
8. Zaidi HA, Awad AW, Bohl MA, Chapple K, Knecht L, Jahnke H, et al. Comparison of outcomes between a less experienced surgeon using a fully endoscopic technique and a very experienced surgeon using a microscopic transsphenoidal technique for pituitary adenoma. J Neurosurg. (2016) 124:596–604. doi: 10.3171/2015.4.JNS15102
PubMed Abstract | Crossref Full Text | Google Scholar
9. Kim JH, Lee JH, Lee JH, Hong AR, Kim YJ, Kim YH. Endoscopic transsphenoidal surgery outcomes in 331 nonfunctioning pituitary adenoma cases after a single surgeon learning curve. World Neurosurg. (2018) 109:e409–16. doi: 10.1016/j.wneu.2017.09.194
PubMed Abstract | Crossref Full Text | Google Scholar
10. Mortini P, Nocera G, Roncelli F, Losa M, Formenti AM, Giustina A. The optimal numerosity of the referral population of pituitary tumors centers of excellence (PTCOE): A surgical perspective. Rev Endocr Metab Disord. (2020) 21:527–36. doi: 10.1007/s11154-020-09564-7
PubMed Abstract | Crossref Full Text | Google Scholar
11. Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. (1997) 40:225–36. doi: 10.1097/00006123-199702000-00001
PubMed Abstract | Crossref Full Text | Google Scholar
12. Leach P, Abou-Zeid AH, Kearney T, Davis J, Trainer PJ, Gnanalingham KK. Endoscopic transsphenoidal pituitary surgery: evidence of an operative learning curve. Neurosurgery. (2010) 67:1205–12. doi: 10.1227/NEU.0b013e3181ef25c5
PubMed Abstract | Crossref Full Text | Google Scholar
13. Bokhari AR, Davies MA, Diamond T. Endoscopic transsphenoidal pituitary surgery: a single surgeon experience and the learning curve. Br J Neurosurg. (2013) 27:44–9. doi: 10.3109/02688697.2012.709554
PubMed Abstract | Crossref Full Text | Google Scholar
14. Casanueva FF, Barkan AL, Buchfelder M, Klibanski A, Laws ER, Loeffler JS, et al. Criteria for the definition of pituitary tumor centers of excellence (PTCOE): A pituitary society statement. Pituitary. (2017) 20:489–98. doi: 10.1007/s11102-017-0838-2
PubMed Abstract | Crossref Full Text | Google Scholar
15. Giustina A, Uygur MM, Frara S, Barkan A, Biermasz NR, Chanson P, et al. Pilot study to define criteria for Pituitary Tumors Centers of Excellence (PTCOE): results of an audit of leading international centers. Pituitary. (2023) 26:583–96. doi: 10.1007/s11102-023-01345-0
PubMed Abstract | Crossref Full Text | Google Scholar
16. Drexler R, Rotermund R, Smith TR, Kilgallon JL, Honegger J, Nasi-Kordhishti I, et al. Defining benchmark outcomes for transsphenoidal surgery of pituitary adenomas: a multicenter analysis. Eur J Endocrinol. (2023) 189:379–86. doi: 10.1093/ejendo/lvad124
PubMed Abstract | Crossref Full Text | Google Scholar
17. Baussart B, Venier A, Jouinot A, Reuter G, Gaillard S. Closure strategy for endoscopic pituitary surgery: Experience from 3015 patients. Front Oncol. (2023) 12:1067312. doi: 10.3389/fonc.2022.1067312
PubMed Abstract | Crossref Full Text | Google Scholar
18. Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2014) 99:3933–51. doi: 10.1210/jc.2014-2700
PubMed Abstract | Crossref Full Text | Google Scholar
19. Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of cushing's syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2015) 100:2807–31. doi: 10.1210/jc.2015-1818
PubMed Abstract | Crossref Full Text | Google Scholar
20. Mattogno PP, D'Alessandris QG, Chiloiro S, Bianchi A, Giampietro A, Pontecorvi A, et al. Reappraising the role of trans-sphenoidal surgery in prolactin-secreting pituitary tumors. Cancers (Basel). (2021) 13:3252. doi: 10.3390/cancers13133252
PubMed Abstract | Crossref Full Text | Google Scholar
21. Fajardo-Montañana C, Villar R, Gómez-Ansón B, Brea B, Mosqueira AJ, Molla E, et al. Recommendations for the diagnosis and radiological follow-up of pituitary neuroendocrine tumours. Endocrinol Diabetes Nutr (Engl Ed). (2022) 69:744–61. doi: 10.1016/j.endien.2021.10.014
PubMed Abstract | Crossref Full Text | Google Scholar
22. Fountas A, Coulden A, Fernández-García S, Tsermoulas G, Allotey J, Karavitaki N. Central diabetes insipidus (vasopressin deficiency) after surgery for pituitary tumours: a systematic review and meta-analysis. Eur J Endocrinol. (2024) 191:S1–S13. doi: 10.1093/ejendo/lvae084
留言 (0)