Glioblastoma (GBM) is the most malignant type of glioblastoma featuring fast proliferation, strong invasion, and poor prognosis (Peng et al., 2018). Patients with GBM usually show a variety of clinical manifestations that are caused by dysfunction of affected areas of the brain, such as lethargy, apathy, blindness, seizures, and language changes (McKinnon et al., 2021). Besides, it often grows in the brain parenchyma in an invasive manner (Lah et al., 2020).
Cells in biological tissues are subject to mechanical forces, including hydrostatic pressure, shear stress, compression, and tension (Humphrey et al., 2014). In biological systems, tissue stiffness varies considerably between organs and between healthy and diseased states of the same organ (Kai et al., 2016). In some regions, solid tumor tissue is harder than its untransformed counterpart, e.g., normal brain tissue is usually less than 100 Pa, whereas human low to high-grade glioblastomas (LGG and HGG) have progressively stiffer tissues ranging from 100 to 104 Pa. This is due to the uncontrolled proliferation of cells in confined spaces, vascular hyper-permeability, insufficient lymphatic drainage, and increased deposition of extracellular matrix (ECM) protein deposition increases. Miroshnikova et al. used atomic force microscopy to quantify the hardness of non-tumor glial proliferation as well as glial cell tissue with different levels of malignant transformation in frozen human brain biopsies. The results showed that glial tissues have the lowest ECM hardness (Young’s modulus, E; 10–180 Pa), which increases progressively with the level of malignancy (50-1,400 Pa for LGGs and 70-13,500 Pa for GBM). NanoString nCounter gene expression analysis of prognostic predictor gene clusters from human GBM tissue biopsies (aggressiveness was categorized on a scale of 1–10, with 1 indicating the most aggressive) indicated that there was a significant correlation between ECM hardness and prognosis of glioblastomas, with higher hardness being associated with a worse prognosis (Miroshnikova et al., 2016). Chauvet et al. used intraoperative shear wave elastography to measure the elasticity of tumors and surrounding tissues in patients undergoing brain tumor resection. The study showed that the mean elasticity of meningiomas, low-grade gliomas, high-grade gliomas, and brain metastases was 33.1 ± 5.9 kPa (3.3 ± 0.6 m/s), 23.7 ± 4.9 kPa (2.8 ± 0.6 m/s), 11.4 ± 3.6 kPa (1.9 ± 0.6 m/s), and 16.7 ± 2.5 kPa (2.4 ± 0.4 m/s), respectively, demonstrating that the greater the tissue stiffness, the higher the malignancy., again demonstrating that the greater the tissue hardness, the higher the malignancy of the glioma (Chauvet et al., 2016). Increased tissue stiffness leads to tumor progression by modulating proliferation, invasion, apoptosis evasion, drug resistance, angiogenesis, metabolism, and growth-promoting signaling pathways (Northey et al., 2017; Oudin and Weaver, 2016).
Cells can convert mechanomechanical signals into biological or electrical signals, a process known as biomechanical signal transduction. Mechanical signal transduction pathways are often classified into three categories: cell membrane receptors and surface mechanosensitive ion channels, the cytoskeleton (stress fibers, microtubules, etc.), and the nucleoskeleton and cytoskeletal linker complexes (Chaudhuri et al., 2018). Mechanosensitive ion channels are protein pores embedded in the plasma membrane. When the channel is stimulated to open by a mechanical signal, ions enter the cell from the pore in the direction of the electrochemical gradient, without the need for energy from adenosine triphosphate (ATP) hydrolysis.
Piezo1 channels are a member of the Piezo family of mechanosensitive ion channels, the first mechanically gated cation channel family identified and established in mammals, which can be directly activated by mechanical stimuli alone to mediate mechanosensitive cationic currents (Coste et al., 2012). Piezo1 is mainly found in tissue cells that are sensitive to mechanical tension stimuli, such as the lung, bladder, and skin, and is involved in the development of various tumors (Coste et al., 2010). Studies have shown that Piezo1 is largely unexpressed in normal brain tissue, but is expressed at high levels within glioblastomas. However, their role in the pathogenesis of GBM remains unclear. This paper aims to summarise the role of Piezo1 in the development of GBM and the role of Piezo1 in the treatment of GBM and to discuss the role of Piezo1 inhibitors in the treatment of GBM.
2 Piezo1The Piezo1 gene is located on human chromosome 16p24.3 and consists of 51 exons, 2547 amino acids and 3 transcripts (Yu and Liao, 2021). In plan view, it looks like a propeller blade or a tricuspid with an ion-penetrating pore in the middle and a cap c-terminal extracellular structural domain (CED) at the top. When viewed from the side, the Piezo1 channels distributed across the cell membrane interact with the lipid membranes surrounding the channels to form a dome-like structure (Guo and MacKinnon, 2017; Lin et al., 2019; Yang et al., 2022) and use the aforementioned trimeric structure to mediate mechanical force transfer via a lever principle mechanism (Wang et al., 2018; Thien et al., 2024). The central ion-conducting pore and the three peripheral propeller-like blades are two important regions for the function of Piezo1 as a mechanosensitive channel. Two transmembrane (TM) helices, called outer helix-inner helix (OH - IH) pairs, the C-terminal extracellular structural domain (CED) and the intracellular C-terminal structural domain (CTD) trimer form the transmembrane channel of Piezo1. The CED is located on top of the central ion pore and has been shown to regulate channel inactivation kinetics as well regulating cation specificity for the channels (Wu et al., 2017; Zhao and Zlokovic, 2020). The transmembrane portion of the channel is lined by hydrophobic amino acid residues of IH, which contract in the middle of the membrane to form the neck of the channel (Zhao et al., 2018). The channel has two transmembrane gates surrounded by an OH and two adjacent IHs. Below the transmembrane channel, cations enter the cytoplasm through two intracellular channels, a 10 Å-long constriction site, and three lateral channels closed by side bolts, triggering subsequent different signaling pathways (Saotome et al., 2018; Geng et al., 2020). (Figure 1).
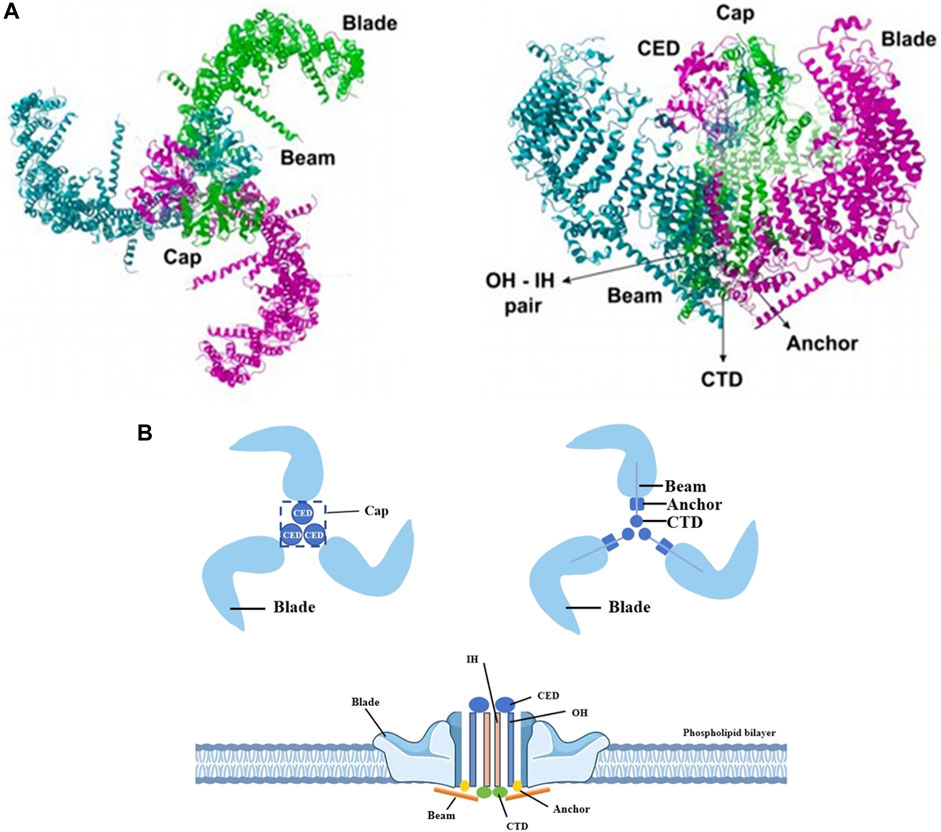
Figure 1. Different views of piezo1. (A) The Piezo1 protein exhibits a propeller-like three-bladed structure, with the 38 transmembrane helices of the three subunits coming together in the membrane plane to form a signature nanobowl-like structure. Adapted from (Thien et al., 2024). (B) Two transmembrane helices (OH - IH), the extracellular C-terminal structural domain (CED) and the intracellular C-terminal structural domain (CTD) trimer form the transmembrane channel of Piezo1. The CED, located between the OH and the IH, is the tip of the ion channel, and cations enter the channel through the open window (Cap) located directly above the membrane. The IH constricts in the middle of the membrane and forms the neck of the channel. When an external mechanical force stimulates the membrane, the stress causes the Piezo1 channel to change from a closed state to an open state, which induces Ca2+ influx into the interior of the cell and triggers different signaling pathways to follow.
The transmembrane pore is followed on the inner side of the cell by two ion-permeable pathways, including a 10-Å- long vertical constriction neck along the central axis and three lateral channels extending from the central pore through three lateral openings. It is now generally accepted that the lateral channels, rather than the vertical constriction necks, may serve as the primary intracellular cation conductance pathway. The fact that the outlets of the lateral channels and the central constriction neck are surrounded by negative and positive surface electrostatic potentials, respectively, further supports the structural suitability of the lateral channels for cation permeation.
One of the key issues for piezo1 channels is how to precisely determine and regulate their precise mechanical sensitivity and cation permeation properties. Each lateral channel has a lateral “plug”. This “plug” physically controls the on/off switching of the lateral ion channel, thus affecting both the ion permeation and the mechanical sensitivity of the piezo1. The lateral cation channels, the lateral plugs, the gating element and the central constrictor neck together form the “plug and latch” mechanism of piezo1, which coordinates the switching of the lateral channels using the plugs, which are connected to the central axis to form a lever-like mechanical transduction mechanism. The three lateral ion channels are equipped with three independently positioned lateral plugs that allow the piezo1 to move asymmetrically when external mechanical forces are applied asymmetrically to the force sensing vanes, and ultimately convert the conformational changes induced by peripheral vane tension into on/off switching of the ion channels inside the cell (Geng et al., 2020).
Piezo1 is embedded in lipid bilayers and is sensitive to local and global stresses in the bilayer. It is located in the endoplasmic reticulum, the cytoplasmic compartment, and the nuclear membrane close to the nucleus (Eisenhoffer et al., 2012; Gudipaty et al., 2017). Piezo1 enables cells to sense a variety of mechanical forces, including radial pressure, membrane stretch, compression, shear stress, matrix stiffness, ultrasound, and matrix pressure (Nourse and Pathak, 2017; Ridone et al., 2019). When a mechanical stimulus impinges on the cell membrane, the stress is distributed to all components including the bilayer, cytoskeleton (CSK), and extracellular matrix (ECM), which converge on the Piezo1 channel, inducing a change from a closed to an open state of the Piezo1 channel. The Piezo1 channel opens to allow Ca2+, K+, and Na+ plasma flow. Under mechanical stress, Piezo1 regulates a variety of functions such as protein synthesis, secretion, migration, proliferation, and apoptosis (Hong et al., 2023). Therefore, the main factors affecting Piezo1 gating include cell membrane tension and stiffness, cytoskeletal proteins, and other channels or proteins that interact with Piezo1.
3 Physiological role of piezo1 in different tissues and organs and its expression in different tumorsPiezo1 channel protein is widely expressed in a variety of cells, including osteoblasts, immune cells, and cancer cells, among others. It is associated with a variety of mechanotransduction processes under different physiological and pathophysiological conditions. To emphasize the effectiveness of Piezo1 as a novel drug target for disease intervention, we summarize its physiological roles in different tissues (Table 1) and organs and its expression in different tumor tissues (Table 2).
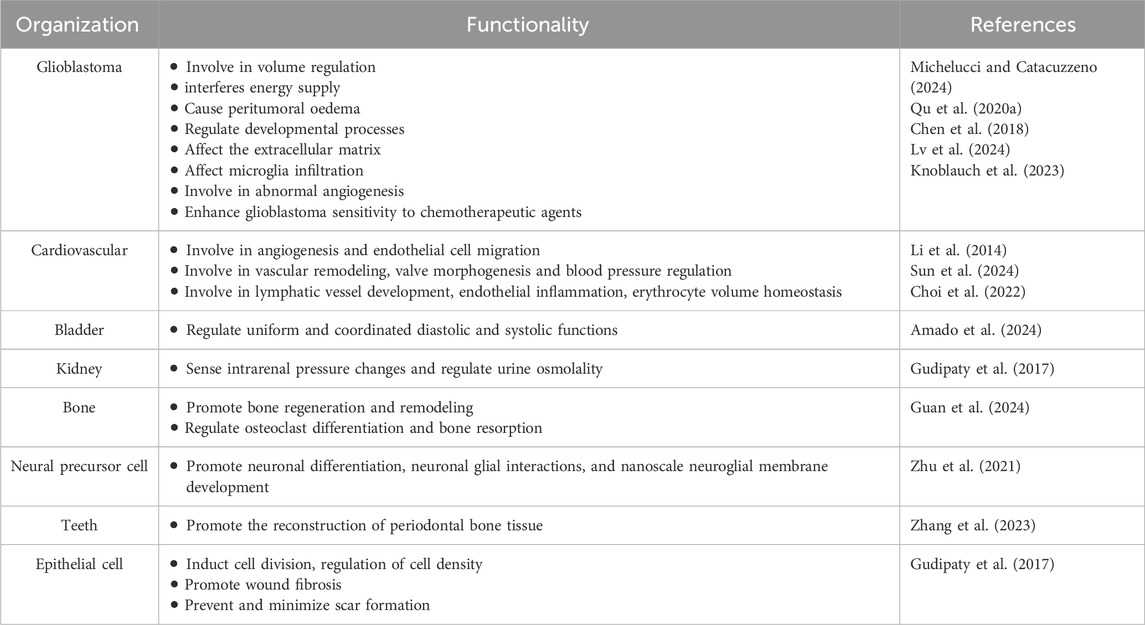
Table 1. Comparison of piezo1 function in glioblastoma versus other normal tissues.
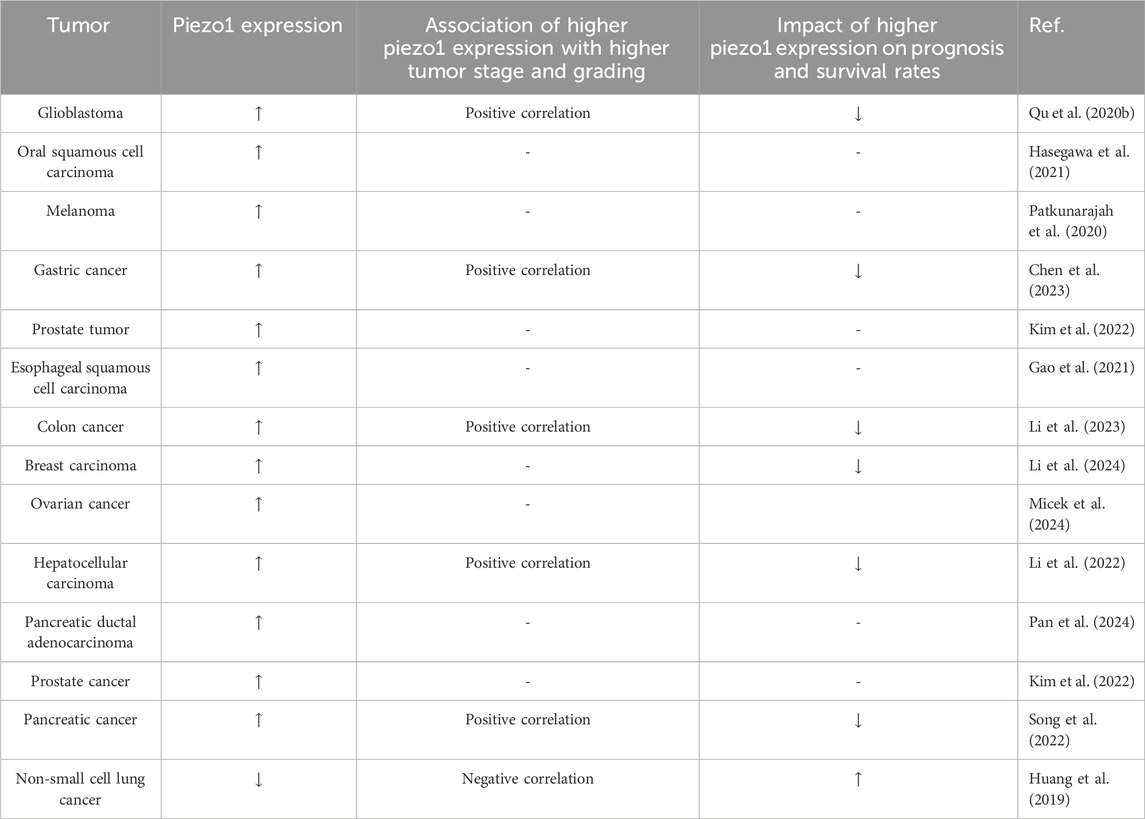
Table 2. Expression of piezo1 in different tumor tissues and its relationship with different tumor grades and prognosis.
4 Relationship between Piezo1 and glioblastoma4.1 Piezo1 expressed in gliomas of different gradesNeurons and glial cells in the central nervous system exhibit significant mechanosensitivity and can sense mechanical stimuli from the surrounding environment and convert them into biochemical signals to regulate their function (Cox et al., 2016; Tortorella et al., 2022). Piezo1 is expressed in neuronal cells, microglia, astrocytes, oligodendrocytes, and neural stem cells, and is activated when neuronal cells are stimulated by external tensile forces, activated when neuronal cells are stimulated by external changes in stretch force, osmotic pressure, and matrix stiffness (Hong et al., 2023), and participates in cellular physiological activities in a variety of ways, such as increasing cytoplasmic Ca2+ signaling or initiating Ca2+ inward flow and affecting the levels of downstream Ca2+ signaling proteins involved in neuronal function, through the c-Jun N-terminal kinase (JNK1) and mammalian target of rapamycin (mTOR) signaling pathways (Liu et al., 2021).
It was demonstrated that all histological subtypes of gliomas, except oligodendrogliomas, showed Piezo1 overexpression compared to normal brain tissue and that the expression level of Piezo1 was positively correlated with World Health Organization (WHO) grade and histopathology (Chen et al., 2018). Piezo1 expression was more significantly upregulated in high-grade gliomas (WHO grade III and IV) than in low-grade gliomas (WHO grade II). Isocitrate dehydrogenase (IDH) mutation, CpG island methylation (G-CIMP) phenotype, and 1p/19q co-deletion are important molecular biomarkers in gliomas to guide prognosis and treatment. Piezo1 expression was significantly higher in the IDH1 wild-type group of gliomas than in the IDH1 mutant group and was elevated in the 1p/19q-non-co-deletion group. Gliomas with non-G-CIMP phenotypes had higher levels of Piezo1 expression compared to G-CIMP phenotypes. Grade II oligodendrogliomas with IDH1 mutation and 1p/19q co-deletion had the lowest level of Piezo1 protein expression. IDH mutation established a glioma hypermethylation phenotype, and the deoxyribonucleic acid (DNA) structural domains spanning the Piezo1 promoter and 8000 bp upstream of the Piezo1 transcriptional start site (TSS) were also hypermethylated in IDH mutant gliomas. The methylation status of these probes was negatively correlated with Piezo1 messenger ribonucleic acid (mRNA) expression. Thus more aggressive IDH wild-type gliomas are epigenetically more inclined to upregulate Piezo1 at the transcriptional level (Zhou et al., 2020).
Kaplan-Meier survival analysis of multiple human glioblastoma datasets showed that patients with elevated Piezo1 expression had significantly worse overall survival (Figure 2). Multifactorial Cox regression analysis showed that Piezo1 overexpression was an independent prognostic factor for overall survival (OS) in glioblastoma patients (Qu et al., 2020b). These results suggest that Piezo1 can be used as a prognostic biomarker for glioblastoma patients in conjunction with molecular markers such as IDH wild-type, 1p/19q non-coding, and non-G-CIMP phenotypes.
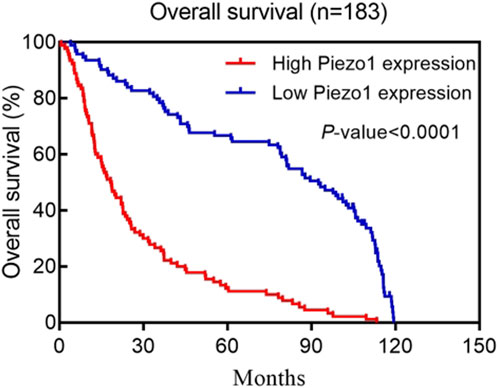
Figure 2. Kaplan–Meier postoperative survival curve for patterns of patients with glioma and Piezo1 expression. Adapted from (Qu et al., 2020b).
Altered matrix stiffness in glioblastomas promotes elevated expression of piezo1 in glioblastomas, and Piezo1 is physically localized to the focal adhesions of glioblastoma cells, catalyzing the maturation and growth of focal adhesions through a force-dependent calcium signaling pathway. Piezo1 is one of the major ion channels conferring mechanosensitivity to GBM stem cells, and RNA of Piezo1 knockdown glioblastoma cell lines sequencing and dual-assay TCGA database analysis of Piezo1-related genes showed that Piezo1 is associated with activation of extracellular matrix (ECM), actin cytoskeleton remodeling and integrin adhesion signaling. In addition, Piezo1 expression transduces mechanical stimuli between glioblastoma cells, promoting tumorigenesis and progression (Hong et al., 2023). This finding could explain why the self-stiffening changes in glioblastomas are mainly caused by pressure gradients generated by the tumor itself rather than collagen deposition or cross-linking.
Piezo1 knockdown inhibited clonal growth of GBM cell lines. Tumor ball formation was blocked in GBM stem cells after Piezo1 knockdown. In vitro experiments, GBM cells were able to express Piezo1 short hairpin RNA (shRNA) in a doxycycline-dependent manner. Knockdown of Piezo1 expression by doxycycline-induced Piezo1 shRNA could be observed after treatment of glioblastoma model mice with doxycycline, which significantly suppressed GBM tumor growth and prolonged the survival time of the mice, suggesting that Piezo1 is critical for the maintenance and progression of glioblastomas once tumors form (Chen et al., 2018).
4.2 Involvement of piezo1 in glioblastoma cell volume regulationRegulated volume reduction (RVD) is an evolutionarily conserved process used by animal cells to restore normal volume when osmotic shock-induced swelling occurs. RVD plays an important role in a number of physiological processes, including the prevention of necrotic cell death induced by persistent cellular swelling, and is mediated primarily by the concerted activity of ion channels that mediate the passage of CI− and K+ ions (Miyazaki et al., 2007). Volume-regulated anion channel (VRAC), a heterodimeric protein that mediates swelling-activated CI− currents (ICl), has been identified during hypotonic cell swelling as the common and major channel responsible for CI− transport during RVD in almost all vertebrate cells. Tension-activated K+ channels, voltage-dependent K+ channels, and Ca2+-activated K+ (KCa) large-, medium-, and small-conductance channels (BK, IK, and SK, respectively) also play key roles in volume regulation in many cell types (Caramia et al., 2019).
In addition to CI− and K+ channels, nonselective Ca2+-permeable mechanosensitive channels (MSC) are also involved in cell volume regulation. During hypotonic cell swelling, MSCs sense changes in cell membrane tension and activate as a result. Their activation then triggers intracellular Ca2+ signaling, which in turn regulates various effectors. (Arniges et al., 2004).
In human glioblastoma cells, Piezo1 is the main component of MSCs activated by cell swelling in glioblastoma cells, and is also primarily responsible for mechanotransduction during glioblastoma cell volume regulation. Ca2+ signaling can be amplified by activation of the mechanism, which is activated by the influx of MSCs activated by hypertonic cell swelling to activate IK and BK channels and VRAC, which are essential for RVD development. The cascade mechanism of Piezo1 activation from Ca2+ influx to IK/BK channel activation, which ultimately regulates cell volume, is an important signaling pathway for Piezo1 regulation of glioblastoma cell spreading, migration, and death (Michelucci and Catacuzzeno, 2024).
In glioblastoma tissues, the infiltration of isolated tumor cells into the peritumoral parenchyma often culminates in tumor migration and invasion. This situation greatly limits the success of surgical resection. The invasion of GBM cells into the confined space of healthy brain parenchyma requires that they be able to sense the presence of external barriers and forces from the environment and adjust their size and shape accordingly to pass through. It has been shown that glioblastoma cells use piezo1 channels to sense mechanical stimuli from the tumor microenvironment and deform the cell membrane (stretching, indentation, invagination, etc.), inducing an increase in cytoplasmic Ca2+ through, for example, activation of KCa channels and modulation of actin cytoskeletal polymerization, which allows for a change in the volume and shape of the cells required to invade healthy brain parenchyma.
KCa3.1 channels (also known as KCNN4, IK1 or SK4 channels) are moderately conductive calcium-activated potassium channels that regulate membrane potential and maintain calcium homeostasis. It has been shown to be involved in regulating GBM migration in response to C-X-C motif chemokine ligand (CXCL), serum and bradykinin in vitro. Studies have shown that the volume of invasive glioma cells is reduced by a maximum of 30%–35% compared to the volume of normal glial cells, which requires glioma cells to release all free unbound cytoplasmic water as well as K+ channels. Water permeably follows the release of ions through ion channels and cotransporter proteins, with a consequent change in cell volume. Inhibition of KCa3.1 channels retards cell invasion and migration (Watkins and Sontheimer, 2011).
Volume changes are critical in cell death, such as in apoptotic cell death, which is preceded by a contraction of cell volume called apoptotic volume decrease (AVD). In GBM cells, KCa channels are directly involved in AVD induced by the addition of astrocystin or TNF-α-related apoptosis-inducing ligand (TRAIL), which activate intrinsic or extrinsic pathways of apoptosis, respectively.
4.3 Piezo1 interferes with the energy supply in glioblastomasCa2+ regulates glioblastoma maintenance, proliferation, migration, and cell cycle (Leclerc et al., 2016; Satir and Christensen, 2007). Most cancer cells obtain higher glucose utilization under aerobic conditions by producing ATP using glycolysis, a phenomenon known as the Warburg effect (Vander Heiden et al., 2009). When intracellular glucose is deficient, free fatty acid oxidation replaces glucose for cellular energy and maintains intracellular reactive oxygen species (ROS) levels and calcium homeostasis, Ca2+ is closely related to lipid metabolism (Li et al., 2018; Wires et al., 2017).
Sonodynamic therapy (SDT) is a non-invasive technique based on the combination of acoustic sensitizers and acoustic activation that disrupts the mitochondrial respiratory chain, leading to an increase in intracellular ROS levels and calcium overload, as well as inhibition of proliferation, invasion, and promotion of apoptosis in the biologically more aggressive grade IV glioblastomas (Chen et al., 2022). On the one hand, the mechanical force introduced by ultrasound activated the opening of the transient calcium channel Piezo1 on the membrane, maintained the open state of the Piezo1 channel by increasing the level of intracellular oxidative stress, prolonged the opening time of Piezo1, and increased the Ca2+ inward flow, which caused the cell to swell and activated the calcium overload pathway of the death mechanism. On the other hand, ROS generated by ultrasound-activated intracellular ultrasound sensitizers prolonged the opening time of Piezo1 and promoted Ca2+ entry into the cell to bind to lipid droplets to form complexes, thereby affecting lipid metabolism and preventing it from providing energy to glioblastoma cells, leading to cell death (Chen et al., 2022).
ROS generated by SDT activates the mitochondrial apoptotic pathway (Chen et al., 2017), leading to mitochondrial dysfunction and DNA damage, which sustains mitochondrial calcium uniporter (MCU) channel activity and induces Ca2+ overload in the mitochondria, further aggravating the mitochondrial dysfunction, generating more ROS, and forming a vicious cycle that ends in death.
4.4 Peritumoral oedema in glioblastoma caused by Piezo1Peritumoral oedema is an independent risk factor for poor prognosis and recurrence of glioblastoma (Schoenegger et al., 2009). Peritumoral oedema can exacerbate neurological signs and clinical symptoms in glioblastoma patients, such as seizures, neurological deficits, and increased intracranial pressure, and can even lead to brain herniation. In addition, peritumoral oedema can affect tumor exposure during surgery and make surgical resection more difficult (Wang et al., 2011; Hou et al., 2013). A recent study reported a linear correlation between the expression level of Piezo1 and the severity of peritumoral oedema, with preoperative magnetic resonance imaging (MRI) and corresponding Piezo1 immunohistochemical staining results in patients with different degrees of oedema. The higher the degree of oedema, the higher the Piezo1 expression (Qu et al., 2020a).
Peritumoral brain oedema (PTBE) includes vasogenic and cytotoxic oedema (Qu et al., 2020a; Liang et al., 2007). Vasogenic oedema is usually due to the degradation of tight junctions between endothelial cells (e.g., E-cadherin, N-cadherin, and b-catenin), which increases vascular permeability. Intravascular fluid then leaks out into the tissue space, further causing tissue oedema. The Piezo1 protein acts as a calcium channel to promote calcium ion influx into vascular endothelial cells, which in turn activates calcium-dependent calpain. Calpain further promotes the degradation of tight junctions between vascular endothelial cells and increases vascular permeability (Friedrich et al., 2019). High vascular permeability allows the extravasation of protein-rich fluid, leading to cerebral oedema. Extracellular cations enter neurons and glial cells through cation channels and accumulate intracellularly, while cation in-flow drives anion inflow, leading to increased intracellular osmotic pressure and entry of extracellular interstitial water molecules into the cells, resulting in cellular swelling and the formation of cytotoxic oedema occurs. Thus, inhibition of Piezo1 reduces clinical symptoms in glioblastoma patients and may be a therapeutic target for diseases involving blood-brain barrier collapse (Qu et al., 2020a).
4.5 Pathways regulated by piezo1 during glioblastoma development4.5.1 JNK1/mTORThe ROS-c-JunN-terminalkinase 1 (JNK1) and AKT-mammalian target of rapamycin (mTOR) signaling pathways are the main molecular mechanisms involved in glioblastoma cell survival, proliferation, motility, and differentiation, and the activation and activity of this signaling pathway is regulated by intracellular Ca2+ signaling (Mohamed et al., 2022; Park et al., 2008). Activation of cell cycle proteins D1 and cyclin-dependent kinases 4 (CDK4) and assembly of the cell cycle protein D1-CDK4 complex are essential for promoting the cell cycle transition from G1 to S phase. Highly expressed and aberrantly activated piezo1 in glioblastoma induces calcium inward flow. Large amounts of intracellular Ca2+ promote phosphorylation of Akt and mTOR, followed by activation of the cell cycle protein D1/CDK4 complex, which promotes cell survival, cell cycle progression, cell proliferation, and migration. In addition, since Akt stabilizes the mature cell cycle protein D1, piezo1-induced activation of Akt can promote the transition of tumor cells from G1 to S phase by activating the cell cycle protein D1. knockdown of Piezo1 may inactivate Akt, inhibit the activation of the cell cycle protein D1, and cause the cells to stagnate in the G1 phase (Han et al., 2019). Meanwhile, studies have shown that inhibition of Piezo1 further increases the expression of P-JNK1 (T183/Y185), promotes the expression of pro-apoptotic proteins such as c-Jun N-terminal kinase (cJUN), Bcl2-associated X (BAX), etc., inhibits tumor cell proliferation, and induces apoptosis of glioma cells (Liu et al., 2019).
Celastrol is a major active natural product extracted from the root bark of the traditional Chinese medicine Rehmannia glutinosa, which possesses a wide range of biological properties such as anti-tumor, immunosuppression, and weight loss (Liu et al., 2015; Hu et al., 2017b). Previous studies have shown that celastrol inhibits the growth of rat glioblastoma cells and human glioblastoma cells by inhibiting VEGFR expression, inducing apoptosis and cell cycle arrest (Huang et al., 2008; Ge et al., 2010). Recent studies have shown that celastrol triggers apoptosis and autophagy by activating the ROS/JNK signaling pathway and blocking the AKT/mTOR signaling pathway, inducing G2/M phase block, and in a dose-dependent manner. In vivo experiments showed that celastrol increased the levels of cleaved Caspase-3, LC3B, and p-JNK, decreased the expression of p-AKT and mTOR, and inhibited the growth of tumors in a mouse glioblastoma in situ transplantation model. Naïve T cells are relatively quiescent until exposed to antigenic stimulation, they are metabolically quiescent and have low nutrient uptake, glycolytic rate and biosynthesis. Calcium store-operated calcium entry (SOCE) directs the proliferation of naïve T cells and adaptive immune responses by activating the nuclear factor of activated T cells (NFA T) and the PI3K-AKT-mTOR pathway regulating the expression of glucose transporter proteins, glycolytic enzymes, and metabolic regulators (Vaeth et al., 2017). It was shown that both channels are regulated by intracellular Ca2+ signaling and were shown to be involved in the downstream mechanisms of Piezo1 (Liu et al., 2019).
4.5.2 FAKsAdhesion zones are hubs of cytoskeletal structures connecting the extracellular matrix to the intracellular skeleton, and they detect and transmit external mechanical forces. Focal adhesion kinase (FAK), a member of the adhesion zone proteins, is a key regulator of adhesion plaque dynamics and cytoskeletal remodeling, which exhibits high expression in a wide range of tumors and correlates with poor prognosis. FAK activation promotes tumor growth, invasion, metastasis, and angiogenesis through kinase-dependent (such as PI3K/AKT signaling pathway, P53 signaling pathway, RAS/RAF/MEK/ERK signaling pathway, YAP signaling pathway) and kinase-independent pathways (e.g., inhibition of T-cells, B-cells, and dendritic cells in the immune microenvironment. Promote myeloid-derived suppressor cells (MDSC), tumor-associated macrophages (TAM), cancer-associated fibroblasts (CAF), and so on.) (Hu et al., 2024; Kim et al., 2011). Piezo1 interacts with FAK and signals to transcriptional co-activators through the pdz-binding motif (TAZ), leading to chromatin remodeling and changes in transcript levels (Hu et al., 2017a). Tumor tissue sclerosis provides a mechanistic microenvironment for the activation of Piezo1, which promotes adhesion plaque assembly and activates the β1-integrin-FAK signaling pathway, regulates cell proliferation and participates in extracellular matrix remodeling, which further regulates tissue stiffness and the stiffer environment further enhances Piezo1 expression, which increases mechanosensory and mechanotransduction capacities of tumor cells (Chen et al., 2018). Tumor cells thus form a feedback loop between Piezo1-dependent mechanosensing and abnormal tissue mechanics that interact and exacerbate disease. Integrin inhibitors (such as cilengitide or etanercept) have been tested in clinical trials. A randomized phase II trial demonstrated that cilengitide monotherapy was well tolerated and had modest anti-tumor activity against recurrent glioblastoma (Chen et al., 2018). However, a phase III trial demonstrated that cilengitide in combination with standard temozolomide radiotherapy did not have better efficacy than conventional treatment in glioblastoma, particularly in tumors with methylated O6 -methylguanine-DNA methyltransferase (MGMT) promoters, and the use of cilengitide as a subunit antagonist of the integrins αvβ3 and αvβ5 is currently still controversial (Desgrosellier and Cheresh, 2010).
4.5.3 YAPYes-associated protein (YAP), an important co-transcription factor of the Hippo signaling pathway, plays an important role in mechanistic effects and the malignant proliferation and invasion of tumor cells. YAP/transcriptional coactivator with PDZ-binding motif (TAZ) is an effector of mechanistic signaling stimuli of matrix stiffness, stretch, and cell density. Mechanically relevant events such as altering mechanical stimuli inside and outside the cell, inoculating cells with different matrix stiffnesses, exposing cells to periodic stretching motions, and adjusting the degree of cell adhesion to the extracellular matrix can cause activation of YAP/TAZ in the nucleus (Zanconato et al., 2016). Abnormalities in YAP function have been closely associated with glioblastomagenesis, cellular proliferation, maintenance of tumor cell stemness, and invasive metastasis. Matrix stiffness can promote glioblastoma cell proliferation, EMT, and angiogenic mimicry formation through YAP, etc (Castellan et al., 2021). Piezo1 acts as a mechanosignal receptor, transmits extracellular matrix stiffness signals, and promotes glioblastoma cell proliferation, invasion, and epithelial-mesenchymal transition by regulating the nuclear expression and activity of YAP and subsequent TAZ activation. The study demonstrated that the piezo1 agonist Yoda1 significantly increased intracellular Ca2+ concentration in the cytoplasm of valvular mesenchymal stromal cells, whereas piezo1 knockdown reduced Ca2+ influx. Simultaneous immunofluorescence determination of YAP subcellular localization demonstrated that Yoda1 triggered YAP nuclear translocation, whereas piezo1 knockdown prevented cytoplasmic YAP from migrating to the nucleus. Using the calcium chelator1,2-bis (2-aminophenoxy)ethane-n,n,n0,n0-tetraacetic Acid Tetrakis (acetoxymethyl Ester) (BAPTA-AM) to reduce intracellular Ca2+ concentration, it was observed that BAPTA-AM eliminated Yoda1-induced intracellular Ca2+ accumulation and that BAPTA-AM inhibited Yoda1-induced YAP nuclear translocation, resulting in YAP being mostly localized in the cytoplasm. These results suggest that intracellular calcium accumulation is responsible for the YAP nuclear translocation induced by piezo1 (Zhong et al., 2023). Meanwhile, it was found that YAP was located downstream of β1-integrin-RhoA, which was an important effector of cellular response to matrix signals and mechanical stimuli (Zoi et al., 2022). Downregulation of the expression of Piezo1 had a significant effect on the β1-integrin/FAK pathway, and also significantly affected the nuclear localization and activity of YAP, suggesting that Piezo1 promotes the progression of glioblastoma invasion through the β1-integrin/FAK/YAP axis. invasion progression. It was shown that knockdown of the Piezo1 gene in glioblastomas using interfering shRNA lentiviral vectors resulted in a significant increase in the expression of the cellular epithelial marker E-cadherin, and a significant decrease in the expression of the mesenchymal markers waveform protein, SNAIL, and Slug. This suggests that the expression of Piezo1 regulates EMT, and maintains mesenchymal characteristics of glioblastoma cells. characteristics of glioblastoma cells (Figure 3).
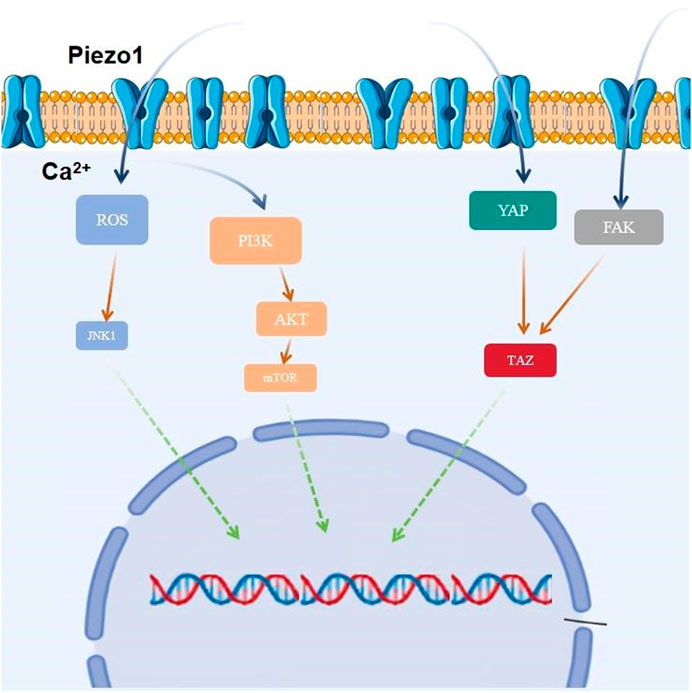
Figure 3. Piezo1 is involved in glioblastoma proliferation by regulating multiple signaling pathways.
4.6 Relationship between Piezo1 and glioblastoma exosomesExosomes are small extracellular vesicles (EVs) with a diameter of 40–160 nm (average ∼100 nm) that mediate intercellular communication (Kalluri and LeBleu, 2020). Exosomes contain components such as proteins, lipids, DNA, mRNA, and non-coding RNAs, which play important roles in tumor angiogenesis, invasion, metastasis, and drug resistance. Cyclic RNAs are a novel class of RNA transcripts produced by head-to-tail splicing of exons, which are produced and structured differently from mRNAs and therefore have unique cellular functions and potential biomedical applications (Memczak et al., 2013). Studies have shown that circular RNAs play an important regulatory role in glioblastoma. circZNF800 is a recently identified circular RNA that is highly expressed in glioblastoma stem cells. Studies have shown that glioblastoma stem cells secrete exosomes to enhance GBM cell proliferation and migration and inhibit apoptosis in glioblastoma. highly expressed circZNF800 regulates the expression of tumor-associated genes through the circRNA/PI3K/AKT axis and promotes glioblastoma progression (Zhang et al., 2024).
Micro RNAs (miRNAs) are small, highly conserved, small non-coding RNA molecules that control gene expression at the post-transcriptional level. It inhibits translation or promotes degradation of mRNAs by binding to complementary binding sites within the 3‘ untranslated region (3’UTR) of target messenger RNAs (mRNAs)(Bartel, 2004). miRNA-139 is located at 11q13.4 and exhibits anti-tumor and anti-metastatic activities in humans. It was shown that miR-139-5p was significantly reduced and negatively correlated with circZNF800 expression in GBM tissues compared with normal brain tissues. miR-139-5p inhibitor significantly promoted the expression of p-AKT protein, which further promoted the invasion of U251 and U87 cells and inhibited apoptosis of GBM cells, whereas the downregulation of circZNF800 eliminated this effect. Piezo1, a direct target of miR - 1395p, was regulated by circZNF800, and correlation analysis showed a positive correlation between the expression levels of circZNF800 and Piezo1 mRNA. In GBM cells, miR-139-5p significantly reduced the expression of Piezo1 mRNA. Whereas overexpression of circZNF800 significantly increased Piezo1 mRNA expression. CircZNF800 activated the Piezo1/AKT signaling pathway through phagocytosis of miR-139-5p and regulated proliferation, migration, and apoptosis of GBM cells (Zhang et al., 2024).
4.7 Piezo1 affects the extracellular matrix of glioblastomasThe extracellular matrix is the general scaffold that maintains the homeostasis of tissues and organs in vivo. It is also a key component of the cancer microenvironment that supports tumorigenesis. During tumor development and progression, a complex ECM network is established by fibrous or nonfibrous collagen, elastin, proteoglycans, glycoproteins, laminin, fibronectin, and other matrix proteins. The extracellular matrix not only provides nests for cancer cells and stromal cells, but also serves as a reservoir for growth factors and cytokines. In addition, the ECM interacts with stromal, parenchymal, precancerous or cancer cells to initiate different cell signaling cascades that stimulate cellular differentiation metastasis, autophagy, epithelial-mesenchymal transition (EMT), cell migration, invasion and metabolic reprogramming to promote cancer cell proliferation, migration, invasion, angiogenesis, and immune evasion. mechanical stimuli induced by both the ECM per se and by ECM sclerosis can activate cell membrane receptors and mechanosensors such as integrins, piezo1 and transient receptor potential ion channel subfamily V 4 (TRPV4), thereby modulating the malignant phenotype of tumor and stromal cells. In the glioblastoma tumor microenvironment, Piezo1 plays a particularly strong role in the ECM signaling pathway.
As a therapeutic target for glioblastomas, Piezo1 senses microenvironmental stiffness and converts mechanical stimuli into electrical and chemical signals that promote calcium influx. Elevated intracellular Ca2+ concentration catalyzes the assembly and maturation of focal adhesions and directly activates the integrin-focal adhesion pathway, which stimulates cell proliferation and regulates extracellular matrix remodeling, further increasing glioblastoma tissue stiffness in positive feedback. Enhanced stiffness between glioblastoma cells and the ECM microenvironment in turn regulates the activation of Piezo1.
Tumor cells and stromal cells can respond to mechanical signals induced by matrix sclerosis. ECM sclerosis typically induces mechanical perturbation of the lipid bilayer and activation of transient receptor potential (TRP) family channels and piezo1 channels, evolutionarily conserved ion channels that link ECM sclerosis-associated mechanical forces to cellular signaling pathways, particularly Ca2+ signaling in tumor and stromal cells. The cell surface receptor integrin is a mechanosignal transducer that can be activated by Piezo1 to promote cancer stem cells and drug resistance. Physical interactions between the extracellular structural domains of integrins and ECM proteins induce the assembly of cytoplasmic complexes consisting of scaffolding proteins (neuregulin, talin, stumpins, etc.), adhesion plaque kinase (FAK), Src, and PI3K/Akt, which orchestrate adhesion plaque and cytoskeletal assembly with stromal mechanical cues. The Rap1 GTPase also responds to the cytoplasmic signaling of integrins by stabilizing integrins and recruiting neuregulin to the adhesion plaque. Rap1 GTPase also responds to matrix sclerosis by stabilizing integrins and recruiting connexins to adhesion patches. In addition, Rho-associated coiled-coil kinases (ROCK) activation may be induced by ECM sclerosis, which then promotes integrin signaling, mitogen-activated protein kinase (MAPK) activation, and SNAIL stabilization. Integrins, integrin-linked kinase (ILK), SNAIL, and Src upregulate YAP expression and activation, thereby upregulating piezo1 expression.
Piezo1 senses the stiffness of the microenvironment and converts mechanical stimuli into electronic and chemical signals that promote calcium influx. Elevated intracellular Ca2+ concentrations catalyze the assembly and maturation of focal adhesions, and piezo1 activates the integrin-FAK pathway when the extracellular structural domains of integrins interact with ECM proteins such as collagen, laminin, and tendon proteins, whose cytoplasmic structural domains are complexed with scaffolding proteins, including ankyrin and stumpyrin, as well as kinases, such as adhesion patch kinase (FAK) and Src. Through integrin-taIin, talin-FAK interactions, multiple FAK molecules accumulate at the focal adhesion site, resulting in the activation of FAK by phosphorylation and its association with other signaling molecules. Upon activation of FAK, its phosphorylated Tyr397 binds to the Src family of kinases, causing the PAK/Src complex by forming the FAK/Src complex. Src complex and causes phosphorylation of Paxillin and clustered regularly interspaced shortpalindromic repeats (CRISPR)-associated systems (Cas), which activate mitogen-activated protein kinase (MAPK), a kinase downstream of the rat sarcoma (Ras) pathway, via the junction proteins cysteine-rich receptor-like protein kinases (Crk) and growth factor receptor-bound protein 2 (Grb2) to control lesion adhesion and cytoskeleton assembly, regulate extracellular matrix remodeling, and increase the stiffness of glioblastoma tissues. and activate integrin-dependent intracellular kinase signaling to regulate cell adhesion, motility, proliferation, survival and differentiation (Chen et al., 2018).
It was shown that GBM stem cells were cultured in polyacrylamide hydrogels at different stiffness levels (100-5,000 Pa) in the normal human brain, low-grade glioblastomas, and high-grade glioblastomas. The percentage of proliferatively active GBM stem cells and the total number of cells increased with increasing stiffness, while Piezo1 knockdown eliminated this stiffness-dependent tumor cell growth (Chen et al., 2018).
Furthermore, many signaling pathways such as matrix metalloproteinase (MMP) family, tissue inhibitor of metalloproteinases (TIMP) family, mitogen-activated protein kinase (MAPK) family, phosphoinositide 3 kinase (PI3K) family, and others are positively correlated with high expression of Piezo1 during the pathology of glioblastoma development. Various pathways such as the mitotic cell cycle, focal adhesion, ECM, and tumor cell response to mechanical stimuli were altered after the knockdown of Piezo1. In the CGGA and TCGA databases, Piezo1 expression was positively correlated with the genes TIMP1, MMP2, MMP9, MAPK13, MAPKAPK2, PIK3R6, PIK3CD, PLOD1, and AKT2, and was also positively correlated with the genes TIMP1, MMP2, MMP9, MAPK13, MAPKAPK2, PIK3R6, PIK3CD, PLOD1, and AKT2, as opposed to low-grade glioblastomas (WHO classification II and WHO classification III) in glioblastomas (WHO grade IV) had significantly higher expression of all ECM-related genes positively associated with Piezo1. Among these genes, TIMP1, MAPK13, MAPKAPK2, and MAPKAPK3 regulate cell proliferation, PLOD1, MMP14, ADAM9, and PLAU control ECM remodeling, MMP2, and MMP9 control tumor-associated tissue remodeling; PIK3CD is involved in immune response, AKT2 regulates tumorigenesis, FHL3 controls actin cytoskeleton, and TAZ regulates the mechanosensitive HIPPO signaling pathway (Figure 4). Taken together, these data suggest that Piezo1 not only delivers mechanical inputs to promote glioblastoma growth but also positively regulates the ECM and other mechanotransduction mechanisms in tumors (Zhou et al., 2020).
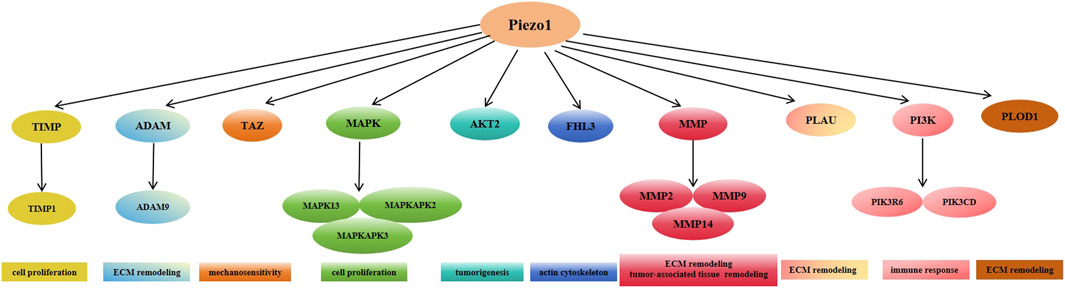
Figure 4. Expression of Piezo1 positively correlates with multiple genes mediating extracellular matrix remodeling in glioblastoma.
4.8 Piezo1 contributes to the immunosuppressive tumor microenvironment in glioblastomaIn healthy brain tissue, glioblastoma-associated microglia (GAM) are highly branched, and branched microglia usually exhibit properties of M1-type macrophages that secrete pro-inflammatory factors to destroy pathogens. When inflammation, infection, trauma, or other neurologic diseases occur in the brain, microglia transform to the M2-type macrophage form. The transformed microglia have enlarged cell bodies, shortened protrusions, amoeboid morphology and activated phagocytosis. (Gieryng et al., 2017). GAM is the predominant inflammatory cell in the tumor micro-environment (TME), which not only exerts an immunosuppressive function and induces immune tolerance, but also promotes tumor growth and metastasis (Lin et al., 2024). Additionally, studies have shown that the immunosuppressive phenotype of GAMs correlates with GBM grade, with higher grades showing greater prevalence of tolerant or protumor GAMs (De et al., 2016). Neovascularisation in malignant tumors is one of the features that distinguish them from benign tumors, and the results of both clinical and experimental studies support the production of angiogenic factors [e.g., VEGF] by GAM to stimulate angiogenesis in glioblastomas. Studies have shown that IL-6 released from GAM keeps the blood-brain barrier in glioblastoma patients highly permeable by activating the Janus kinase 2/signal transducer and activator of transcription 3 (JAK/STAT3) pathway in endothelial cells and down-regulating the levels of intercellular junction proteins, which is responsible for the formation of vasogenic brain oedema (Couto et al., 2019). GAM-released IL-1β can also be used to stimulate the formation of vasculogenic brain oedema in glioblastoma cells by promoting glycerol-3-phosphate dehydrogenase 2 (GPD2) phosphorylation and glycolysis, thereby accelerating tumor proliferation and growth (Lu et al., 2020). In addition, microglia induce platelet-derived growth factor receptor (PDGFR) expression in mouse glioblastoma models and tumor cells from human patients, and the expression of this receptor stimulates glioblastoma cell migration, thereby accelerating tumor progression (Sherafat et al., 2021; Wallmann et al., 2018). Piezo1-mediated increases in cytoplasmic Ca2+ are involved in the inhibition of microglia proliferation. Piezo1 opening promotes extracellular Ca2+ endocytosis and releases Ca2+ stored in the endoplasmic reticulum into the cytoplasm, creating a cytoplasmic Ca2+ peak that interferes with the homeostasis of endoplasmic reticulum-mitochondrial Ca2+ communication and induces microglial dysfunction. Piezo1 is recruited to the endoplasmic reticulum via the R-Ras (which is most likely in an activated state), and then stimulates calcineurin signaling to maintain integrin activation, thereby enhancing cell adhesion. In vitro, microglia cultured on hardness-gradient hydrogels migrate to harder regions and are more responsive in these regions, suggesting that microglia may migrate towards and function in harder tumor tissue in vivo. Inhibition of Piezo1 expression modulates the affinity for integrins within microglia, thereby increasing the rate of cell migration (Hu et al., 2023). Bone marrow-derived macrophages (BMDM) migrate to the tumor site and accumulate in the central region of the GBM, exerting a predominantly immunosuppressive effect, whereas resident microglia have little or no immunosuppressive function. In grade II and III glioblastomas, microglia accounted for the majority of macrophages and lacked significant immunosuppressive activity (Miller and Hjelmeland, 2023). Activation of Piezo1 channel proteins by mechanical stimulation regulates macrophage functional differentiation, modulates dendritic cells (DC) responses triggers inflammation, and participates in T-cell activation, among many other ways, in immune cell infiltration in the tumor microenvironment (Pinton et al., 2019).
Mechanical signaling has been identified as a potential trigger for inflammatory activation in microglia. Piezo1 has the ability to regulate microglial migration patterns and immune responses, and has been suggested to act as a mechanosensor for microglial cell lines. Piezo1 regulates the pro-inflammatory response of microglial cells upon activation by lipopolysaccharide (LPS), a commonly used surrogate for bacterial infections, inducing a transient changes. In vitro and in vivo, deletion of the Piezo1 gene in microglia reduced LPS-induced proinflammatory cytokine production, whereas Yoda1 increased LPS-stimulated proinflammatory cytokine production in microglia. Similar to microglia, macrophages lacking the Piezo1 gene exhibited reduced inflammation under both resting and endotoxin-stimulated conditions, accompanied by decreased proinflammatory cytokine production. These alterations may be related to Piezo1-mediated Ca2+influx and crosstalk between cytoskeletal and integrin proteins and piezo1 (Hu et al., 2023). While at the same time, some studies have shown that LPS can lead to microglia activation via Toll-like receptor 4 (TLR4), leading to activation of NF-κB signaling via the extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 mitogen-activated protein kinase (MAPK) pathways, which ultimately drives TNF-α and IL-6 expression and production. However Yoda1 inhibits LPS-induced NF-κB activation by activating piezo1-Ca2+ signaling. These results suggest that activation of piezo1 channels reduces LPS-induced microglia activation and proinflammatory cytokine production, and that NF-κB signaling may act as a major mediator of (Malko et al., 2023).
In addition to this, ROS generated by SDT stimulation interferes with mitochondrial oxidative respiration, allowing more Ca2+ to enter the mitochondria, leading to mitochondrial calcium overload and dysfunction. MCU inhibition due to mitochondrial dysfunction prevents mitochondrial calcium uptake and promotes M1 polarization (Tedesco et al., 2019). Early intervention of Ca2+ channels in combination with SDT increases the proportion of M1-type macrophages with pro-inflammatory and anti-tumor effects.
Piezo1, integrins, and YAP are also strongly associated with cancer immunity. High collagen density and piezo1 activation promote macrophage polarization and enhance their immunosuppressive phenotype, leading to a decrease in cytotoxic T cell numbers and proliferation and inducing the expansion of immunosuppressive myeloid cells. Studies have shown that deletion of piezo1 in T cells does not affect effector T cell function but increases immunosuppressive myeloid cell, suggesting that activation of piezo1 in T cells may enhance the immune response in autoimmune diseases. In addition, increased matrix stiffness impedes the ability of dendritic cells to elicit an immune response in vitro and inhibits dendritic cell migration, which is essential for activating T cells and eliciting an immune response. necessary for T cells and eliciting an immune response (Jiang et al., 2022).
4.9 Involvement of Piezo1 in abnormal angiogenesis in glioblastomaIncreased intracellular Ca2+ concentration plays a crucial role in angiogenesis and arterial remodeling (Moccia et al., 2014; Moccia et al., 2012). Growth factors and cytokines, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), angiopoietin, and stromal-derived factor-1α (SDF-1α), trigger potent Ca2+ signaling in vascular endothelial cells (Potenza et al., 2014; Moccia et al., 2003; Noren et al., 2016; Munaron and Fiorio Pla, 2000) recruiting large numbers of downstream Ca2+-dependent pro-angiogenic transcription factors. These transcription factors include but are not limited to the nuclear factor of activated t-cells (NFAT), nuclear factor κ b (NF-κB) and cyclic adenosine monophosphate (cAMP) -response element binding protein (CREB) (Noren et al., 2016; Zhu et al., 2008; Chen et al., 2016), myosin li
留言 (0)