Branched-chain Amino Acids (BCAAs) — namely isoleucine, leucine, and valine — are essential amino acids that play a crucial role in metabolic homeostasis through nutritional signaling (1). Elevated levels of BCAAs and their related metabolites are now recognized as metabolic markers for obesity, insulin resistance, and type 2 diabetes in humans (2, 3). Given the close link between metabolic disorders and the pathogenesis of cardiovascular disease (CVD), research suggests that BCAAs may directly contribute to heart failure (HF) (4), vascular disease (5, 6), hypertension (7), and arrhythmias (8).
Currently, the mechanisms underlying the association between BCAAs and cardiovascular disease (CVD) are not fully understood. Known mechanisms include the activation of the serine/threonine protein kinase mTOR by BCAAs (9), mitochondrial dysfunction (10), alterations in cardiac substrate utilization, and platelet activation (11).
Additional studies have found that cardiovascular disease (CVD) can induce changes in tissues that regulate BCAA homeostasis, such as skeletal muscle, liver, and adipose tissue. These changes may contribute to elevated circulating level of BCAAs in individuals with CVD (12). Therefore, further research is needed to elucidate the causal relationship between BCAA dysregulation and CVD.
Currently, there are limited large-scale, prospective clinical studies investigating the causal relationship between BCAAs - including isoleucine, leucine, and valine - and cardiovascular disease (CVD) risk (13). While most studies suggest that high levels of amino acids (AAs) predict increased CVD risk (14), many of these studies have primarily focused on aromatic amino acids and specific populations (such as heart failure patients, women, and the elderly) (13, 15–18), and typically involve relatively small sample sizes. Some studies have even suggested that reduced BCAAs levels are associated with an increased risk of major adverse cardiovascular events (MACE), particularly in populations of elderly men over 70 years of age (17). Given the notable differences in BCAA levels between men and women, as well as across age groups, there is a need for large-sample studies that systematically explore how BCAA levels correlate with CVD risk across various demographics.
Our study aimed to investigate the relationship between baseline BCAAs and the risk of major adverse cardiovascular events (MACE) in a large prospective cohort. Additionally, we explored whether gender and age influence this relationship.
2 Materials and methods2.1 Study design and sampleUK Biobank recruited participants aged between 40 and 70 years from across the UK between 2006 to 2010 (19). At enrollment, comprehensive data were collected on sociodemographic factors (e.g., age, sex), lifestyle behaviors (smoking and drinking status, diet, and physical activity level), medical history, and genetic information through touchscreen questionnaires, physical examinations, and sample analyses. Baseline biochemical assays included amino acids, HbA1c, and blood lipids measurements.
Health-related outcomes were monitored through regular linkages with various national datasets, including primary care records, hospital admissions, and mortality registries (20).
2.2 Standard protocol approvals, registrations, and patient consentsAssessments were conducted at 22 centers across 22 Scotland, England, and Wales as part of the UK Biobank study. The assessment process consisted of five components: written consent, touchscreen questionnaires (including detailed dietary recall), face-to-face interviews with study nurses, physical measurements (such as hand grip strength, spirometry, and bone density scans), and collection of blood, urine, and saliva samples.
The UK Biobank database contains information from 502,359 participants. In this study, participants who had prevalent MACE at the time of recruitment (n=13,546) or had missing values for BCAAs, isoleucine, leucine, and valine (n=222,003) were excluded from the analysis. Ultimately, 266,840 participants were included in the study. The UK Biobank has obtained ethical approval from the NHS National Research Ethics Service (16/NW/0274), and all participants provided informed consent before data collection.
2.3 Outcome measure: MACEThe primary outcome of this study was the incidence of MACE, defined as a composite endpoint including cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and hospitalization for unstable angina. Cases were identified using hospital inpatient records (primary or secondary hospital diagnosis) or death registry records (underlying or contributory cause of death). Diagnosis were based on the International Cleucinesification of Diseases coding system (ICD-9 and ICD-10).
2.4 BCAAs and covariatesGiven the inconsistencies in prior research regarding the correlation between BCAAs levels and the risk of MACE, particularly in males, we hypothesize a potential J-shaped relationship. To more effectively explore this relationship, we have categorized the levels of BCAAs, isoleucine, leucine, and valine into quintiles,Q2 was used as the reference category. Covariates included gender, age, body mass index (BMI) categorized as underweight (<18.5kg/m2), normal (18.5-23.9 kg/m2), overweight (24.0-28.0 kg/m2), and obese (≥28.0 kg/m2) (21). Other covariates included HbA1c, Low-density lipoprotein cholesterol(LDL-c), systolic blood pressure(SBP), smoking and drinking status (never, current, and past), and physical activity level (measured in MET).
2.5 Statistical analysisCumulative incidence of MACE was computed according to maternal HDP status. We examined the association of BCAAs level - including isoleucine, leucine, and valine - with MACE using a Cox regression model with follow-up time as the time scale. Adjusted hazard ratios and corresponding 95% confidence intervals were calculated after adjusting for gender, age, body mass index (BMI)(underweight: <18.5, normal: 18.5-23.9, overweight: 24.0-28.0, obese: ≥28.0), HbA1c, LDL-c, SBP, smoking and drinking status(never, current and past), and activity (Metabolic Equivalent of Task, MET).
To evaluate the influence of gender and age on the relationship between BCAAs and MACE, we conducted separate analyses for men and women, as well as for individuals aged 65 and above compared to those below this age threshold.
3 Results3.1 Baseline characteristicsA total of 266840 individuals were included in the final study, 121,067(45.37%) males and 145,773 (54.63%) females. The baseline average age was 56.95 ± 8.08 yrs. During a follow-up period of 13.80 ± 0.83 years, MACE occurred in 52,598(10.47%) participants.
Baseline characteristics showed that BMI, HbA1c, triglyceride, and C-reactive protein (CRP) increased progressively across the quintile of BCAAs, while HDL-C level decreased across the quintiles. Age, SBP, and DBP (diastolic blood pressure) showed consistent trends(Table 1). Similar trends were observed for BMI, blood pressure, HbA1c, triglycerides, CRP, HDL, Age, SBP, and DBP across the quintiles of isoleucine, leucine, and valine.
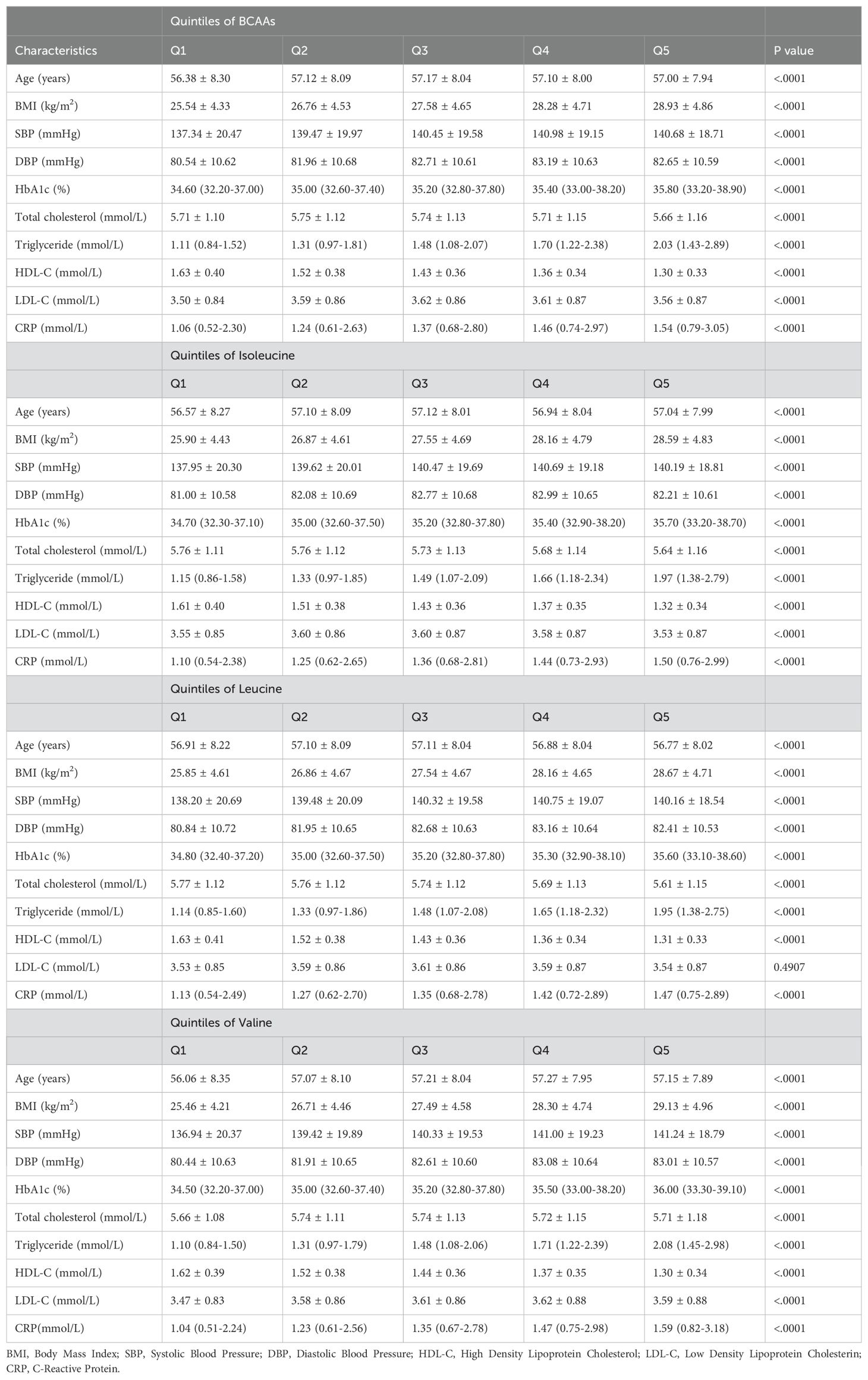
Table 1. Baseline characteristics according to quintiles of BCAAs, isoleucine, leucine and valine.
3.2 Incidence of MACEIn the overall population (Figure 1A), the incidence of MACE increased progressively across the quintiles for each BCAA:
● For BCAAs: 6.34% (1st quintile), 7.18% (2nd quintile), 8.08% (3rd quintile), 8.79% (4th quintile), and 9.79% (5th quintile).
● For isoleucine: 6.39% (1st quintile), 7.24% (2nd quintile), 7.99% (3rd quintile), 8.97% (4th quintile), and 9.58% (5th quintile).
● For leucine: 6.63% (1st quintile), 7.13% (2nd quintile), 8.02% (3rd quintile), 8.65% (4th quintile), and 9.74% (5th quintile).
● For valine: 6.42% (1st quintile), 7.16% (2nd quintile), 7.98% (3rd quintile), 8.85% (4th quintile), and 9.76% (5th quintile).
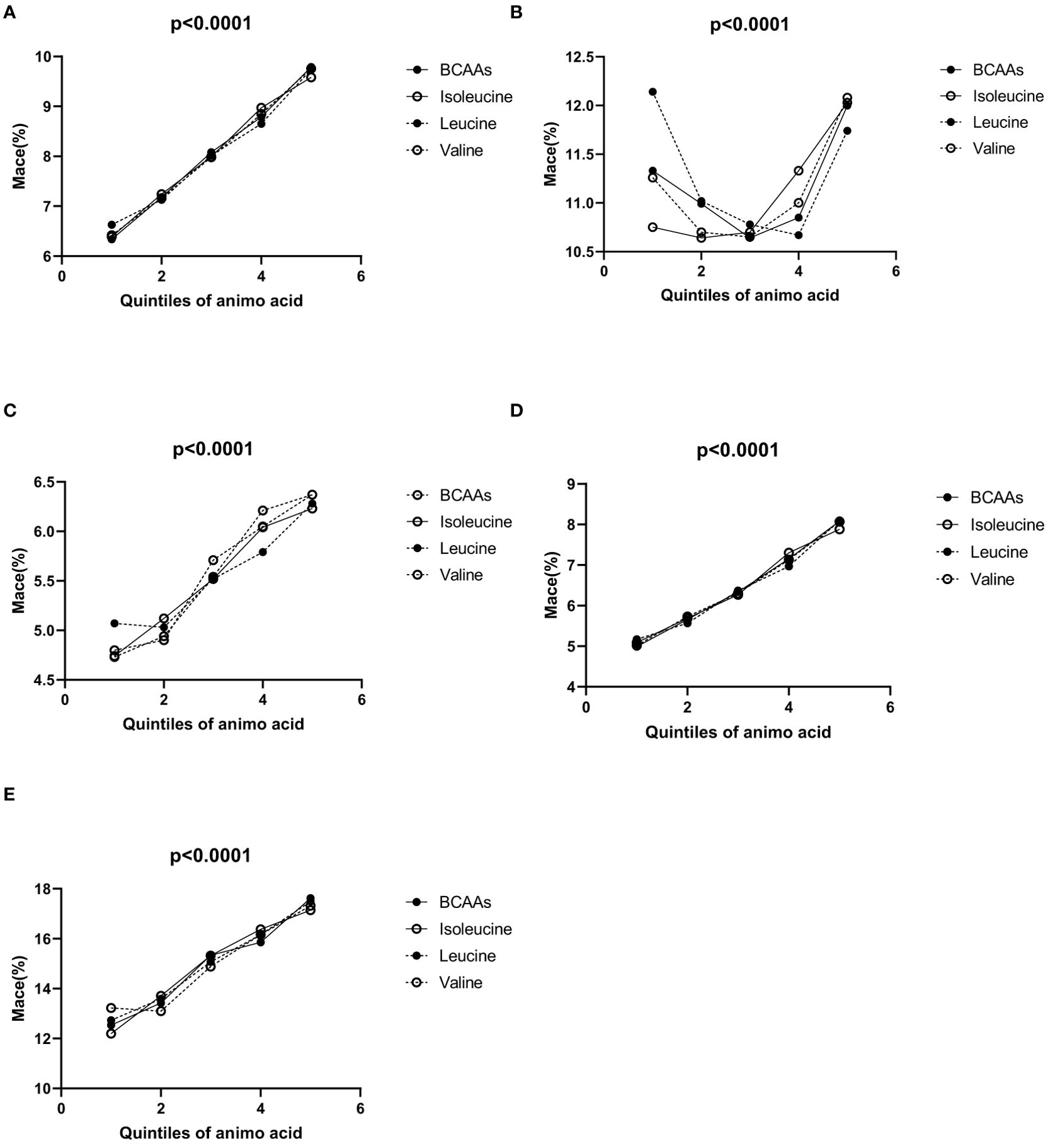
Figure 1. (A–E) Incidence of MACE according to the quintiles of BCAAs, isoleucine, leucine and valine. (A) Incidence of MACE according to the quintiles of BCAAs, isoleucine, leucine and valine in overall population. (B) Incidence of MACE according to the quintiles of BCAAs, isoleucine, leucine and valine in males. (C) Incidence of MACE according to the quintiles of BCAAs, isoleucine, leucine and valine in females. (D) Incidence of MACE according to the quintiles of BCAAs, isoleucine, leucine and valine in participants under 65. (E) Incidence of MACE according to the quintiles of BCAAs, isoleucine, leucine and valine in 65 and older.
In males (Figure 1B), for BCAAs, using the second quintile (10.99%) as a reference, the incidence of MACE was higher in the first quintile (11.33%), and then varied slightly in the third (10.64%), fourth (10.85%), and fifth quintiles (12%). For isoleucine, with the second quintile (10.64%) as a reference, the incidence of MACE was higher in the first quintile (10.75%), and continued to increase in the third (10.70%), fourth (11.33%), and fifth quintiles (12.03%). For leucine, taking the second quintile (11.02%) as a reference, the first quintile showed a higher incidence of MACE (12.14%), while the third (10.78%), fourth (10.67%), and fifth quintiles (11.74%) exhibited variations. For valine, using the second quintile (10.7%) as a reference, the incidence of MACE was higher in the first quintile (11.26%) and increased slightly in the third (10.65%), fourth (11%), and fifth quintiles (12.08%).
In females (Figure 1C), for BCAAs, the incidence of MACE increased across the quintiles: 4.8% (1st quintile), 4.9% (2nd quintile), 5.71% (3rd quintile), 6.05% (4th quintile), and 6.37% (5th quintile). For isoleucine, the incidence of MACE rosed gradually across the quintiles: 4.74% (1st quintile), 5.12% (2nd quintile), 5.52% (3rd quintile), 6.04% (4th quintile), and 6.23% (5th quintile). For leucine, the incidence of MACE increased with the quintiles: 5.07% (1st quintile), 5.03% (2nd quintile), 5.52% (3rd quintile), 5.79% (4th quintile), and 6.28% (5th quintile). For valine, the incidence of MACE increased across the quintiles: 4.73% (1st quintile), 4.94% (2nd quintile), 5.54% (3rd quintile), 6.21% (4th quintile), and 6.37% (5th quintile).
In participants under 65 (Figure 1D), the incidence of MACE increased progressively across the quintiles for each BCAA:
● For BCAAs: 4.99% (1st quintile), 5.66% (2nd quintile), 6.34% (3rd quintile), 7.15% (4th quintile), and 8.08% (5th quintile).
● For isoleucine: 5.09% (1st quintile), 5.69% (2nd quintile), 6.27% (3rd quintile), 7.30% (4th quintile), and 7.88% (5th quintile).
● For leucine: 5.17% (1st quintile), 5.57% (2nd quintile), 6.37% (3rd quintile), 6.97% (4th quintile), and 8.10% (5th quintile).
● For valine: 5.02% (1st quintile), 5.73% (2nd quintile), 6.30% (3rd quintile), 7.13% (4th quintile), and 8.07% (5th quintile).
In all groups, the p-values were <0.05, indicating statistical significance.
In participants aged 65 and older (Figure 1E), the incidence of MACE also increased across the quintiles for each BCAA:
● For BCAAs: 12.54% (1st quintile), 13.42% (2nd quintile), 15.34% (3rd quintile), 15.86% (4th quintile), and 17.62% (5th quintile).
● For isoleucine: 12.20% (1st quintile), 13.71% (2nd quintile), 15.32% (3rd quintile), 16.38% (4th quintile), and 17.14% (5th quintile).
● For leucine: 12.73% (1st quintile), 13.59% (2nd quintile), 15.07% (3rd quintile), 16.16% (4th quintile), and 17.49% (5th quintile).
● For valine: 13.22% (1st quintile), 13.10% (2nd quintile), 14.88% (3rd quintile), 16.16% (4th quintile), and 17.32% (5th quintile).
In the overall population (Figures 2A–D), the cumulative incidence of MACE was lowest in the first quintile for BCAAs, isoleucine, leucine, and valine, with a gradual increase across the higher quintiles. The log-rank P values for the survival curves in Figures 2A–D were all <0.001.
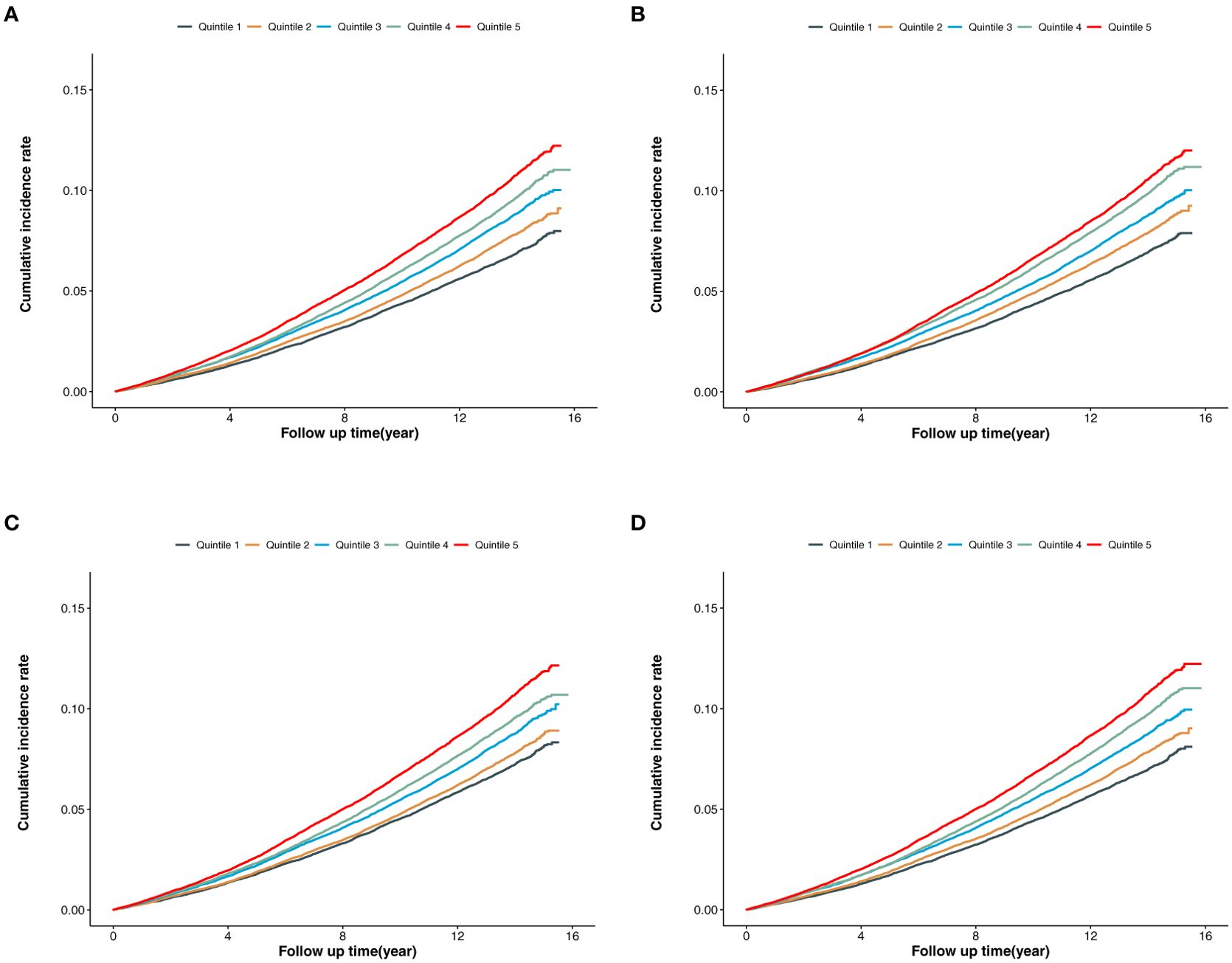
Figure 2. (A) Cumulative incidence of MACE according to the quintiles of BCAAs. (B) Cumulative incidence of MACE according to the quintiles of isoleucine. (C) Cumulative incidence of MACE according to the quintiles of leucine. (D) Cumulative incidence of MACE according to the quintiles of valine.
3.3 Association between BCAAs and MACEIn the overall population (Table 2A), compared to the second quintile, individuals in the fifth quintile of BCAAs had a 7% increased risk of MACE (95% CI 1.02-1.13) after adjusting for age, gender, BMI, HbA1c, LDL, SBP, smoking and drinking status, and physical activity. For isoleucine, the increased risk of MACE was 8% in the fourth quintile (95% CI 1.03-1.14), and 11% in the fifth quintile (95% CI 1.06-1.16). For leucine, participants in the first quintile had a 5% increased risk of MACE (95% CI 1.00-1.11), while those in the fifth quintile had an 8% increased risk (95% CI 1.03-1.13). For valine, the increased risk was 6% in both the first quintile (95% CI 1.01-1.12) and the fifth quintile (95% CI 1.01-1.11).
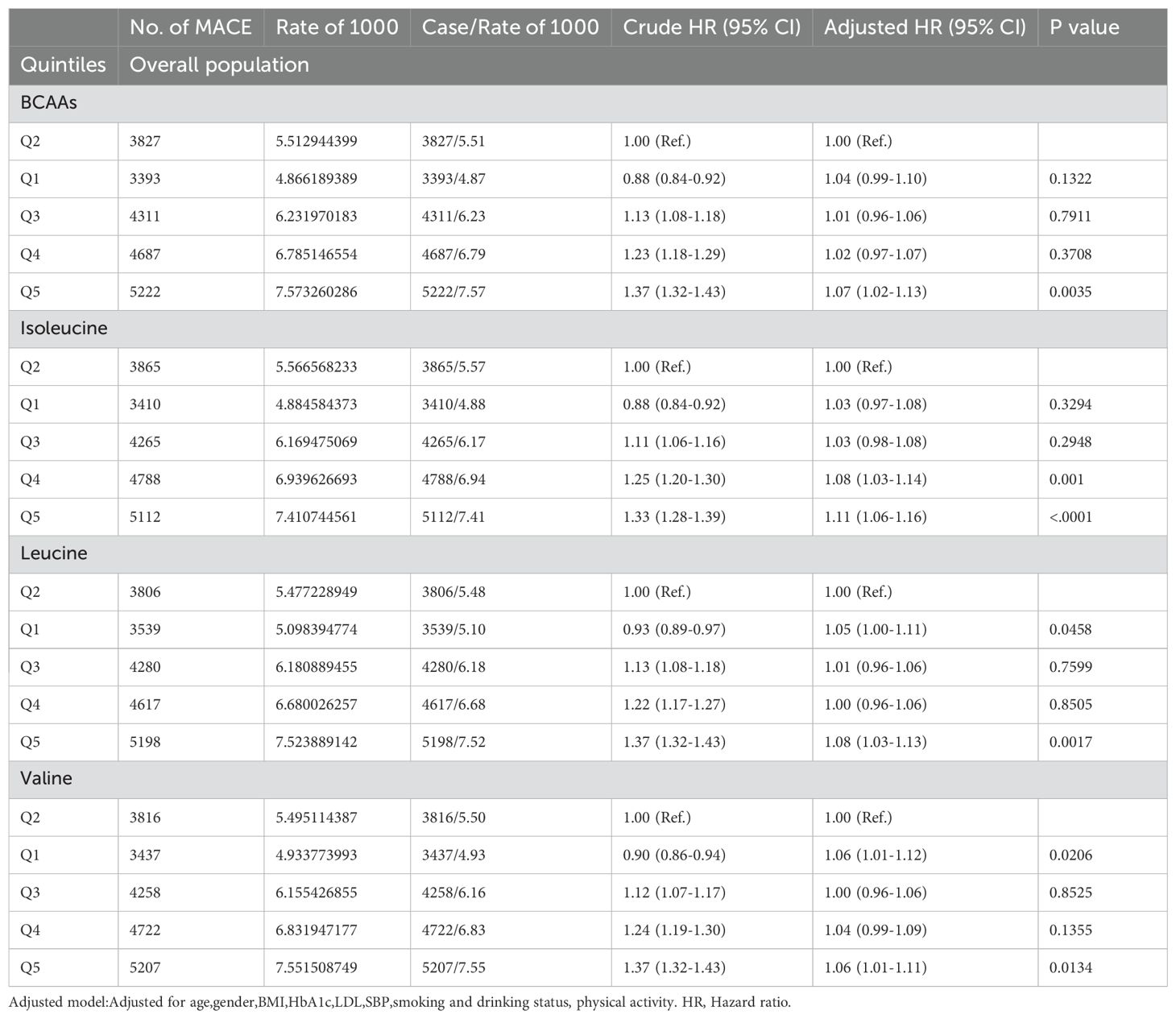
Table 2A. Hazard ratios for associations between MACE and BCAAs in overall population.
In males (Table 2B), BCAAs didn’t show any significant association with the risk of MACE. For isoleucine, the risk of mace increased by 8% (95% CI 1.02-1.15) in the fourth quintile and by 12% (95% CI 1.05-1.19) in the fifth quintile. For leucine, the risk of mace increased by 9% (95% CI 1.01-1.18) in the first quintile and 6% (95% CI 1.00-1.12) in the fifth quintile. For valine, the risk of mace increased by 8% (95% CI 1.00-1.16) in the first quintile.
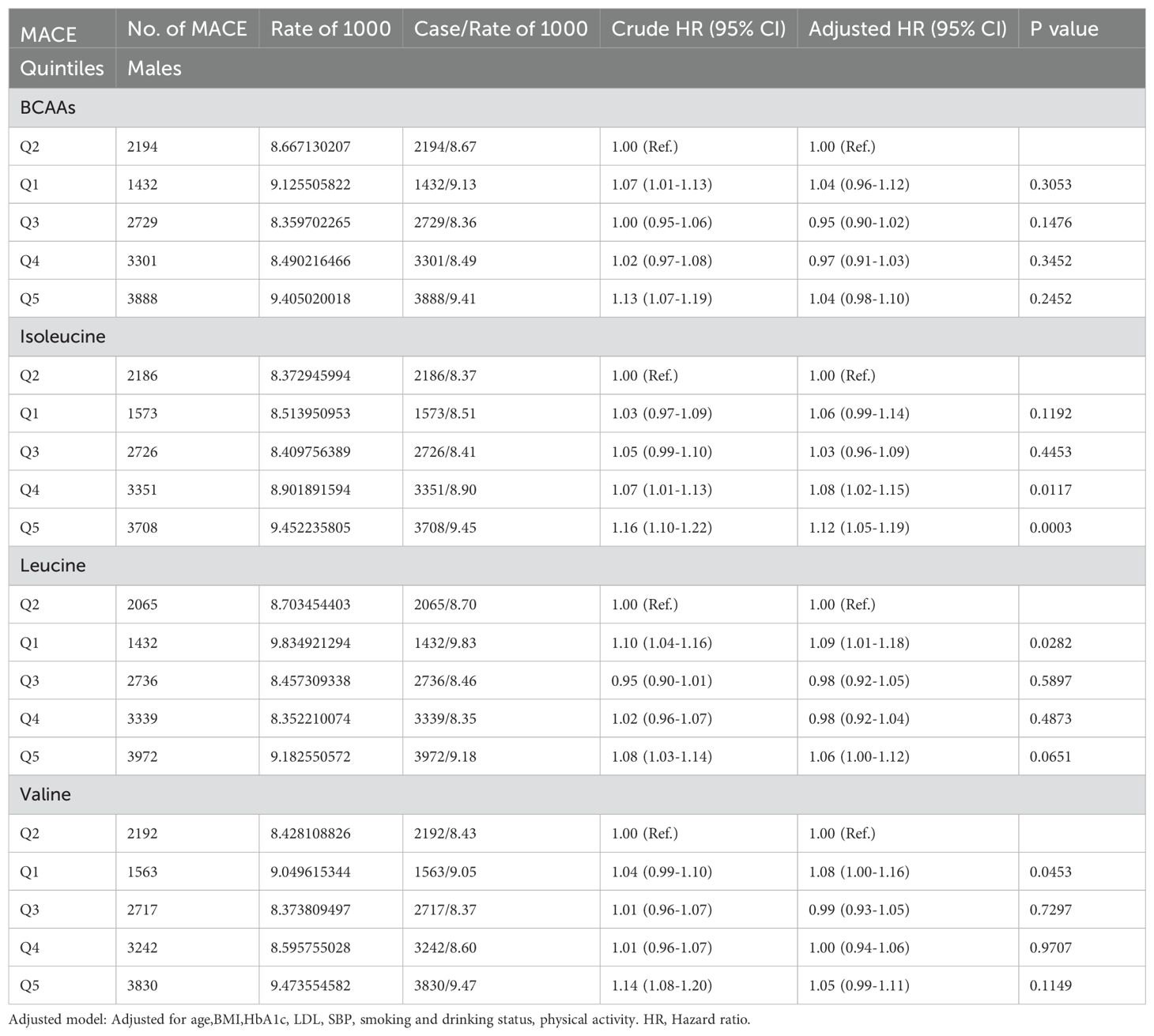
Table 2B. Hazard ratios for associations between MACE and BCAAs in males.
In females (Table 2C), compared to the second quintile, the third, fourth, and fifth quintiles of BCAAs had an increased risk of MACE by 9% (95% CI 1.00-1.18), 11% (95% CI 1.02-1.21) and 12% (95% CI 1.03-1.22), respectively. For isoleucine, the fourth and fifth quintiles showed increased risks of 10% (95% CI 1.01-1.19) and 9% (95% CI 1.00-1.18), respectively. The fifth quintile of leucine was associated with an 11% (95% CI 1.02-1.20) increased risk. For valine, the third quintile had an increased risk of MACE by 11% (95% CI 1.02-1.20). All findings were adjusted for several potential confounders.
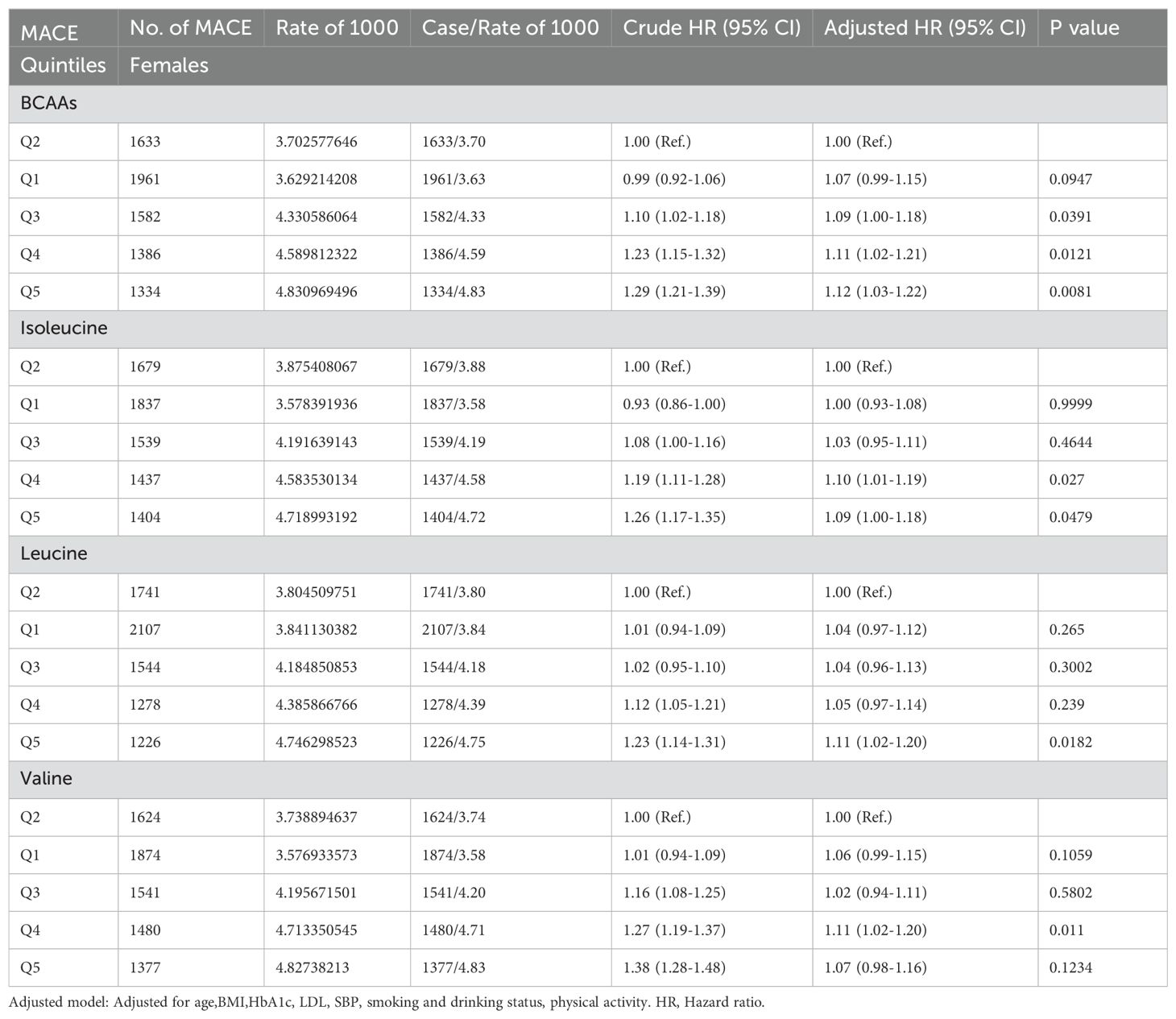
Table 2C. Hazard ratios for associations between MACE and BCAAs in females.
In participants younger than 65 (Table 2D), compared to the second quintile, the fifth quintile of BCAAs had an increased risk of MACE by 6% (95% CI 1.00-1.13). For isoleucine, the fourth and fifth quintiles showed increased risks of 8% (95% CI 1.02-1.15) and 12% (95% CI 1.06-1.19), respectively. The fifth quintile of leucine was associated with a 6% (95% CI 1.00-1.13) increased risk. Valine did not show an association with the risk of MACE.
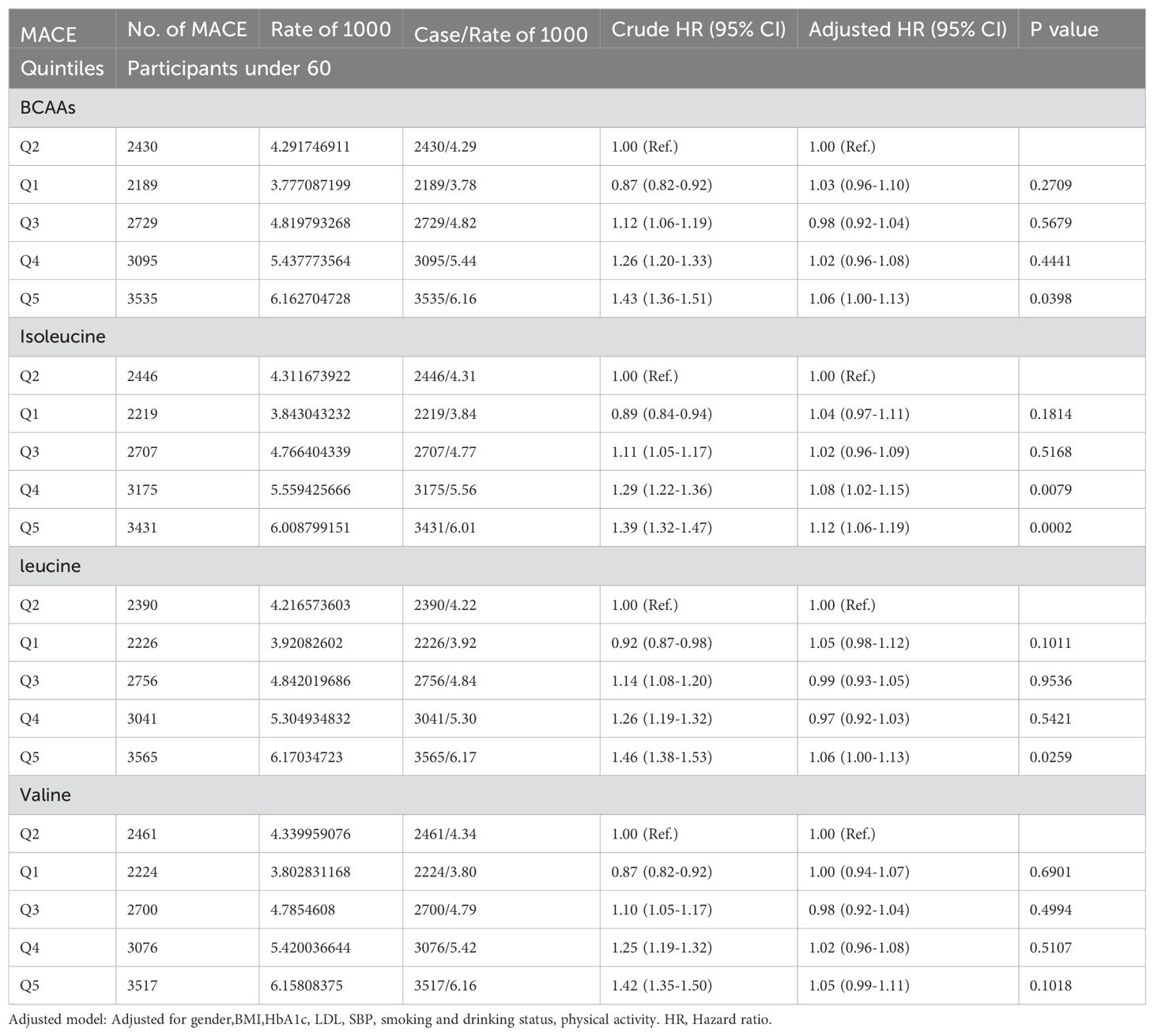
Table 2D. Hazard ratios for associations between MACE and BCAAs in participants under 60.
In participants older than 65 (Table 2E), neither BCAAs nor the individual amino acids—isoleucine, leucine, and valine—showed an association with MACE.
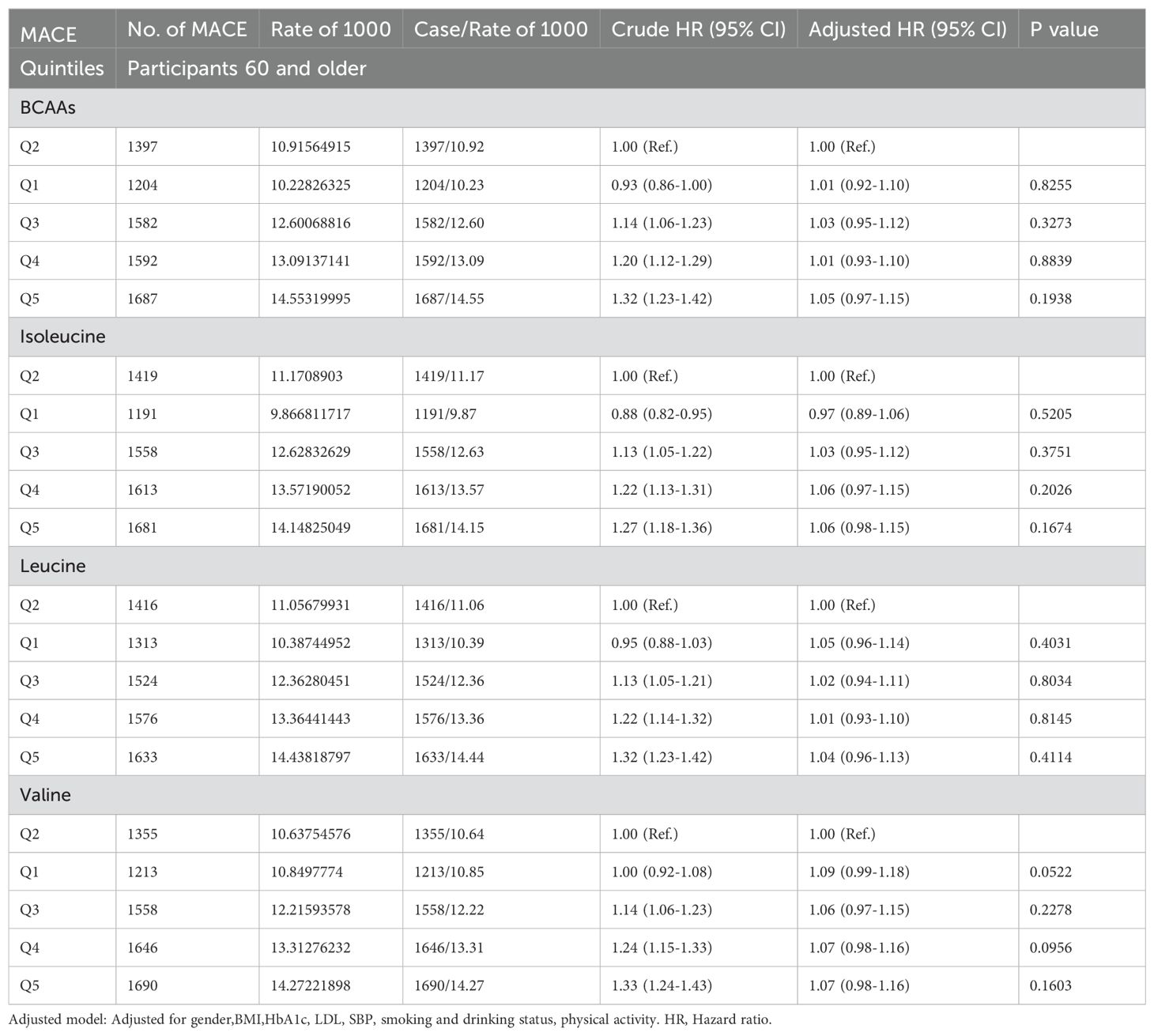
Table 2E. Hazard ratios for associations between MACE and BCAAs in 60 and older.
4 DiscussionIn this cohort study using the UK Biobank, individuals in the highest quintiles of BCAAs, isoleucine, leucine, and valine had an increased risk of future MACE,except for those aged over 65. This elevated risk was independent of age, gender, BMI, HbA1c, LDL, systolic blood pressure (SBP), smoking and drinking status, and activity level. Additionally, the lowest quintiles of isoleucine and valine also showed elevated MACE risk compared to the second quintiles. In males, higher quintiles of isoleucine, the first and fifth quintiles of leucine, and the first quintile of valine were associated with a higher future risk of MACE. In females, increased MACE risk was observed in the third, fourth, and fifth quintiles of BCAAs; the fourth and fifth quintiles of isoleucine; the fifth quintile of leucine; and the fourth quintile of valine. For individuals under 65, the highest quintiles of BCAAs, isoleucine, and leucine were also associated with a higher risk of MACE. However, in those aged 65 and older, no significant association was found between BCAAs and MACE.
Previous studies have also suggested that high levels of BCAAs are associated with a higher risk of CVD. A nested case-control study conducted by Olle Melander et al. involving 253 pairs of subjects used a baseline AA-score to assess future CVD risk (14). However, this study focused primarily on the DM-AA score and did not address specific amino acid levels. Additionally, the amino acid score only included isoleucine and aromatic amino acids. Another study using data from American and European cohorts indicated that certain amino acids and their gut metabolites might signal the risk of major adverse cardiovascular events (MACE), but it only involved aromatic amino acids (15). A study of 138 heart failure patients found that leucine, valine, and their derivatives predicted mortality risk better than NT-proBNP, though the impact of heart failure itself on amino acid levels remains unclear (13). A study of 700 European Americans (16) suggested a positive correlation between BCAA levels and coronary artery disease (CAD) risk. However, as a cross-sectional study, it could not establish causality. Conversely, a study of 2,346 African Americans found that high leucine levels were associated with a reduced risk of coronary heart disease. This discrepancy may be due to unique socioeconomic factors affecting African Americans, which could potentially influence a range of diseases, including cancer, anxiety, and cardiovascular conditions. Additionally, reduced BCAA levels could be associated with frailty, MACE, and mortality (17).
Our research also conducted subgroup analyses across different gender and age groups. The findings suggests that in males, high levels of isoleucine and leucine, as well as low levels of leucine and valine, are positively correlated with an increased risk of MACE. The subgroup analysis in females reveals that high levels of BCAAs, isoleucine, and leucine are positively correlated with the risk of MACE. Additionally, the fourth quintile of valine, rather than the fifth, was associated with an increased risk of MACE. However, a study involving 27,041 women suggests that the predictive ability of BCAAs for CVD is similar to that of LDL, with isoleucine, leucine, and valine all showing positive association with CVD (18). Additionally, a Finnish study found that isoleucine and leucine are positively associated with cardiovascular events in women, whereas valine did not exhibit a significant correlation (22). Consequently, the association between valine levels and MACE risk in females remains contentious.
The differences in results between genders may be due to the following reasons. Our study suggests that BCAAs are positively correlated with the risk of MACE in the general population. In males, BCAAs levels are negatively correlated with age(the β=-0.04558,p<0.001), meaning that BCAAs levels are lower in elderly males. This may be related to muscle catabolism, which significantly affects circulating BCAAs levels. Muscle atrophy in elderly males and the decrease in BCAAs levels may indicate a more frail state. Therefore, in males, BCAAs are “J”-shaped correlated with the risk of MACE. In contrast, In females included in our study, BCAAs level are positively correlated with age(the β=0.06863,p<0.001). We believed that females inherently have lower muscle mass, and the effect of age-related muscle atrophy on circulating BCAA levels is relatively small. This may explain the differing correlation between BCAAs and MACE risk across genders. Females inherently have lower muscle mass, and the effect of age-related muscle atrophy on circulating BCAA levels is relatively small.
The inconsistent results regarding parity between the US study and ours may be attributed to differences in study period, sample size, and population sociodemographic characteristics, particularly age distribution.
In the subgroup analysis by age, high levels of BCAAs, isoleucine, and leucine were positively correlated with MACE in individuals under 65 years old, but no such correlation was observed in those over 65. Limited research on BCAA levels and aging exists, but one study found that decreased BCAA levels in elderly men over 70 were associated with frailty, MACE, and mortality (23). This discrepancy might be due to a decline in BCAA levels with age (24–26) or frailty (27).
We conducted an analysis in men over 65y and found a J-shaped or J-like correlation between the incidence of MACE and the quintile levels of total BCAAs, isoleucine, leucine, and valine. This could be attributed to muscle catabolism, which has a substantial impact on circulating BCAA levels. The muscle atrophy observed in elderly men, along with the decline in BCAA levels, might signal a more frail state. As a result, in men, BCAAs exhibit a “J”-shaped correlation with the risk of MACE.
Compared to previous studies with smaller sample sizes, our study with a larger sample size more comprehensively demonstrates the correlation between BCAA levels and MACE in men. This does not conflict with previous studies but rather serves as a complement.
Research suggests that in cardiovascular diseases, BCAA metabolism is disrupted due to downregulation of Krüppel-like factor 15 (KLF15), mediated by TAK1 and p38 MAPK signaling (28, 29). This disruption leads to decreased expression of BCAA metabolic enzymes such as BCAT2 (30, 31), BCKDH (10, 12), and PPM1K (32), causing BCAAs and BCKAs to accumulate in the heart. Cardiac injury further disrupts BCAA metabolism in peripheral tissues, increasing circulating BCAA and BCKA levels and their delivery to the heart. Leucine activates mTOR in the heart, inhibiting autophagy through ULK1, promoting insulin resistance via S6K-mediated phosphorylation of insulin receptor substrate 1 (33), and stimulating protein synthesis by phosphorylating 4E-BP1 (34). BCKAs also increase 4E-BP1 phosphorylation and activate the MEK-ERK MAPK pathway, but impair mitochondrial complex I (3, 35), causing oxidative stress. In ischemia-reperfusion injury, Ppm1k deletion leads to BCAA and BCKA accumulation (36), worsening the injury by reducing glucose transport and oxidation (37). In obesity and insulin resistance, BCAA accumulation decreases fatty acid oxidation and increases triglyceride storage. Elevated BCKA levels in obesity and type 2 diabetes impair AKT (38) and PDH in the heart, affecting fuel selection. It’s unclear whether these metabolic changes cause cardiac dysfunction or if cardiac alterations impact BCAA metabolism in other tissues. BCAAs also hinder vascular relaxation through mTOR-dependent ROS generation (39, 40) and promote thrombosis by stimulating tropomodulin 3 propionylation. The valine-derived metabolite 3-HIB (41) increases lipid transport via FATP3 and FATP4. The impact of BCAA-lipid interactions on atherosclerosis is still uncertain.
Our investigation has several strengths. Firstly, it is one of the few large-scale prospective studies to systematically analyze the correlation between branched-chain amino acid levels and the risk of MACE across diverse populations. Secondly, it accounts for lifestyle factors such as glucose and lipid metabolism markers, smoking, alcohol consumption, and physical activity.
However, there are limitations to our study. Firstly, our results are limited to white European participants to avoid genetic heterogeneity, which may restrict the generalizability sof our findings to other ethnic groups. Secondly, the study did not include data on the metabolic products of amino acids and their potential impact on MACE. Future research could address this gap by testing serum samples for these metabolic products and analyzing their correlation with MACE. Lastly, the diagnosis of MACE was based on past medical history, which may have led to the omission of patients with asymptomatic cardiovascular disease.
5 ConclusionOur study consistently observed a positive association between BCAAs, as well as the individual amino acids, isoleucine, leucine, and valine, and the risk of MACE in the overall population. Additionally, a higher risk of MACE was found in females with the lowest quintile of valine and in individuals younger than 65 with the lowest quintile of isoleucine.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The UK Biobank data were accessed under application number 96511.
Ethics statementThe studies involving humans were approved by NHS National Research Ethics Service (16/NW/0274). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsWS: Formal analysis, Funding acquisition, Investigation, Writing – original draft. RL: Data curation, Validation, Writing – review & editing. YL: Resources, Supervision, Writing – review & editing. YEY: Data curation, Methodology, Supervision, Writing – review & editing. BL: Methodology, Supervision, Writing – review & editing. YFY: Data curation, Methodology, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82300954), China International Medical Exchange Foundation(No. KY2024-056) and Huashan Hospital Internal Startup Fund (No.2021QD023).
AcknowledgmentsThe authors would like to thank the staff at the UK Biobank for their efforts in designing, collecting, and organizing the UK Biobank data, as well as for creating the public database.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References3. White PJ, McGarrah RW, Herman MA, Bain JR, Shah SH, Newgard CB. Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Mol Metab. (2021) 52:101261. doi: 10.1016/j.molmet.2021.101261
PubMed Abstract | Crossref Full Text | Google Scholar
4. Hunter WG, Kelly JP, McGarrah RW 3rd, Khouri MG, Craig D, Haynes C, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc. (2016) 5. doi: 10.1161/JAHA.115.003190
PubMed Abstract | Crossref Full Text | Google Scholar
5. Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circulation. Cardiovasc Genet. (2010) 3:207–14. doi: 10.1161/CIRCGENETICS.109.852814
PubMed Abstract | Crossref Full Text | Google Scholar
6. Bhattacharya S, Granger CB, Craig D, Haynes C, Bain J, Stevens RD, et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. (2014) 232:191–6. doi: 10.1016/j.atherosclerosis.2013.10.036
PubMed Abstract | Crossref Full Text | Google Scholar
7. Flores-Guerrero JL, Groothof D, Connelly MA, Otvos JD, Bakker SJL, Dullaart RPF. Concentration of branched-chain amino acids is a strong risk marker for incident hypertension. Hypertension. (2019) 74:1428–35. doi: 10.1161/HYPERTENSIONAHA.119.13735
PubMed Abstract | Crossref Full Text | Google Scholar
8. Portero V, Nicol T, Podliesna S, Marchal GA, Baartscheer A, Casini S, et al. Chronically elevated branched chain amino acid levels are pro-arrhythmic. Cardiovasc Res. (2022) 118:1742–57. doi: 10.1093/cvr/cvab207
PubMed Abstract | Crossref Full Text | Google Scholar
9. Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Sci (New York N.Y.). (2016) 351:43–8. doi: 10.1126/science.aab2674
PubMed Abstract | Crossref Full Text | Google Scholar
10. Walejko JM, Christopher BA, Crown SB, Zhang GF, Pickar-Oliver A, Yoneshiro T, et al. Branched-chain α-ketoacids are preferentially reaminated and activate protein synthesis in the heart. Nat Commun. (2021) 12:1680. doi: 10.1038/s41467-021-21962-2
PubMed Abstract | Crossref Full Text | Google Scholar
11. Xu Y, Jiang H, Li L, Chen F, Liu Y, Zhou M, et al. Branched-chain amino acid catabolism promotes thrombosis risk by enhancing tropomodulin-3 propionylation in platelets. Circulation. (2020) 142:49–64. doi: 10.1161/CIRCULATIONAHA.119.043581
PubMed Abstract | Crossref Full Text | Google Scholar
12. Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. (2019) 29:417–29.e4. doi: 10.1016/j.cmet.2018.10.013
PubMed Abstract | Crossref Full Text | Google Scholar
13. Lanfear DE, Gibbs JJ, Li J, She R, Petucci C, Culver JA, et al. Targeted metabolomic profiling of plasma and survival in heart failure patients. JACC Heart Fail. (2017) 5:823–32. doi: 10.1016/j.jchf.2017.07.009
PubMed Abstract | Crossref Full Text | Google Scholar
14. Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engstrom G, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. (2013) 34:1982–9. doi: 10.1093/eurheartj/ehs424
PubMed Abstract | Crossref Full Text | Google Scholar
15. Nemet I, Li XS, Haghikia A, Li L, Wilcox J, Romano KA, et al. Atlas of gut microbe-derived products from aromatic amino acids and risk of cardiovascular morbidity and mortality. Eur Heart J. (2023) 44:3085–96. doi: 10.1093/eurheartj/ehad333
PubMed Abstract | Crossref Full Text | Google Scholar
16. Chevli PA, Freedman BI, Hsu FC, Xu J, Rudock ME, Ma L, et al. Plasma metabolomic profiling in subclinical atherosclerosis: the Diabetes Heart Study. Cardiovasc Diabetol. (2021) 20:231. doi: 10.1186/s12933-021-01419-y
PubMed Abstract | Crossref Full Text | Google Scholar
17. Cruz DE, Tahir UA, Hu J, Ngo D, Chen ZZ, Robbins JM, et al. Metabolomic analysis of coronary heart disease in an African American cohort from the jackson heart study. JAMA Cardiol. (2022) 7:184–94. doi: 10.1001/jamacardio.2021.4925
PubMed Abstract | Crossref Full Text | Google Scholar
18. Tobias DK, Lawler PR, Harada PH, Demler OV, Ridker PM, Manson JE, et al. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ Genom Precis Med. (2018) 11:e002157. doi: 10.1161/CIRCGEN.118.002157
PubMed Abstract | Crossref Full Text | Google Scholar
19. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. (2018) 562:203–09. doi: 10.1038/s41586-018-0579-z
PubMed Abstract | Crossref Full Text | Google Scholar
20. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PloS Med. (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
PubMed Abstract | Crossref Full Text | Google Scholar
21. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ sciences: BES. (2002) 15:83–96.
PubMed Abstract | Google Scholar
22. Wurtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. (2015) 131:774–85. doi: 10.1161/CIRCULATIONAHA.114.013116
PubMed Abstract | Crossref Full Text | Google Scholar
23. Le Couteur DG, Ribeiro R, Senior A, Hsu B, Hirani V, Blyth FM, et al. Branched chain amino acids, cardiometabolic risk factors and outcomes in older men: the concord health and ageing in men project. J Gerontol A Biol Sci Med Sci. (2020) 75:1805–10. doi: 10.1093/gerona/glz192
PubMed Abstract | Crossref Full Text | Google Scholar
24. Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M. Individual variability in human blood metabolites identifies age-related differences. Proc Natl Acad Sci United States America. (2016) 113:4252–9. doi: 10.1073/pnas.1603023113
PubMed Abstract | Crossref Full Text | Google Scholar
25. Kouchiwa T, Wada K, Uchiyama M, Kasezawa N, Niisato M, Murakami H, et al. Age-related changes in serum amino acids concentrations in healthy individuals. Clin Chem Lab Med. (2012) 50:861–70. doi: 10.1515/cclm-2011-0846
PubMed Abstract | Crossref Full Text | Google Scholar
27. Adachi Y, Ono N, Imaizumi A, Muramatsu T, Andou T, Shimodaira Y, et al. Plasma amino acid profile in severely frail elderly patients in Japan. Int J Gerontology. (2018) 12:290–93. doi: 10.1016/j.ijge.2018.03.003
留言 (0)