Intracranial pressure (ICP) refers to the pressure exerted by the contents within the cranial cavity against its walls. Elevated ICP, known as intracranial hypertension, can lead to brain tissue compression, resulting in ischemia, hypoxia, coma, brain dysfunction, or even fatality. This condition is a common cause of fatality in clinical settings (1). Predicting ICP elevation is challenging, and traditional invasive ICP monitoring techniques, such as lumbar puncture and craniotomy, require advanced physician skills and carry risks of complications including intracranial infection and hemorrhage (2). Consequently, there is significant clinical value in identifying a simple, rapid, and non-invasive method for assessing ICP changes. Recent literature indicates that ultrasound measurement of optic nerve sheath diameter (ONSD) can effectively assess ICP levels due to its simplicity, non-invasiveness, and ability to provide real-time, dynamic data (3). While current research has primarily examined the correlation between invasive ICP monitoring and ONSD measurements via ultrasound, studies on the impact of dynamic ONSD monitoring for evaluating ICP reduction through dehydration in patients with craniocerebral injuries are limited. The aim of this study is to explore the effectiveness of ONSD measurement by critical care bedside ultrasound in guiding ICP reduction through dehydration in such patients.
2 Data and methods 2.1 Clinical dataA total of 188 patients with craniocerebral injuries, admitted to the intensive care unit of our hospital between January 2021 and November 2022, were included in the study. The cohort comprised 116 males and 72 females, aged 18 to 88 years, with a mean age of 56 years. Diagnoses among these patients primarily included craniocerebral trauma, intracranial hemorrhage, subarachnoid hemorrhage, and extensive cerebral infarction. Exclusion criteria encompassed patients with glaucoma, severe ocular trauma, optic neuritis, optic nerve tumors, or other ocular diseases, as well as those with a hospital stay of < 24 h. Ultimately, 130 patients met the inclusion criteria.
2.2 Treatment methodsBoth patient groups were administered a standard dehydration regimen, which typically included mannitol at 0.5 g/kg every 8 h or hypertonic saline. The frequency of osmotic therapy could be adjusted based on clinical conditions, and furosemide and protein could be added as needed. Analgesia and sedation were managed strictly according to the Critical Care Pain Observation Tool (COPT) and the Richmond Agitation-Sedation Scale (RASS). Vital signs, blood routine, electrolytes, and other relevant indicators were continuously monitored. Fluid balance was carefully maintained, and active nerve nutritional therapy was provided.
In the test group, while patients received standard treatment, qualified physicians from the Department of Critical Care Medicine performed ONSD measurements using Sonosite ultrasound equipment (model: M-Turbo). Measurement procedure: Patients were placed in the supine position with eyes gently closed. A transparent film was placed over the eyelids, and a high-frequency probe, coated with an appropriate coupling agent, was held like a pen and positioned at the center of the eyelid for a transverse examination. This procedure visualized the optic nerve in its long axis, with the optic nerve sheath appearing as a strip-shaped hypoechoic structure extending from the optic nerve head into the cranium. ONSD was measured 3 mm behind the optic nerve head. Each eye was measured three times, and the average value was calculated. ONSD measurements were taken 30 min prior to the daily administration of mannitol or hypertonic saline. An ONSD ≥ 4.8 mm indicated elevated ICP, prompting immediate adjustment of the osmotic therapy to maintain the ONSD below the target value of < 4.8 mm (Figure 1).
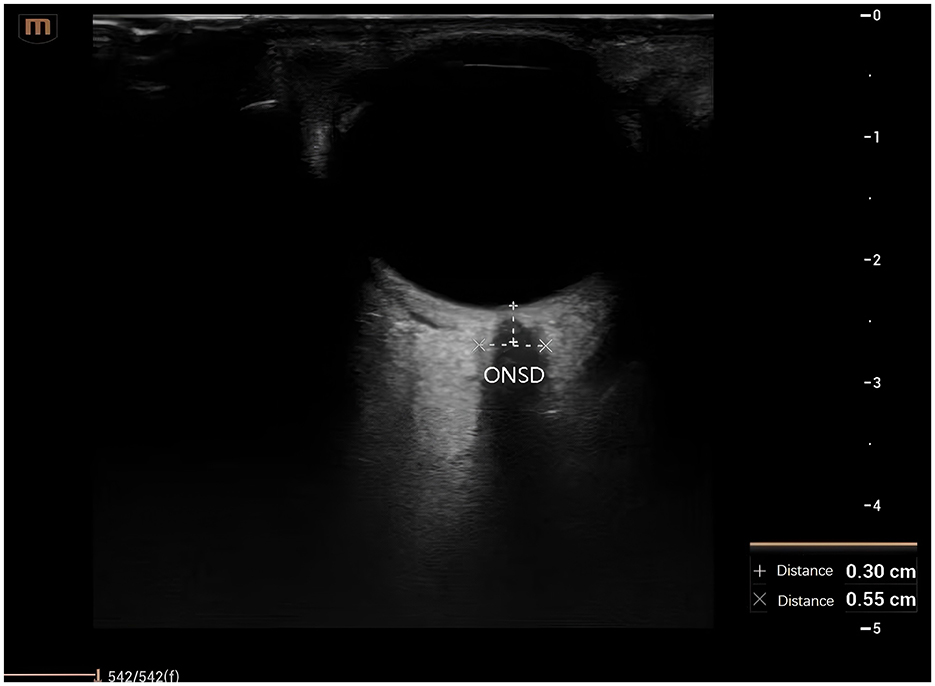
Figure 1. Ultrasound assessment and measurement of the optic nerve. ONSD, optic nerve sheath diameter.
In the control group, ICP was monitored using conventional methods, with ICP assessment based on clinical symptoms or brain CT imaging. Patients underwent brain CT scans at 12, 24, and 72 h post-surgery. Any clinical changes, such as anisocoria, deterioration in consciousness, headache, or alterations in cranial imaging (e.g., significant brain tissue swelling, midline shift, brainstem injury, or cisterna ambiens compression), indicated elevated ICP. In such cases, osmotic therapy regimen of the patient was adjusted empirically.
2.3 Observation indicators(1) APACHE II scores and Glasgow Coma Scale (GCS) scores of patients upon admission and 28 days after admission.
(2) Prognostic indicators for patients include the Glasgow Outcome Scale (GOS) and the treatment effectiveness rate.
(3) Additional evaluation indicators include the duration of ICU stay and the length of mechanical ventilation.
2.4 Criteria for determining patients' prognosis and efficacyIn this study, GOS was utilized to assess the prognosis of patients with craniocerebral injuries (4). The scale is categorized as follows: (1) Good (5 points) indicates that the patient has fully recovered and can return to normal work and study activities, with follow-up CT scans showing no abnormalities; (2) Moderate disability (4 points) denotes that the patient experiences some neurological symptoms but remains self-sufficient, with follow-up CT scans appearing largely normal; (3) Severe disability (3 points) implies that the patient maintains a clear conscious state but requires assistance from others, with follow-up CT scans showing improvement compared to previous results; (4) Vegetative survival (2 points) represents a state of coma or persistent vegetative state, with no improvement or deterioration in condition; (5) Fatality (1 point). A GOS score of ≤ 3 indicates an unfavorable prognosis, while a score of >3 reflects a favorable prognosis.
2.5 Statistical processingData analysis was conducted using SPSS version 23.0. Measurement data, which met the criteria for normal distribution and homogeneity of variance, were expressed as mean ± standard deviation (). Comparisons between two groups were performed using paired t-tests or independent samples t-tests. Categorical data were presented as frequencies (n) or percentages (%), and comparisons between groups were made using the χ2 test. A P < 0.05 was considered indicative of statistical significance.
3 Results 3.1 Comparison of APACHE II score and GCS score between the two groups on admission and 28d after admissionAt admission, there were no statistical differences in APACHE II scores and GCS scores between the two groups of patients with intracranial hypertension (P > 0.05). Compared to admission values, both groups exhibited significant reductions in APACHE II scores and significant increases in GCS scores, with these changes being statistically significant (P < 0.05). Twenty-eight days after admission, the observation group showed a more pronounced decrease in APACHE II scores and a greater increase in GCS scores compared to the control group, with these differences also being statistically significant (P < 0.05; see Table 1).
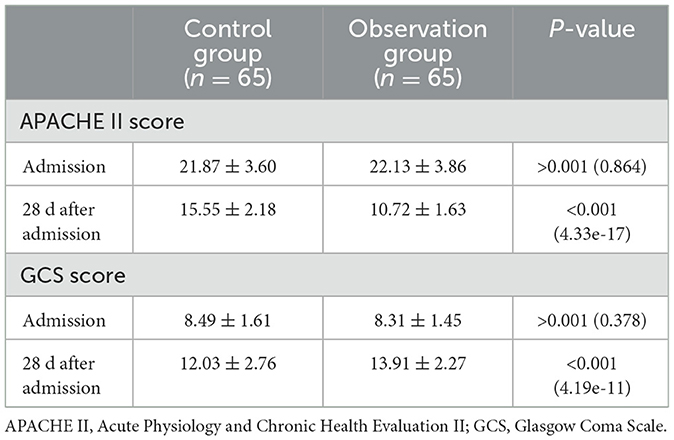
Table 1. Comparison of APACHE II scores and GCS scores between the two patient groups at admission and 28 days after admission.
3.2 Comparison of ICU stay time and mechanical ventilation time between the two groupsCompared to the control group, the observation group experienced a significantly shorter duration of ICU stay and mechanical ventilation time, with these differences being statistically significant (see Table 2).
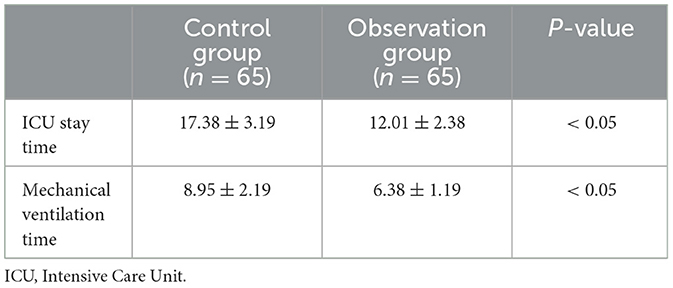
Table 2. Comparison of ICU length of stay and duration of mechanical ventilation between the two patient groups.
3.3 Comparison of GOS between the two groupsIn comparison to the control group, the observation group had a significantly higher proportion of patients with a favorable prognosis, with the difference being statistically significant (P < 0.05). Additionally, the proportions of severe disability and vegetative survival were significantly lower in the observation group, with these differences also being statistically significant (P < 0.05). No statistical differences were observed between the two groups regarding moderate disability, mortality, and overall treatment effectiveness (see Table 3).
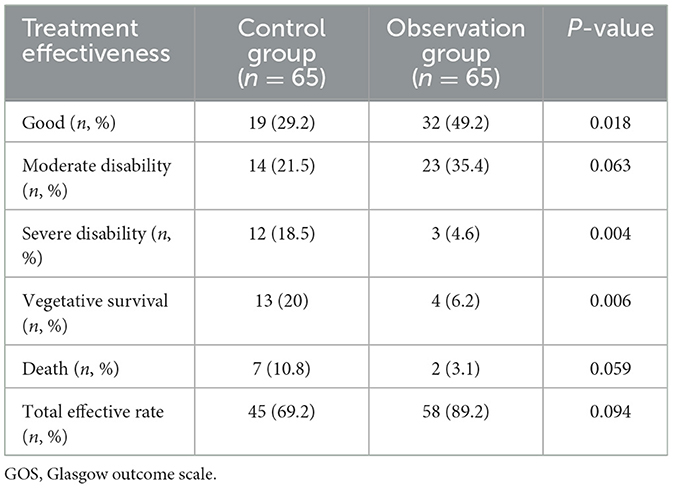
Table 3. Comparison of GOS scores between the two patient groups.
4 DiscussionElevated ICP can alter cerebral perfusion pressure and cerebral blood flow, leading to cerebral ischemia, hypoxia, and potential brain herniation, which significantly impacts prognosis of patient. Effective dehydration to reduce ICP can improve patient conditions and enhance survival rates (5). Consequently, employing an effective dehydration regimen is crucial. The aim of this study was to compare the effectiveness of dehydration regimens guided by clinical manifestations or intracranial imaging versus those guided by ONSD measurements in patients with intracranial hypertension, to identify an optimal dehydration strategy for managing this condition (6).
ICP is challenging to predict and can progress rapidly. In current clinical practice, ICP is primarily assessed using both invasive and non-invasive methods, with invasive monitoring being considered the gold standard for diagnosing elevated ICP (7). Invasive techniques include lumbar puncture, ventricular manometry, and similar procedures. Non-invasive methods encompass cranial CT, MRI, evoked potentials, and transcranial Doppler ultrasonography. However, invasive tests are complex and carry risks of complications such as infection, hemorrhage, and brain damage, which may exacerbate the condition of patient (8). Non-invasive clinical methods also have limitations, including the need for patient transfer, high subjectivity, or lack of available equipment. Bedside ultrasound, as a non-invasive diagnostic tool, offers significant advantages due to its ability to provide continuous monitoring and better diagnostic value (9).
The subarachnoid space of the optic nerve is in continuous communication with the cerebrospinal fluid (CSF) in the cranial subarachnoid space, and the optic nerve sheath is an extension of the dura mater. A transverse subarachnoid space allows for the gradual drainage of CSF (10). When ICP rises, CSF leakage from the arachnoid can lead to persistent dilation of the interstitial space surrounding the optic nerve. This dilation reduces blood flow, impairs circulation, causes venous stasis, and results in an increase in ONSD (11, 12).
Research has shown that assessing ONSD can offer a more accurate evaluation of intracranial hypertension (13). Bedside measurement of ONSD using critical care ultrasound has proven to be highly effective and feasible for diagnosing intracranial hypertension (14). Furthermore, patients with intracranial hypertension admitted to the ICU typically present with higher APACHE II scores and lower GCS scores. Following dehydration aimed at reducing ICP, restoring consciousness, and providing cranial nerve support, both APACHE II scores and GCS scores improved, making these metrics valuable for evaluating the severity of intracranial hypertension. In this study, APACHE II scores in both the observation and control groups were significantly lower, and GCS scores were significantly higher 28 days after admission compared to initial scores, indicating the effectiveness of both dehydration regimens based on conventional monitoring and ONSD monitoring. However, the APACHE II score in the observation group was notably lower and the GCS score notably higher compared to the control group after 28 days, suggesting that the ONSD-based regimen was more effective. In clinical practice, conventional dehydration regimens based on clinical symptoms or CT imaging lack real-time monitoring and exhibit greater subjectivity. In contrast, ONSD examination enables real-time dynamic monitoring at the bedside, allowing for timely and accurate adjustments to dehydration treatment while minimizing external interference, thereby enhancing therapeutic outcomes.
The GOS score is a key indicator for assessing the prognosis of patients with craniocerebral injuries. According to this study, the proportion of patients with a favorable prognosis in the observation group was higher compared to the control group. Additionally, the observation group had a significantly reduced proportion of severe disability and vegetative survival. The ICU stay duration and mechanical ventilation time were also significantly shorter in the observation group than in the control group. These findings suggest that modifying the dehydration regimen based on ONSD measurements can enhance treatment efficacy and improve prognosis of patient.
This study further validates the effectiveness and clinical utility of critical care ultrasound for measuring ONSD in diagnosing and managing intracranial hypertension. However, there are several limitations to consider. First, no statistically significant differences in fatality rates and overall treatment efficacy between the two patient groups were observed in this study. This lack of difference may be attributed to the relatively small sample size, suggesting that further research with a larger cohort is needed to address this issue. Second, the ultrasound measurement of ONSD involves a degree of subjectivity. Despite the operators being blinded to intracranial pressure values and adhering to standardized measurement procedures, variability in the interpretation of high-density and low-density shadows of the optic nerve sheath among different operators remains a potential source of error. This subjectivity may impact the broader clinical adoption of ultrasound-based ONSD monitoring (15–17). To address the limitations of B-scan ultrasound, the Standardized A-scan ultrasound technique has been proposed. This technique provides a more objective and reproducible method for measuring ONSD by analyzing the amplitude of the reflected sound waves. Studies have shown that A-scan ultrasound can reduce inter-operator variability and improve the accuracy of ONSD measurements. Future research should aim to validate the use of A-scan ultrasound in clinical settings and compare its performance with B-scan ultrasound.
5 ConclusionDynamic monitoring of ONSD using bedside critical care ultrasound has proven to be an effective tool in guiding osmotic therapy for patients with intracranial hypertension. This approach significantly reduces ICP and provides a reliable basis for subsequent treatment decisions, potentially lowering the rate of disability and improving patient prognosis.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThis study was conducted in accordance with the declaration of Helsinki and with approval from the Ethics Committee of Affiliated Hospital of Hebei University (HDFYLL-KY-2024-139). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsNZ: Conceptualization, Data curation, Funding acquisition, Writing – original draft. ML: Formal analysis, Writing – review & editing. TSh: Data curation, Formal analysis, Writing – review & editing. NL: Formal analysis, Writing – review & editing. KW: Data curation, Formal analysis, Writing – review & editing. SS: Conceptualization, Data curation, Writing – review & editing. TSu: Conceptualization, Formal analysis, Writing – original draft.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant from the therapeutic value of real-time monitoring of optic nerve sheath width by severe ultrasound in patients with severe traumatic brain injury (2021Q007).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsGCS, Glasgow coma scale; GOS, Glasgow outcome scale; ONSD, Optic nerve sheath diameter; APACHE II, A cute physiology and chronic health evaluation II; ICP, Intracranial pressure.
References1. Shi J, Tan L, Ye J, Hu L. Hypertonic saline and mannitol in patients with traumatic brain injury: a systematic and meta-analysis. Medicine. (2020) 99:e21655. doi: 10.1097/MD.0000000000021655
PubMed Abstract | Crossref Full Text | Google Scholar
2. Fernando SM, Tran A, Cheng W, Rochwerg B, Taljaard M, Kyeremanteng K, et al. Diagnosis of elevated intracranial pressure in critically ill adults: systematic review and meta-analysis. BMJ. (2019) 366:l4225. doi: 10.1136/bmj.l4225
PubMed Abstract | Crossref Full Text | Google Scholar
3. Gupta N, Singh VK, Jafa S. Correlation of positive end-expiratory and intracranial pressure using the ultrasonographic-guided measurement of optic nerve sheath diameter in traumatic brain injury patients. Neurol India. (2021) 69:1670–4. doi: 10.4103/0028-3886.333532
PubMed Abstract | Crossref Full Text | Google Scholar
4. Reith FC, Van den Brande R, Synnot A, Gruen R, Maas AI. The reliability of the glasgow coma scale: a systematic review. Intensive Care Med. (2016) 42:3–15. doi: 10.1007/s00134-015-4124-3
PubMed Abstract | Crossref Full Text | Google Scholar
5. Jahns FP, Miroz JP, Messerer M, Daniel RT, Taccone FS, Eckert P, et al. Quantitative pupillometry for the monitoring of intracranial hypertension in patients with severe traumatic brain injury. Crit Care. (2019) 23:155. doi: 10.1186/s13054-019-2436-3
PubMed Abstract | Crossref Full Text | Google Scholar
6. Radu RA, Terecoasă EO, Tiu C, Ghi?ă C, Nicula AI, Marinescu AN, et al. Neutrophil-to-lymphocyte ratio as an independent predictor of in-hospital mortality in patients with acute intracerebral hemorrhage. Medicina. (2021) 57:622. doi: 10.3390/medicina57060622
PubMed Abstract | Crossref Full Text | Google Scholar
7. Zhu Z, Waxman S, Wang B, Wallace J, Schmitt SE, Tyler-Kabara E, et al. Interplay between intraocular and intracranial pressure effects on the optic nerve head in vivo. Exp Eye Res. (2021) 213:108809. doi: 10.1016/j.exer.2021.108809
PubMed Abstract | Crossref Full Text | Google Scholar
9. Su L, Li YC, Yu QL, Wang DH, Chen SM. The relationship between the diameter of the optic nerve sheath and intracranial pressure measured by bedside ultrasound and CT reconstruction. Chin J CT MRI. (2020) 18:16–8. doi: 10.3969/j.issn.1672-5131.2020.01.006
Crossref Full Text | Google Scholar
10. Robba C, Cardim D, Tajsic T, Pietersen J, Bulman M, Donnelly J, et al. Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: a prospective observational study. PLoS Med. (2017) 14:e1002356. doi: 10.1371/journal.pmed.1002356
PubMed Abstract | Crossref Full Text | Google Scholar
11. Hanafi MG, Verki MM, Parei SN. Ultrasonic assessment of optic nerve sheath to detect increased intracranial pressure. J Med Ultrasound. (2019) 27:69–74. doi: 10.4103/JMU.JMU_54_18
PubMed Abstract | Crossref Full Text | Google Scholar
12. Montorfano L, Giambartolomei G, Funes DR, Lo Menzo E, Dip F, White KP, et al. The cushing reflex and the vasopressin-mediated hemodynamic response to increased intracranial pressure during acute elevations in intraabdominal pressure. Surgery. (2020) 167:478–83. doi: 10.1016/j.surg.2019.10.006
PubMed Abstract | Crossref Full Text | Google Scholar
13. Robba C, Santori G, Czosnyka M, Corradi F, Bragazzi N, Padayachy L, et al. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. (2018) 44:1284–94. doi: 10.1007/s00134-018-5305-7
PubMed Abstract | Crossref Full Text | Google Scholar
14. Beare NA, Kampondeni S, Glover SJ, Molyneux E, Taylor TE, Harding SP, et al. Detection of raised intracranial pressure by ultrasound measurement of optic nerve sheath diameter in African children. Trop Med Int Health. (2008) 13:1400–4. doi: 10.1111/j.1365-3156.2008.02153.x
PubMed Abstract | Crossref Full Text | Google Scholar
15. De Bernardo M, Vitiello L, Rosa N. Optic nerve sheath diameter ultrasonography in differentiation of ischemic and hemorrhagic strokes. Am J Emerg Med. (2019) 37:1384–5. doi: 10.1016/j.ajem.2018.12.048
PubMed Abstract | Crossref Full Text | Google Scholar
16. De Bernardo M, Vitiello L, De Pascale I, Capasso L, Cornetta P, Rosa N. Optic nerve ultrasound evaluation in idiopathic intracranial hypertension. Front Med. (2022) 9:845554. doi: 10.3389/fmed.2022.845554
PubMed Abstract | Crossref Full Text | Google Scholar
17. De Bernardo M, Vitiello L, Capone M, Rosa N. A-scan ultrasonography and optic nerve sheath diameter evaluation in children with acute liver failure. Liver Int. (2020) 40:1504. doi: 10.1111/liv.14372
留言 (0)